Ductility Control via Nano-Precipitation at Grain Boundaries in Ti-Zr-Hf-Nb-Ta Multi-Principal Element Alloys
Abstract
1. Introduction
2. Materials and Methods
3. Results
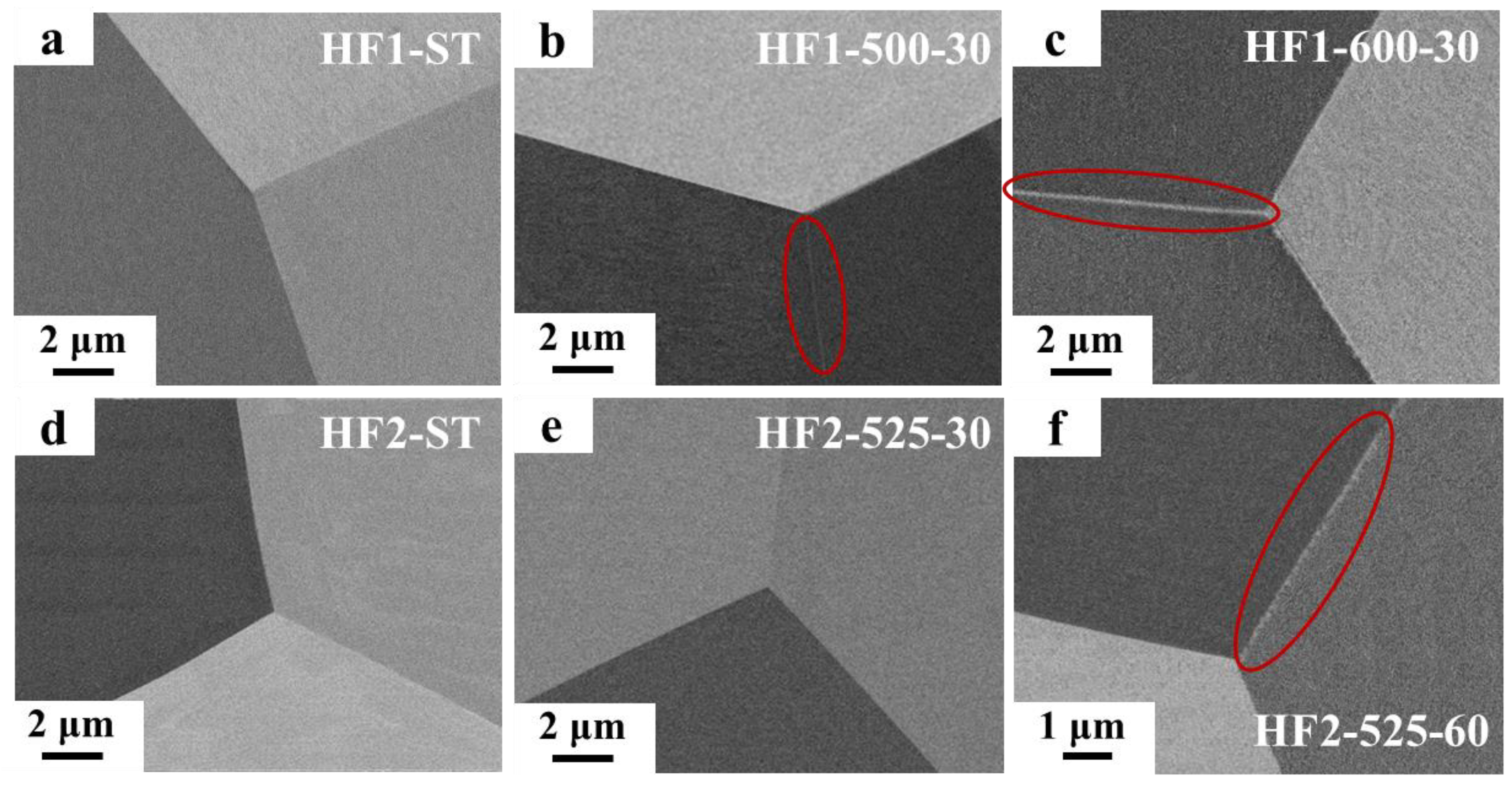
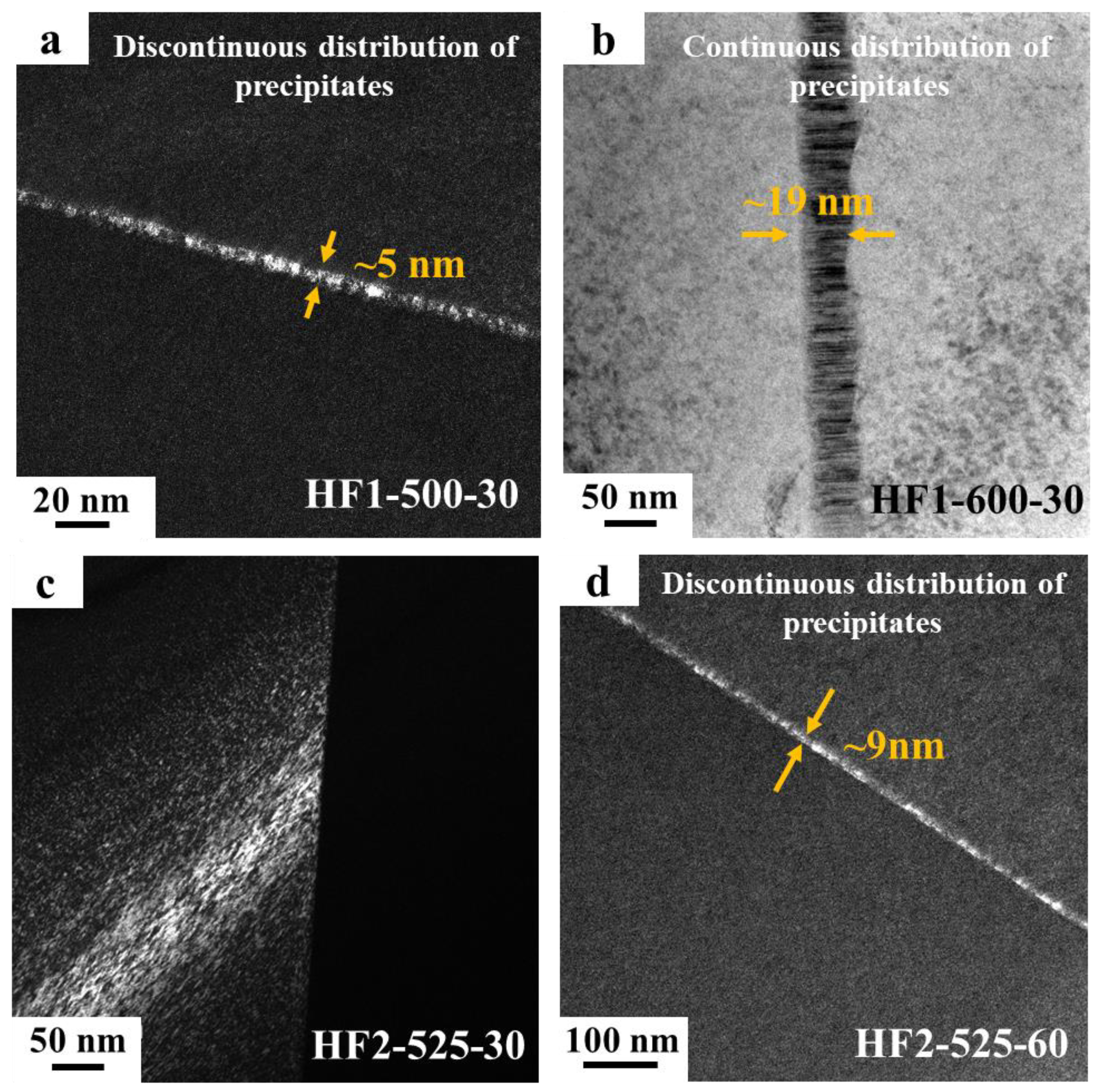
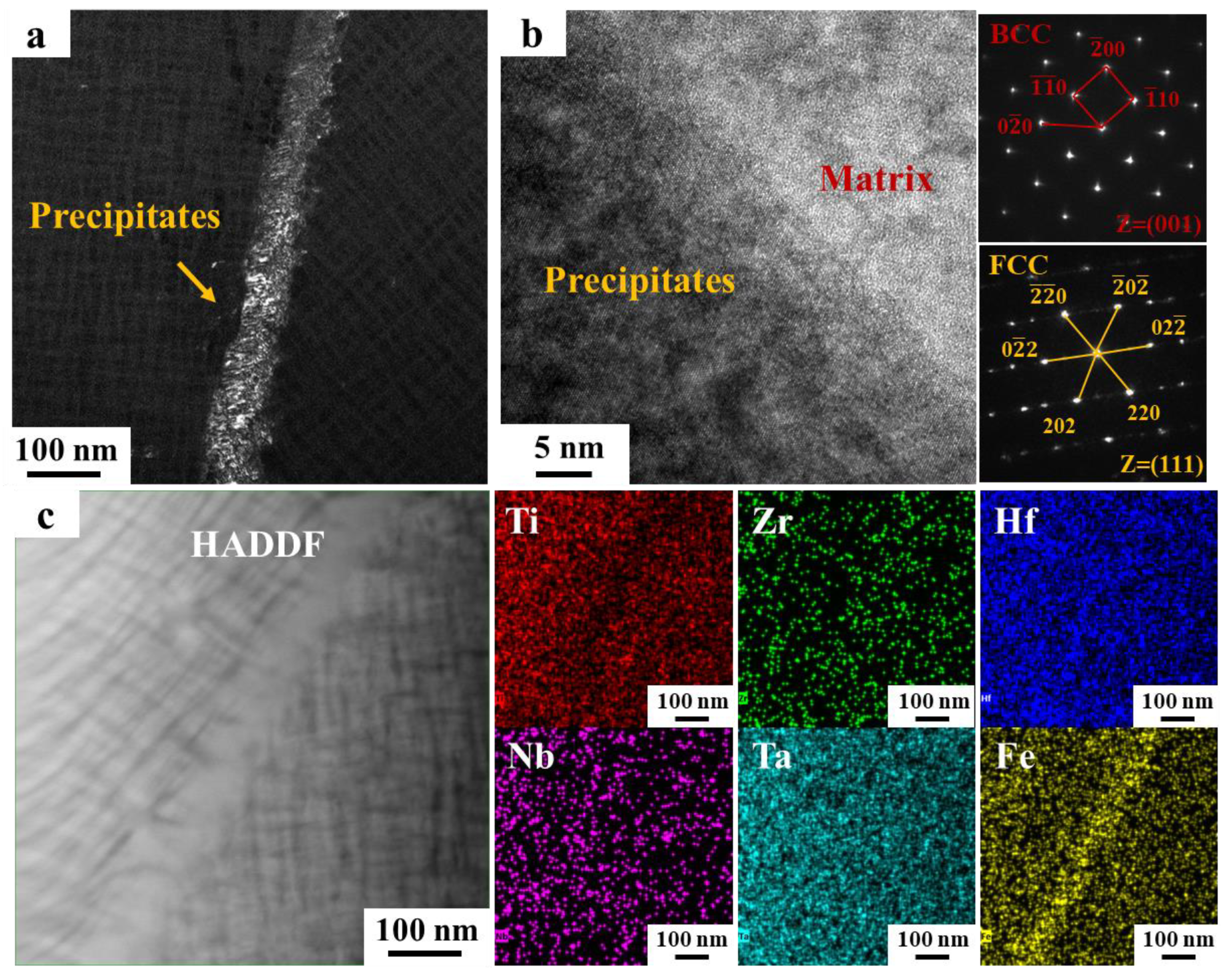
3.1. Microstructural Characterization
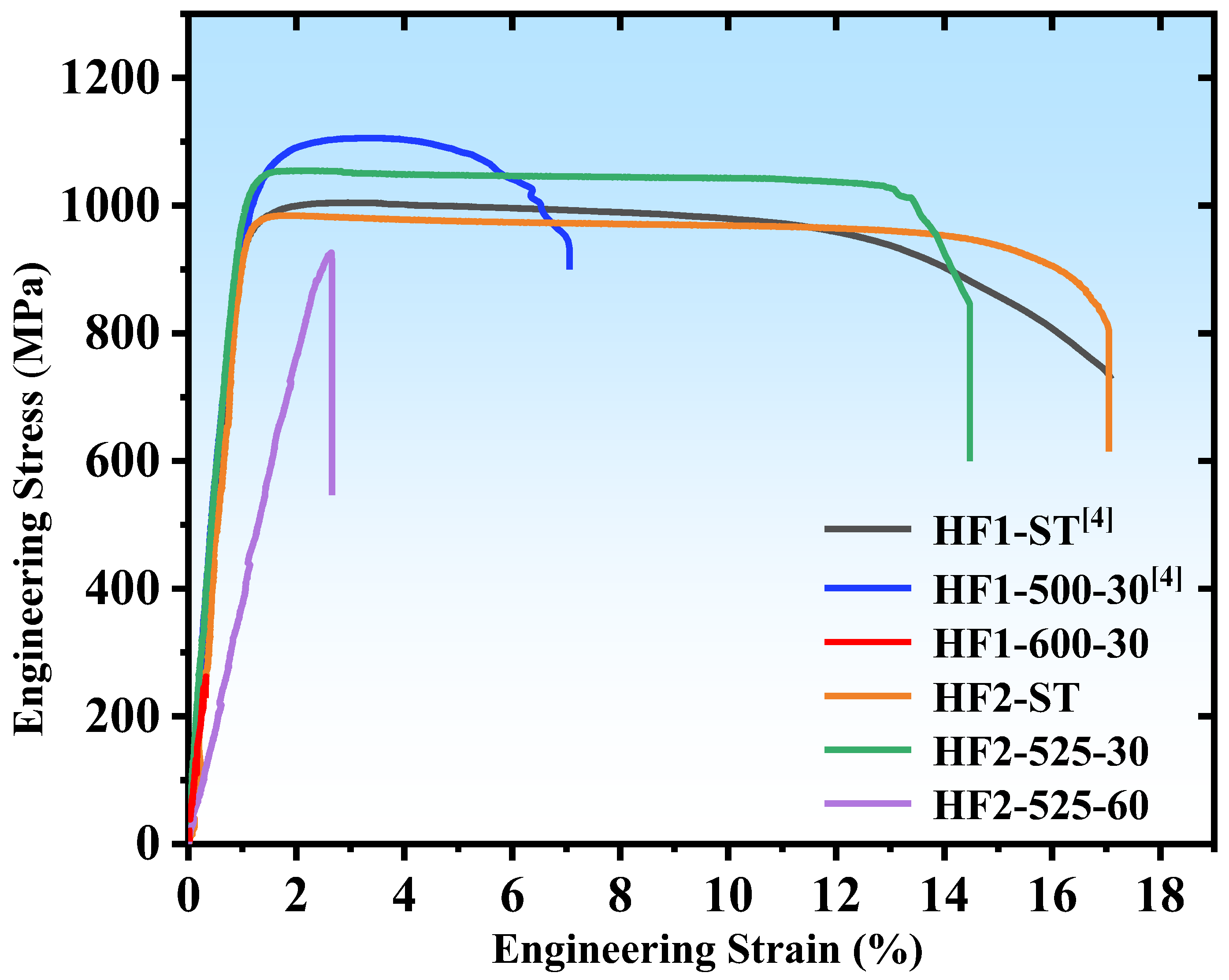
3.2. Mechanical Property
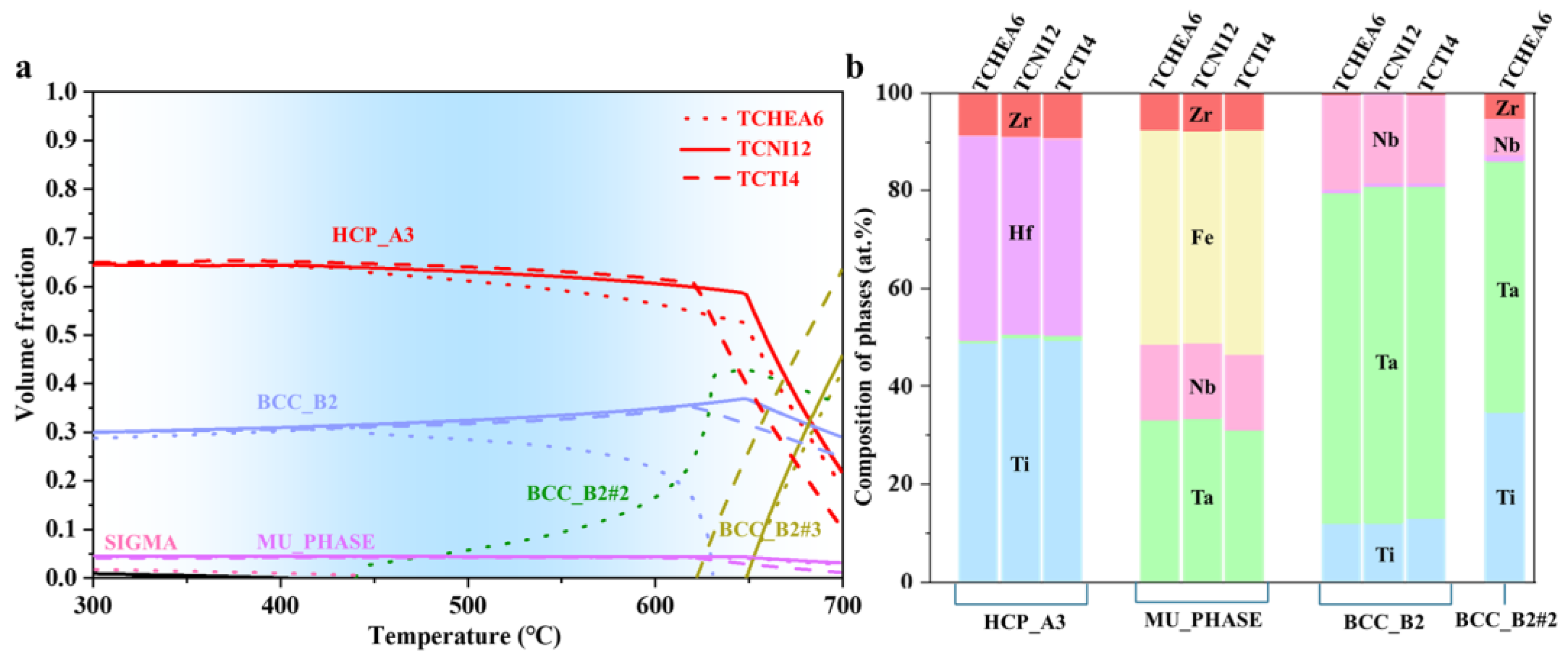
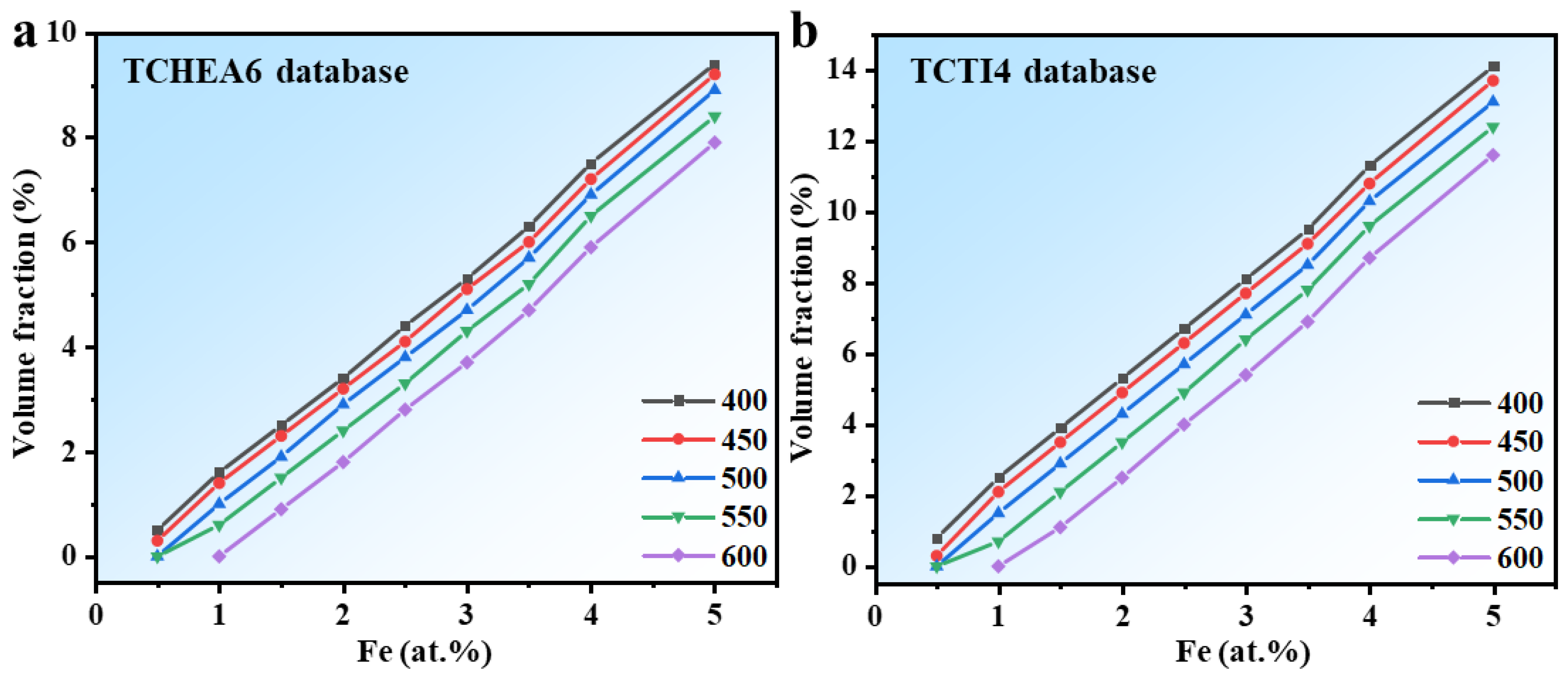
3.3. Thermodynamic Calculation
4. Discussion
4.1. The Influence of Nano-Sized Precipitates on the Mechanical Properties
4.2. Effect of Thermodynamics and Kinetics on Precipitation Evolution
5. Conclusions
- (1)
- The size and distribution of nano-sized precipitates at the grain boundaries are sensitive to both alloy composition and heat treatment parameters and have a critical impact on the mechanical properties of MPEAs. By slightly adjusting the alloy composition (0.15 at.%) and varying the heat treatment parameters, a series of alloys with a wide range of elongation from 2.65% to 19% were developed, while maintaining alloy strengths between 955 and 1081 MPa, providing design flexibility for diverse applications.
- (2)
- The formation of nano-sized precipitates below the TEM detection threshold plays a crucial role in balancing the strength and ductility. The HF2-525-30 alloy, in which no precipitates were observed in either SEM or TEM, exhibited a balanced combination of 1037 MPa strength and approximately 14% elongation.
- (3)
- Thermodynamic calculations using Thermo-Calc, validated against experimental data, provided reliable phase stability information. By selecting specified phases (BCC, FCC, NITI2) within the TCHEA6, TCTI4, and TCNI12 databases, the equilibrium solute products of the Hf2Fe phase at 400–600 °C were obtained, along with the variation of its equilibrium volume fraction with Fe content at different temperatures.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yeh, J.-W.; Chen, S.-K.; Lin, S.-J.; Gan, J.-Y.; Chin, T.-S.; Shun, T.-T.; Tsau, C.-H.; Chang, S.-Y. Nanostructured High-Entropy Alloys with Multiple Principal Elements: Novel Alloy Design Concepts and Outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Cantor, B.; Chang, I.T.H.; Knight, P.; Vincent, A.J.B. Microstructural Development in Equiatomic Multicomponent Alloys. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process. 2004, 375, 213–218. [Google Scholar] [CrossRef]
- Tsai, M.-H.; Yeh, J.-W. High-Entropy Alloys: A Critical Review. Mater. Res. Lett. 2014, 2, 107–123. [Google Scholar] [CrossRef]
- Liu, F.; Wang, B.; Wang, L.; Xue, Y. Solid-Solution Strengthening of Ti-Zr-Hf-Nb-Ta-Fe Refractory High-Entropy Alloy. J. Phys. Conf. Ser. 2022, 2383, 012141. [Google Scholar] [CrossRef]
- Colombini, E.; Rosa, R.; Trombi, L.; Zadra, M.; Casagrande, A.; Veronesi, P. High Entropy Alloys Obtained by Field Assisted Powder Metallurgy Route: SPS and Microwave Heating. Mater. Chem. Phys. 2018, 210, 78–86. [Google Scholar] [CrossRef]
- Bracq, G.; Laurent-Brocq, M.; Varvenne, C.; Perriere, L.; Curtin, W.A.; Joubert, J.-M.; Guillot, I. Combining Experiments and Modeling to Explore the Solid Solution Strengthening of High and Medium Entropy Alloys. Acta Mater. 2019, 177, 266–279. [Google Scholar] [CrossRef]
- Lu, Y.; Huang, H.; Gao, X.; Ren, C.; Gaob, J.; Zhang, H.; Zheng, S.; Jin, Q.; Zhao, Y.; Lu, C.; et al. A Promising New Class of Irradiation Tolerant Materials: Ti2ZrHfV0.5Mo0.2 High-Entropy Alloy. J. Mater. Sci. Technol. 2019, 35, 369–373. [Google Scholar] [CrossRef]
- Stepanov, N.; Tikhonovsky, M.; Yurchenko, N.; Zyabkin, D.; Klimova, M.; Zherebtsov, S.; Efimov, A.; Salishchev, G. Effect of Cryo-Deformation on Structure and Properties of CoCrFeNiMn High-Entropy Alloy. Intermetallics 2015, 59, 8–17. [Google Scholar] [CrossRef]
- Lei, Z.; Liu, X.; Wu, Y.; Wang, H.; Jiang, S.; Wang, S.; Hui, X.; Wu, Y.; Gault, B.; Kontis, P.; et al. Enhanced Strength and Ductility in a High-Entropy Alloy via Ordered Oxygen Complexes. Nature 2019, 565, 546. [Google Scholar] [CrossRef]
- Gludovatz, B.; Hohenwarter, A.; Catoor, D.; Chang, E.H.; George, E.P.; Ritchie, R.O. A Fracture-Resistant High-Entropy Alloy for Cryogenic Applications. Science 2014, 345, 1153–1158. [Google Scholar] [CrossRef]
- George, E.P.; Curtin, W.A.; Tasan, C.C. High Entropy Alloys: A Focused Review of Mechanical Properties and Deformation Mechanisms. Acta Mater. 2020, 188, 435–474. [Google Scholar] [CrossRef]
- Zaddach, A.J.; Scattergood, R.O.; Koch, C.C. Tensile Properties of Low-Stacking Fault Energy High-Entropy Alloys. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process. 2015, 636, 373–378. [Google Scholar] [CrossRef]
- Licavoli, J.J.; Gao, M.C.; Sears, J.S.; Jablonski, P.D.; Hawk, J.A. Microstructure and Mechanical Behavior of High-Entropy Alloys. J. Mater. Eng. Perform. 2015, 24, 3685–3698. [Google Scholar] [CrossRef]
- Wu, Z.; Bei, H.; Pharr, G.M.; George, E.P. Temperature Dependence of the Mechanical Properties of Equiatomic Solid Solution Alloys with Face-Centered Cubic Crystal Structures. Acta Mater. 2014, 81, 428–441. [Google Scholar] [CrossRef]
- He, Q.; Yoshida, S.; Yasuda, H.; Tsuji, N. Effect of Elemental Combination on Microstructure and Mechanical Properties of Quaternary Refractory Medium Entropy Alloys. Mater. Trans. 2020, 61, 577–586. [Google Scholar] [CrossRef]
- Juan, C.-C.; Tsai, M.-H.; Tsai, C.-W.; Hsu, W.-L.; Lin, C.-M.; Chen, S.-K.; Lin, S.-J.; Yeh, J.-W. Simultaneously Increasing the Strength and Ductility of a Refractory High-Entropy Alloy via Grain Refining. Mater. Lett. 2016, 184, 200–203. [Google Scholar] [CrossRef]
- An, Z.; Li, A.; Mao, S.; Yang, T.; Zhu, L.; Wang, R.; Wu, Z.; Zhang, B.; Shao, R.; Jiang, C.; et al. Negative Mixing Enthalpy Solid Solutions Deliver High Strength and Ductility. Nature 2024, 625. [Google Scholar] [CrossRef]
- Pang, J.; Zhang, H.; Zhang, L.; Zhu, Z.; Fu, H.; Li, H.; Wang, A.; Li, Z.; Zhang, H. Simultaneous Enhancement of Strength and Ductility of Body-Centered Cubic TiZrNb Multi-Principal Element Alloys via Boron-Doping. J. Mater. Sci. Technol. 2021, 78, 74–80. [Google Scholar] [CrossRef]
- Liao, Y.-C.; Chen, P.-S.; Li, C.-H.; Tsai, P.-H.; Jang, J.S.C.; Hsieh, K.-C.; Chen, C.-Y.; Lin, P.-H.; Huang, J.C.; Wu, H.-J.; et al. Development of Novel Lightweight Dual-Phase Al-Ti-Cr-Mn-V Medi-um-Entropy Alloys with High Strength and Ductility. Entropy 2020, 22, 74. [Google Scholar] [CrossRef]
- Abuzaid, W.; Sehitoglu, H. Plastic Strain Partitioning in Dual Phase Al13CoCrFeNi High Entropy Alloy. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process. 2018, 720, 238–247. [Google Scholar] [CrossRef]
- Wang, K.; Shang, S.-L.; Wang, Y.; Liu, Z.-K.; Liu, F. Martensitic Transition in Fe via Bain Path at Finite Temperatures: A Comprehensive First-Principles Study. Acta Mater. 2018, 147, 261–276. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, Y.; Zhang, Z.; Liu, L.; Sun, L. Two Novel Zr-Rich Refractory High-Entropy Alloys with Excellent Tensile Mechanical Properties. Intermetallics 2023, 157, 107872. [Google Scholar] [CrossRef]
- Zhao, Z.; Shen, Z.; Dong, P.; He, Y.; Ma, J.; Ma, Y.; Yang, J.; Li, W. Temperature-Dependent Yield Strength of Nanoprecipitate-Strengthened Face-Centered Cubic High Entropy Alloys: Prediction and Analysis. Met. Mater.-Int. 2023, 29, 1723–1738. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, Y.; Han, J.; Wang, X.; Jiang, W.; Liu, C.-T.; Zhang, Z.; Liaw, P.K. Nanoprecipitate-Strengthened High-Entropy Alloys. Adv. Sci. 2021, 8, 2100870. [Google Scholar] [CrossRef]
- Wang, X.; Wang, W.; Reddy, K.M.; Zhou, W.; Liu, P.; Chen, B.; Zhao, Y.; Song, S. Low and Room Temperatures Tensile Properties of a Nanoprecipitate-Strengthened (FeCoCr)40Ni40Al10Cu10 High-Entropy Alloy. Mater. Charact. 2018, 145, 177–184. [Google Scholar] [CrossRef]
- Ming, K.; Bi, X.; Wang, J. Realizing Strength-Ductility Combination of Coarse-Grained Al0.2Co1.5CrFeNi1.5Ti0.3 Alloy via Nano-Sized, Coherent Precipitates. Int. J. Plast. 2018, 100, 177–191. [Google Scholar] [CrossRef]
- Rao, J.C.; Diao, H.Y.; Ocelik, V.; Vainchtein, D.; Zhang, C.; Kuo, C.; Tang, Z.; Guo, W.; Poplawsky, J.D.; Zhou, Y.; et al. Secondary Phases in Al CoCrFeNi High-Entropy Alloys: An in-Situ TEM Heating Study and Thermodynamic Appraisal. Acta Mater. 2017, 131, 206–220. [Google Scholar] [CrossRef]
- Li, J.; Ouyang, D.; Wang, Q.; Teng, Q.; Cai, C.; Wei, Q. Achieving Superior Tensile Strength of CoCrFeNiTi0.3 High-Entropy Alloy via in-Situ Laser Powder Bed Fusion of CoCrFeNi and Ti. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process. 2023, 886, 145649. [Google Scholar] [CrossRef]
- Tong, Y.; Chen, D.; Han, B.; Wang, J.; Feng, R.; Yang, T.; Zhao, C.; Zhao, Y.L.; Guo, W.; Shimizu, Y.; et al. Outstanding Tensile Properties of a Precipitation-Strengthened FeCoNiCrTi0.2 High-Entropy Alloy at Room and Cryogenic Temperatures. Acta Mater. 2019, 165, 228–240. [Google Scholar] [CrossRef]
- Andersson, J.O.; Helander, T.; Höglund, L.; Shi, P.F.; Sundman, B. Thermo-Calc and DICTRA, Computational tools for materials science. Calphad 2002, 26, 273–312. [Google Scholar]
- Thermo-Calc Software TCHEA6/High Entropy Alloys Database. Available online: https://thermocalc.com/products/databases/high-entropy-alloys/ (accessed on 15 June 2024).
- Thermo-Calc Software TCTI4/Ti-Alloys Database. Available online: https://thermocalc.com/products/databases/titanium-and-titanium-aluminide-based-alloys/ (accessed on 15 June 2024).
- Thermo-Calc Software TCNI12/Ni-Alloys Database. Available online: https://thermocalc.com/products/databases/nickel-based-alloys/ (accessed on 15 June 2024).
- Ye, X.; Cui, S.; Liu, T.; Ma, Q.; Liu, G.; Huang, Z.; Guo, J.; Yin, S. Microstructure Characterization and Strengthening Mechanism Analysis of X100 Pipeline Steel. Coatings 2023, 13, 706. [Google Scholar] [CrossRef]
- Mehranpour, M.S.; Shahmir, H.; Asghari-Rad, P.; Hosseinzadeh, M.; Rasooli, N.; Kim, H.S.; Nili-ahmadabadi, M. Upgrading of Superior Strength-Ductility Trade-off of CoCrFeNiMn Microstructural. Materialia 2022, 22, 101394. [Google Scholar] [CrossRef]
- Qin, F.; Li, Y.; He, W.; Zhao, X.; Chen, H. Aging Precipitation Behavior and Its Influence on Mechanical Properties of Mn18Cr18N Austenitic Stainless Steel. Met. Mater.-Int. 2017, 23, 1087–1096. [Google Scholar] [CrossRef]


| Alloy Name | Aging Temperature (°C) | Aging Time (min) |
|---|---|---|
| HF1-ST | —— | —— |
| HF1-500-30 | 500 | 30 |
| HF1-600-30 | 600 | 60 |
| HF2-ST | —— | —— |
| HF2-525-30 | 525 | 30 |
| HF2-525-60 | 525 | 60 |
| HF2-525-24h | 525 | 24 h |
| Alloy Name | Yield Strength (MPa) | Elongation (%) | References |
|---|---|---|---|
| HF1-ST | 971 | 17.5 | [4] |
| HF1-500-30 | 1081 | 7.5 | [4] |
| HF1-600-30 | 271 ± 6 | 0.35 ± 0.15 | This work |
| HF2-ST | 955 ± 25 | 19 ± 2 | This work |
| HF2-525-30 | 1037 ± 17 | 14 ± 1 | This work |
| HF2-525-60 | 960 ± 50 | 2.65 ± 0.15 | This work |
| T/°C | Equilibrium Solute Product | |||
|---|---|---|---|---|
| TCHEA6 Database | TCTI4 Database | |||
| Average Value/10−2 | Variance/10−6 | Average Value/10−2 | Variance/10−6 | |
| 400 | 4.25 | 0.95 | 4.39 | 1.00 |
| 425 | 4.69 | 1.22 | 4.85 | 1.26 |
| 450 | 5.13 | 1.58 | 5.32 | 1.60 |
| 475 | 5.58 | 1.90 | 5.78 | 2.03 |
| 500 | 6.04 | 2.38 | 6.26 | 2.49 |
| 525 | 6.51 | 2.91 | 6.75 | 3.00 |
| 550 | 6.98 | 3.45 | 7.25 | 3.49 |
| 575 | 7.46 | 4.08 | 7.76 | 4.07 |
| 600 | 7.96 | 4.68 | 8.28 | 4.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Ke, H.; Wang, B.; Wang, L.; Xue, Y. Ductility Control via Nano-Precipitation at Grain Boundaries in Ti-Zr-Hf-Nb-Ta Multi-Principal Element Alloys. Materials 2025, 18, 1463. https://doi.org/10.3390/ma18071463
Li J, Ke H, Wang B, Wang L, Xue Y. Ductility Control via Nano-Precipitation at Grain Boundaries in Ti-Zr-Hf-Nb-Ta Multi-Principal Element Alloys. Materials. 2025; 18(7):1463. https://doi.org/10.3390/ma18071463
Chicago/Turabian StyleLi, Jiaying, Huibin Ke, Benpeng Wang, Liang Wang, and Yunfei Xue. 2025. "Ductility Control via Nano-Precipitation at Grain Boundaries in Ti-Zr-Hf-Nb-Ta Multi-Principal Element Alloys" Materials 18, no. 7: 1463. https://doi.org/10.3390/ma18071463
APA StyleLi, J., Ke, H., Wang, B., Wang, L., & Xue, Y. (2025). Ductility Control via Nano-Precipitation at Grain Boundaries in Ti-Zr-Hf-Nb-Ta Multi-Principal Element Alloys. Materials, 18(7), 1463. https://doi.org/10.3390/ma18071463