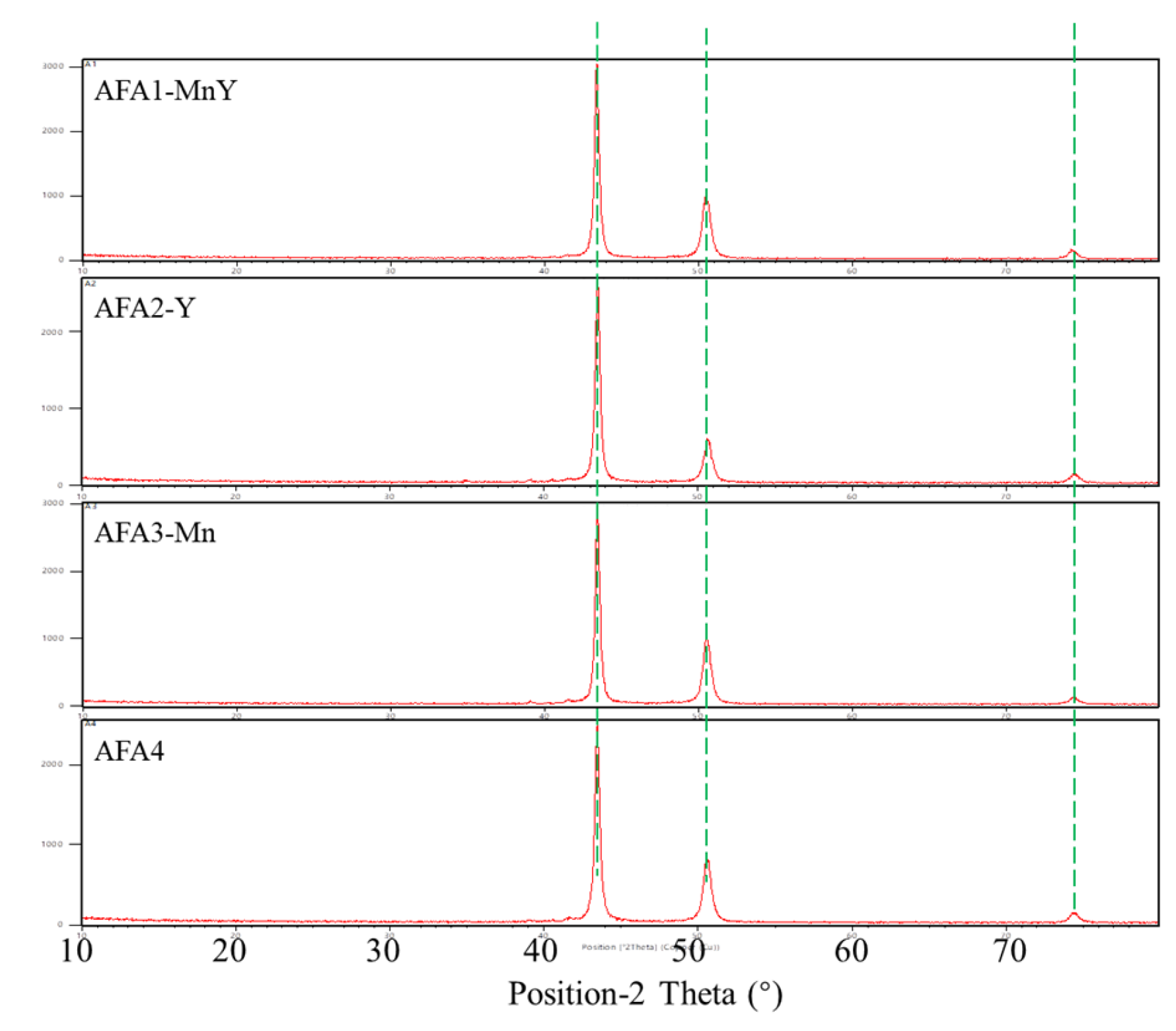
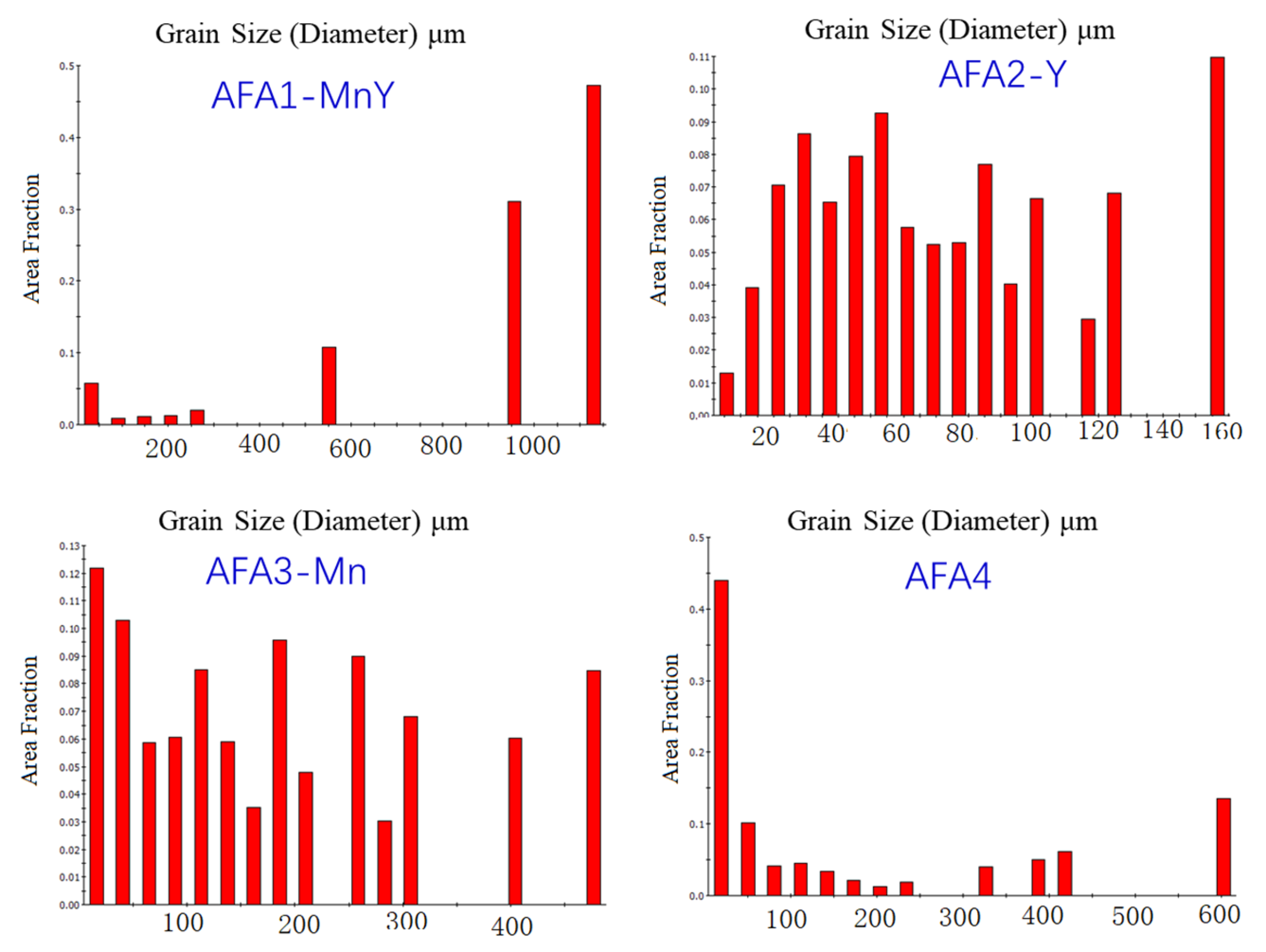
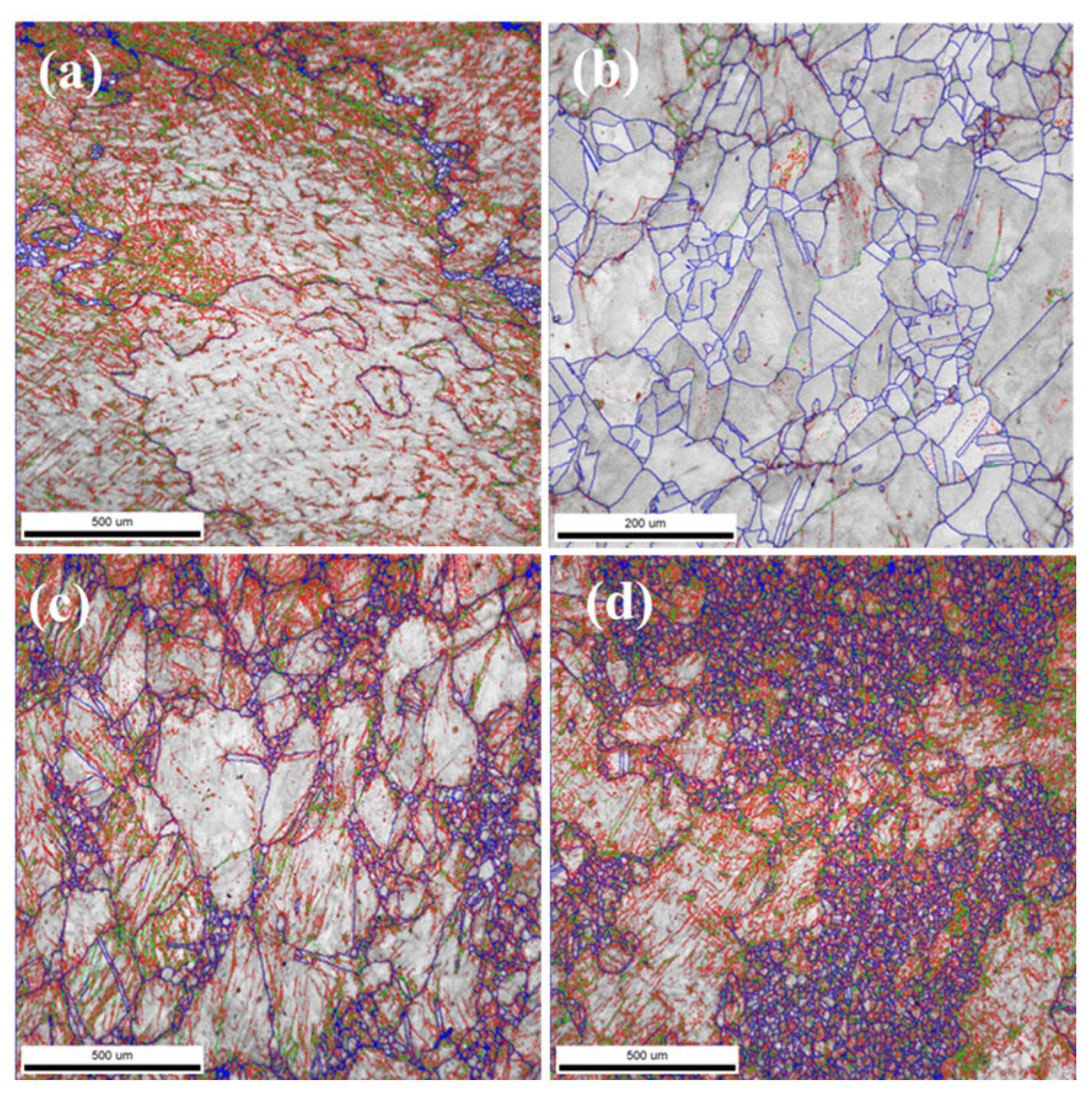
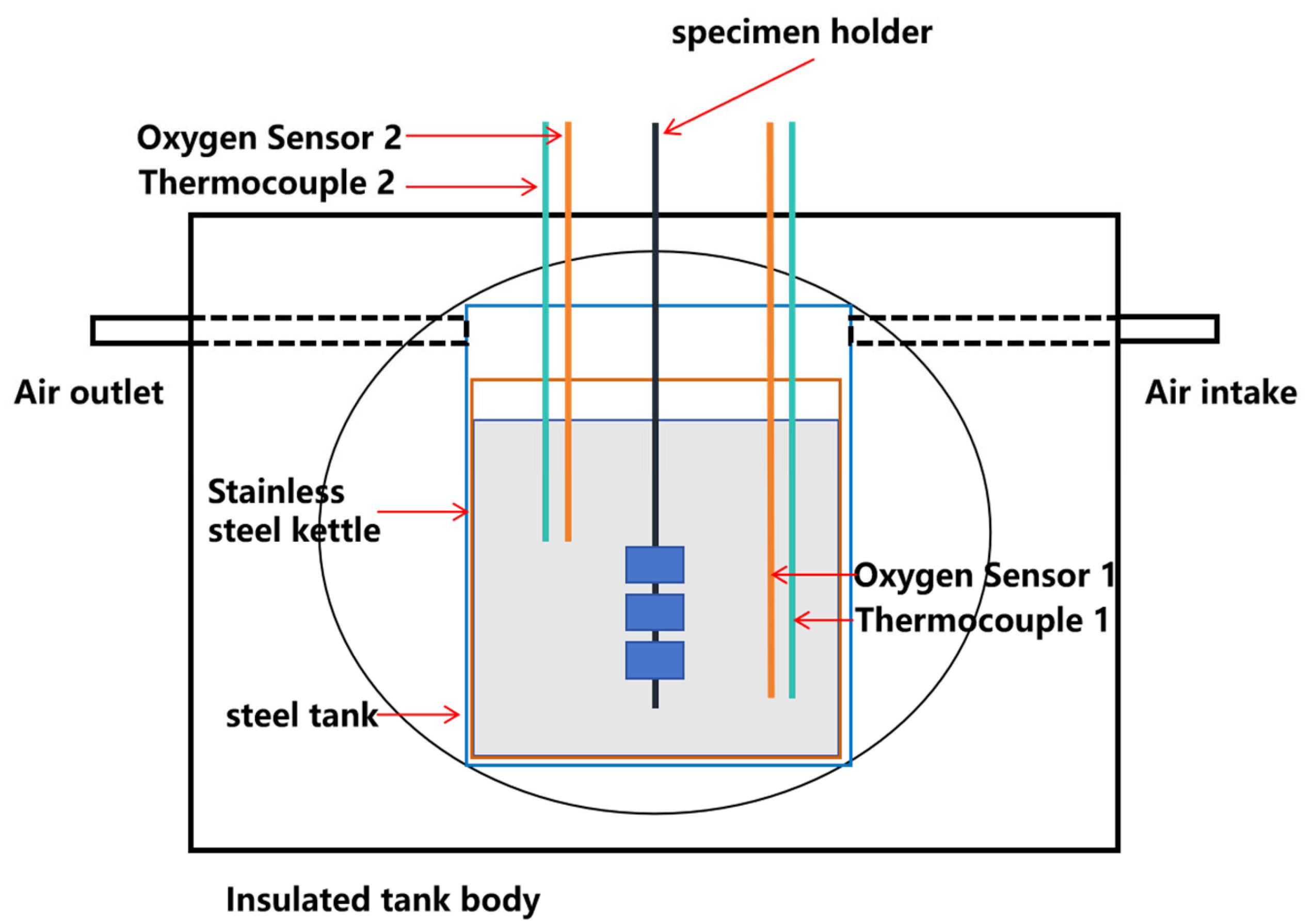
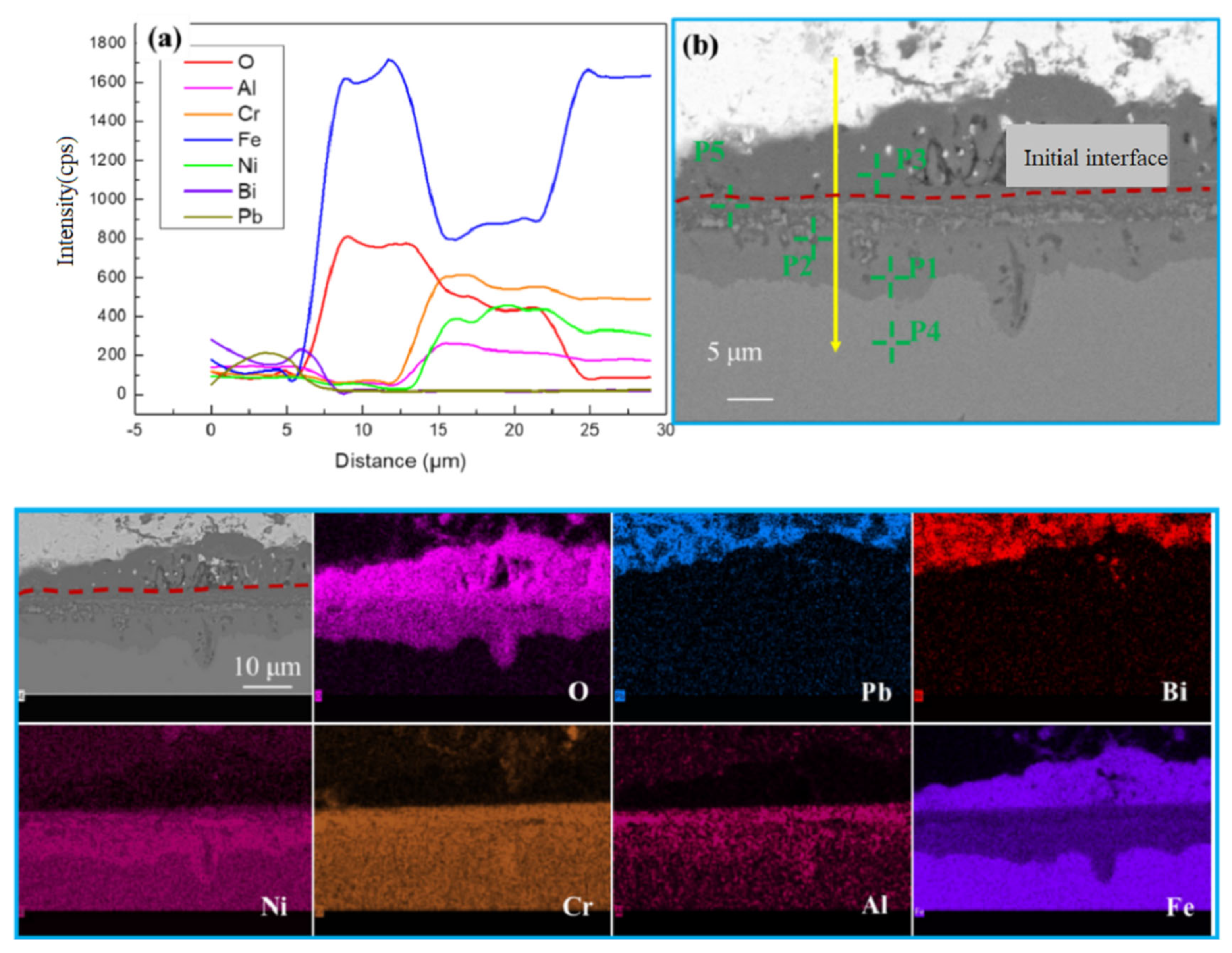
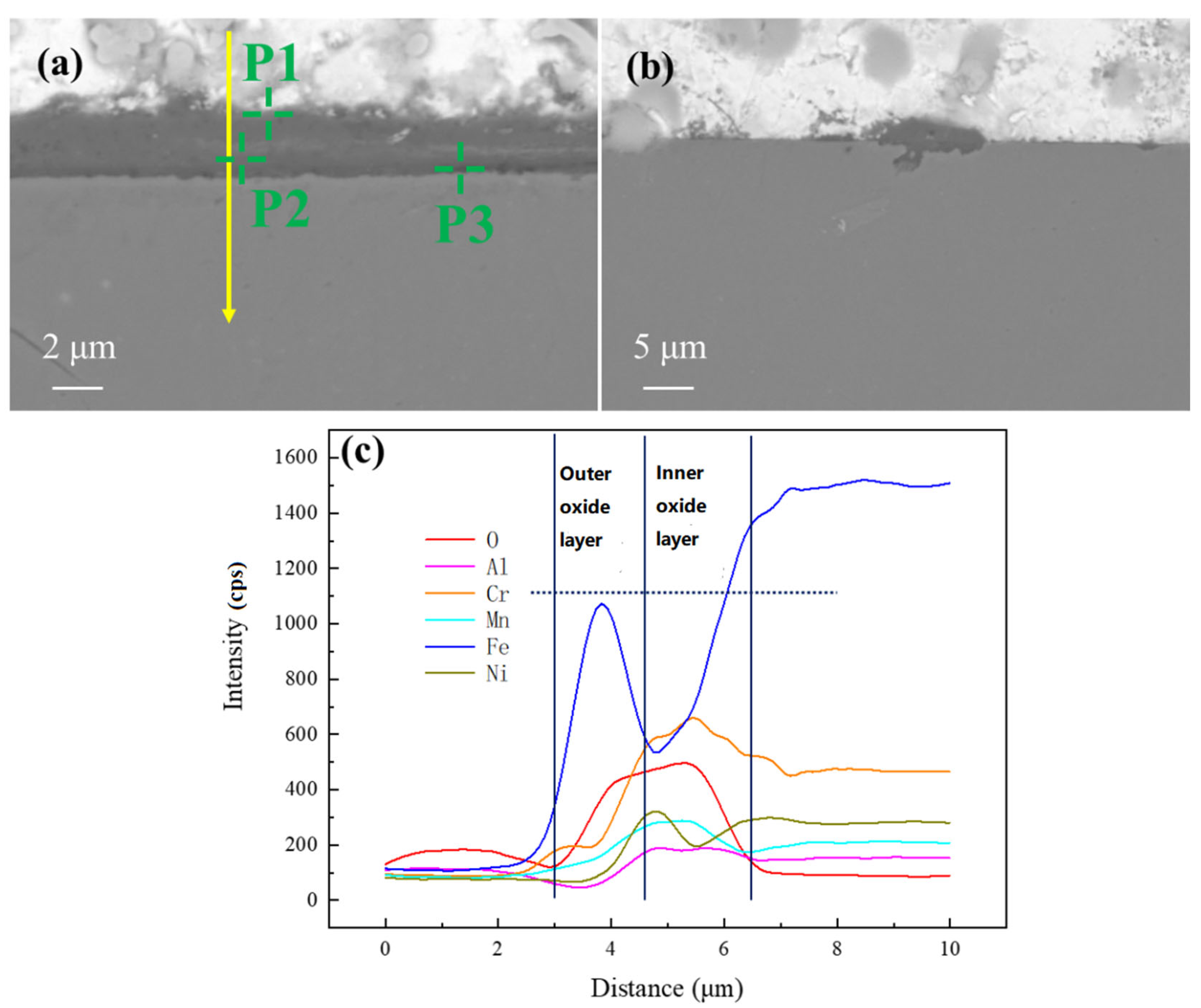
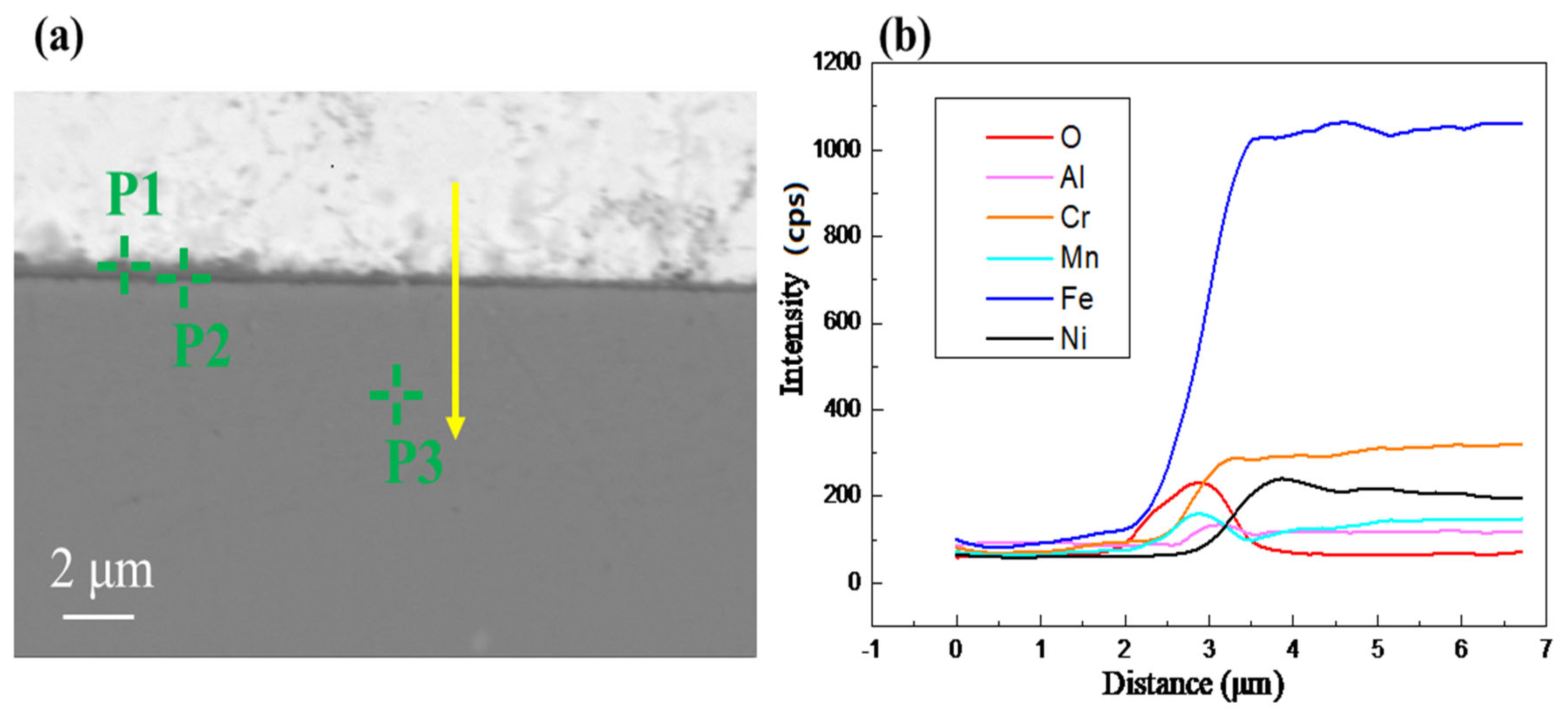
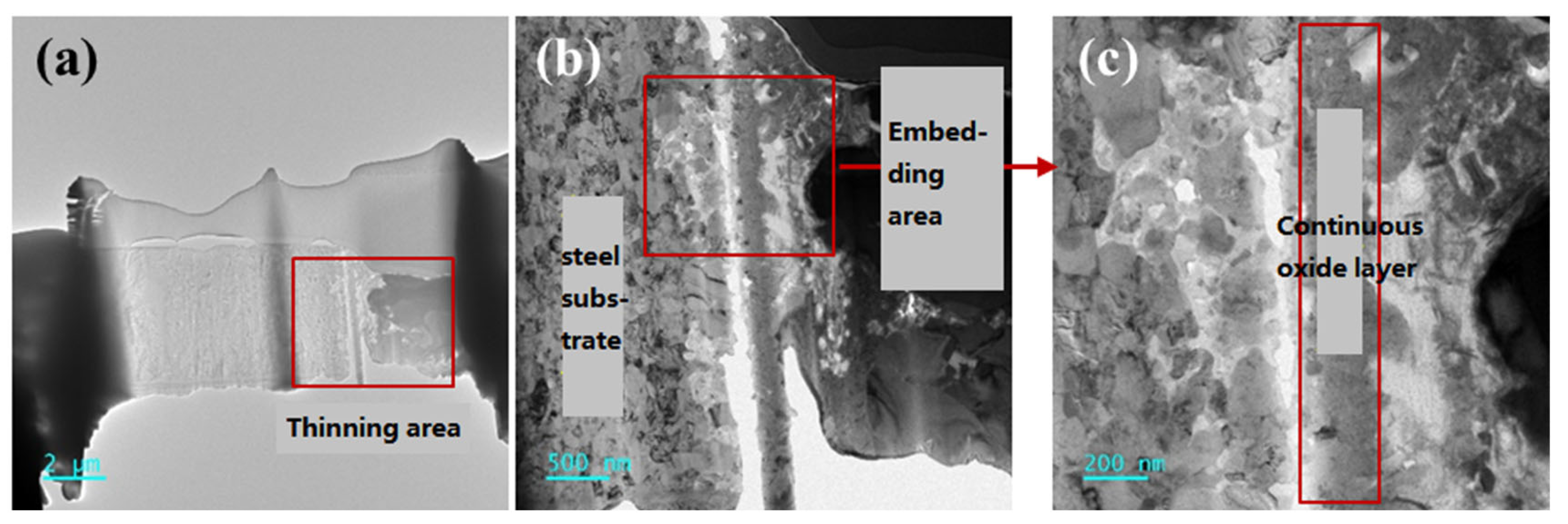
1. Introduction
The lead–bismuth eutectic (LBE) coolant possesses excellent neutronic, thermohydraulic, chemical inertness, and inherent safety properties, which make it highly promising for applications in lead-cooled reactors, accelerator-driven subcritical nuclear energy systems (ADS), and fusion reactors [
1]. However, the severe corrosion of structural materials caused by liquid metal LBE in lead-cooled reactors poses significant challenges. Conventional commercial austenitic stainless steels (e.g., 316L, 304L, and 15-5Ti) and ferritic/martensitic steels (e.g., T91, HT9, P22, and EP823) fail to meet the service performance requirements under the high-temperature operational conditions of future lead-cooled reactors.
The severity of corrosion in lead-cooled reactors lies in the co-occurrence of dissolution and oxidation corrosion of structural materials in liquid LBE. Research by Valentyn Tsisar et al. [
2,
3] on austenitic steels (1.4970, 316L, and 1.4571) in 550 °C LBE with an oxygen concentration of 10
−6 wt.% shows that although oxidation dominates, dissolution corrosion caused by selective leaching of elements remains significant. Over time, beneath the initially formed oxide layer composed of α-Cr
2O
3 and spinel (Fe,Cr)
xO
y, Pb and Bi penetration is also observed, indicating that Cr-containing oxide layers at elevated temperatures are insufficient to resist LBE corrosion. Developing new materials with excellent resistance to high-temperature LBE corrosion is one of the critical challenges for enabling the engineering application of lead-cooled reactors [
4].
The standard Gibbs free energy of formation for Al oxides is lower compared to Fe, Cr, and Si, allowing for the formation of a dense and thermodynamically stable Al
2O
3 film. Alumina-forming austenitic (AFA) alloys were proposed by Oak Ridge National Laboratory in 2007 [
5,
6]. These alloys are novel materials developed by adding Al and Nb to ultrafine-grained steels. The bulk contains numerous fine precipitates that enhance the material’s strength and resistance to radiation damage. AFA alloys exhibit excellent corrosion resistance and high-temperature creep resistance.
Research on the application of AFA alloys in lead-cooled reactors remains relatively limited. Ejenstam et al. [
7] reported that after nearly one year of corrosion testing in liquid lead, 15Cr-Nb AFA alloys exhibited significantly reduced corrosion compared to 15-15Ti austenitic steel, forming an ultrathin oxide layer containing k-Al
2O
3 or θ-Al
2O
3. Al-containing oxide films [
8] comprise Fe/Cr oxides, Cr/Al oxides, and Al
2O
3. Beneath the oxide film, a NiAl phase acts as a reservoir for Al diffusion to sustain the growth of the Al
2O
3 protective layer, providing long-term self-healing corrosion resistance [
9,
10]. Studies in 600 °C liquid lead showed that the AFA alloy surface formed an ultrathin oxide layer (~100 nm) of (Al
0.77Cr
0.23)
2O
3 [
11].
The oxidation mechanism of oxygen-controlled LBE is similar to that of metal oxidation in other oxygen-containing environments [
12]. The “available space model” can explain the formation of oxidation zones and ion migration on the alloy surface in LBE [
13]. This model suggests that Al can reduce oxygen ingress into the material by forming a continuous and dense oxide film, thereby enhancing corrosion resistance. The rate of alumina formation is controlled by the diffusion of oxygen ions through the oxide layer to the substrate and the migration of metal ions to the oxide–liquid interface [
14]. The migration of oxygen through the Al
2O
3 layer is governed by the concentration of oxygen vacancies [
15]. The presence of multivalent cations with valences higher than Al
3+ reduces the concentration of oxygen vacancies to compensate for the increased positive charge caused by aliovalent cation impurities. This suppresses the growth rate of the alumina film. These findings suggest that the formation of a stable oxide film requires not only a high thermodynamic driving force but also consideration of the oxide layer formation rate. The resistance of materials to lead–bismuth corrosion can be improved kinetically by promoting the formation of Al
2O
3 in a competitive relationship between oxidation and dissolution.
Since Ni has a high solubility in liquid lead and Mn, which stabilizes the austenitic structure in a manner similar to Ni, has a solubility in LBE that is about ten times lower than that of Ni, replacing part of the Ni with Mn appears beneficial for mitigating dissolution corrosion. Additionally, Mn substitution for Ni is expected to improve the alloy’s radiation resistance and significantly reduce costs. Mn-substituted AFA alloys exhibit great potential and demand for corrosion resistance in LBE. Studies have shown that in high-temperature air oxidation environments, Mn readily forms external oxide layers [
16]. For Mn-containing AFA alloys with approximately 50% reduced Ni content, creep-rupture lifetimes under harsh conditions (1023 K and 100 MPa) range from 7.2 × 10
5 to 1.8 × 10
6 s (200–500 h), demonstrating improved performance compared to 347 stainless steel, indicating that mechanical properties remain at a high level after Mn substitution for Ni [
17].
However, the substitution of Mn for Ni in AFA steels presents certain challenges. Manganese is an element prone to segregation and exhibits relatively high solubility in Cr
2O
3 oxide scales, which can facilitate the transformation of the oxide into a spinel-structured CrMn
1.5O
4. In AFA steels, the formation rate of Cr
2O
3 oxide scales is faster than that of Al
2O
3 oxide scales. Under such conditions, the Cr
2O
3 oxide scale may transform into the spinel-structured CrMn
1.5O
4 before the formation of the Al
2O
3 oxide scale, significantly compromising the oxidation resistance of AFA steels [
18,
19,
20].
Nevertheless, studies have also indicated that the solubility of Mn in Al
2O
3 oxide scales is nearly negligible, which does not impair the protective effect of the alumina oxide scale. Additionally, the other properties of austenitic stainless steels with Mn substituting for Ni remain acceptable [
21,
22,
23,
24]. This makes it feasible to develop cost-effective, high-performance AFA steels.
A critical issue in developing Mn-containing AFA steels is determining the appropriate Mn content. An increase in Mn content can inhibit the precipitation of the NiAl phase, which is one of the sources of aluminum for the formation of Al
2O
3 oxide scales. Consequently, higher Mn content may suppress the formation of alumina oxide scales [
25]. Moreover, if Mn-containing AFA steels are to be used in liquid metal environments, electrochemical corrosion cannot be overlooked. Research has shown that Mn can form non-metallic inclusions such as MnS with sulfur, and the potential difference between these inclusions and the matrix can lead to severe electrochemical reactions, degrading the corrosion resistance of the matrix [
26,
27].
This study prepared various novel Mn-containing AFA alloys with low Cr and Ni content, investigating their corrosion resistance in high-temperature LBE (liquid bismuth–lead eutectic) environments, with the aim of identifying Mn-containing AFA steels suitable for application in high-temperature LBE conditions.