The Effect of Manufacturing Factors on the Material Properties and Adhesion of C. albicans and S. mutans on Additive Denture Base Material
Abstract
1. Introduction
1.1. Candida albicans and Streptococcus mutans
1.2. The Impact of Manufacturing Parameters on Material Properties
1.3. The Influence of Material Properties on C. albicans and S. mutans
1.4. Research Aim
2. Materials and Methods
2.1. Specime Preparation
2.2. Surface Characterization
2.3. Adhesion
2.4. Statistical Analysis
3. Results
3.1. Adhesion of C. albicans
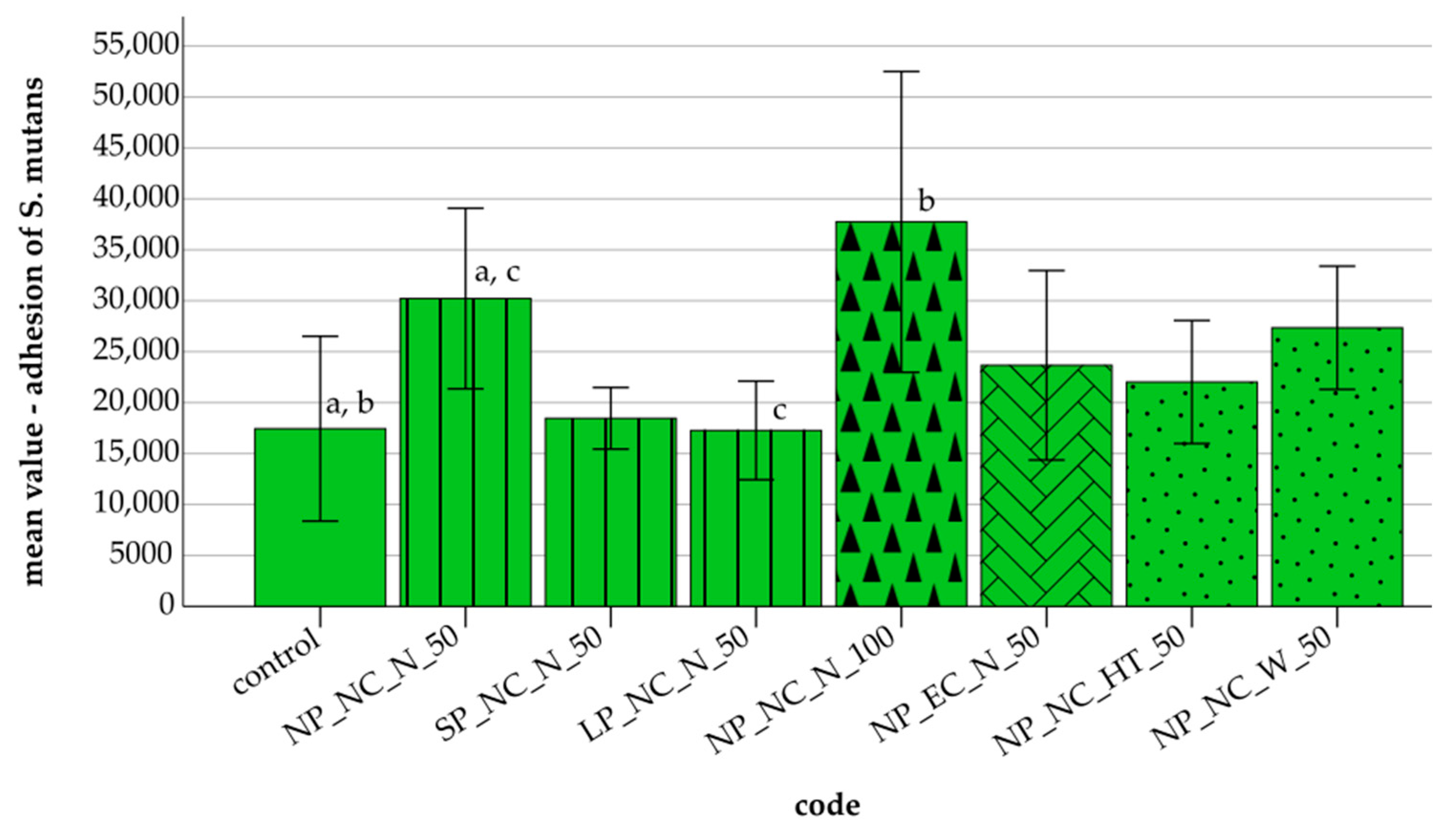
3.2. Adhesion of S. mutans
3.3. Surface Parameters
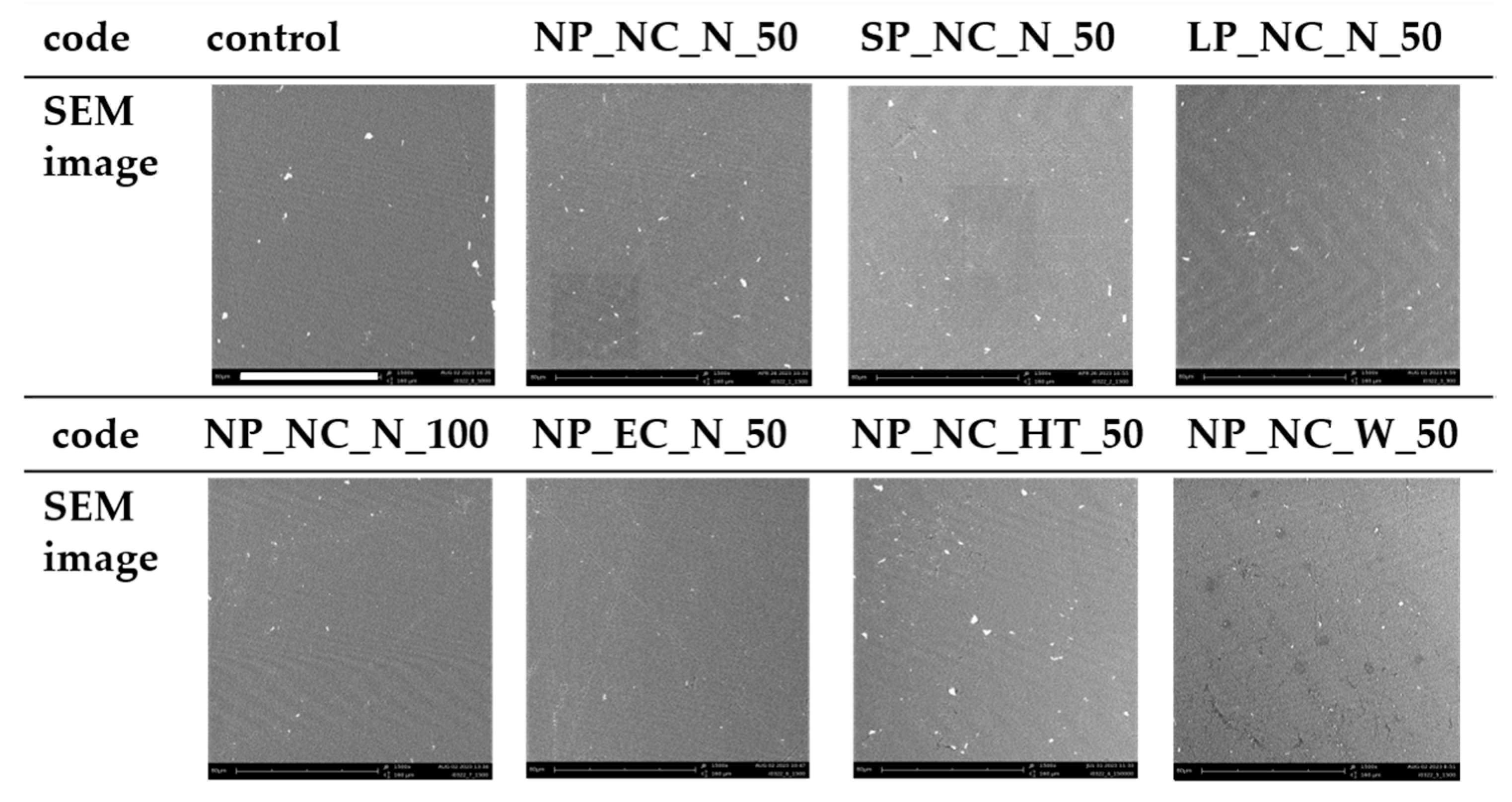
3.4. SEM Images
4. Discussion
4.1. C. albicans
4.1.1. Polymerization
4.1.2. Layer Thickness
4.1.3. Cleaning
4.1.4. Storage
4.2. S. mutans
4.2.1. Polymerization
4.2.2. Layer Thickness
4.2.3. Cleaning
4.2.4. Storage
4.3. Limitation of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Valm, A.M. The Structure of Dental Plaque Microbial Communities in the Transition from Health to Dental Caries and Periodontal Disease. J. Mol. Biol. 2019, 431, 2957–2969. [Google Scholar] [CrossRef] [PubMed]
- Pflughoeft, K.J.; Versalovic, J. Human microbiome in health and disease. Annu. Rev. Pathol. 2012, 7, 99–122. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Roza, M.P.; Colli, K.G.; Dalben, Y.R.; Maifrede, S.B.; Valiatti, T.B.; Novo, V.M.; Cayô, R.; Grão-Velloso, T.R.; Gonçalves, S.S. Candida-associated denture stomatitis: Clinical, epidemiological, and microbiological features. Braz. J. Microbiol. 2023, 54, 841–848. [Google Scholar] [CrossRef]
- Odds, F.C. Mycology in oral pathology. Acta Stomatol. Belgica 1997, 75–80. [Google Scholar]
- Gendreau, L.; Loewy, Z.G. Epidemiology and etiology of denture stomatitis. J. Prosthodont. 2011, 20, 251–260. [Google Scholar] [CrossRef]
- Song, F.; Koo, H.; Ren, D. Effects of Material Properties on Bacterial Adhesion and Biofilm Formation. J. Dent. Res. 2015, 94, 1027–1034. [Google Scholar] [CrossRef]
- Teughels, W.; Van Assche, N.; Sliepen, I.; Quirynen, M. Effect of material characteristics and/or surface topography on biofilm development. Clin. Oral Implant. Res. 2006, 17 (Suppl. 2), 68–81. [Google Scholar] [CrossRef]
- Perić, M.; Miličić, B.; Kuzmanović Pfićer, J.; Živković, R.; Arsić Arsenijević, V. A Systematic Review of Denture Stomatitis: Predisposing Factors, Clinical Features, Etiology, and Global Candida spp. Distribution. J. Fungi 2024, 10. [Google Scholar] [CrossRef]
- Latib, Y.O.; Owen, C.P.; Patel, M. Viability of Candida albicans in Denture Base Resin After Disinfection: A Preliminary Study. Int. J. Prosthodont. 2018, 31, 436–439. [Google Scholar] [CrossRef]
- Da Silva, M.D.D.; Nunes, T.S.B.S.; Viotto, H.E.d.C.; Coelho, S.R.G.; de Souza, R.F.; Pero, A.C. Microbial adhesion and biofilm formation by Candida albicans on 3D-printed denture base resins. PLoS ONE 2023, 18, e0292430. [Google Scholar] [CrossRef]
- Li, Y.; Huang, S.; Du, J.; Wu, M.; Huang, X. Current and prospective therapeutic strategies: Tackling Candida albicans and Streptococcus mutans cross-kingdom biofilm. Front. Cell. Infect. Microbiol. 2023, 13, 1106231. [Google Scholar] [CrossRef] [PubMed]
- Mayer, F.L.; Wilson, D.; Hube, B. Candida albicans pathogenicity mechanisms. Virulence 2013, 4, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Van Noort, R. The future of dental devices is digital. Dent. Mater. 2012, 28, 3–12. [Google Scholar] [CrossRef]
- Kurzendorfer, L.; Hahnel, S.; Rosentritt, M.; Schmidt, M. Die digitale Prothesenfertigung. Quintessenz J. 2023, 49, 612–620. [Google Scholar]
- Andreescu, C.F.; Ghergic, D.L.; Botoaca, O.; Hancu, V.; Banateanu, A.M.; Patroi, D.N. Evaluation of Different Materials Used for Fabrication of Complete Digital Denture. Mater. Plast. 2018, 55, 124–128. [Google Scholar] [CrossRef]
- Valenti, C.; Federici, M.I.; Masciotti, F.; Marinucci, L.; Xhimitiku, I.; Cianetti, S.; Pagano, S. Mechanical and Biological Properties of 3D Printing Additive Manufacturing dental Prosthesis Materials: A Systematic Review; OSF: Charlottesville, VA, USA, 2021. [Google Scholar] [CrossRef]
- Aati, S.; Akram, Z.; Shrestha, B.; Patel, J.; Shih, B.; Shearston, K.; Ngo, H.; Fawzy, A. Effect of post-curing light exposure time on the physico-mechanical properties and cytotoxicity of 3D-printed denture base material. Dent. Mater. 2022, 38, 57–67. [Google Scholar] [CrossRef]
- Bayarsaikhan, E.; Lim, J.-H.; Shin, S.-H.; Park, K.-H.; Park, Y.-B.; Lee, J.-H.; Kim, J.-E. Effects of Postcuring Temperature on the Mechanical Properties and Biocompatibility of Three-Dimensional Printed Dental Resin Material. Polymers 2021, 13. [Google Scholar] [CrossRef]
- Alageel, O.; Wazirian, B.; Almufleh, B.; Tamimi, F. Fabrication of Dental Restorations Using Digital Technologies: Techniques and Materials. In Digital Restorative Dentistry; Tamimi, F., Hirayama, H., Eds.; Springer International Publishing: Cham, Switzerland, 2019. [Google Scholar]
- Cheah, C.; Nee, A.; Fuh, J.; Lu, L.; Choo, Y.; Miyazawa, T. Characteristics of photopolymeric material used in rapid prototypes. J. Mater. Process. Technol. 1997, 67, 41–45. [Google Scholar] [CrossRef]
- Fuh, J.; Lu, L.; Tan, C.; Shen, Z.; Chew, S. Processing and characterising photo-sensitive polymer in the rapid prototyping process. J. Mater. Process. Technol. 1999, 89–90, 211–217. [Google Scholar] [CrossRef]
- Wulff, J.; Schmid, A.; Huber, C.; Rosentritt, M. Dynamic fatigue of 3D-printed splint materials. J. Mech. Behav. Biomed. Mater. 2021, 124, 104885. [Google Scholar] [CrossRef]
- Reymus, M.; Lümkemann, N.; Stawarczyk, B. 3D-printed material for temporary restorations: Impact of print layer thickness and post-curing method on degree of conversion. Int. J. Comput. Dent. 2019, 22, 231–237. [Google Scholar] [PubMed]
- Li, P.; Lambart, A.-L.; Stawarczyk, B.; Reymus, M.; Spintzyk, S. Postpolymerization of a 3D-printed denture base polymer: Impact of post-curing methods on surface characteristics, flexural strength, and cytotoxicity. J. Dent. 2021, 115, 103856. [Google Scholar] [CrossRef]
- Al-Dulaijan, Y.A.; Alsulaimi, L.; Alotaibi, R.; Alboainain, A.; Alalawi, H.; Alshehri, S.; Khan, S.Q.; Alsaloum, M.; AlRumaih, H.S.; Alhumaidan, A.A.; et al. Comparative Evaluation of Surface Roughness and Hardness of 3D Printed Resins. Materials 2022, 15. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Fernandez, P.K.; Spintzyk, S.; Schmidt, F.; Yassine, J.; Beuer, F.; Unkovskiy, A. Effects of layer thickness and build angle on the microbial adhesion of denture base polymers manufactured by digital light processing. J. Prosthodont. Res. 2023, 67, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Machado-Santos, L.; Baroudi, K.; Silikas, N.; Tribst, J.P.M.; Coelho Sinhoreti, M.A.; Brandt, W.C.; Liporoni, P.C.S. Physical analysis of an acrylic resin modified by metal and ceramic nanoparticlesles. Dent. Med. Probl. 2023, 60, 657–664. [Google Scholar] [CrossRef]
- Teixeira, A.B.V.; Da Valente, M.L.C.; Sessa, J.P.N.; Gubitoso, B.; Schiavon, M.A.; Dos Reis, A.C. Adhesion of biofilm, surface characteristics, and mechanical properties of antimicrobial denture base resin. J. Adv. Prosthodont. 2023, 15, 80–92. [Google Scholar] [CrossRef]
- Koch, C.; Bürgers, R.; Hahnel, S. Candida albicans adherence and proliferation on the surface of denture base materials. Gerodontology 2013, 30, 309–313. [Google Scholar] [CrossRef]
- Wulff, J.; Rauch, A.; Schmidt, M.B.; Rosentritt, M. Biaxial Flexural Strength of Printed Splint Materials. Materials 2024, 17, 1112. [Google Scholar] [CrossRef]
- Perea-Lowery, L.; Gibreel, M.; Vallittu, P.K.; Lassila, L.V. 3D-Printed vs. Heat-Polymerizing and Autopolymerizing Denture Base Acrylic Resins. Materials 2021, 14, 5781. [Google Scholar] [CrossRef]
- Schubert, A.; Bürgers, R.; Baum, F.; Kurbad, O.; Wassmann, T. Influence of the Manufacturing Method on the Adhesion of Candida albicans and Streptococcus mutans to Oral Splint Resins. Polymers 2021, 13, 1534. [Google Scholar] [CrossRef]
- Berli, C.; Thieringer, F.M.; Sharma, N.; Müller, J.A.; Dedem, P.; Fischer, J.; Rohr, N. Comparing the mechanical properties of pressed, milled, and 3D-printed resins for occlusal devices. J. Prosthet. Dent. 2020, 124, 780–786. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-M.; Park, J.-M.; Kim, S.-K.; Heo, S.-J.; Koak, J.-Y. Flexural Strength of 3D-Printing Resin Materials for Provisional Fixed Dental Prostheses. Materials 2020, 13, 3970. [Google Scholar] [CrossRef] [PubMed]
- Falahchai, M.; Ghavami-Lahiji, M.; Rasaie, V.; Amin, M.; Neshandar Asli, H. Comparison of mechanical properties, surface roughness, and color stability of 3D-printed and conventional heat-polymerizing denture base materials. J. Prosthet. Dent. 2023, 130, 266.e1–266.e8. [Google Scholar] [CrossRef]
- Tokarz, Z.; Krzysciak, P.; Wieczorek, A. Effectiveness of methods for removing the Candida albicans biofilm from the dental acrylic surface. Dent. Med. Probl. 2023, 60, 665–671. [Google Scholar] [CrossRef]
- International Organization for Standardization. Geometrical Product Specifications (GPS)—Surface Texture: Areal: Part 2: Terms, Definitions and Surface Texture Parameters; IOS: Geneva, Switzerland, 2019. [Google Scholar]
- International Organization for Standardization. Metallic Materials–Vickers Hardness Test: Part 1: Test Method; IOS: Geneva, Switzerland, 2023. [Google Scholar]
- International Organization for Standardization. Paints and Varnishes–Wettability: Part 2: Determination of the Surface Free Energy of Solid Surfaces by Measuring the Contact Angle; IOS: Geneva, Switzerland, 2024. [Google Scholar]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polymer. Sci 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Bächle, J.; Merle, C.; Hahnel, S.; Rosentritt, M. Bacterial Adhesion on Dental Polymers as a Function of Manufacturing Techniques. Materials 2023, 16, 2373. [Google Scholar] [CrossRef]
- Yacob, N.; Ahmad, N.A.; Safii, S.H.; Yunus, N.; Razak, F.A. Is microbial adhesion affected by the build orientation of a 3-dimensionally printed denture base resin? J. Prosthet. Dent. 2023, 130, e1–e131. [Google Scholar] [CrossRef]
- Meirowitz, A.; Rahmanov, A.; Shlomo, E.; Zelikman, H.; Dolev, E.; Sterer, N. Effect of Denture Base Fabrication Technique on Candida albicans Adhesion In Vitro. Materials 2021, 14, 221. [Google Scholar] [CrossRef]
- Mazurek-Popczyk, J.; Nowicki, A.; Arkusz, K.; Pałka, Ł.; Zimoch-Korzycka, A.; Baldy-Chudzik, K. Evaluation of biofilm formation on acrylic resins used to fabricate dental temporary restorations with the use of 3D printing technology. BMC Oral Health 2022, 22, 442. [Google Scholar] [CrossRef]
- Xu, Y.; Xepapadeas, A.B.; Koos, B.; Geis-Gerstorfer, J.; Li, P.; Spintzyk, S. Effect of post-rinsing time on the mechanical strength and cytotoxicity of a 3D printed orthodontic splint material. Dent. Mater. 2021, 37, e314–e327. [Google Scholar] [CrossRef]
- Rashid, H.; Sheikh, Z.; Vohra, F. Allergic effects of the residual monomer used in denture base acrylic resins. Eur. J. Dent. 2015, 9, 614–619. [Google Scholar] [CrossRef] [PubMed]
- Poker, B.d.C.; Oliveira, V.d.C.; Macedo, A.P.; Gonçalves, M.; Ramos, A.P.; Silva-Lovato, C.H. Evaluation of surface roughness, wettability and adhesion of multispecies biofilm on 3D-printed resins for the base and teeth of complete dentures. J. Appl. Oral Sci. 2024, 32, e20230326. [Google Scholar] [CrossRef] [PubMed]
- Brambilla, E.; Gagliani, M.; Ionescu, A.; Fadini, L.; García-Godoy, F. The influence of light-curing time on the bacterial colonization of resin composite surfaces. Dent. Mater. 2009, 25, 1067–1072. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.-S.; Watts, D.C. Residual monomer concentrations in denture-base acrylic resin after an additional, soft-liner, heat-cure cycle. Dent. Mater. 1999, 15, 296–300. [Google Scholar] [CrossRef]
- Wulff, J.; Schweikl, H.; Rosentritt, M. Cytotoxicity of printed resin-based splint materials. J. Dent. 2022, 120, 104097. [Google Scholar] [CrossRef]
- Lee, W.-J.; Jo, Y.-H.; Yilmaz, B.; Yoon, H.-I. Effect of build angle, resin layer thickness and viscosity on the surface properties and microbial adhesion of denture bases manufactured using digital light processing. J. Dent. 2023, 137, 104608. [Google Scholar] [CrossRef]
- Jang, W.; Kook, G.-S.; Kang, J.-H.; Kim, Y.; Yun, Y.; Lee, S.-K.; Park, S.-W.; Lim, H.-P.; Yun, K.-D.; Park, C. Effect of Washing Condition on the Fracture Strength, and the Degree of Conversion of 3D Printing Resin. Appl. Sci. 2021, 11, 11676. [Google Scholar] [CrossRef]
- Vallittu, P.K.; Miettinen, V.; Alakuijala, P. Residual monomer content and its release into water from denture base materials. Dent. Mater. 1995, 11, 338–342. [Google Scholar] [CrossRef]
- Stafford, G.D.; Brooks, S.C. The loss of residual monomer from acrylic orthodontic resins. Dent. Mater. 1985, 1, 135–138. [Google Scholar] [CrossRef]
- Bural, C.; Aktaş, E.; Deniz, G.; Ünlüçerçi, Y.; Kızılcan, N.; Bayraktar, G. Effect of post-polymerization heat-treatments on degree of conversion, leaching residual MMA and in vitro cytotoxicity of autopolymerizing acrylic repair resin. Dent. Mater. 2011, 27, 1135–1143. [Google Scholar] [CrossRef]

| Abbreviation | Influence |
|---|---|
| NP | Normal polymerization |
| SP | Short polymerization |
| LP | Long polymerization |
| NC | Normal cleaning (isopropanol) |
| EC | Extended cleaning (isopropanol) |
| 50 | Layer thickness of 50 µm |
| 100 | Layer thickness of 100 µm |
| N | No storage |
| HT | Heat treatment in an incubator at 37 °C (7 days) |
| W | Storage in water at 37 °C (30 days) |
| Code | Material | Parameter |
|---|---|---|
| Control | Poured denture base material | Manufacturer’s recommendations |
| Polymerization | ||
| NP_NC_N_50 | Additive denture base material | NP-cure: Seq. 1: Wavelength: upper, 720 s, 80% Seq. 2: Wavelength: lower, 60 s, 100% NC: Pre-cleaning (4 min), cleaning (4 min), and drying (10 min) with isopropanol (99%) |
| SP_NC_N_50 | Additive denture base material | SP-cure: Seq. 1: 360 s, 80% Seq. 2: 30 s, 100% |
| LP_NC_N_50 | Additive denture base material | LP-Cure: Seq. 1: 1200 s (max. cure), 80% Seq. 2: 120 s, 100% |
| Layer thickness | ||
| NP_NC_N_100 | Additive denture base material | Layer thickness: 100 µm |
| Cleaning | ||
| NP_EC_N_50 | Additive denture base material | EC: Pre-cleaning (4 min), cleaning (4 min), and drying (10 min) with isopropanol (99%) |
| Storage | ||
| NP_NC_HT_50 | Additive denture base material | Heat treatment in an incubator: 7 d, 37 °C |
| NP_NC_W_50 | Additive denture base material | Water storage in an incubator: 30 d, 37 °C |
| Code | Sa (µm) | Sz (µm) |
|---|---|---|
| Control | 3.35 (0.54) | 24.80 (3.26) |
| NP_NC_N_50 | 3.34 (0.48) | 25.78 (3.78) |
| SP_NC_N_50 | 3.48 (0.53) | 27.48 (4.70) |
| LP_NC_N_50 | 3.02 (0.14) | 22.23 (1.49) |
| NP_NC_N_100 | 3.26 (0.52) | 24.99 (3.87) |
| NP_EC_N_50 | 2.98 (0.37) | 22.74 (2.55) |
| NP_NC_HT_50 | 3.01 (0.31) | 22.70 (4.25) |
| NP_NC_W_50 | 3.13 (0.29) | 24.11 (2.13) |
| Code | HM (N/mm2) | HIT (N/mm2) | EIT (kN/mm2) | ηIT (%) |
|---|---|---|---|---|
| control | 111.20 (4.10) | 145.10 (5.72) | 3.97 (0.12) | 28.49 (0.32) |
| NP_NC_N_50 | 96.60 (5.10) | 131.00 (7.62) | 3.06 (0.16) | 35.92 (1.31) |
| SP_NC_N_50 | 99.00 (4.50) | 133.10 (6.37) | 3.19 (0.14) | 34.34 (0.51) |
| LP_NC_N_50 | 90.40 (8.13) | 122.90 (11.71) | 2.89 (0.31) | 36.43 (1.82) |
| NP_NC_N_100 | 97.00 (3.74) | 130.70 (5.68) | 3.14 (0.11) | 35.05 (0.40) |
| NP_EC_N_50 | 98.30 (8.63) | 132.10 (12.96) | 3.20 (0.19) | 35.07 (1.36) |
| NP_NC_HT_50 | 94.10 (8.72) | 128.40 (12.07) | 2.93 (0.37) | 36.82 (1.97) |
| NP_NC_W_50 | 85.30 (12.16) | 118.80 (15.22) | 2.47 (0.49) | 39.16 (2.74) |
| Code | SFE (mJ/m2) |
|---|---|
| Control | 34.16 (1.08) |
| NP_NC_N_50 | 30.66 (4.19) |
| SP_NC_N_50 | 35.44 (1.52) |
| LP_NC_N_50 | 31.91 (1.97) |
| NP_NC_N_100 | 36.28 (1.66) |
| NP_EC_N_50 | 26.61 (2.29) |
| NP_NC_HT_50 | 34.31 (4.40) |
| NP_NC_W_50 | 35.82 (1.98) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurzendorfer-Brose, L.; Rosentritt, M. The Effect of Manufacturing Factors on the Material Properties and Adhesion of C. albicans and S. mutans on Additive Denture Base Material. Materials 2025, 18, 1323. https://doi.org/10.3390/ma18061323
Kurzendorfer-Brose L, Rosentritt M. The Effect of Manufacturing Factors on the Material Properties and Adhesion of C. albicans and S. mutans on Additive Denture Base Material. Materials. 2025; 18(6):1323. https://doi.org/10.3390/ma18061323
Chicago/Turabian StyleKurzendorfer-Brose, Laura, and Martin Rosentritt. 2025. "The Effect of Manufacturing Factors on the Material Properties and Adhesion of C. albicans and S. mutans on Additive Denture Base Material" Materials 18, no. 6: 1323. https://doi.org/10.3390/ma18061323
APA StyleKurzendorfer-Brose, L., & Rosentritt, M. (2025). The Effect of Manufacturing Factors on the Material Properties and Adhesion of C. albicans and S. mutans on Additive Denture Base Material. Materials, 18(6), 1323. https://doi.org/10.3390/ma18061323






