A Study on the Microstructure and Properties of Cu-Fe-Mg-Ti Alloys Based on Composition Regulation
Abstract
1. Introduction
2. Materials and Methods
3. Results
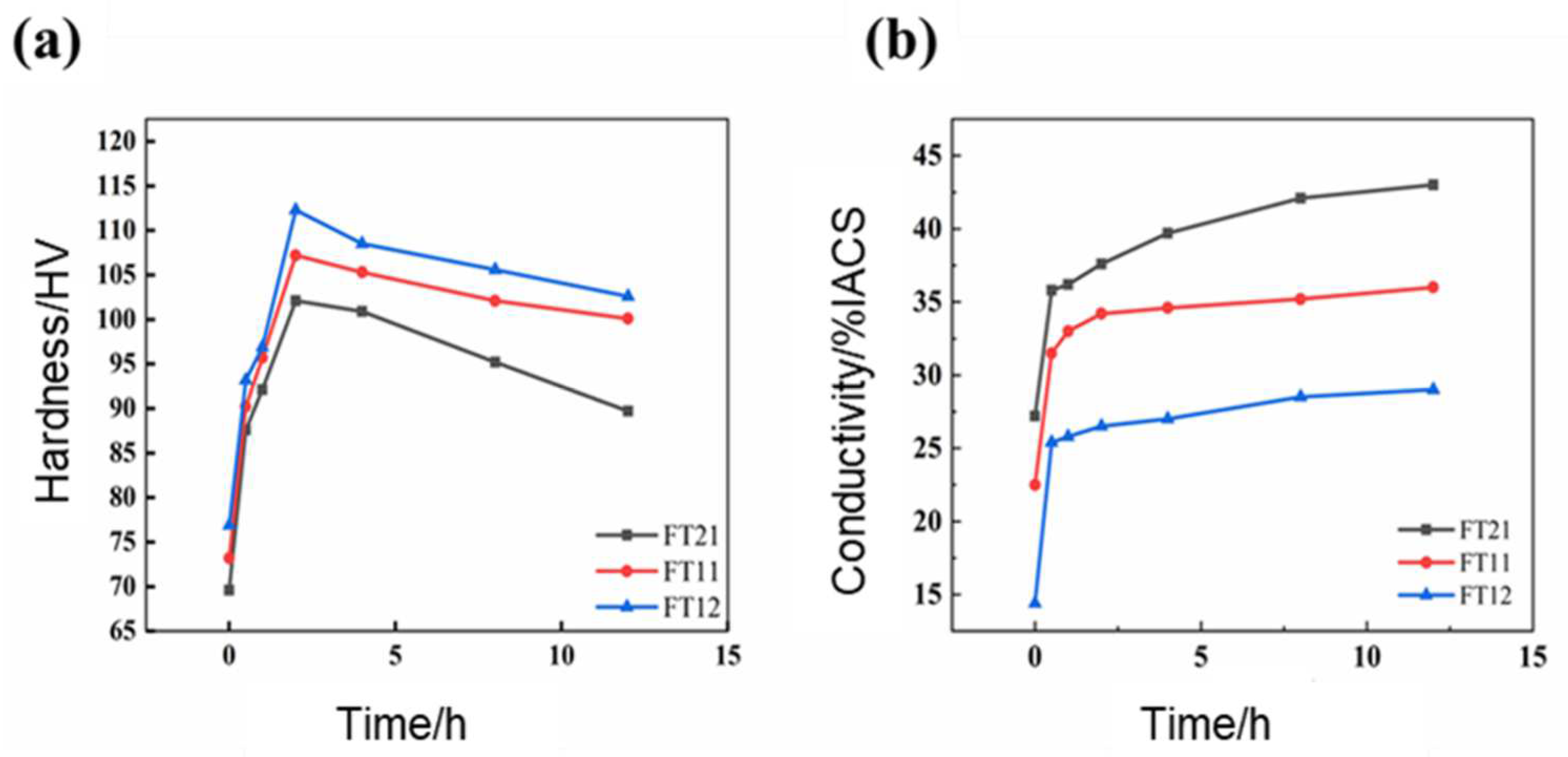
3.1. Mechanical Properties
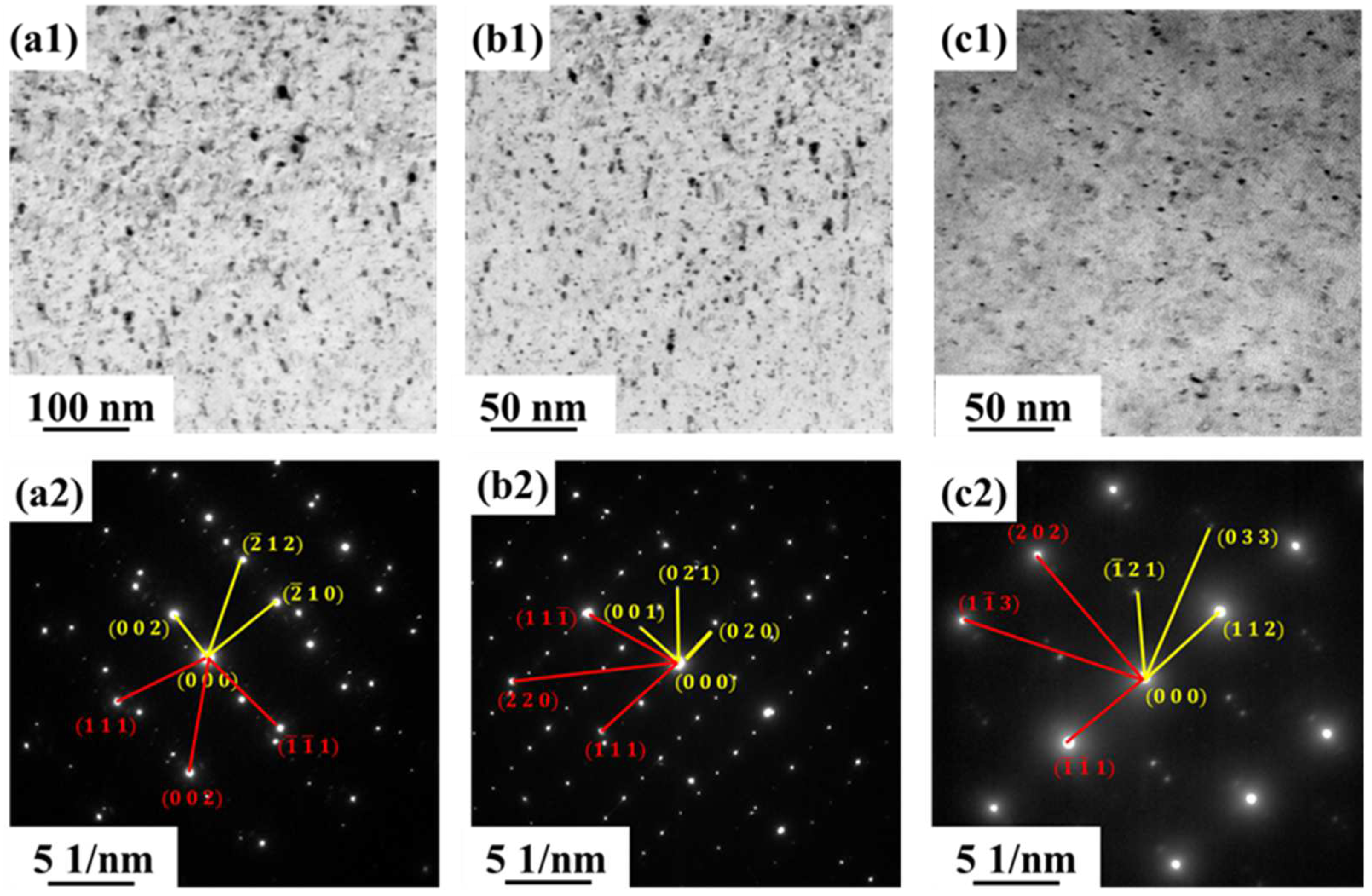
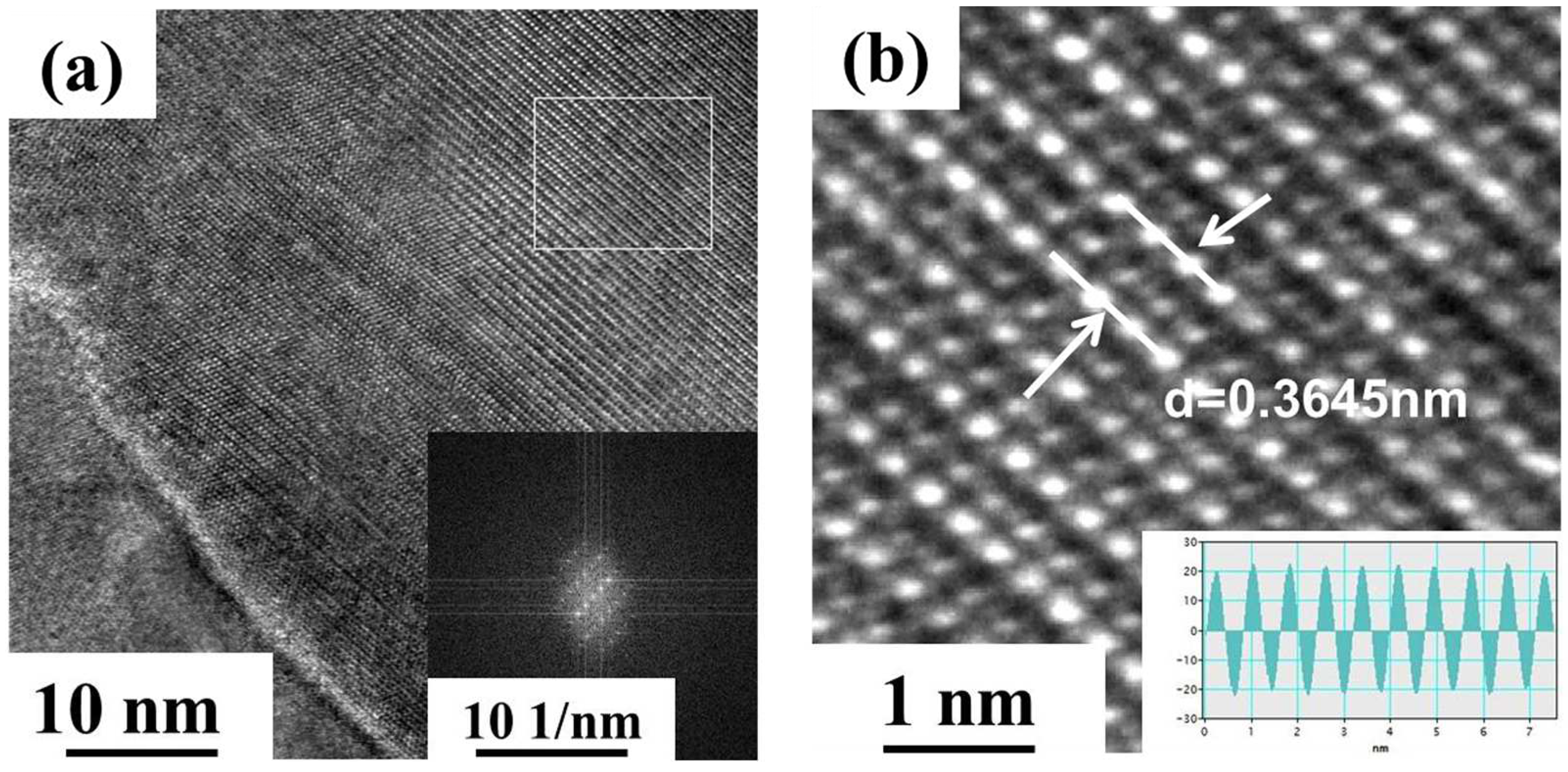
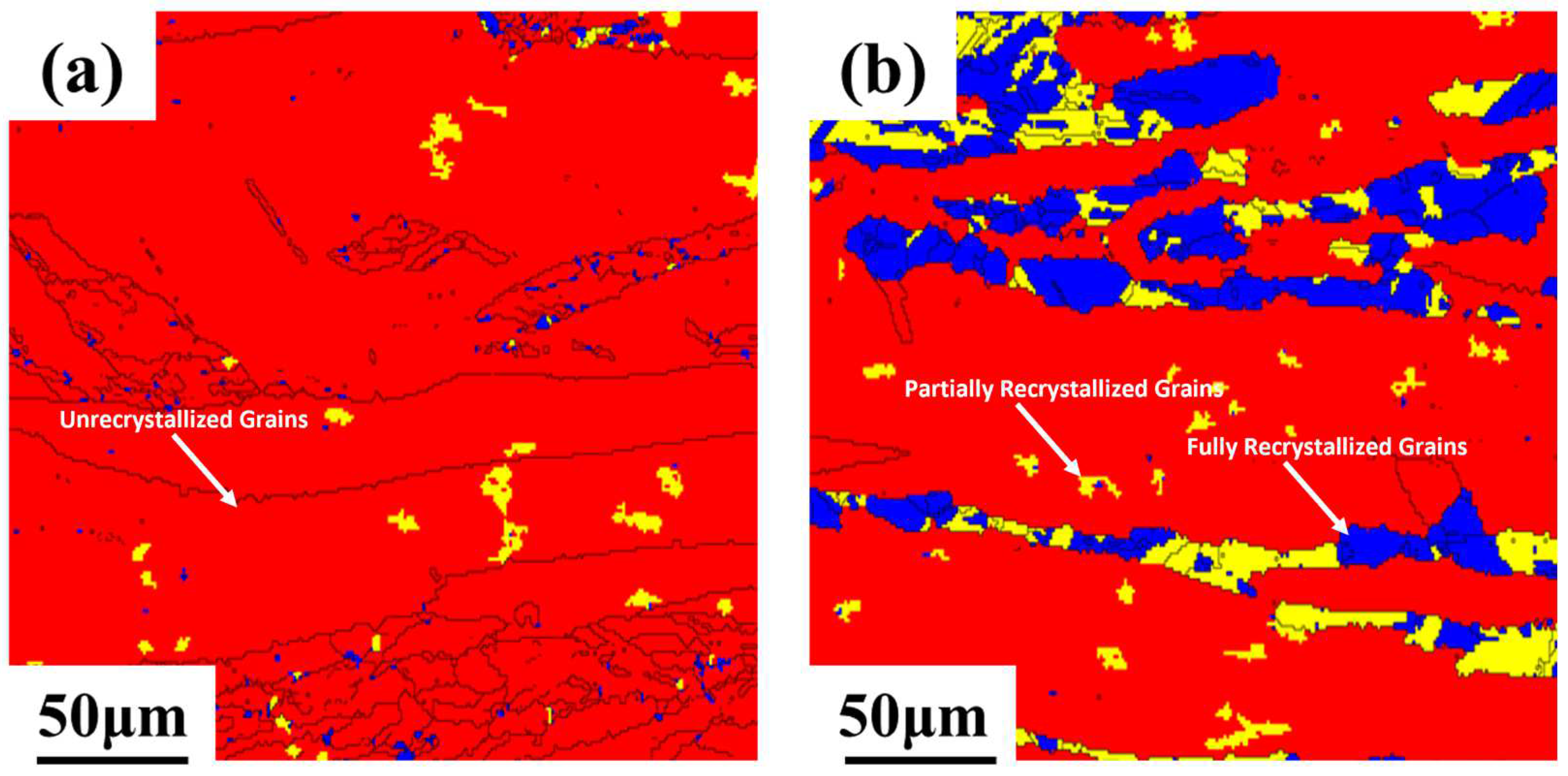
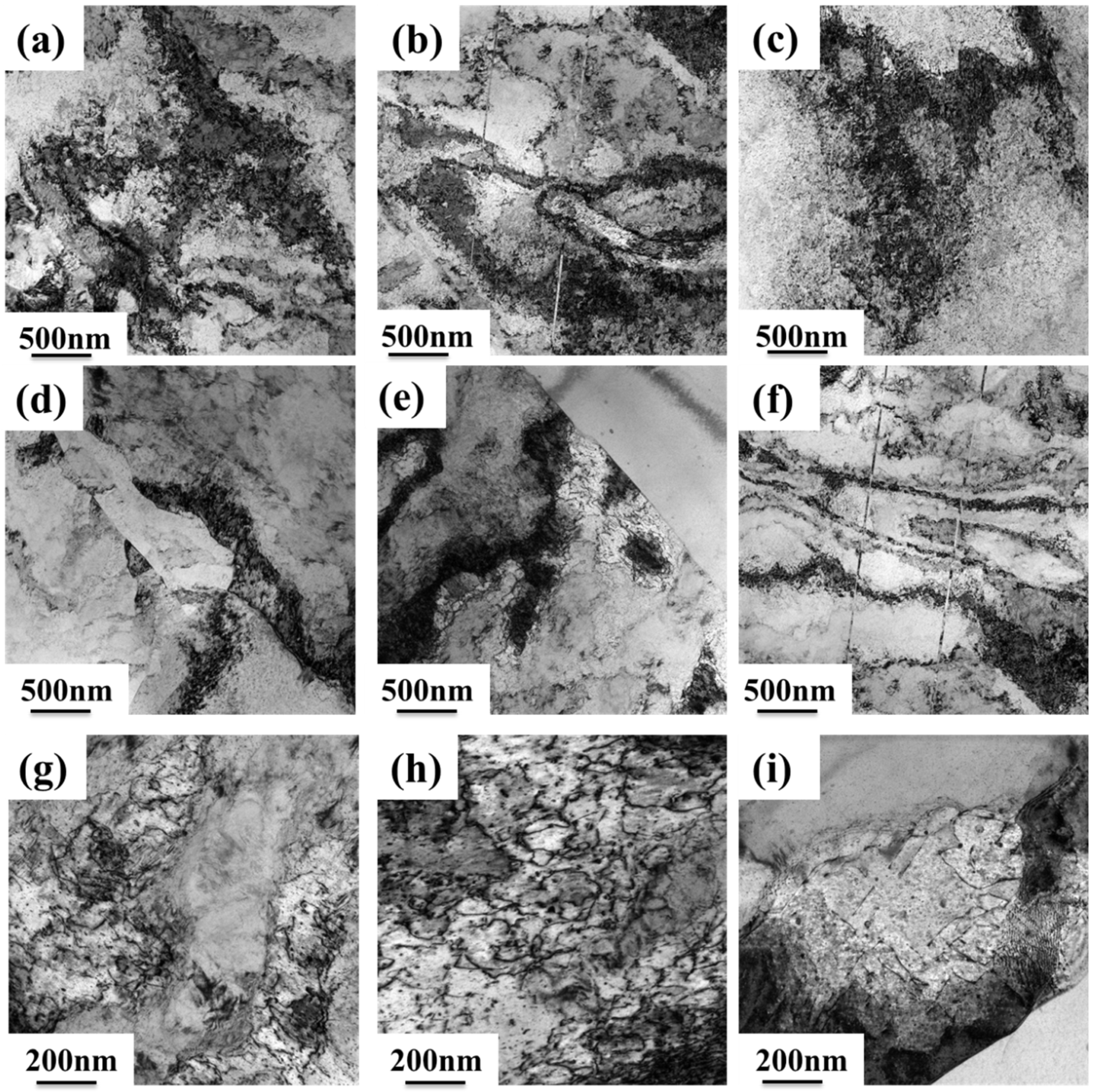
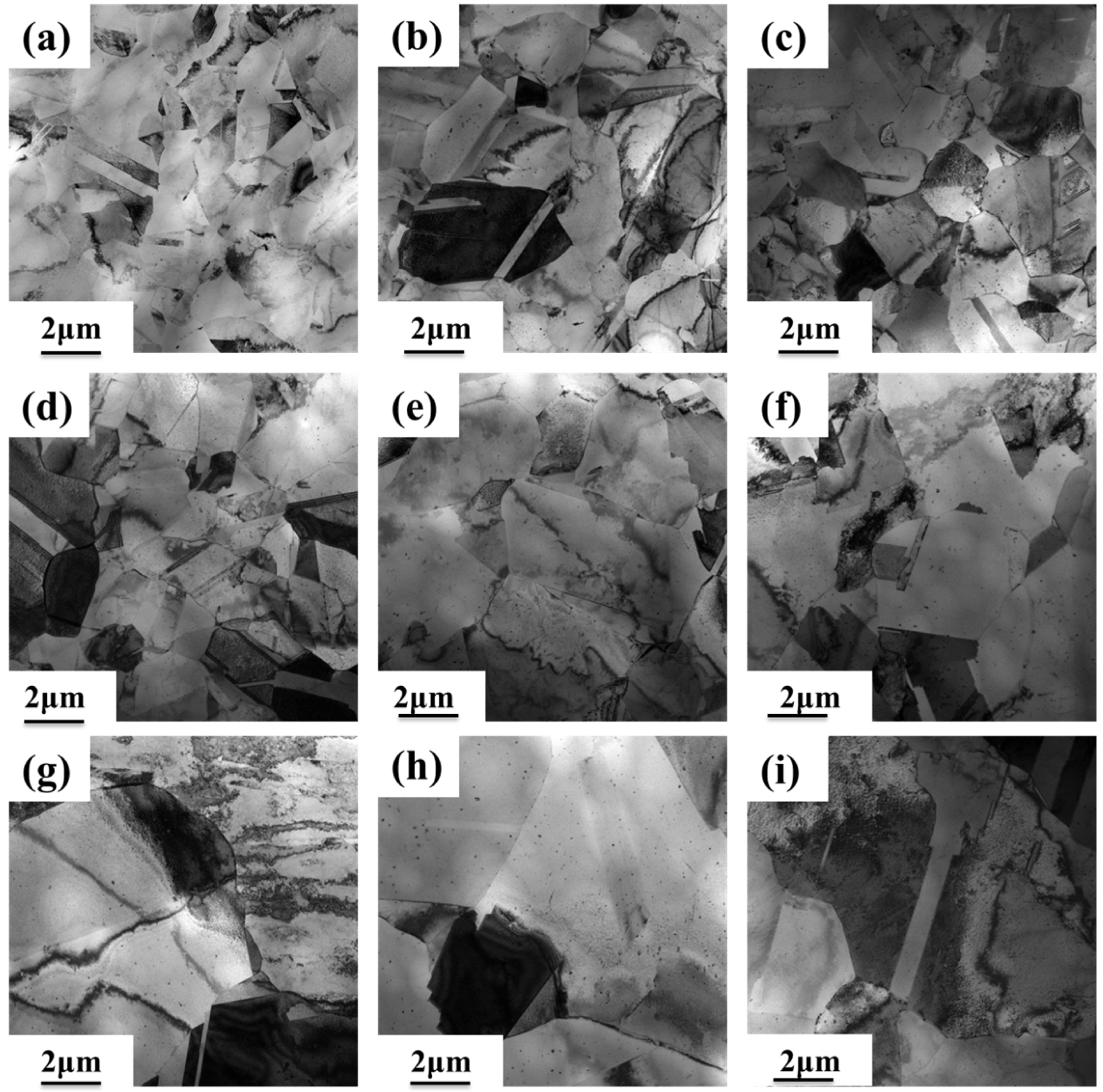
3.2. Microstructure of the Alloy
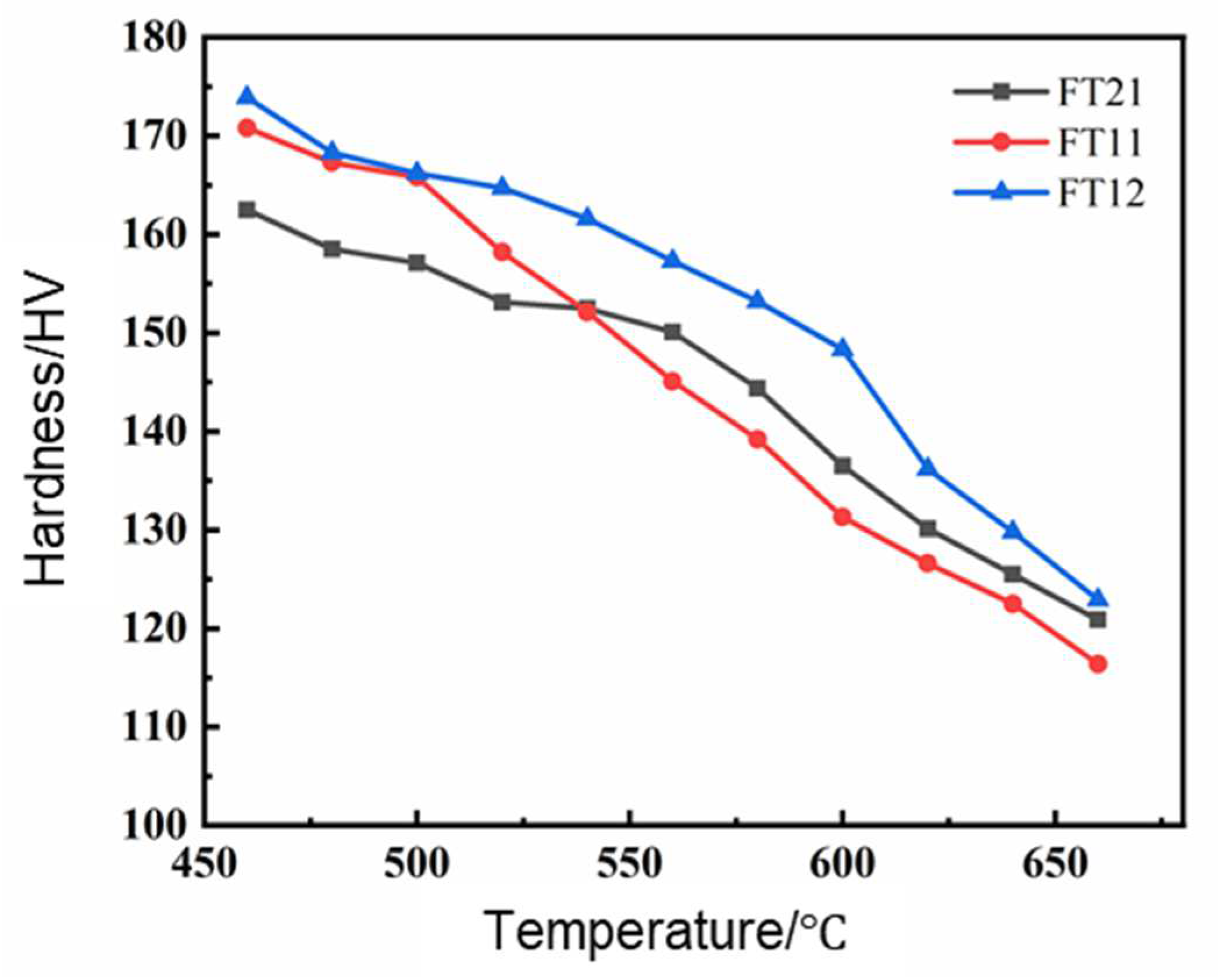
3.3. High-Temperature Resistance to Softening
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lei, Q.; Yang, Y.; Zhu, X.; Jiang, Y.; Gong, S.; Li, Z. Research Progress and Prospect on High Strength, High Conductivity, and High Heat Resistance Copper Alloys. Cailiao Daobao/Mater. Rep. 2021, 35, 15153–15161. [Google Scholar]
- Jiang, Y.; Lou, H.; Xie, H.; Li, T.; Song, K.; Liu, X.; Yun, X.; Wang, H.; Xiao, Z.; Li, Z. Development Status and Prospects of Advanced Copper Alloy. Strateg. Study Chin. Acad. Eng. 2020, 22, 84–92. [Google Scholar] [CrossRef]
- Božić, D.; Dimčić, O.; Dimčić, B.; Cvijović, I.; Rajković, V. The combination of precipitation and dispersion hardening in powder metallurgy produced Cu–Ti–Si alloy. Mater. Charact. 2008, 59, 1122–1126. [Google Scholar] [CrossRef]
- Li, J.; Ding, H.; Li, B.; Gao, W.; Bai, J.; Sha, G. Effect of Cr and Sn additions on microstructure, mechanical-electrical properties and softening resistance of Cu–Cr–Sn alloy. Mater. Sci. Eng. A 2021, 802, 140628. [Google Scholar] [CrossRef]
- Fan, J.; Cao, J.; Zheng, L. Effect of heat treatment on properties of Cu-0.4Co-0.2Ni-0.2Sn copper alloy with large deformation cold working. Jinshu Rechuli/Heat Treat. Met. 2019, 44, 165–168. [Google Scholar]
- Liu, J.; Wang, X.; Chen, J.; Liu, J. The effect of cold rolling on age hardening of Cu-3Ti-3Ni-0.5Si alloy. J. Alloys Compd. 2019, 797, 370–379. [Google Scholar] [CrossRef]
- Li, L.; Pan, D.A.; Li, B.; Wu, Y.; Wang, H.; Gu, Y.; Zuo, T. Patterns and challenges in the copper industry in China. Resour. Conserv. Recycl. 2017, 127, 1–7. [Google Scholar] [CrossRef]
- Chen, W.; Hu, X.; Guo, W.; Zou, J.; Liu, K.; Lu, D.; Tan, D. Effects of C Addition on the Microstructures of As-Cast Cu-Fe-P Alloys. Materials 2019, 12, 2772. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, K.H.; Lee, S.B.; Kim, B.M. Study of edge-cracking characteristics during thin-foil rolling of Cu-Fe-P strip. Int. J. Precis. Eng. Manuf. 2013, 14, 2109–2118. [Google Scholar] [CrossRef]
- Lu, D.; Wang, J.; Lu, L.; Liu, Y.; Xie, S.; Sun, B. Effect of Boron and Cerium on Microstructures and Properties of Cu-Fe-P Alloy. J. Rare Earths 2006, 24, 602–606. [Google Scholar] [CrossRef]
- Chen, J.; Wang, J.; Xiao, X.; Wang, H.; Chen, H.; Yang, B. Contribution of Zr to strength and grain refinement in Cu–Cr–Zr alloy. Mater. Sci. Eng. A 2019, 756, 464–473. [Google Scholar] [CrossRef]
- Bodyakova, A.I.; Mishnev, R.V.; Kaibyshev, R.O. Microstructure and Properties of Cu–Cr–Zr Alloys After Plastic Deformation and Aging. Met. Sci. Heat Treat. 2024, 66, 343–354. [Google Scholar] [CrossRef]
- Wang, H.; Gong, L.; Liao, J.; Chen, H.; Xie, W.; Yang, B. Retaining meta-stable fcc-Cr phase by restraining nucleation of equilibrium bcc-Cr phase in CuCrZrTi alloys during ageing. J. Alloys Compd. 2018, 749, 140–145. [Google Scholar] [CrossRef]
- Xiao, X.; Yi, Z.; Chen, T.; Liu, R.; Wang, H. Suppressing spinodal decomposition by adding Co into Cu–Ni–Si alloy. J. Alloys Compd. 2016, 660, 178–183. [Google Scholar] [CrossRef]
- Suzuki, S.; Shibutani, N.; Mimura, K.; Isshiki, M.; Waseda, Y. Improvement in strength and electrical conductivity of Cu–Ni–Si alloys by aging and cold rolling. J. Alloys Compd. 2006, 417, 116–120. [Google Scholar] [CrossRef]
- Huang, J.; Xiao, Z.; Dai, J.; Li, Z.; Jiang, H.; Wang, W.; Zhang, X. Microstructure and Properties of a Novel Cu–Ni–Co–Si–Mg Alloy with Super-high Strength and Conductivity. Mater. Sci. Eng. A 2019, 744, 754–763. [Google Scholar] [CrossRef]
- Sun, Y.; Xu, G.; Feng, X.; Peng, L.; Huang, G.; Xie, H.; Mi, X.; Liu, X. Effect of Ag on Properties, Microstructure, and Thermostability of Cu-Cr Alloy. Materials 2020, 13, E5386. [Google Scholar] [CrossRef]
- Liu, K.; Huang, Z.; Zhang, X.; Lu, D.; Atrens, A.; Zhou, H.; Yin, Y.; Yu, J.; Guo, W. Influence of Ag micro-alloying on the thermal stability and ageing characteristics of a Cu–14Fe in-situ composite. Mater. Sci. Eng. A 2016, 673, 1–7. [Google Scholar] [CrossRef]
- Garbacz-Klempka, A.; Czekaj, E.; Kozana, J.; Perek-Nowak, M.; Piękoś, M. Influence of Al and Fe additions on structure and properties of Cu-Sn alloys. Key Eng. Mater. 2016, 682, 226–235. [Google Scholar] [CrossRef]
- Wendt, H.; Wagner, R. Mechanical properties of copper-iron alloys in the transition from solid solution to precipitation hardening. Acta Metall. 1982, 30, 1561–1570. [Google Scholar] [CrossRef]
- Nagarjuna, S.; Balasubramanian, K.; DS, S. Effects of cold work on precipitation hardening of Cu-4.5 mass% Ti alloy. Mater. Trans. JIM 1995, 36, 1058–1066. [Google Scholar] [CrossRef]
- Dong, Q.; Wang, M.; Shen, L.; Jia, Y.; Li, Z. Diffraction analysis of α-Fe precipitates in a polycrystalline Cu-Fe alloy. Mater. Charact. 2015, 105, 129–135. [Google Scholar] [CrossRef]
- Miura, H.; Tsukawaki, H.; Sakai, T.; Jonas, J.J. Effect of particle/matrix interfacial character on the high-temperature deformation and recrystallization behavior of Cu with dispersed Fe particles. Acta Mater. 2008, 56, 4944–4952. [Google Scholar] [CrossRef]
- Shi, G.; Chen, X.; Jiang, H.; Wang, Z.; Tang, H.; Fan, Y. Strengthening mechanisms of Fe nanoparticles for single crystal Cu-Fe alloy. Mater. Sci. Eng. A 2015, 636, 43–47. [Google Scholar] [CrossRef]
- Datta, A.; Soffa, W.A. The structure and properties of age hardened Cu-Ti alloys. Acta Metall. 1976, 24, 987–1001. [Google Scholar] [CrossRef]
- Nagarjuna, S.; Balasubramanian, K.; Sarma, D.S. Effect of prior cold work on mechanical properties, electrical conductivity and microstructure of aged Cu-Ti alloys. J. Mater. Sci. 1999, 34, 2929–2942. [Google Scholar] [CrossRef]
- Zhang, P.; Jie, J.; Gao, Y.; Li, H.; Wang, T.; Li, T. Influence of cold deformation and Ti element on the microstructure and properties of Cu-Cr system alloys. J. Mater. Res. 2015, 30, 2073–2080. [Google Scholar] [CrossRef]
- Heuer, R.P. The Effect of Iron and Oxygen on the Electrical Conductivity of Copper. J. Am. Chem. Soc. 1927, 49, 2711–2720. [Google Scholar] [CrossRef]
- Murray, J.L. The Fe–Ti (Iron-Titanium) system. J. Phase Equilibria 1981, 2, 320–334. [Google Scholar] [CrossRef]


| Fe/Ti Atomic Ratio | Number | Fe | Ti | Co | Mg | Cu |
|---|---|---|---|---|---|---|
| 1:2 | FT12 | 0.34 | 0.59 | / | 0.09 | Bal |
| 1:1 | FT11 | 0.45 | 0.55 | / | 0.08 | Bal |
| 2:1 | FT21 | 0.69 | 0.31 | / | 0.11 | Bal |
| Temperature | FT12 |
|---|---|
| 500 °C | 2.8 |
| 620 °C | 28.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, Y.; Xiao, X.; Yuan, D.; Chen, J. A Study on the Microstructure and Properties of Cu-Fe-Mg-Ti Alloys Based on Composition Regulation. Materials 2025, 18, 1325. https://doi.org/10.3390/ma18061325
Ding Y, Xiao X, Yuan D, Chen J. A Study on the Microstructure and Properties of Cu-Fe-Mg-Ti Alloys Based on Composition Regulation. Materials. 2025; 18(6):1325. https://doi.org/10.3390/ma18061325
Chicago/Turabian StyleDing, Yu, Xiangpeng Xiao, Dawei Yuan, and Jinshui Chen. 2025. "A Study on the Microstructure and Properties of Cu-Fe-Mg-Ti Alloys Based on Composition Regulation" Materials 18, no. 6: 1325. https://doi.org/10.3390/ma18061325
APA StyleDing, Y., Xiao, X., Yuan, D., & Chen, J. (2025). A Study on the Microstructure and Properties of Cu-Fe-Mg-Ti Alloys Based on Composition Regulation. Materials, 18(6), 1325. https://doi.org/10.3390/ma18061325







