Prediction Models for Radiological Characterization of Natural Aggregates Based on Chemical Composition and Mineralogy
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
- S: 8 siliceous gravels and sands of quartzitic composition (S1, S3, S5, S6, S7, S10, S11, and S12) and 4 feldspathic ones (S2, S4, S8, and S9).
- C: 5 carbonates, with a calcitic composition (C3 and C5) and dolomitic composition (C1, C2, and C4).
- V: 7 rocks of volcanic origin (V1–V7).
- G: 15 gravels and sands of granitic origin (G1–G15).
2.2. Methods
2.2.1. Chemical and Mineralogical Compositions of the Aggregates
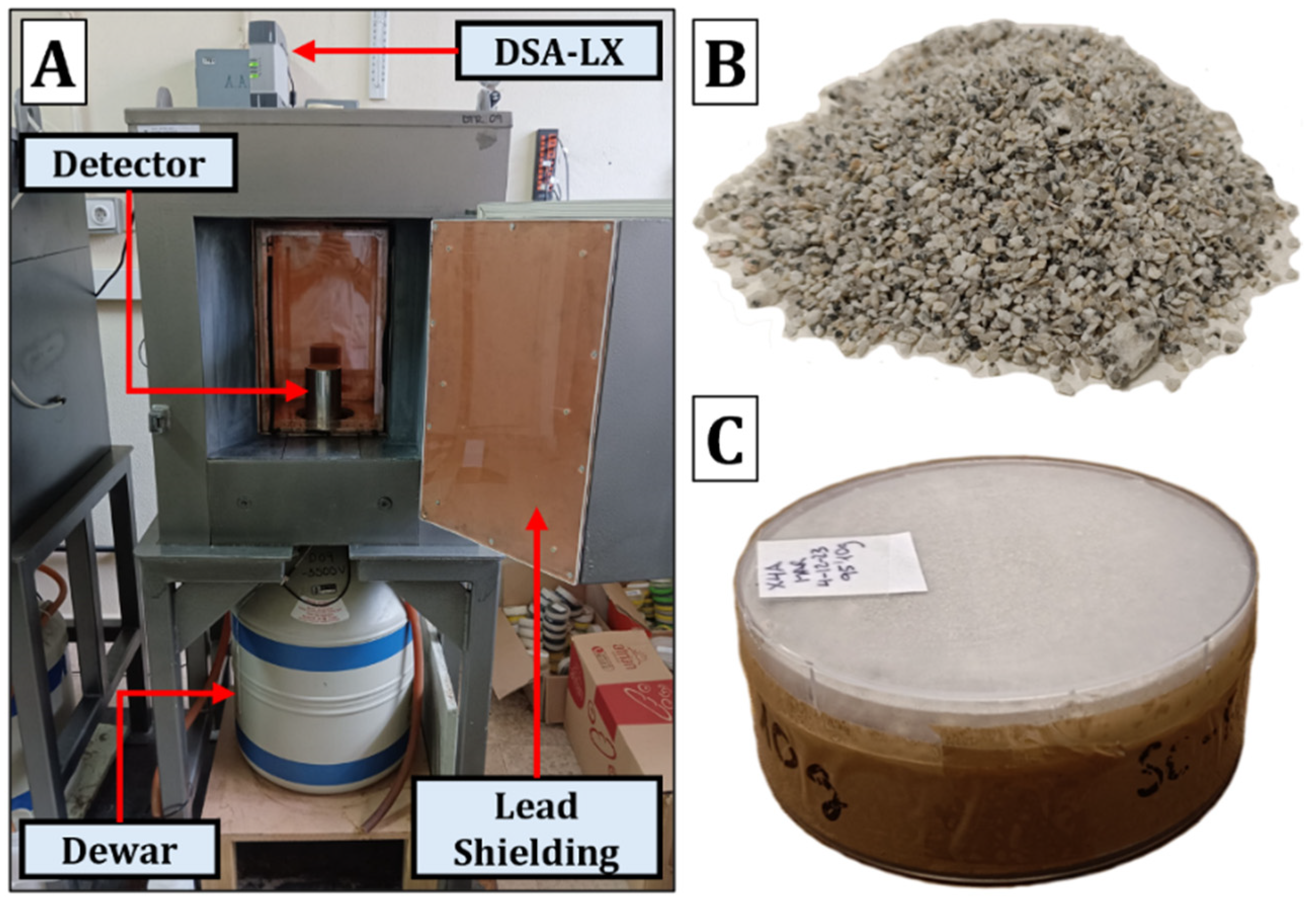
2.2.2. Gamma Spectrometry
2.2.3. Data Analysis
- Variables with a percentage of values above the limit of detection (LoD) of less than 25% were removed to prevent zero-inflated data, which could potentially introduce biases that distort the model [42];
- Variables whose variance exceeded the significance level of 0.05 were eliminated when applying backward stepwise regression, as they were not statistically significant.
2.2.4. Validation of the Models for the Estimation of the Activity Concentrations of 226Ra, 232Th (212Pb), and 40K
3. Results and Discussion
3.1. Chemical Composition and Activity Concentrations of Natural Radionuclides in Aggregates
3.2. Mineralogy of Aggregates
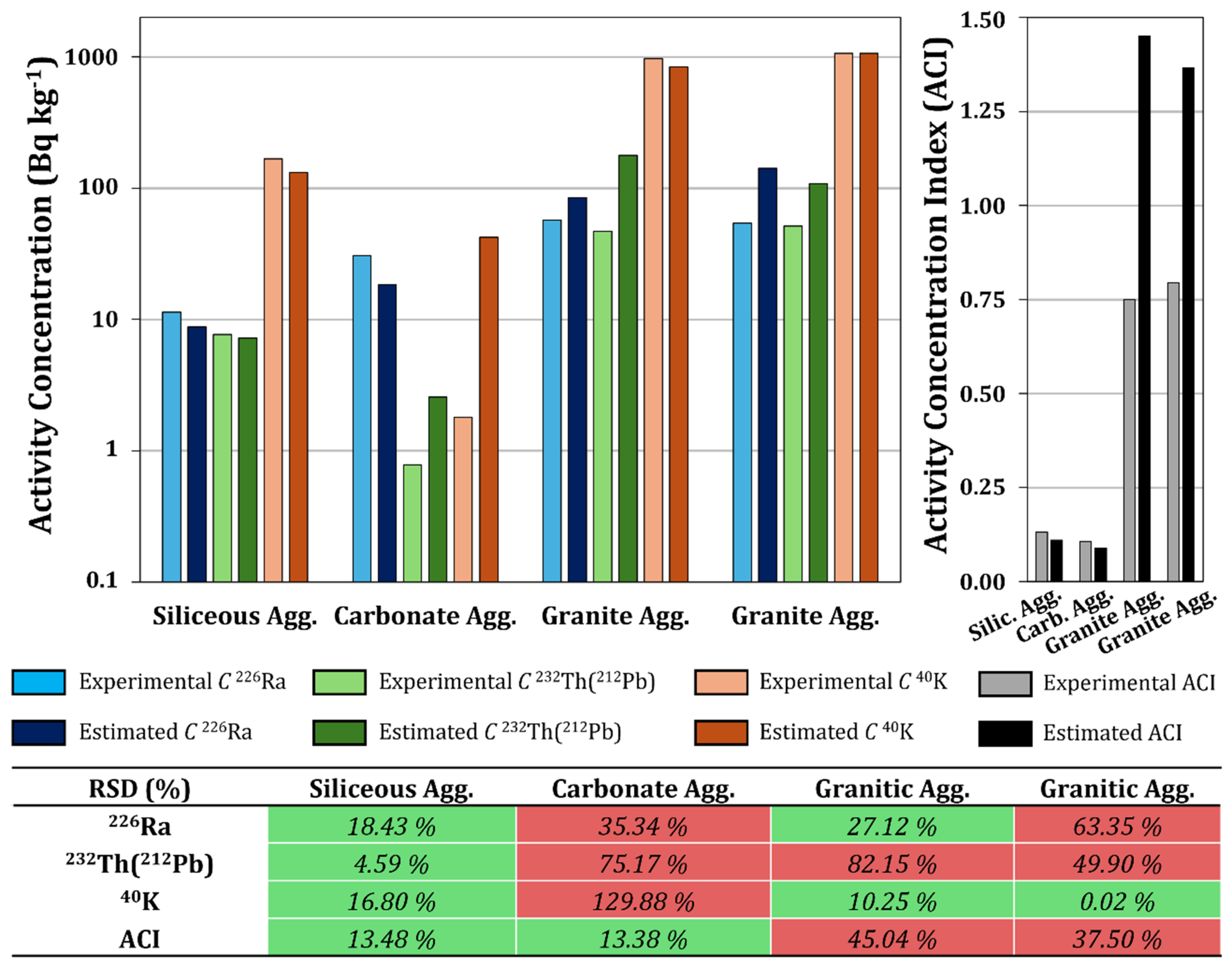
3.3. Correlation Between Chemical Composition and Activity Concentration
3.4. Predictive Models of Activity Concentrations of 226Ra, 232Th (212Pb), and 40K Based on Chemical Composition Parameters
3.5. Validation of the Achieved Models
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cardoso, R.; Silva, R.V.; de Brito, J.; Dhir, R. Use of recycled aggregates from construction and demolition waste in geotechnical applications: A literature review. Waste Manag. 2016, 49, 131–145. [Google Scholar] [CrossRef] [PubMed]
- de Andrade Salgado, F.; de Andrade Silva, F. Recycled aggregates from construction and demolition waste towards an application on structural concrete: A review. J. Build. Eng. 2022, 52, 104452. [Google Scholar] [CrossRef]
- Brioschi, N.; Cordero, E.G.; Ng, S.; Paglia, C. Low-cement eco-concrete and the role of recycled concrete aggregates (RCA), supplementary cementitious materials (SCMs), and other additives in improving sustainability and durability. Mater. Constr. 2024, 74, e359. [Google Scholar] [CrossRef]
- Naderi, M.; Kaboudan, A. Experimental study of the effect of aggregate type on concrete strength and permeability. J. Build. Eng. 2021, 37, 101928. [Google Scholar] [CrossRef]
- An, J.; Kim, S.S.; Nam, B.H.; Durham, S.A. Effect of aggregate mineralogy and concrete microstructure on thermal expansion and strength properties of the normal strength concrete. Appl. Sci. 2017, 7, 1307. [Google Scholar] [CrossRef]
- Piasta, W.; Góra, J.; Turkiewicz, T. Properties and durability of coarse igneous rock aggregates and concretes. Constr. Build. Mater. 2016, 126, 119–129. [Google Scholar] [CrossRef]
- Menadi, B.; Kenai, S.; Khatib, J.; Aït-Mokhtar, A. Strength and durability of concrete incorporating crushed limestone sand. Constr. Build. Mater. 2009, 23, 625–633. [Google Scholar] [CrossRef]
- Aquino, C.; Inoue, M.; Miura, H.; Mizuta, M.; Okamoto, T. The effects of limestone aggregate on concrete properties. Constr. Build. Mater. 2010, 24, 2363–2368. [Google Scholar] [CrossRef]
- Suárez-Navarro, J.A.; Alonso, M.D.M.; Gascó, C.; Pachón, A.; Carmona-Quiroga, P.M.; Argiz, C.; Sanjuán, M.; Puertas, F. Effect of particle size and composition of granitic sands on the radiological behaviour of mortars. Boletín De La Soc. Española De Cerámica Y Vidr. 2022, 61, 561–573. [Google Scholar] [CrossRef]
- Anjos, R.; Veiga, R.; Soares, T.; Santos, A.; Aguiar, J.; Frascá, M.; Brage, J.; Uzêda, D.; Mangia, L.; Facure, A.; et al. Natural radionuclide distribution in Brazilian commercial granites. Radiat. Meas. 2005, 39, 245–253. [Google Scholar] [CrossRef]
- Moghazy, N.M.; El-Tohamy, A.M.; Fawzy, M.M.; Awad, H.A.; Zakaly, H.M.H.; Issa, S.A.M.; Ene, A. Natural radioactivity, radiological hazard and petrographical studies on aswan granites used as building materials in Egypt. Appl. Sci. 2021, 11, 6471. [Google Scholar] [CrossRef]
- Guillén, J.; Tejado, J.; Baeza, A.; Corbacho, J.; Muñoz, J. Assessment of radiological hazard of commercial granites from Extremadura (Spain). J. Environ. Radioact. 2014, 132, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.-J.; Ryu, Y.-H.; Dong, K.-R.; Cho, J.-H.; Lee, H.-K.; Chung, W.-K. Study on measurement and quantitative analysis of Radon-222 emitted from construction materials. Ann. Nucl. Energy 2012, 49, 88–95. [Google Scholar] [CrossRef]
- Shabek, F.; Obradović, B.; Čeliković, I.; Đurašević, M.; Samolov, A.; Kolarž, P.; Janićijević, A. The Radon Exhalation Rate and Dose Assessment of Granite Used as a Building Material in Serbia. Atmosphere 2024, 15, 1495. [Google Scholar] [CrossRef]
- Martín-Rodríguez, P.; Fernández-Jiménez, A.; Alonso, M.d.M.; Palomo, A.; García-Lodeiro, I. Valorisation of “La Palma” Volcanic Ash for Making Portland-Blended, Alkaline and Hybrid Portland–Alkaline Cements. Materials 2024, 17, 242. [Google Scholar] [CrossRef]
- Capaccioni, B.; Cinelli, G.; Mostacci, D.; Tositti, L. Long-term risk in a recently active volcanic system: Evaluation of doses and indoor radiological risk in the quaternary Vulsini Volcanic District (Central Italy). J. Volcanol. Geotherm. Res. 2012, 247, 26–36. [Google Scholar] [CrossRef]
- Turhan, Ş.; Atıcı, E.; Varinlioğlu, A. Radiometric analysis of volcanic tuff stones used as ornamental and structural building materials in Turkey and evaluation of radiological risk. Radioprotection 2015, 50, 273–280. [Google Scholar] [CrossRef]
- Hanfi, M.Y.; Gawad, A.E.A.; Ali, K.G.; Abu-Donia, A.; Alsafi, K.G.; Khafaji, M.; Albahiti, S.K.; Alqahtani, M.S.; Khalil, M.; Wahed, A.A.A. Environmental risk assessment associated with acidic volcanics in Egypt. Appl. Radiat. Isot. 2022, 188, 110413. [Google Scholar] [CrossRef]
- EU. Laying down Basic Safety Standards for Protection against the Dangers Arising from Exposure to Ionising Radiation, and Repealing Directives 89/618/Euratom, 90/641/Euratom, 96/29/Euratom, 97/43/Euratom and 2003/122/Euratom. Council Directive 2013/59/Euratom of 5 Dec; European Commission: Brussels, Belgium, 2013; Volume 57. [Google Scholar]
- Suárez-Navarro, J.; Moreno-Reyes, A.; Gascó, C.; Alonso, M.; Puertas, F. Gamma spectrometry and LabSOCS-calculated efficiency in the radiological characterisation of quadrangular and cubic specimens of hardened portland cement paste. Radiat. Phys. Chem. 2020, 171, 108709. [Google Scholar] [CrossRef]
- Caño, A.; Suárez-Navarro, J.; García-Calvo, J.; García, Q.M.; Alonso, M. Determination of a standard efficiency using the ISOCS uncertainty estimator for calculating the activity concentration of 226Ra, 232Th, and 40K in concrete. Measurement 2025, 242, 115856. [Google Scholar] [CrossRef]
- Suresh, G.; Ramasamy, V.; Meenakshisundaram, V.; Venkatachalapathy, R.; Ponnusamy, V. A relationship between the natural radioactivity and mineralogical composition of the Ponnaiyar river sediments, India. J. Environ. Radioact. 2011, 102, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Hanfi, M.Y.; Gawad, A.E.A.; Eliwa, H.; Ali, K.; Taki, M.M.; Sayyed, M.; Khandaker, M.U.; Bradley, D.A. Assessment of radioactivity in Granitoids at Nikeiba, Southeastern Desert, Egypt; radionuclides concentrations and radiological hazard parameters. Radiat. Phys. Chem. 2022, 200, 110113. [Google Scholar] [CrossRef]
- Taalab, S.A.; Ismail, A.M.; El Maadawy, W.; Abdelrahman, K.; Khandaker, M.U.; Sakr, A.K.; Hanfi, M.Y. Natural radioactivity, mineralogy and hazard assessment of syenogranites (ornamental stones) using a statistical approach. Nucl. Eng. Technol. 2024, 56, 4141–4148. [Google Scholar] [CrossRef]
- Coletti, C.; Brattich, E.; Cinelli, G.; Cultrone, G.; Maritan, L.; Mazzoli, C.; Mostacci, D.; Tositti, L.; Sassi, R. Radionuclide concentration and radon exhalation in new mix design of bricks produced reusing NORM by-products: The influence of mineralogy and texture. Constr. Build. Mater. 2020, 260, 119820. [Google Scholar] [CrossRef]
- Suárez-Navarro, J.A.; Sanjuán, M.A.; Argiz, C.; Hernáiz, G.; Barragán, M.; Estévez, E. Radiological assessment of iron silicate as a potential aggregate in concrete and mortars. Cem. Concr. Compos. 2022, 129, 104490. [Google Scholar] [CrossRef]
- Gawad, A.E.A.; Hanfi, M.Y.; Alqahtani, M.S.; Ramadan, A.A. Geochemical features and radiological risk assessment of Wadi El-Regeita granites, South Central Sinai, Egypt. Radiat. Phys. Chem. 2025, 226, 112161. [Google Scholar] [CrossRef]
- Caño, A.; Suárez-Navarro, J.A.; Puertas, F.; Fernández-Jiménez, A.; Alonso, M.D.M. New Approach to Determine the Activity Concentration Index in Cements, Fly Ashes, and Slags on the Basis of Their Chemical Composition. Materials 2023, 16, 2677. [Google Scholar] [CrossRef]
- Mastori, H.; Piluso, P.; Haquet, J.-F.; Denoyel, R.; Antoni, M. Limestone-siliceous and siliceous concretes thermal damaging at high temperature. Constr. Build. Mater. 2019, 228, 116671. [Google Scholar] [CrossRef]
- Binici, H.; Shah, T.; Aksogan, O.; Kaplan, H. Durability of concrete made with granite and marble as recycle aggregates. J. Mater. Process. Technol. 2008, 208, 299–308. [Google Scholar] [CrossRef]
- Demirdag, S.; Gunduz, L. Strength properties of volcanic slag aggregate lightweight concrete for high performance masonry units. Constr. Build. Mater. 2008, 22, 135–142. [Google Scholar] [CrossRef]
- Korkanç, M.; Tuğrul, A. Evaluation of selected basalts from Niğde, Turkey, as source of concrete aggregate. Eng. Geol. 2004, 75, 291–307. [Google Scholar] [CrossRef]
- Li, P.; Yu, Q.; Brouwers, H. Effect of coarse basalt aggregates on the properties of Ultra-high Performance Concrete (UHPC). Constr. Build. Mater. 2018, 170, 649–659. [Google Scholar] [CrossRef]
- EN 196-2:2014; Method of Testing Cement-Part 2: Chemical Analysis of Cement. AENOR: Madrid, Spain, 2014. (In Spanish)
- Alonso, M.; Suárez-Navarro, J.; Pérez-Sanz, R.; Gascó, C.; Reyes, A.M.d.L.; Lanzón, M.; Blanco-Varela, M.; Puertas, F. Data on natural radionuclide’s activity concentration of cement-based materials. Data Brief 2020, 33, 106488. [Google Scholar] [CrossRef]
- EN ISO/IEC 17025:2017; General Requirements for the Competence of Testing and Calibration Laboratories. ISO: Geneva, Switzerland, 2017.
- CANBERRA. Genie 2000 Operations Manual; Canberra Industries: Meriden, CT, USA, 2012. [Google Scholar]
- Suárez-Navarro, J.; Benavente, J.; Expósito-Suárez, V.; Caño, A.; Hernaiz, G.; Alonso, M.M. 226Ra activity concentration determined directly from the 186 keV photopeak using gamma spectrometry and a neural network. Radiat. Phys. Chem. 2024, 217, 111486. [Google Scholar] [CrossRef]
- Bé, M.M.; Chisté, V.; Dulieu, C.; Kellett, M.; Mougeot, X.; Arzu, A.; Chechev, V.; Kuzmenko, N.; Kibédi, T.; Luca, A. Table of Radionuclides (Vol. 8-A= 41 to 198), Bureau International Des Poids et Mesures (BIPM), Sevres. 2016. Available online: https://cea.hal.science/cea-02476462/ (accessed on 15 September 2024).
- Kassambara, A.; Mundt, F. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses. R package Version 1.0.7. 2020. Available online: https://cran.r-project.org/package=factoextra (accessed on 15 September 2024).
- Hill, B.D. Sequential Kaiser-Meyer-Olkin Procedure as an Alternative for Determining the Number of Factors in Common-Factor Analysis: A Monte Carlo Simulation; Oklahoma State University: Stillwater, OK, USA, 2011. [Google Scholar]
- Zuur, A.F.; Ieno, E.N.; Walker, N.J.; Saveliev, A.A.; Smith, G.M. Zero-Truncated and Zero-Inflated Models for Count Data. In Mixed Effects Models and Extensions in Ecology with R. Statistics for Biology and Health; Springer: New York, NY, USA, 2009. [Google Scholar] [CrossRef]
- Méndez-Ramírez, I.; Moreno-Macías, H.; Gómez-Humarán, I.M.; Murata, C. Conglomerados como solución alternativa al problema de la multicolinealidad en modelos lineales. Cienc. Clínicas 2014, 15, 39–46. [Google Scholar] [CrossRef]
- Akinwande, M.O.; Dikko, H.G.; Samson, A. Variance inflation factor: As a condition for the inclusion of suppressor variable (s) in regression analysis. Open J. Stat. 2015, 5, 754. [Google Scholar] [CrossRef]
- Expósito-Suárez, V.M.; Suárez-Navarro, J.A.; Caro, A.; Sanz, M.B.; Hernaiz, G.; González-Sanabria, A.; Suárez-Navarro, M.J.; Jordá-Bordehore, L.; Chamorro-Villanueva, H.; Arlandi, M.; et al. Radiological characterization of the tailings of an abandoned copper mine using a neural network and geostatistical analysis through the Co-Kriging method. Environ. Geochem. Health 2024, 46, 297. [Google Scholar] [CrossRef]
- Schön, J.H. Chapter 1: Rocks—Their Classification and General Properties. In Handbook of Petroleum Exploration and Production; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Sharma, S.; Kumar Vyas, A. A study on use of granite powder and crusher dust as fine aggregate in cement mortar. Mater. Today Proc. 2023, 93, 176–181. [Google Scholar] [CrossRef]
- Tanyıldızı, M.; Gokalp, I. Utilization of pumice as aggregate in the concrete: A state of art. Constr. Build. Mater. 2023, 377, 131102. [Google Scholar] [CrossRef]
- Blatt, H.; Tracy, R.J.; Owens, B.E. Petrology, Igneous, Sedimentary, and Metamorphic, 3rd ed.; W.H. Freeman & Company: New York, NY, USA, 2006; Volume 10, p. 304. [Google Scholar]
- Philpotts, A.R.; Ague, J.J. Principles of Igneous and Metamorphic Petrology; Cambridge University Press: Cambridge, UK, 2009. [Google Scholar] [CrossRef]
- Dziuban, C.D.; Shirkey, E.C. When is a correlation matrix appropriate for factor analysis? Some decision rules. Psychol. Bull. 1974, 81, 358. [Google Scholar] [CrossRef]
- Sayın, N. Radioactive element contents of some granites used as building materials: Insights into the radiological hazards. Bull. Eng. Geol. Environ. 2013, 72, 579–587. [Google Scholar] [CrossRef]
- Khandaker, M.U.; Asaduzzaman, K.; Bin Sulaiman, A.F.; Bradley, D.; Isinkaye, M.O. Elevated concentrations of naturally occurring radionuclides in heavy mineral-rich beach sands of Langkawi Island, Malaysia. Mar. Pollut. Bull. 2018, 127, 654–663. [Google Scholar] [CrossRef]
- Habib, A.; Akhi, S.Z.; Khan, R.; Phoungthong, K.; Basir, S.; Anik, A.H.; Islam, A.R.M.T.; Idris, A.M. Elevated levels of environmental radioactivity in fluvial sediment: Origin and health risk assessment. Environ. Sci. Process. Impacts 2024, 26, 555–581. [Google Scholar] [CrossRef]
- Khan, R.; Parvez, S.; Jolly, Y.N.; Haydar, A.; Alam, F.; Khatun, M.A.; Sarker, M.R.; Habib, A.; Tamim, U.; Das, S.; et al. Elemental abundances, natural radioactivity and physicochemical records of a southern part of Bangladesh: Implication for assessing the environmental geochemistry. Environ. Nanotechnol. Monit. Manag. 2019, 12, 100225. [Google Scholar] [CrossRef]
- Papadopoulos, A.; Koroneos, A.; Christofides, G.; Papadopoulou, L.; Tzifas, I.; Stoulos, S. Assessment of gamma radiation exposure of beach sands in highly touristic areas associated with plutonic rocks of the Atticocycladic zone (Greece). J. Environ. Radioact. 2016, 162, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Pavlidou, S.; Koroneos, A.; Papastefanou, C.; Christofides, G.; Stoulos, S.; Vavelides, M. Natural radioactivity of granites used as building materials. J. Environ. Radioact. 2006, 89, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Snyder, M.; Hunley, L.; Stanberry, J.; Szlamkowicz, I.; Jones, B.; Anagnostopoulos, V. A Preliminary Sorption Study of Uranium on MnO2 (Pyrolusite) in the Presence of Siderophore Desferrioxamine B—The Mechanism of a Ternary System. Water 2023, 15, 3241. [Google Scholar] [CrossRef]
- Shahin, H.A.E.R.A. Occurrence of uraniferous iron and manganese oxides in biotite granite North East Gabal El Sela area, South Eastern Desert, Egypt. Arab. J. Geosci. 2013, 6, 2245–2259. [Google Scholar] [CrossRef]
- Winter, J.D. Principles of Igneous and Metamorphic Petrology; Pearson Education: Harlow, UK, 2014; Volume 2. [Google Scholar]






| Radioactive Series | Radionuclide | Energy (keV) | Photons/100 Disintegrations |
|---|---|---|---|
| Uranium Series | 234Th | 63.30 (2) | 3.75 (8) |
| 226Ra | 186.211 (13) | 3.555 (19) | |
| 214Pb | 351.932 (2) | 35.60 (7) | |
| 214Bi | 609.312 (7) | 45.49 (19) | |
| 1120.287 (10) | 14.91 (3) | ||
| 1764.494 (14) | 15.31 (5) | ||
| 210Pb | 46.539 (1) | 4.252 (40) | |
| Thorium Series | 228Ac | 911.196 (6) | 26.2 (8) |
| 212Pb | 238.632 (2) | 43.6 (5) | |
| 208Tl | 583.187 (2) | 85.0 (5) | |
| Potassium | 40K | 1460.822 (6) | 10.55 (11) |
| Mineral Classification | Phases (%) | Siliceous Agg. | Carbonate Agg. | Volcanic Agg. | Granitic Agg. | ||||
|---|---|---|---|---|---|---|---|---|---|
| S9 | S12 | C2 | C5 | V3 | V6 | G5 | G12 | ||
| Silicates | Quartz | 83.77 | 70.35 | 0.15 | 30.58 | 26.05 | |||
| K Feldspar | 2.46 | 15.70 | 1.14 | 6.52 | 30.72 | 31.69 | |||
| Plagioclase | 13.77 | 9.71 | 16.10 | 13.74 | 22.68 | 27.55 | |||
| Biotite * | 13.36 | 11.53 | |||||||
| Chlorite | 0.32 | 0.42 | |||||||
| Illite * | 0.37 | ||||||||
| Diopside | 37.88 | 37.33 | |||||||
| Nepheline | 0.92 | 0.74 | |||||||
| Olivine | 1.43 | 2.67 | |||||||
| Zircon | 0.14 | 0.20 | |||||||
| Carbonates | Calcite | 4.24 | 0.28 | 99.63 | |||||
| Dolomite | 99.57 | ||||||||
| Oxides | Hematite | 2.83 | 4.18 | ||||||
| Titanomagnetite | 3.29 | ||||||||
| Anatase | 0.80 | 0.78 | |||||||
| Phosphates | Apatite | 1.40 | 1.78 | ||||||
| Amorphous | 36.40 | 34.81 | |||||||
| Rwp | 13.90 | 9.99 | 11.56 | 11.35 | 3.92 | 4.26 | 10.65 | 10.68 | |
| Radionuclide | Student’s t-Test | Fisher’s F-Test | |
|---|---|---|---|
| Siliceous and Carbonate Aggregates | 226Ra | 1.000 | 0.125 |
| 232Th(212Pb) | 1.000 | 0.084 | |
| 40K | 1.000 | 0.528 | |
| Granite Aggregates | 226Ra | 1.000 | 0.261 |
| 232Th(212Pb) | 1.000 | 0.477 | |
| 40K | 0.999 | 0.712 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caño, A.; Alonso, M.d.M.; Pachón-Montaño, A.; Marzal, Q.; Hernáiz, G.; Sousa, L.; Suárez-Navarro, J.A. Prediction Models for Radiological Characterization of Natural Aggregates Based on Chemical Composition and Mineralogy. Materials 2025, 18, 1369. https://doi.org/10.3390/ma18061369
Caño A, Alonso MdM, Pachón-Montaño A, Marzal Q, Hernáiz G, Sousa L, Suárez-Navarro JA. Prediction Models for Radiological Characterization of Natural Aggregates Based on Chemical Composition and Mineralogy. Materials. 2025; 18(6):1369. https://doi.org/10.3390/ma18061369
Chicago/Turabian StyleCaño, Andrés, María del Mar Alonso, Alicia Pachón-Montaño, Queralt Marzal, Guillermo Hernáiz, Luís Sousa, and José Antonio Suárez-Navarro. 2025. "Prediction Models for Radiological Characterization of Natural Aggregates Based on Chemical Composition and Mineralogy" Materials 18, no. 6: 1369. https://doi.org/10.3390/ma18061369
APA StyleCaño, A., Alonso, M. d. M., Pachón-Montaño, A., Marzal, Q., Hernáiz, G., Sousa, L., & Suárez-Navarro, J. A. (2025). Prediction Models for Radiological Characterization of Natural Aggregates Based on Chemical Composition and Mineralogy. Materials, 18(6), 1369. https://doi.org/10.3390/ma18061369









