Enhancement of Fracture Toughness of Inner Liner Material for Type IV Hydrogen Storage Cylinders Based on Molecular Dynamics Method
Abstract
1. Introduction
2. Simulation Method
2.1. Tensile Simulation
2.2. J-Integral Calculation
2.3. Fracture Analysis
3. Results and Discussion
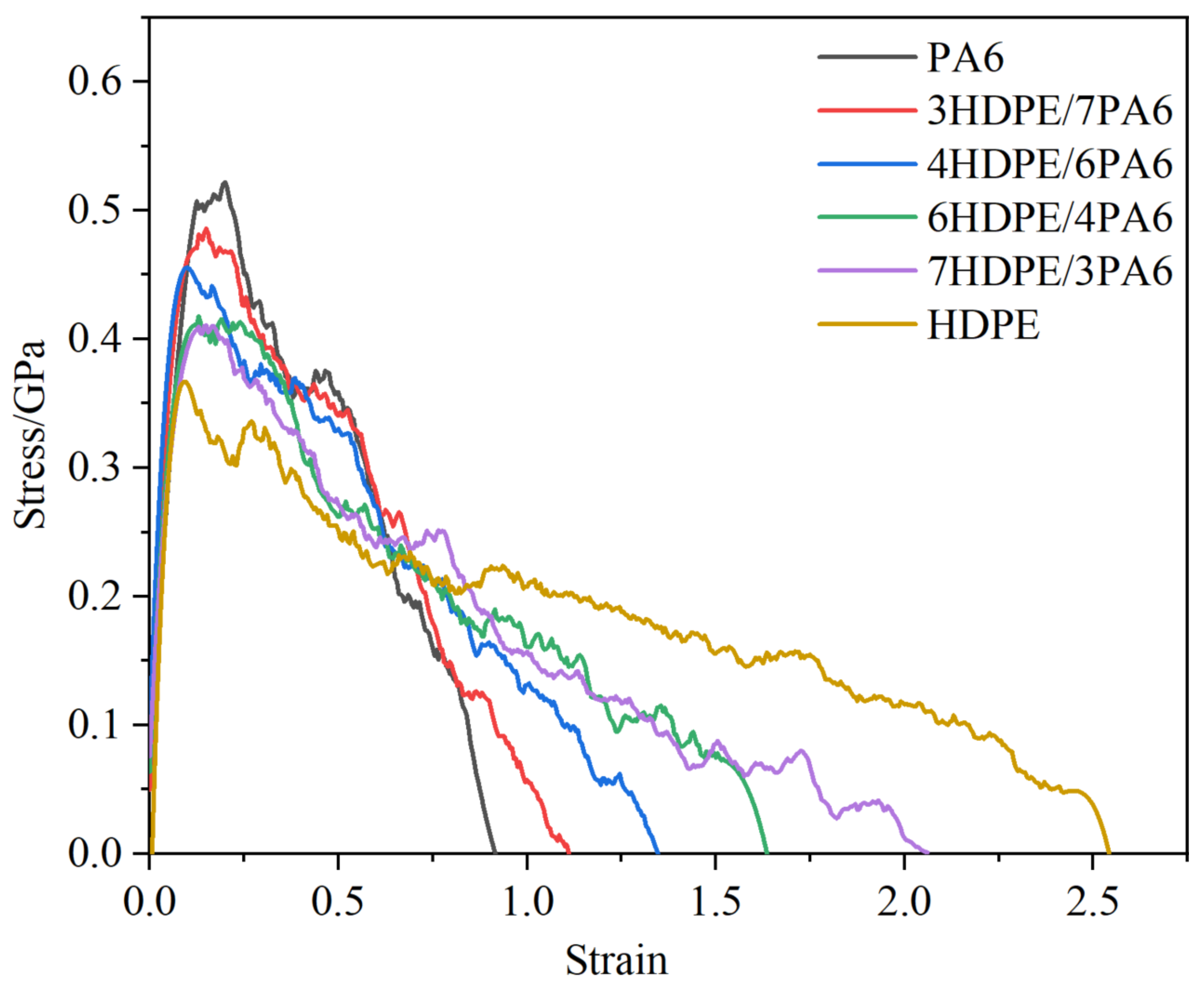
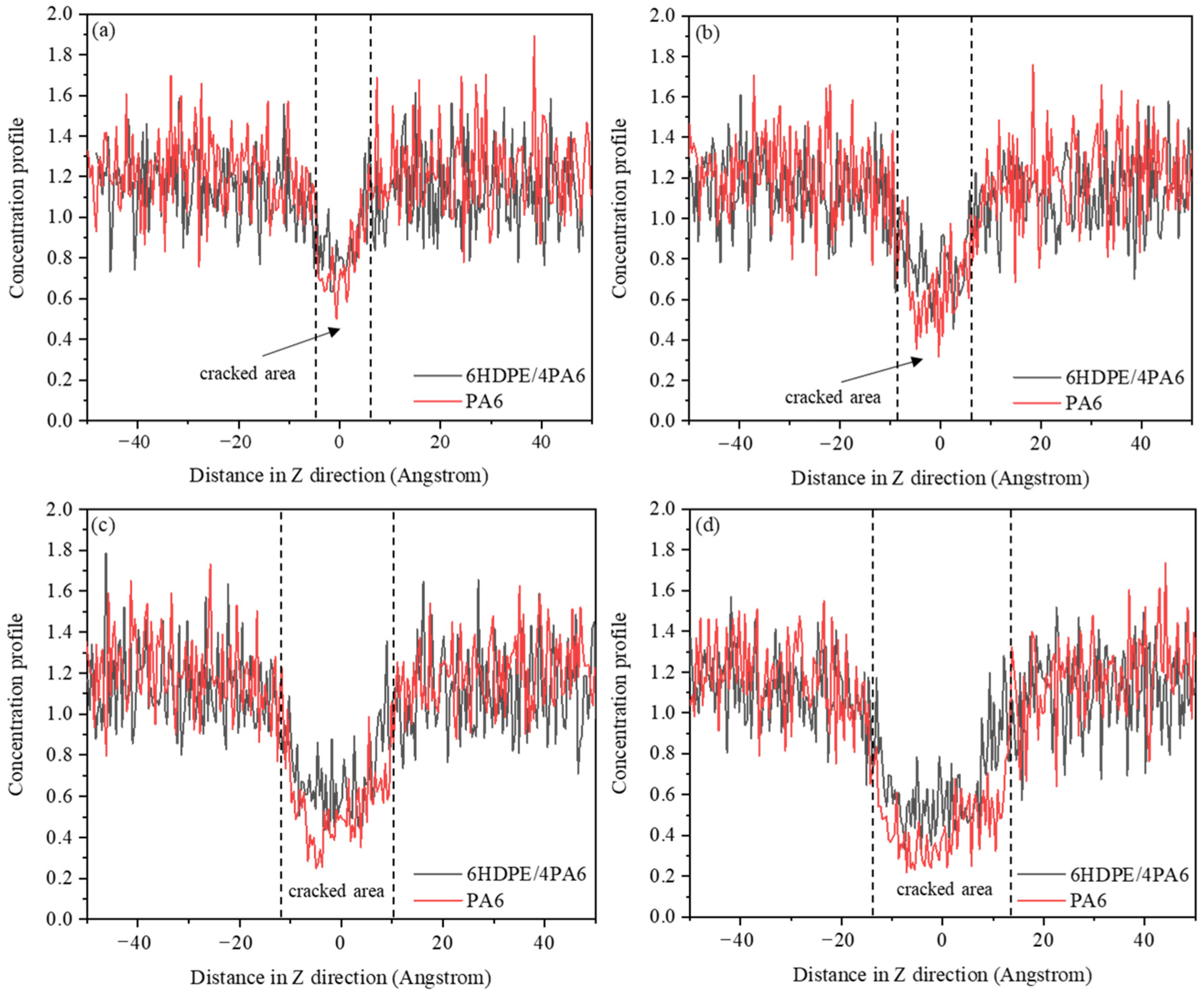
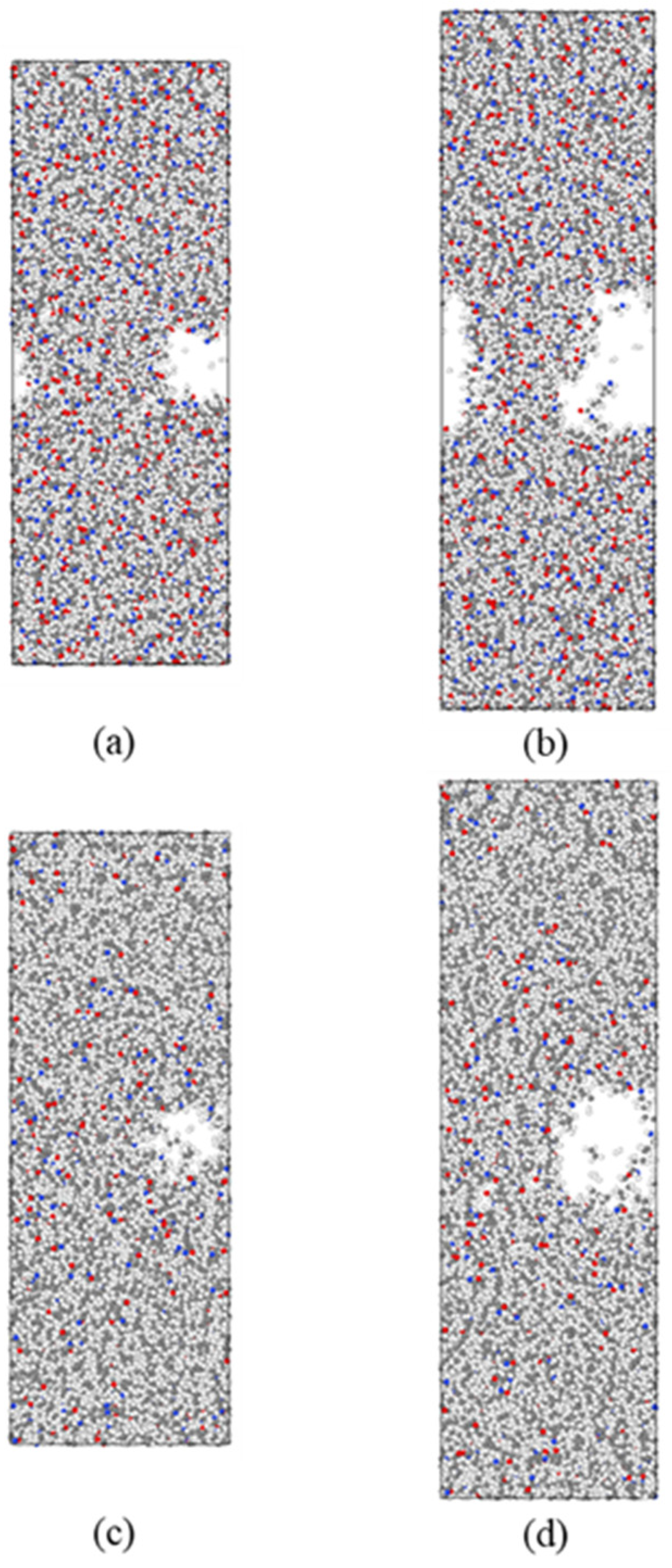
3.1. Fracture Toughness and Crack Extension of Materials
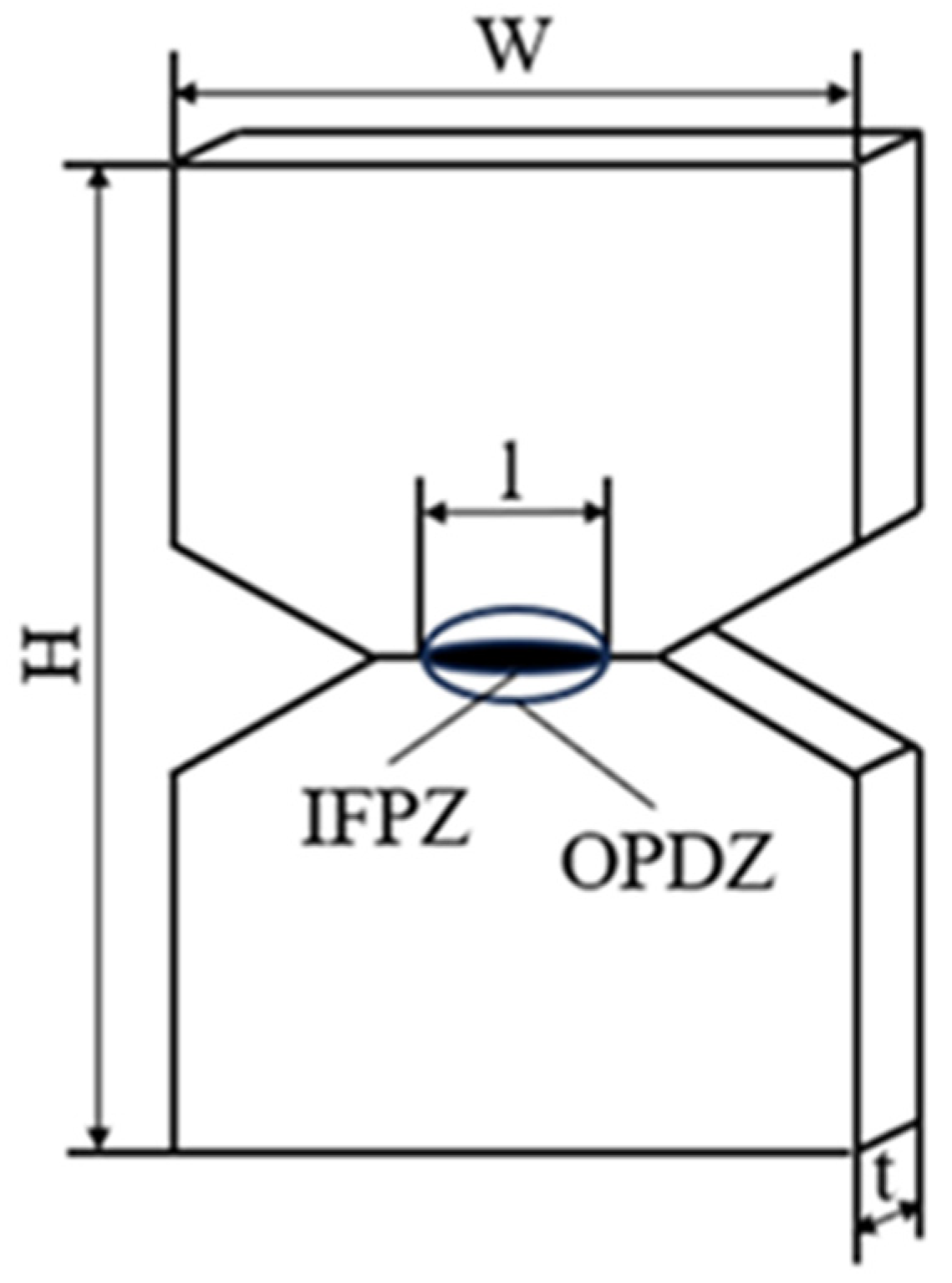
3.2. Experimental Test
3.2.1. EWF Theory
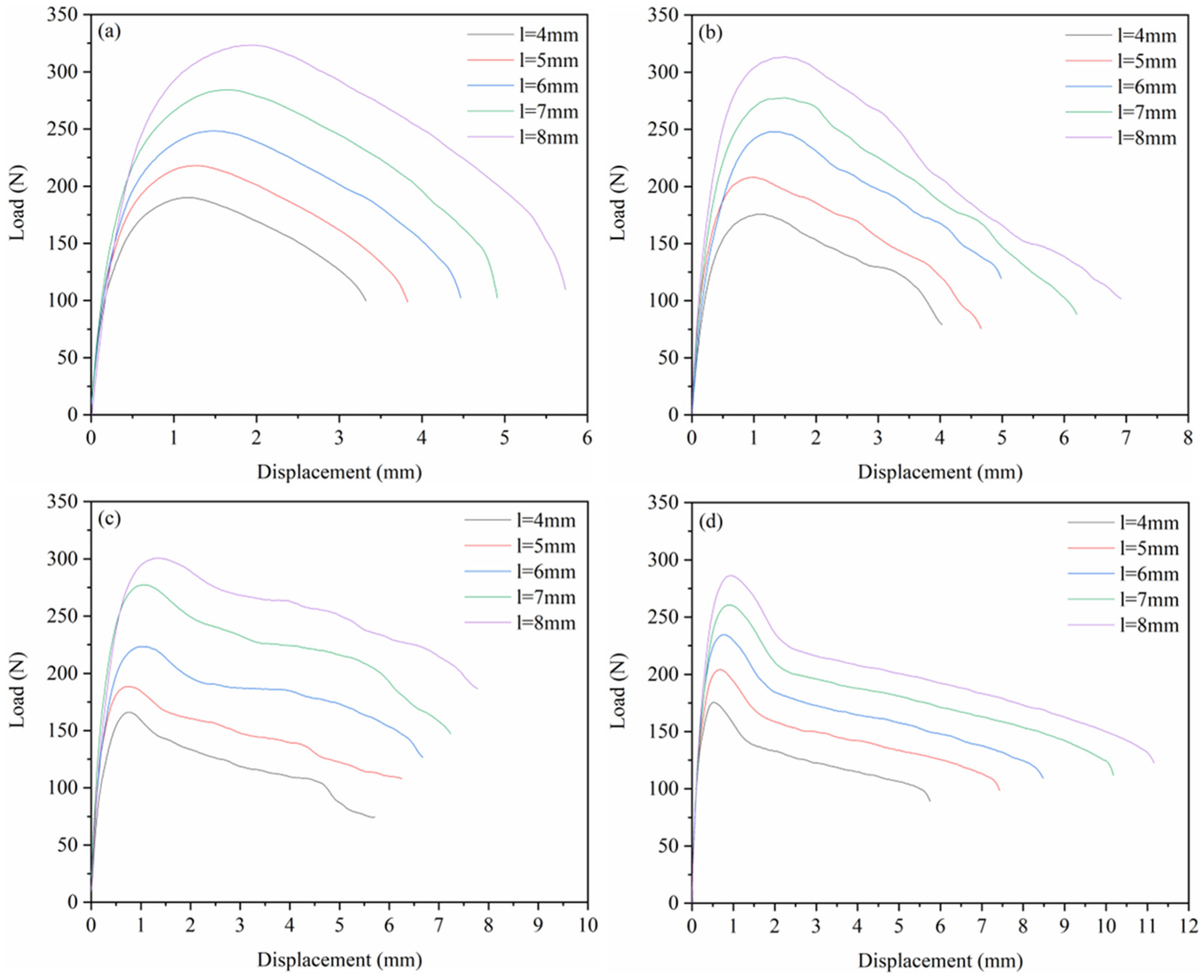
3.2.2. Load–Displacement Curve
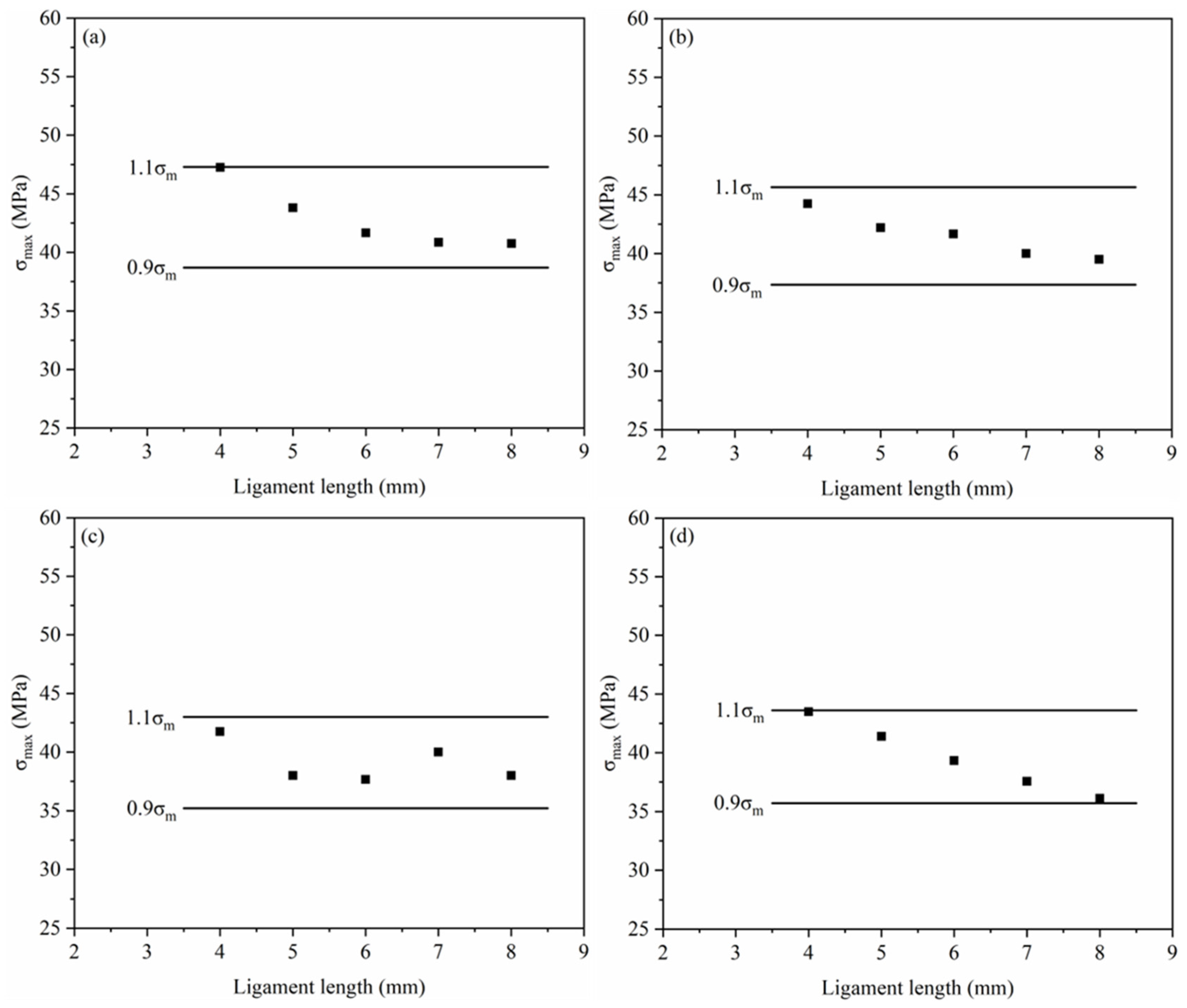
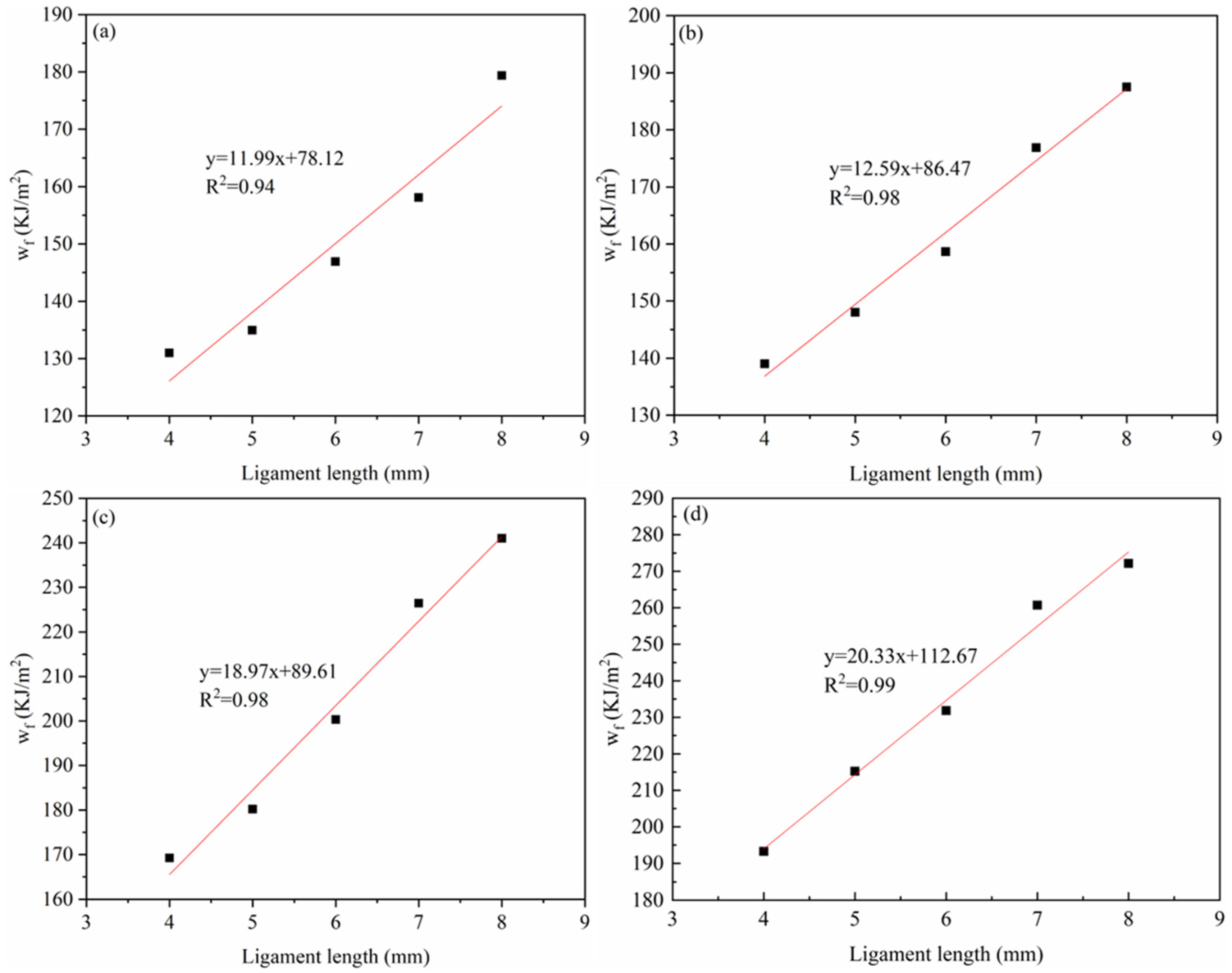
3.2.3. EWF Parameters
4. Conclusions
- With an increase in the HDPE content, the tensile strength of the composites gradually decreases, while the elongation at break and fracture toughness show gradual improvement trends. In the fracture process, the addition of HDPE can make the composite material undergo more plastic deformation and molecular chain slip, which effectively disperses the local stress, thus absorbing more energy and slowing down the expansion of the crack. The decrease in atomic concentration in the crack region is effectively slowed down, which enhances the resistance of the material to crack expansion.
- The fracture toughness of the four materials was calculated by the EWF method. The results reveal that as the HDPE mass ratio increases, the we of the PA6/HDPE composite material also gradually improves. The trend observed in the simulation results was in agreement with the experimental results, verifying the effectiveness of the molecular dynamics simulation method in predicting the fracture toughness of materials.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alasali, F.; Abuashour, M.I.; Hammad, W.; Almomani, D.; Obeidat, A.M.; Holderbaum, W. A review of hydrogen production and storage materials for efficient integrated hydrogen energy systems. Energy Sci. Eng. 2024, 12, 1934–1968. [Google Scholar] [CrossRef]
- Bakker, S.; van Lente, H.; Meeus, M.T.H. Credible expectations—The US Department of Energy’s Hydrogen Program as enactor and selector of hydrogen technologies. Technol. Forecast. Soc. Chang. 2012, 79, 1059–1071. [Google Scholar] [CrossRef]
- Yan, X.R.; Zheng, W.G.; Wei, Y.J.; Yan, Z.Q. Current Status and Economic Analysis of Green Hydrogen Energy Industry Chain. Processes 2024, 12, 315. [Google Scholar] [CrossRef]
- Li, J.; Chai, X.Z.; Gu, Y.P.; Zhang, P.Y.; Yang, X.; Wen, Y.H.; Xu, Z.; Jiang, B.W.; Wang, J.; Jin, G.; et al. Small-Scale High-Pressure Hydrogen Storage Vessels: A Review. Materials 2024, 17, 721. [Google Scholar] [CrossRef]
- Air, A.; Shamsuddoha, M.; Gangadhara Prusty, B. A review of Type V composite pressure vessels and automated fibre placement based manufacturing. Compos. Part B Eng. 2023, 253, 110573. [Google Scholar] [CrossRef]
- Jaber, M.; Yahya, A.; Arif, A.F.; Jaber, H.; Alkhedher, M. Burst pressure performance comparison of type V hydrogen tanks: Evaluating various shapes and materials. Int. J. Hydrogen Energy 2024, 81, 906–917. [Google Scholar] [CrossRef]
- Barthelemy, H.; Weber, M.; Barbier, F. Hydrogen storage: Recent improvements and industrial perspectives. Int. J. Hydrogen Energy 2017, 42, 7254–7262. [Google Scholar] [CrossRef]
- Barthélémy, H. Hydrogen storage—Industrial prospectives. Int. J. Hydrogen Energy 2012, 37, 17364–17372. [Google Scholar] [CrossRef]
- Li, M.X.; Bai, Y.F.; Zhang, C.Z.; Song, Y.X.; Jiang, S.F.; Grouset, D.; Zhang, M.J. Review on the research of hydrogen storage system fast refueling in fuel cell vehicle. Int. J. Hydrogen Energy 2019, 44, 10677–10693. [Google Scholar] [CrossRef]
- Li, X.; Huang, Q.H.; Liu, Y.T.; Zhao, B.D.; Li, J.P. Review of the Hydrogen Permeation Test of the Polymer Liner Material of Type IV On-Board Hydrogen Storage Cylinders. Materials 2023, 16, 5366. [Google Scholar] [CrossRef]
- Wang, D.L.; Liao, B.B.; Zheng, J.Y.; Huang, G.; Hua, Z.L.; Gu, C.H.; Xu, P. Development of regulations, codes and standards on composite tanks for on-board gaseous hydrogen storage. Int. J. Hydrogen Energy 2019, 44, 22643–22653. [Google Scholar] [CrossRef]
- Barboza Neto, E.S.; Chludzinski, M.; Roese, P.B.; Fonseca, J.S.O.; Amico, S.C.; Ferreira, C.A. Experimental and numerical analysis of a LLDPE/HDPE liner for a composite pressure vessel. Polym. Test. 2011, 30, 693–700. [Google Scholar] [CrossRef]
- Dong, C.F.; Liu, Y.T.; Li, J.P.; Bin, G.F.; Zhou, C.L.; Han, W.L.; Li, X. Hydrogen Permeability of Polyamide 6 Used as Liner Material for Type IV On-Board Hydrogen Storage Cylinders. Polymers 2023, 15, 3715. [Google Scholar] [CrossRef]
- Fujiwara, H.; Ono, H.; Ohyama, K.; Kasai, M.; Kaneko, F.; Nishimura, S. Hydrogen permeation under high pressure conditions and the destruction of exposed polyethylene-property of polymeric materials for high-pressure hydrogen devices (2). Int. J. Hydrogen Energy 2021, 46, 11832–11848. [Google Scholar] [CrossRef]
- Sun, Y.; Lv, H.; Zhou, W.; Zhang, C.M. Research on hydrogen permeability of polyamide 6 as the liner material for type Ⅳ hydrogen storage tank. Int. J. Hydrogen Energy 2020, 45, 24980–24990. [Google Scholar] [CrossRef]
- Halldén, Å.; Deriss, M.J.; Wesslén, B. Morphology of LDPE/PA-6 blends compatibilised with poly(ethylene-graft-ethylene oxide)s. Polymer 2001, 42, 8743–8751. [Google Scholar] [CrossRef]
- Lei, Y.P.; Zhang, T.; Zhang, J.F.; Zhang, B.M. Dimensional stability and mechanical performance evolution of continuous carbon fiber reinforced polyamide 6 composites under hygrothermal environment. J. Mater. Res. Technol. 2021, 13, 2126–2137. [Google Scholar] [CrossRef]
- Wang, X.L.; Chen, X.D.; Yang, J.N.; Xie, P.C.; Yang, W.M. Research on torsional laminated extrusion for improving the permeability and mechanical properties of HDPE/PA6 composite for type IV storage tank liners. J. Appl. Polym. Sci. 2023, 140, e54567. [Google Scholar] [CrossRef]
- Lin, T.A.; Lou, C.W.; Lin, J.H. The Effects of Thermoplastic Polyurethane on the Structure and Mechanical Properties of Modified Polypropylene Blends. Appl. Sci. 2017, 7, 1254. [Google Scholar] [CrossRef]
- Salahshoori, I.; Namayandeh Jorabchi, M.; Valizadeh, K.; Yazdanbakhsh, A.; Bateni, A.; Wohlrab, S. A deep insight of solubility behavior, mechanical quantum, thermodynamic, and mechanical properties of Pebax-1657 polymer blends with various types of vinyl polymers: A mechanical quantum and molecular dynamics simulation study. J. Mol. Liq. 2022, 363, 119793. [Google Scholar] [CrossRef]
- Ivancic, R.J.S.; Audus, D.J. Predicting compatibilized polymer blend toughness. Sci. Adv. 2024, 10, eadk6165. [Google Scholar] [CrossRef]
- Tang, J.; Tang, W.H.; Yuan, H.L.; Jin, R.G. Mechanical behaviors of ethylene/styrene interpolymer compatibilized polystyrene/polyethylene blends. J. Appl. Polym. Sci. 2007, 104, 4001–4007. [Google Scholar] [CrossRef]
- Gartner, T.E., III; Jayaraman, A. Modeling and Simulations of Polymers: A Roadmap. Macromolecules 2019, 52, 755–786. [Google Scholar] [CrossRef]
- Li, Y.L.; Wang, S.J.; Wang, Q.; Xing, M. A comparison study on mechanical properties of polymer composites reinforced by carbon nanotubes and graphene sheet. Compos. Part B Eng. 2018, 133, 35–41. [Google Scholar] [CrossRef]
- Liu, F.; Hu, N.; Ning, H.M.; Atobe, S.; Yan, C.; Liu, Y.L.; Wu, L.K.; Liu, X.Y.; Fu, S.Y.; Xu, C.H.; et al. Investigation on the interfacial mechanical properties of hybrid graphene-carbon nanotube/polymer nanocomposites. Carbon 2017, 115, 694–700. [Google Scholar] [CrossRef]
- Venkatesan, K.R.; Koo, B.; Khafagy, K.H.; Chattopadhyay, A. Multiscale modeling of carbon nanotube-reinforced polymer with coarse-grain molecular dynamics informed morphology. Compos. Sci. Technol. 2022, 223, 109412. [Google Scholar] [CrossRef]
- Yang, B.; Wang, S.J.; Li, Y.L.; Tang, L.M.; He, E.Q.; Song, S.Y. Molecular dynamics simulations of mechanical properties of swollen nitrile rubber composites by incorporating carbon nanotubes. Polym. Compos. 2020, 41, 3160–3169. [Google Scholar] [CrossRef]
- Zhao, J.B.; Wu, L.L.; Zhan, C.X.; Shao, Q.; Guo, Z.H.; Zhang, L.Q. Overview of polymer nanocomposites: Computer simulation understanding of physical properties. Polymer 2017, 133, 272–287. [Google Scholar] [CrossRef]
- Wu, T.H.; Firoozabadi, A. Fracture Toughness and Surface Energy Density of Kerogen by Molecular Dynamics Simulations in Tensile Failure. J. Phys. Chem. C 2020, 124, 15895–15901. [Google Scholar] [CrossRef]
- Abhiram, B.R.; Ghosh, D. Atomic investigation on optimal interfacial bonding for enhanced fracture properties in polymer nanocomposites. Eng. Fract. Mech. 2023, 281, 109078. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, J.L.; Chen, H.B.; Li, G.; Hu, J.Q.; Zhang, H.Q.; Wang, B. Toughening mechanism of phthalonitrile polymer: MD simulation and experiment. Compos. Sci. Technol. 2023, 232, 109841. [Google Scholar] [CrossRef]
- Wu, P.; Zhang, J.M.; Yang, Z.H.; Zhao, J.P. Molecular Dynamics Simulation of Helium Barrier Performance of Modified Polyamide 6 Lining of IV Hydrogen Storage Tank with Montmorillonite. Molecules 2023, 28, 3333. [Google Scholar] [CrossRef]
- Fu, Y.; Liao, L.; Lan, Y.; Yang, L.; Mei, L.; Liu, Y.; Hu, S. Molecular dynamics and mesoscopic dynamics simulations for prediction of miscibility in polypropylene/polyamide-11 blends. J. Mol. Struct. 2012, 1012, 113–118. [Google Scholar] [CrossRef]
- BIOVIA Materials Studio 2017, version 17.1.0; Dassault Systemes Biovia Corp.: Waltham, MA, USA, 2017.
- Kowalik, M.; Ashraf, C.; Damirchi, B.; Akbarian, D.; Rajabpour, S.; van Duin, A.C.T. Atomistic Scale Analysis of the Carbonization Process for C/H/O/N-Based Polymers with the ReaxFF Reactive Force Field. J. Phys. Chem. B 2019, 123, 5357–5367. [Google Scholar] [CrossRef]
- Liu, L.C.; Liu, Y.; Zybin, S.V.; Sun, H.; Goddard, W.A., III. ReaxFF-lg: Correction of the ReaxFF Reactive Force Field for London Dispersion, with Applications to the Equations of State for Energetic Materials. J. Phys. Chem. A 2011, 115, 11016–11022. [Google Scholar] [CrossRef]
- van Duin, A.C.T.; Dasgupta, S.; Lorant, F.; Goddard, W.A. ReaxFF: A Reactive Force Field for Hydrocarbons. J. Phys. Chem. A 2001, 105, 9396–9409. [Google Scholar] [CrossRef]
- Lin, K.; Yu, T. Debonding simulation of fibre-matrix interfaces of FRP composites with reactive force field. Constr. Build. Mater. 2021, 312, 125304. [Google Scholar] [CrossRef]
- Broberg, K.B. Critical review of some theories in fracture mechanics. Int. J. Fract. Mech. 1968, 4, 11–19. [Google Scholar] [CrossRef]
- Bárány, T.; Czigány, T.; Karger-Kocsis, J. Application of the essential work of fracture (EWF) concept for polymers, related blends and composites: A review. Prog. Polym. Sci. 2010, 35, 1257–1287. [Google Scholar] [CrossRef]
- Fotoohi, A.; Ashenai Ghasemi, F.; Fasihi, M.; Rajaee, P. Mechanical behavior and essential work of fracture of surface-modified exfoliated graphite filled polypropylene/ethylene-vinyl acetate blend nano-composites. J. Appl. Polym. Sci. 2023, 140, e53841. [Google Scholar] [CrossRef]

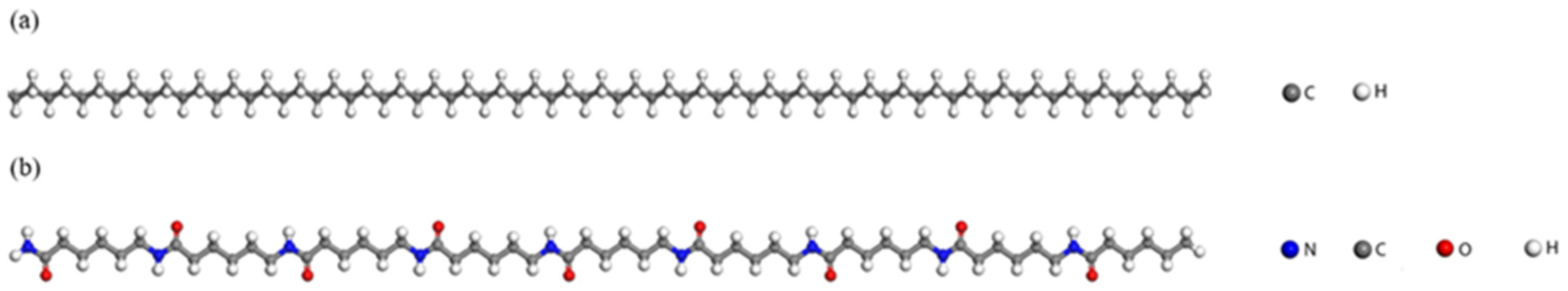

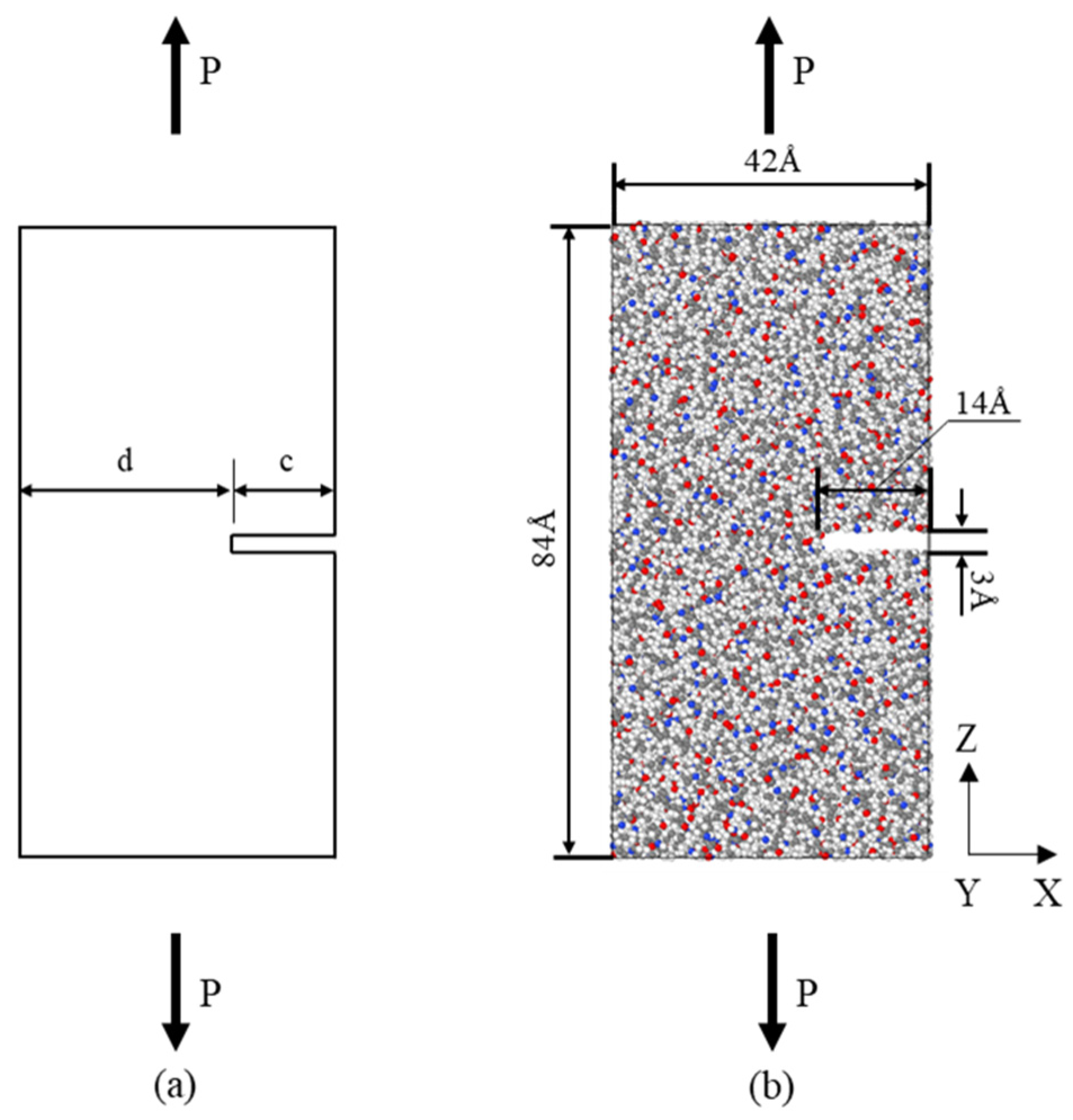
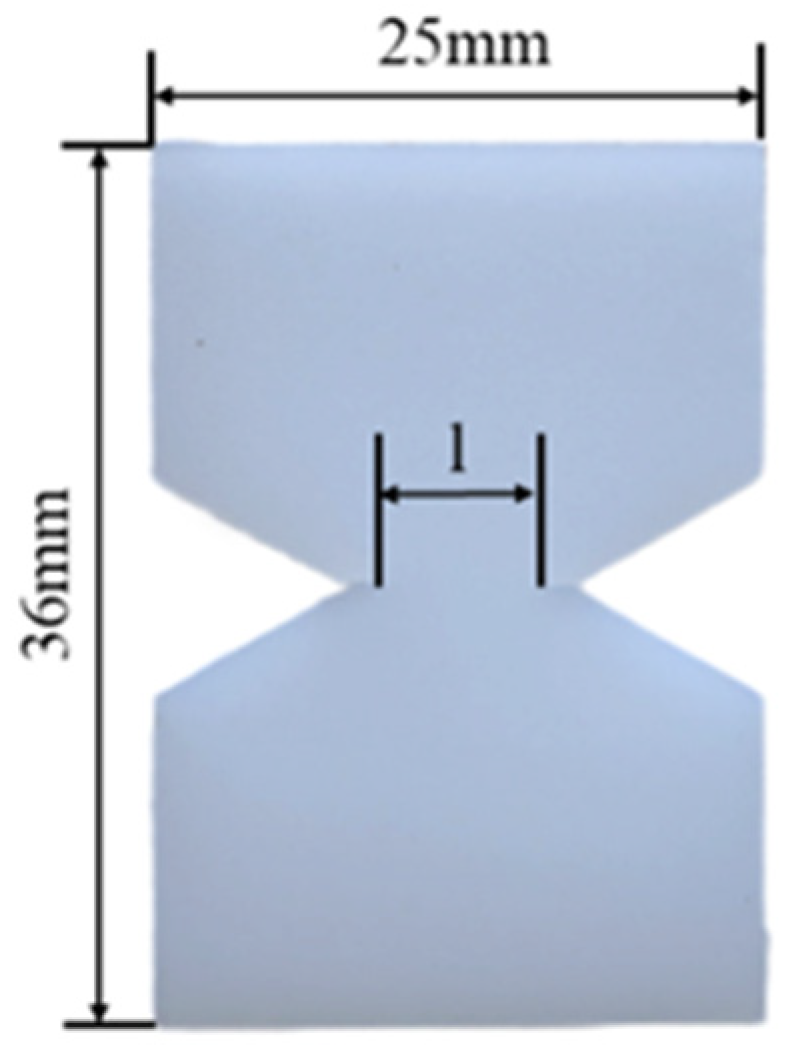
| Models | Number of HDPE Chains | Number of PA6 Chains |
|---|---|---|
| PA6 | 0 | 110 |
| 3HDPE/7PA6 | 15 | 72 |
| 4HDPE/6PA6 | 19 | 59 |
| 6HDPE/4PA6 | 27 | 37 |
| 7HDPE/3PA6 | 31 | 28 |
| HDPE | 43 | 0 |
| Material | Tensile Strength (GPa) | Ultimate Strain (%) | J-Integral (N m−1) | Improvement in J (%) |
|---|---|---|---|---|
| PA6 | 0.522 | 0.915 | 3.035 | Baseline |
| 3HDPE/7PA6 | 0.486 | 1.110 | 3.096 | 2 |
| 4HDPE/6PA6 | 0.456 | 1.345 | 3.291 | 8 |
| 6HDPE/4PA6 | 0.418 | 1.635 | 3.441 | 13 |
| 7HDPE/3PA6 | 0.411 | 2.060 | 3.619 | 19 |
| HDPE | 0.367 | 2.540 | 4.436 | 46 |
| Material | we (kJ m−2) | R2 | Improvement in we (%) |
|---|---|---|---|
| PA6 | 78.12 | 0.94 | Baseline |
| 4HDPE/6PA6 | 86.47 | 0.98 | 11 |
| 7HDPE/3PA6 | 89.61 | 0.98 | 15 |
| HDPE | 112.67 | 0.99 | 44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, B.; Luo, J.; Wu, Y.; Yang, Z.; Zhao, J. Enhancement of Fracture Toughness of Inner Liner Material for Type IV Hydrogen Storage Cylinders Based on Molecular Dynamics Method. Materials 2025, 18, 1363. https://doi.org/10.3390/ma18061363
Yang B, Luo J, Wu Y, Yang Z, Zhao J. Enhancement of Fracture Toughness of Inner Liner Material for Type IV Hydrogen Storage Cylinders Based on Molecular Dynamics Method. Materials. 2025; 18(6):1363. https://doi.org/10.3390/ma18061363
Chicago/Turabian StyleYang, Bingyu, Jinqi Luo, Yuan Wu, Zhenhan Yang, and Jianping Zhao. 2025. "Enhancement of Fracture Toughness of Inner Liner Material for Type IV Hydrogen Storage Cylinders Based on Molecular Dynamics Method" Materials 18, no. 6: 1363. https://doi.org/10.3390/ma18061363
APA StyleYang, B., Luo, J., Wu, Y., Yang, Z., & Zhao, J. (2025). Enhancement of Fracture Toughness of Inner Liner Material for Type IV Hydrogen Storage Cylinders Based on Molecular Dynamics Method. Materials, 18(6), 1363. https://doi.org/10.3390/ma18061363






