Strontium Doping Promotes Low-Temperature Growth of Single-Crystalline Ni-Rich Cathodes with Enhanced Electrochemical Performance
Abstract
1. Introduction
2. Experimental
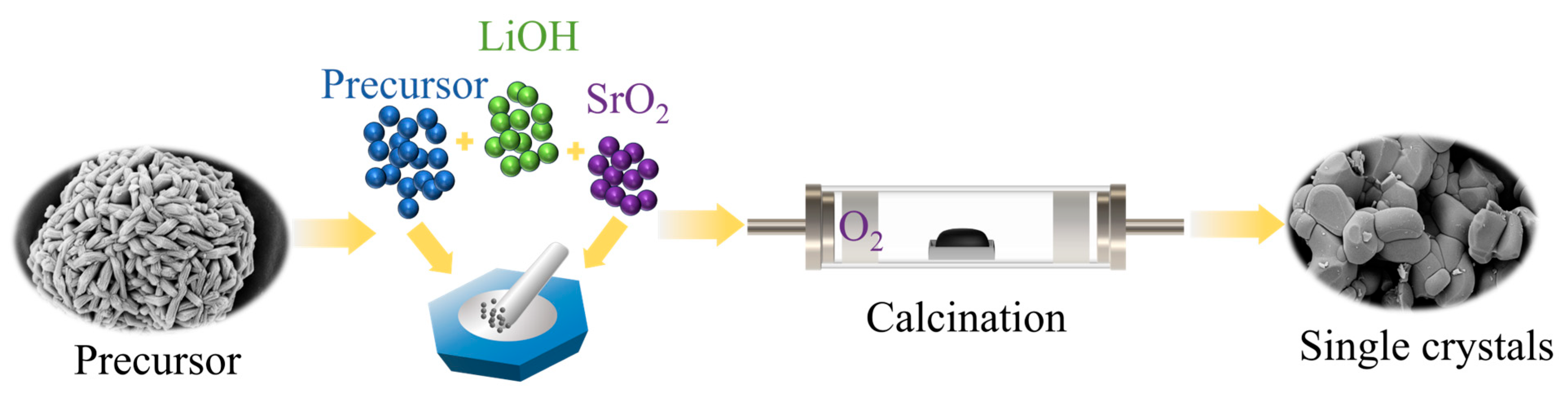
Preparation of Materials
3. Results and Discussion
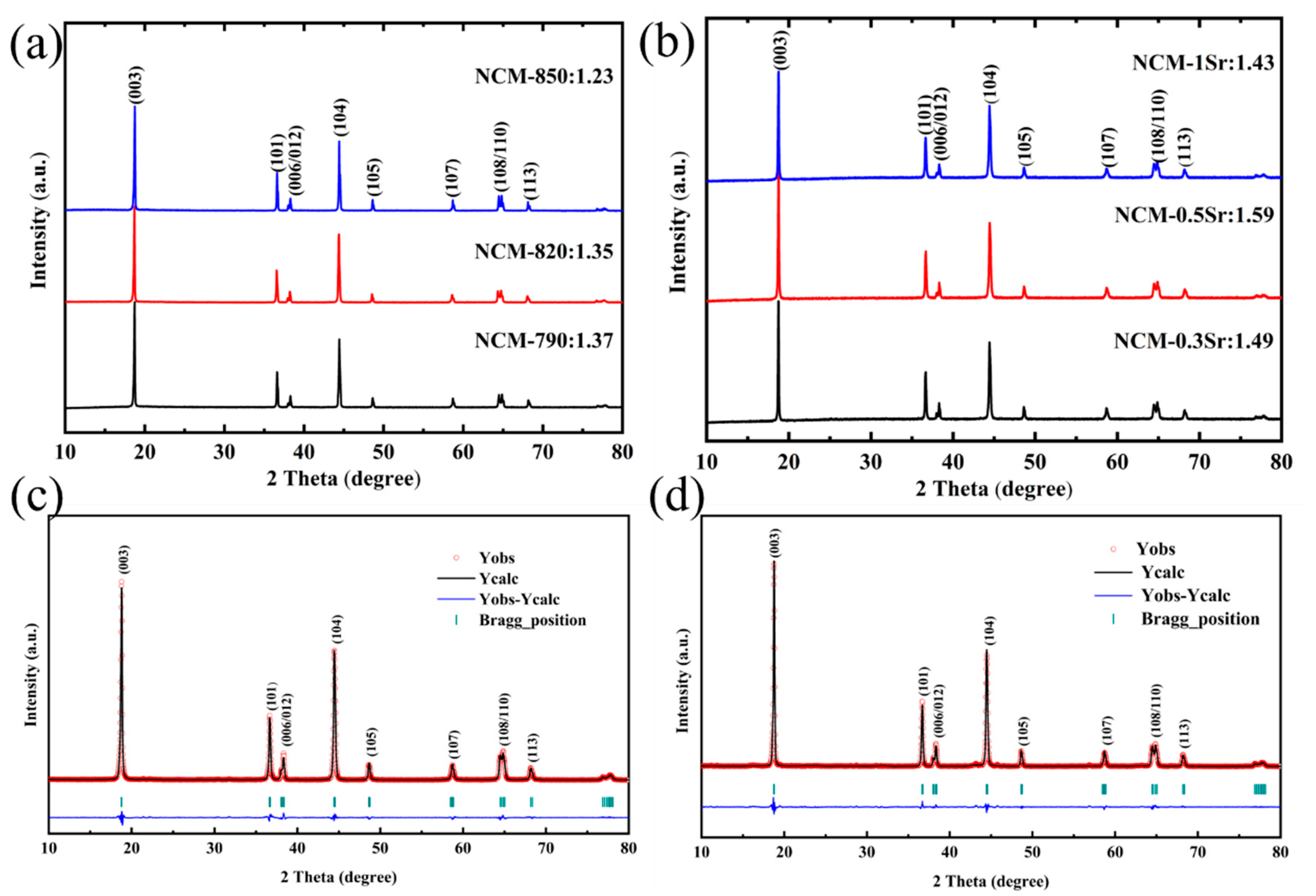
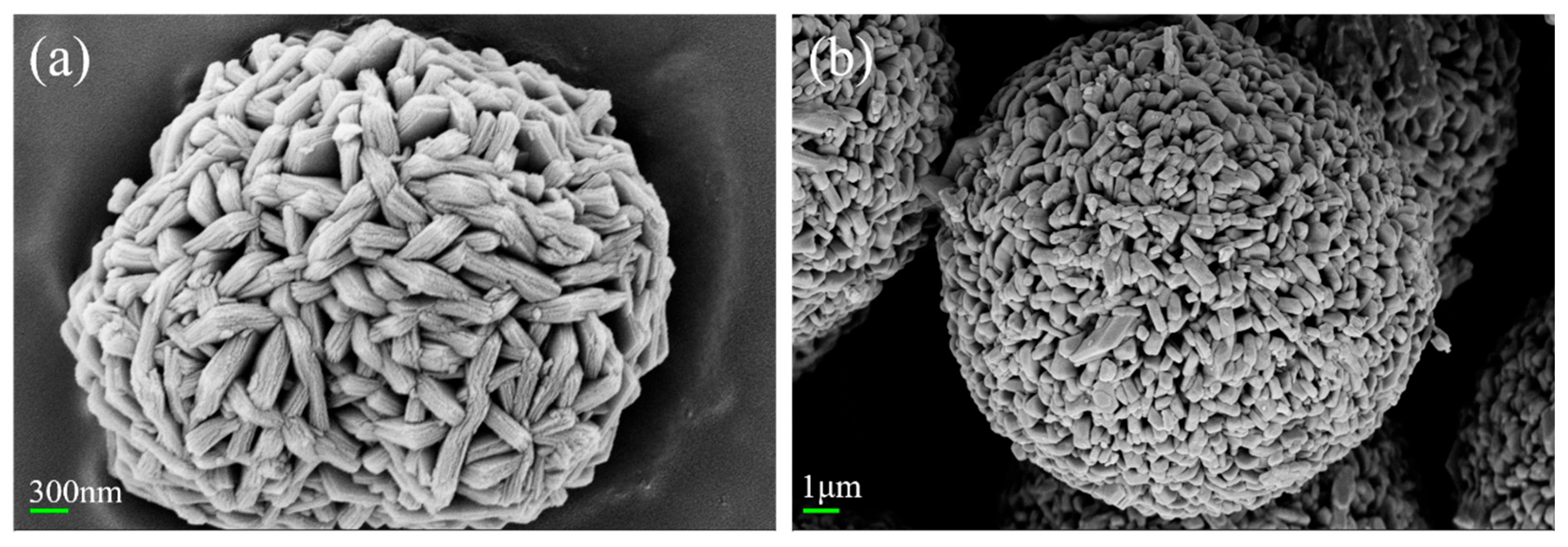
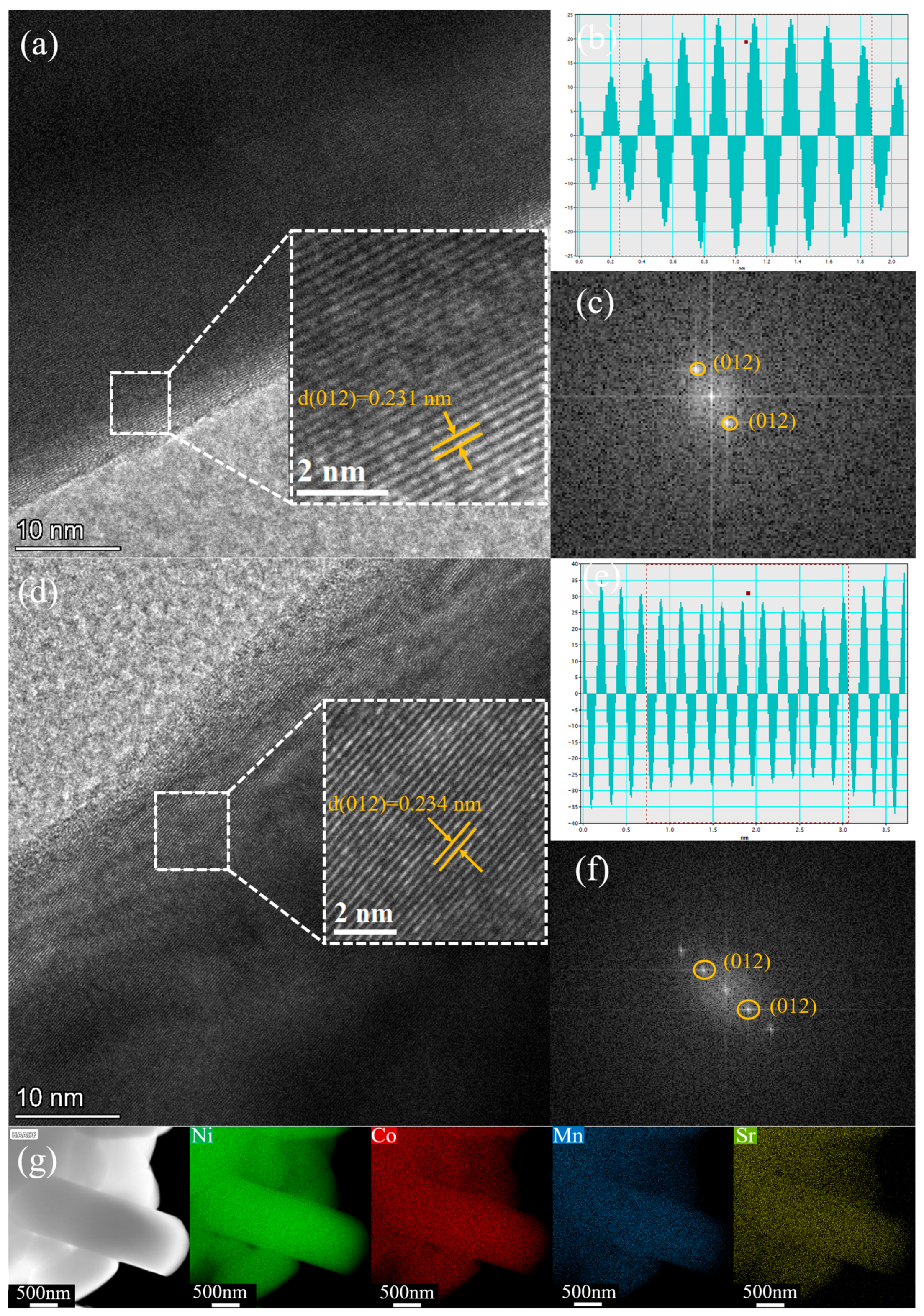
Material Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kaushik, S.; Mehta, T.; Chand, P.; Sharma, S.; Kumar, G. Recent advancements in cathode materials for high-performance Li-ion batteries: Progress and prospects. J. Energy Storage 2024, 97, 112818. [Google Scholar] [CrossRef]
- Li, Y.; Tan, Z.; Liu, Y.; Lei, C.; He, P.; Li, J.; He, Z.; Cheng, Y.; Wu, F.; Li, Y. Past, present and future of high-nickel materials. Nano Energy 2024, 119, 109070. [Google Scholar] [CrossRef]
- Lu, S.-J.; Tang, L.-B.; Wei, H.-X.; Huang, Y.-D.; Yan, C.; He, Z.-J.; Li, Y.-J.; Mao, J.; Dai, K.; Zheng, J.-C. Single-Crystal Nickel-Based Cathodes: Fundamentals and Recent Advances. Electrochem. Energy Rev. 2022, 5, 4. [Google Scholar] [CrossRef]
- Luo, B.; Li, H.; Qi, H.; Liu, Y.; Zheng, C.; Du, W.; Zhang, J.; Chen, L. Effect of crystal morphology of ultrahigh-nickel cathode materials on high temperature electrochemical stability of lithium ion batteries. J. Energy Chem. 2024, 88, 327–335. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, H.; Deng, J.; Han, Z.; Xiao, X.; Wang, L.; Chen, Z.; Deng, Y.; He, X. Insight of Synthesis of Single Crystal Ni-Rich LiNi1−x−yCoxMnyO2 Cathodes. Adv. Energy Mater. 2024, 14, 11. [Google Scholar] [CrossRef]
- You, B.; Wang, Z.; Shen, F.; Chang, Y.; Peng, W.; Li, X.; Guo, H.; Hu, Q.; Deng, C.; Yang, S.; et al. Research Progress of Single-Crystal Nickel-Rich Cathode Materials for Lithium Ion Batteries. Small Methods 2021, 5, 8. [Google Scholar] [CrossRef]
- Choi, J.W.; Aurbach, D. Promise and reality of post-lithium-ion batteries with high energy densities. Nat. Rev. Mater. 2016, 1, 16013. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, T.; Jian, J.; Lin, S.; Sun, D.; Fu, G.; Xu, Y.; Liu, Z.; Li, S.; Huo, H.; et al. Dual Modification Strategy for Enhanced Cycling and Rate Performance of Ni-Rich Cathode Materials in Lithium-Ion Batteries. Small 2024, 20, 2404488. [Google Scholar] [CrossRef]
- Yang, T.; Zhang, K.; Zuo, Y.; Song, J.; Yang, Y.; Gao, C.; Chen, T.; Wang, H.; Xiao, W.; Jiang, Z.; et al. Ultrahigh-nickel layered cathode with cycling stability for sustainable lithium-ion batteries. Nat. Sustain. 2024, 7, 1204–1214. [Google Scholar] [CrossRef]
- Li, W.; Erickson, E.M.; Manthiram, A. High-nickel layered oxide cathodes for lithium-based automotive batteries. Nat. Energy 2020, 5, 26–34. [Google Scholar] [CrossRef]
- Wu, H.; Zhou, X.; Yang, C.; Xu, D.; Zhu, Y.-H.; Zhou, T.; Xin, S.; You, Y. Concentration-Gradient Nb-Doping in a Single-Crystal LiNi0.83Co0.12Mn0.05O2 Cathode for High-Rate and Long-Cycle Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2023, 15, 18828–18835. [Google Scholar] [CrossRef]
- Liu, Z.; Zheng, S.; Zhou, Y.; Tian, X. Enhancing the electrochemical performance of single-crystal LiNi0.8Co0.1Mn0.1O2 cathode material by phosphorus doping. Chem. Eng. Sci. 2024, 285, 119627. [Google Scholar] [CrossRef]
- Liu, Y.; Fan, X.; Luo, B.; Zhao, Z.; Shen, J.; Liu, Z.; Xiao, Z.; Zhang, B.; Zhang, J.; Ming, L.; et al. Understanding the enhancement effect of boron doping on the electrochemical performance of single-crystalline Ni-rich cathode materials. J. Colloid Interface Sci. 2021, 604, 776–784. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Cai, F.; Chu, C.; Fu, B.; Świerczek, K.; Li, L.; Zhao, H. Modification of the Ni-Rich Layered Cathode Material by Hf Addition: Synergistic Microstructural Engineering and Surface Stabilization. ACS Appl. Mater. Interfaces 2024, 16, 12599–12611. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Chen, G. Single-crystal based studies for correlating the properties and high-voltage performance of Li[NixMnyCo1−x−y]O2 cathodes. J. Mater. Chem. A 2019, 7, 5463–5474. [Google Scholar] [CrossRef]
- Shen, J.; Li, H.; Qi, H.; Lin, Z.; Li, Z.; Zheng, C.; Du, W.; Chen, H.; Zhang, S. Enhancing thermodynamic stability of single-crystal Ni-rich cathode material via a synergistic dual-substitution strategy. J. Energy Chem. 2024, 88, 428–436. [Google Scholar] [CrossRef]
- Zhou, W.; Huang, H.; Liu, X.; Gao, J.; Hao, S.M.; Yang, Y.; Qiu, J. Perspective on the Preparation Methods of Single Crystalline High Nickel Oxide Cathode Materials. Adv. Energy Mater. 2023, 13, 2300378. [Google Scholar] [CrossRef]
- Han, Y.; Lei, Y.; Ni, J.; Zhang, Y.; Geng, Z.; Ming, P.; Zhang, C.; Tian, X.; Shi, J.L.; Guo, Y.G.; et al. Single-Crystalline Cathodes for Advanced Li-Ion Batteries: Progress and Challenges. Small 2022, 18, 2107048. [Google Scholar] [CrossRef]
- Hu, Z.; Huang, Q.; Cai, W.; Zeng, Z.; Chen, K.; Sun, Y.; Kong, Q.; Feng, W.; Wang, K.; Wu, Z.; et al. Research Progress on Enhancing the Performance of High Nickel Single Crystal Cathode Materials for Lithium-Ion Batteries. Ind. Eng. Chem. Res. 2023, 62, 2410–2427. [Google Scholar] [CrossRef]
- Lv, F.; Zhang, Y.; Wu, M.; Gu, Y. A Molten-Salt Method to Synthesize Ultrahigh-Nickel Single-Crystalline LiNi0.92Co0.06Mn0.02O2 with Superior Electrochemical Performance as Cathode Material for Lithium-Ion Batteries. Small 2022, 18, 2201946. [Google Scholar] [CrossRef]
- Huang, Y.; Tang, R.; Xiao, F.; Zeng, L.; Wang, Y.; Huang, H. Elevated rate and cycling performance of nickel-rich single-crystal at high voltage enabled by Al/Ce co-doping. J. Power Sources 2024, 597, 234133. [Google Scholar] [CrossRef]
- Ryu, H.-H.; Lee, S.-B.; Sun, Y.-K. Promoting grain growth in Ni-rich single-crystal cathodes for high-performance lithium-ion batteries through Ce doping. J. Solid State Electrochem. 2022, 26, 2097–2105. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, L.; Tian, H.; Yan, J.; Tong, J.; Liu, X.; Zhang, H.; Huang, H.; Hao, S.M.; Gao, J.; et al. Pulse High Temperature Sintering to Prepare Single-Crystal High Nickel Oxide Cathodes with Enhanced Electrochemical Performance. Adv. Energy Mater. 2022, 13, 2203188. [Google Scholar] [CrossRef]
- Chu, B.; Xu, R.; Li, G.; Chen, J.; Xu, Z.; Huang, T.; Wang, B.; Yu, A. Simultaneous B/W dual coating on ultra-high nickel single crystal cathode material for lithium-ion batteries. J. Power Sources 2023, 577, 233260. [Google Scholar] [CrossRef]
- Feng, X.-Y.; Ran, M.-J.; Yuan, M.-M.; Wei, M.-T.; Wu, L.; Hu, Z.-Y.; Chen, L.-H.; Li, Y.; Su, B.-L. Na Doping and CeO2 Nanoparticle Surface Modification Enhancing the Li-Rich Li1.2Mn0.54Ni0.13Co0.13O2 Cathode Material for a Lithium-Ion Battery. ACS Appl. Nano Mater. 2024, 7, 13173–13182. [Google Scholar] [CrossRef]
- Li, F.; Liu, Z.; Liao, C.; Xu, X.; Zhu, M.; Liu, J. Gradient Boracic Polyanion Doping-Derived Surface Lattice Modulation of High-Voltage Ni-Rich Layered Cathodes for High-Energy-Density Li-Ion Batteries. ACS Energy Lett. 2023, 8, 4903–4914. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, Y.; Zhang, B.; Huang, W.; Cheng, L.; Wang, J.; He, X.; Yu, L.; Xiao, Z.; Wen, J.; et al. Optimized In Situ Doping Strategy Stabling Single-Crystal Ultrahigh-Nickel Layered Cathode Materials. ACS Nano 2024, 18, 8002–8016. [Google Scholar] [CrossRef]
- Yu, R.; Zeng, W.; Zhou, L.; Van Tendeloo, G.; Mai, L.; Yao, Z.; Wu, J. Layer-by-layer delithiation during lattice collapse as the origin of planar gliding and microcracking in Ni-rich cathodes. Cell Rep. Phys. Sci. 2023, 4, 101480. [Google Scholar] [CrossRef]
- Wang, J.; Lu, X.; Zhang, Y.; Zhou, J.; Wang, J.; Xu, S. Grain size regulation for balancing cycle performance and rate capability of LiNi0.9Co0.055Mn0.045O2 single crystal nickel-rich cathode materials. J. Energy Chem. 2022, 65, 681–687. [Google Scholar] [CrossRef]
- Zheng, H.-W.; Liu, Z.-C.; Chen, Y.-Z.; Gao, X.-P. La-Doped Ultrahigh-Nickel Layered Oxide Cathode with Enhanced Cycle Stability for Li-Ion Batteries. ACS Appl. Mater. Interfaces 2023, 15, 35043–35051. [Google Scholar] [CrossRef]
- Cheng, L.; Zhou, Y.; Zhang, B.; Wang, W.; Ming, L.; Xiao, Z.; Ou, X. High-rate Ni-rich single-crystal cathodes with highly exposed {0 1 0} active planes through in-situ Zr doping. Chem. Eng. J. 2023, 452, 139336. [Google Scholar] [CrossRef]
- Wang, L.; Chu, Y.; Nong, Y.; Zheng, F.; Li, Y.; Huang, Y.; Li, Y.; Pan, Q.; Wang, H.; Li, Q. Sr-Based Sub/Surface Integrated Layer and Bulk Doping to Enhance High-Voltage Cycling of a Ni-Rich Cathode Material. ACS Sustain. Chem. Eng. 2022, 10, 7883–7895. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, H.; Lu, S.; He, H. Y-Element Doping Improves Electrochemical Performance and Single-Crystal Structural Stability of Cathode LiNi0.8Co0.1Mn0.1O2. ACS Appl. Energy Mater. 2023, 6, 9487–9498. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, Z.; Ma, B.; Li, P.; Zhou, Y.; Tian, X. Improving the single crystal LiNi0.8Co0.1Mn0.1O2 cathode material performance by fluorine doping. Ceram. Int. 2021, 47, 33843–33852. [Google Scholar] [CrossRef]
- Yan, J.; Yang, Z.; Kan, H.; Zha, Y.; Li, C.; Meng, Q.; Dong, P.; Zhang, Y. Microstructure and electrochemical properties of Mg/Nb/Zr co-doped LiNi0.94Mn0.04Al0.02O2 cathode materials. Ceram. Int. 2024, 50, 24872–24880. [Google Scholar] [CrossRef]
- Awasthi, S.; Moharana, S.; Kumar, V.; Wang, N.; Chmanehpour, E.; Sharma, A.D.; Tiwari, S.K.; Kumar, V.; Mishra, Y.K. Progress in doping and crystal deformation for polyanions cathode based lithium-ion batteries. Nano Mater. Sci. 2024, 6, 504–535. [Google Scholar] [CrossRef]
- Lee, S.-B.; Park, N.-Y.; Park, G.-T.; Kim, U.-H.; Sohn, S.-J.; Kang, M.-S.; Ribas, R.M.; Monteiro, R.S.; Sun, Y.-K. Doping Strategy in Developing Ni-Rich Cathodes for High-Performance Lithium-Ion Batteries. ACS Energy Lett. 2024, 9, 740–747. [Google Scholar] [CrossRef]
- Li, H.; Li, J.; Zaker, N.; Zhang, N.; Botton, G.A.; Dahn, J.R. Synthesis of Single Crystal LiNi0.88Co0.09Al0.03O2 with a Two-Step Lithiation Method. J. Electrochem. Soc. 2019, 166, A1956–A1963. [Google Scholar] [CrossRef]
- Sun, X.; Qin, C.; Zhao, B.; Jia, S.; Wang, Z.; Yang, T.; Liu, X.; Pan, L.; Zheng, L.; Luo, D.; et al. A cation and anion dual-doping strategy in novel Li-rich Mn-based cathode materials for high-performance Li metal batteries. Energy Storage Mater. 2024, 70, 103559. [Google Scholar] [CrossRef]
- Liu, J.-K.; Yin, Z.-W.; Zheng, W.-C.; Zhang, J.; Deng, S.-S.; Wang, Z.; Deng, L.; Xie, S.-J.; Liu, Z.-K.; Avdeev, M.; et al. Simple Synchronous Dual-Modification Strategy with Zr4+ Doping and CeO2 Nanowelding to Stabilize Layered Ni-Rich Cathode Materials. ACS Appl. Energy Mater. 2023, 6, 5473–5485. [Google Scholar] [CrossRef]
- He, Z.; Zhang, M.; Zhou, K.; Cheng, Y.; Luo, M.; Su, Y.; Hao, J.; Sun, Y.; Li, Y.; Yang, Y. Enabling Excellent Thermal Stability of an Ultrahigh Nickel-Rich Cathode (LiNi0.90Co0.05Mn0.05O2) by a Magnesium and Titanium Codoping Strategy. ACS Appl. Energy Mater. 2023, 6, 3422–3431. [Google Scholar] [CrossRef]
- Cui, Z.; Li, X.; Bai, X.; Ren, X.; Ou, X. A comprehensive review of foreign-ion doping and recent achievements for nickel-rich cathode materials. Energy Storage Mater. 2023, 57, 14–43. [Google Scholar] [CrossRef]
- Kim, U.-H.; Park, N.-Y.; Park, G.-T.; Kim, H.; Yoon, C.S.; Sun, Y.-K. High-Energy W-Doped Li[Ni0.95Co0.04Al0.01]O2 Cathodes for Next-Generation Electric Vehicles. Energy Storage Mater. 2020, 33, 399–407. [Google Scholar] [CrossRef]
- Zhu, C.; Xu, M.; Huang, K.; Lian, C.; Su, H.; Liu, H. Al- and Nb-Comodified Ni-Rich NCM Cathode for High-Performance Lithium-Ion Batteries. Ind. Eng. Chem. Res. 2024, 63, 2740–2749. [Google Scholar] [CrossRef]
- He, X.; Shen, J.; Zhang, B.; Xiao, Z.; Ye, L.; Mao, Q.; Zhong, Q.; Ou, X. Surface Li+/Ni2+ Antisite Defects Construction for Achieving High-Voltage Stable Single-Crystal Ni-Rich Cathode by Anion/Cation Co-Doping. Adv. Funct. Mater. 2024, 34, 2401300. [Google Scholar] [CrossRef]
- Qiao, L.; You, Q.; Wu, X.; Min, H.; Liu, X.; Yang, H. Mo Doping to Modify Lattice and Morphology of the LiNi0.9Co0.05Mn0.05O2 Cathode toward High-Efficient Lithium-Ion Storage. ACS Appl. Mater. Interfaces 2024, 16, 4772–4783. [Google Scholar] [CrossRef]

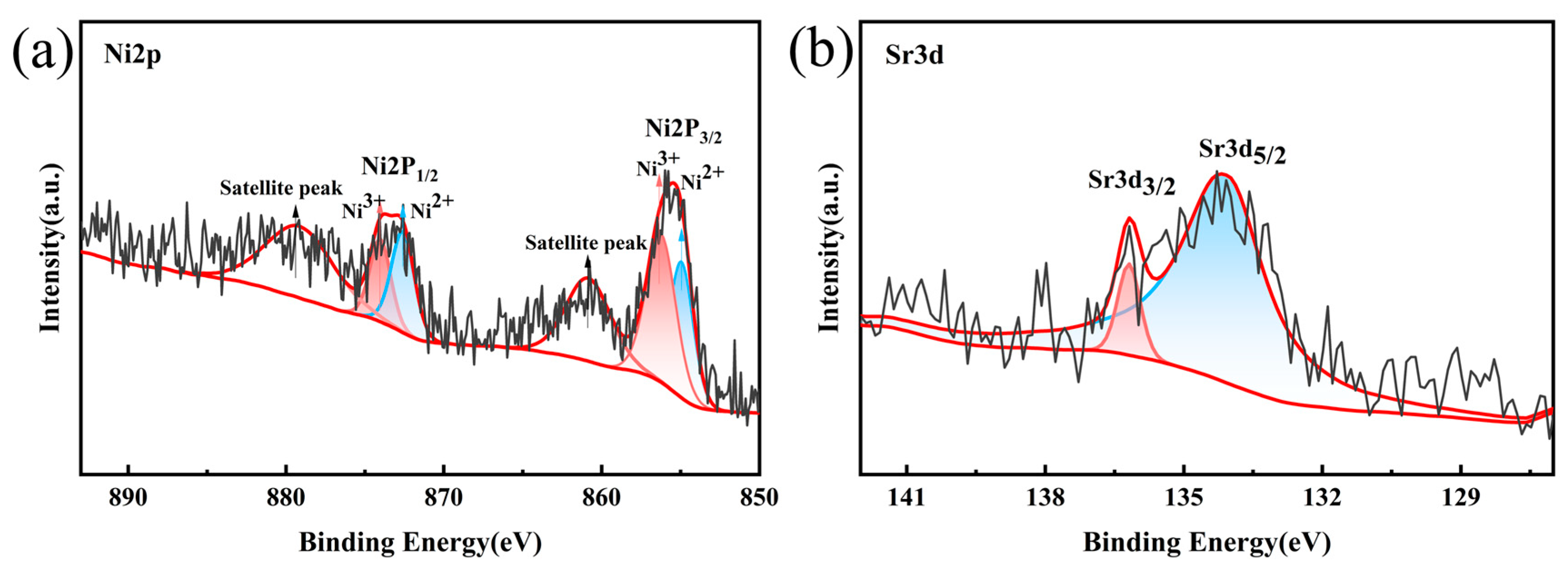
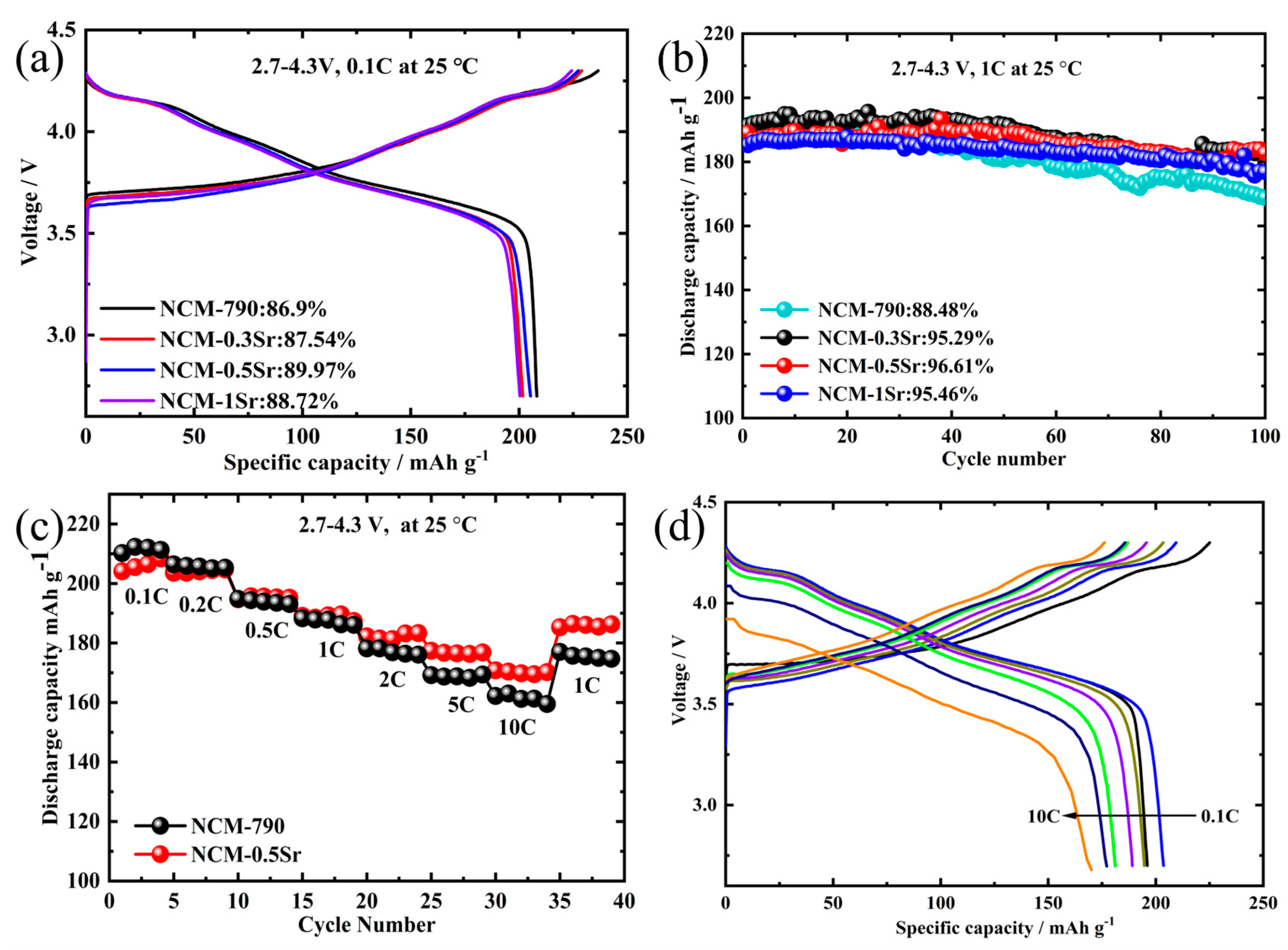

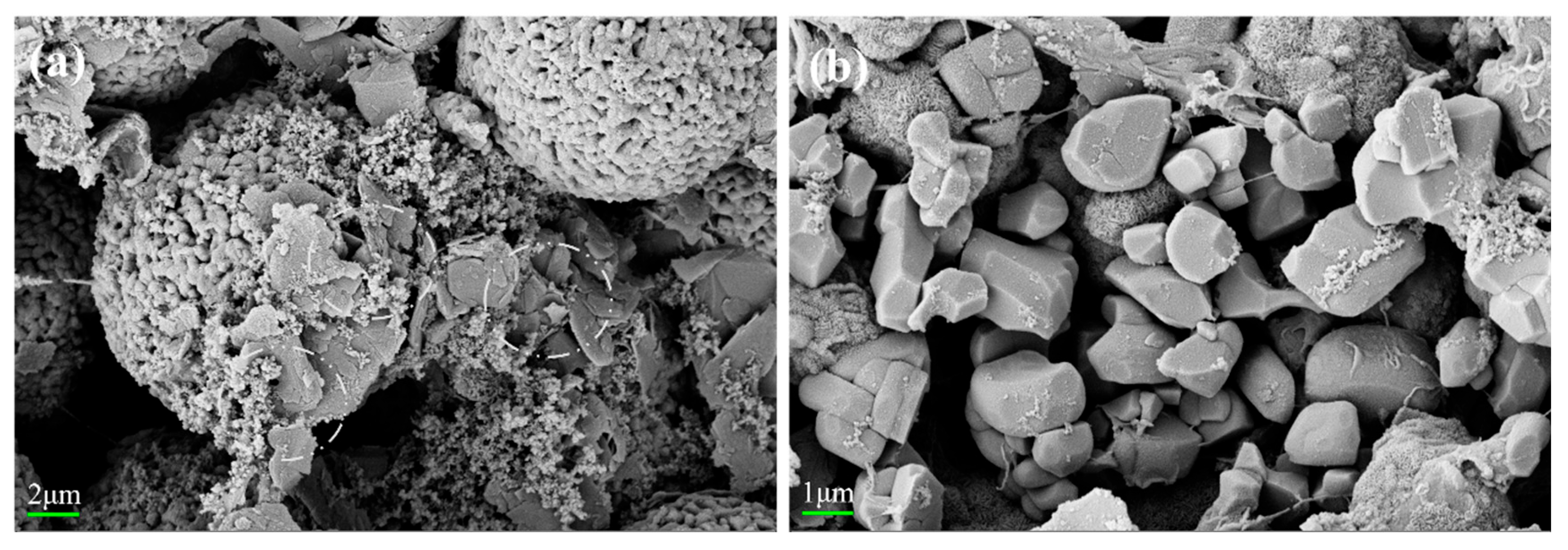
| Sr-Doped Composite Materials | Ni (mol%) | Co (mol%) | Mn (mol%) | Sr (mol%) |
|---|---|---|---|---|
| LiNi0.88Co0.05Mn0.07O2/Sr | 88.13 | 4.83 | 7.31 | 0.496 |
| Sample | a | c | c/a | V(Å3) | R(I003/I104) | Ni in Li Sites (%) | TM Slab | Li Slab |
|---|---|---|---|---|---|---|---|---|
| NCM-790 | 2.8696 | 14.1740 | 4.9404 | 101.03 | 1.37 | 1.5 | 2.1263 | 2.5990 |
| NCM-0.5Sr | 2.8718 | 14.1947 | 4.9429 | 101.38 | 1.59 | 1.23 | 2.1331 | 2.5984 |
| Sample | NCM-790 | NCM-0.5Sr | ||||
|---|---|---|---|---|---|---|
| Ro(Ω) | RSEI(Ω) | Rct(Ω) | Ro(Ω) | RSEI(Ω) | Rct(Ω) | |
| Cycling | ||||||
| 1st | 1.833 | 34.182 | 39.403 | 2.304 | 30.707 | 23.429 |
| 50th | 2.657 | 32.016 | 46.101 | 2.443 | 28.448 | 32.506 |
| 100th | 2.891 | 18.807 | 79.463 | 2.755 | 22.599 | 57.914 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Wang, Y.; Zheng, M.; Cai, F. Strontium Doping Promotes Low-Temperature Growth of Single-Crystalline Ni-Rich Cathodes with Enhanced Electrochemical Performance. Materials 2025, 18, 1320. https://doi.org/10.3390/ma18061320
Wang J, Wang Y, Zheng M, Cai F. Strontium Doping Promotes Low-Temperature Growth of Single-Crystalline Ni-Rich Cathodes with Enhanced Electrochemical Performance. Materials. 2025; 18(6):1320. https://doi.org/10.3390/ma18061320
Chicago/Turabian StyleWang, Jiaqi, Yunchang Wang, Mengran Zheng, and Feipeng Cai. 2025. "Strontium Doping Promotes Low-Temperature Growth of Single-Crystalline Ni-Rich Cathodes with Enhanced Electrochemical Performance" Materials 18, no. 6: 1320. https://doi.org/10.3390/ma18061320
APA StyleWang, J., Wang, Y., Zheng, M., & Cai, F. (2025). Strontium Doping Promotes Low-Temperature Growth of Single-Crystalline Ni-Rich Cathodes with Enhanced Electrochemical Performance. Materials, 18(6), 1320. https://doi.org/10.3390/ma18061320





