A Novel Type II Photoinitiator with Self-Supplied Hydrogen for Anti-Creep Crosslinking Polyethylene Film
Abstract
1. Introduction
2. Experiment
2.1. Materials and Instruments
2.2. Synthesis of BPMD
2.3. Synthesis of DBPMD
2.4. FTIR Spectroscopy
2.5. NMR Hydrogen Spectroscopy
2.6. Mass Spectrometry
2.7. Elemental Analysis
2.8. Differential Scanning Calorimetry (DSC)
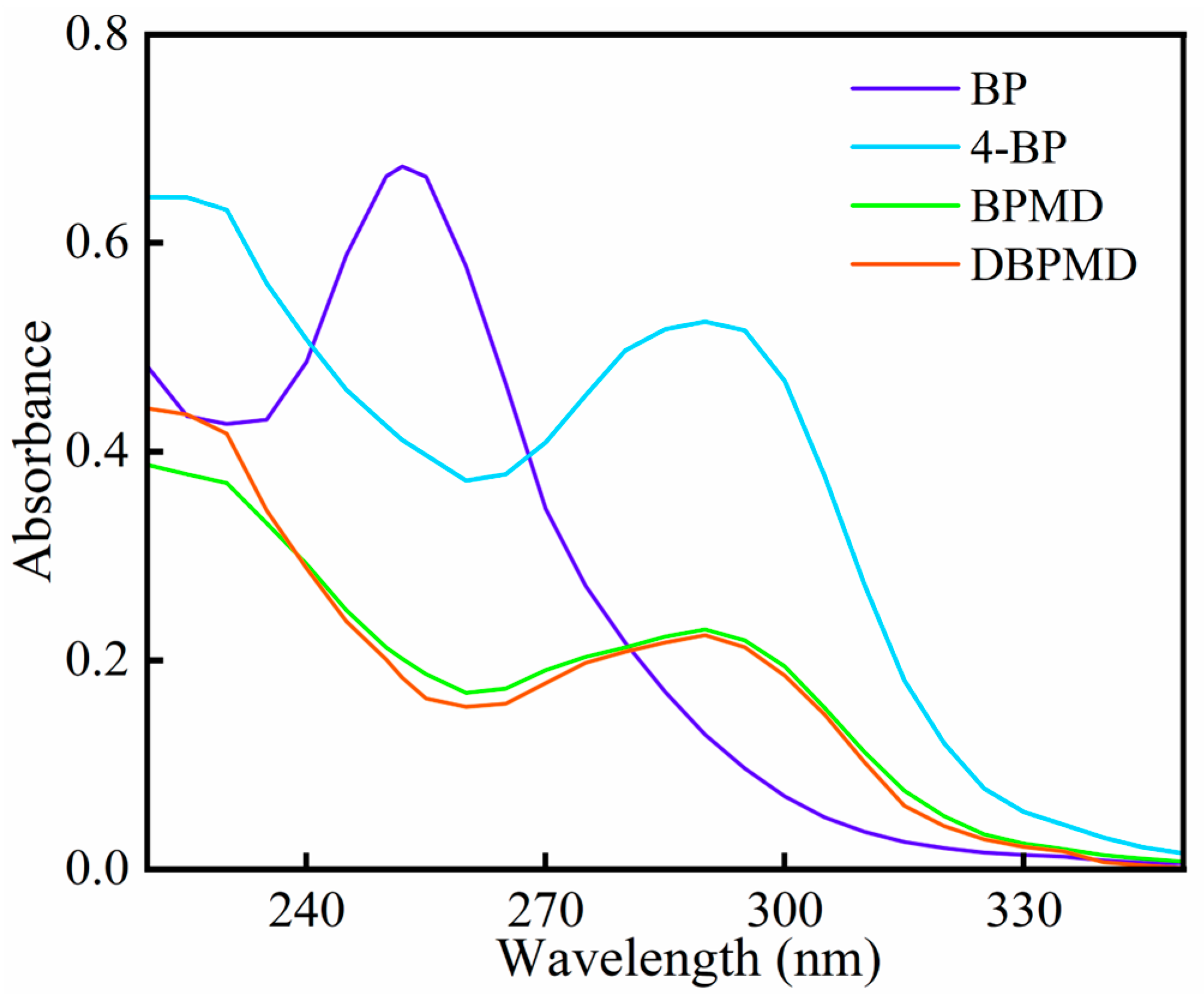
2.9. UV Absorption Behavior
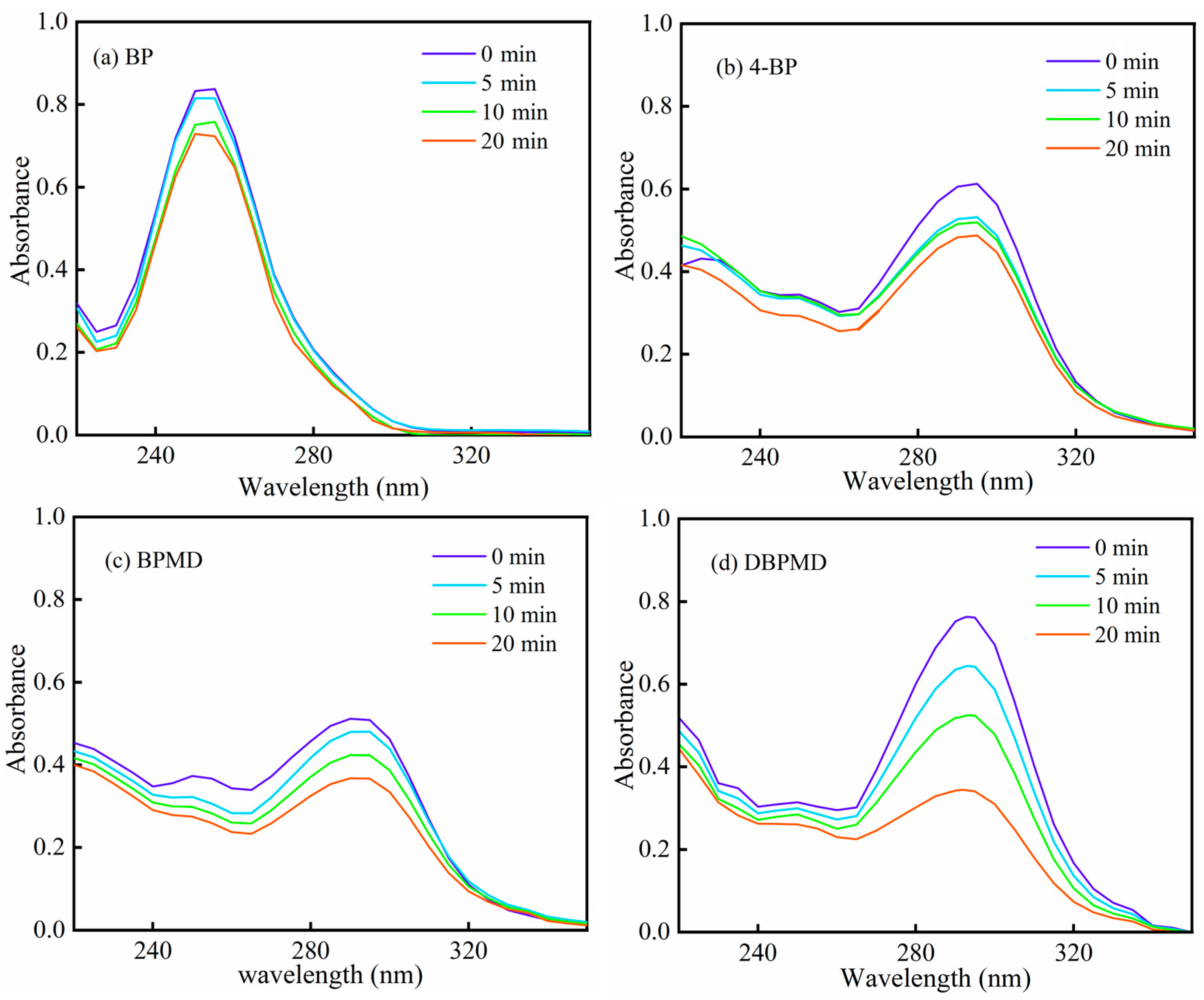
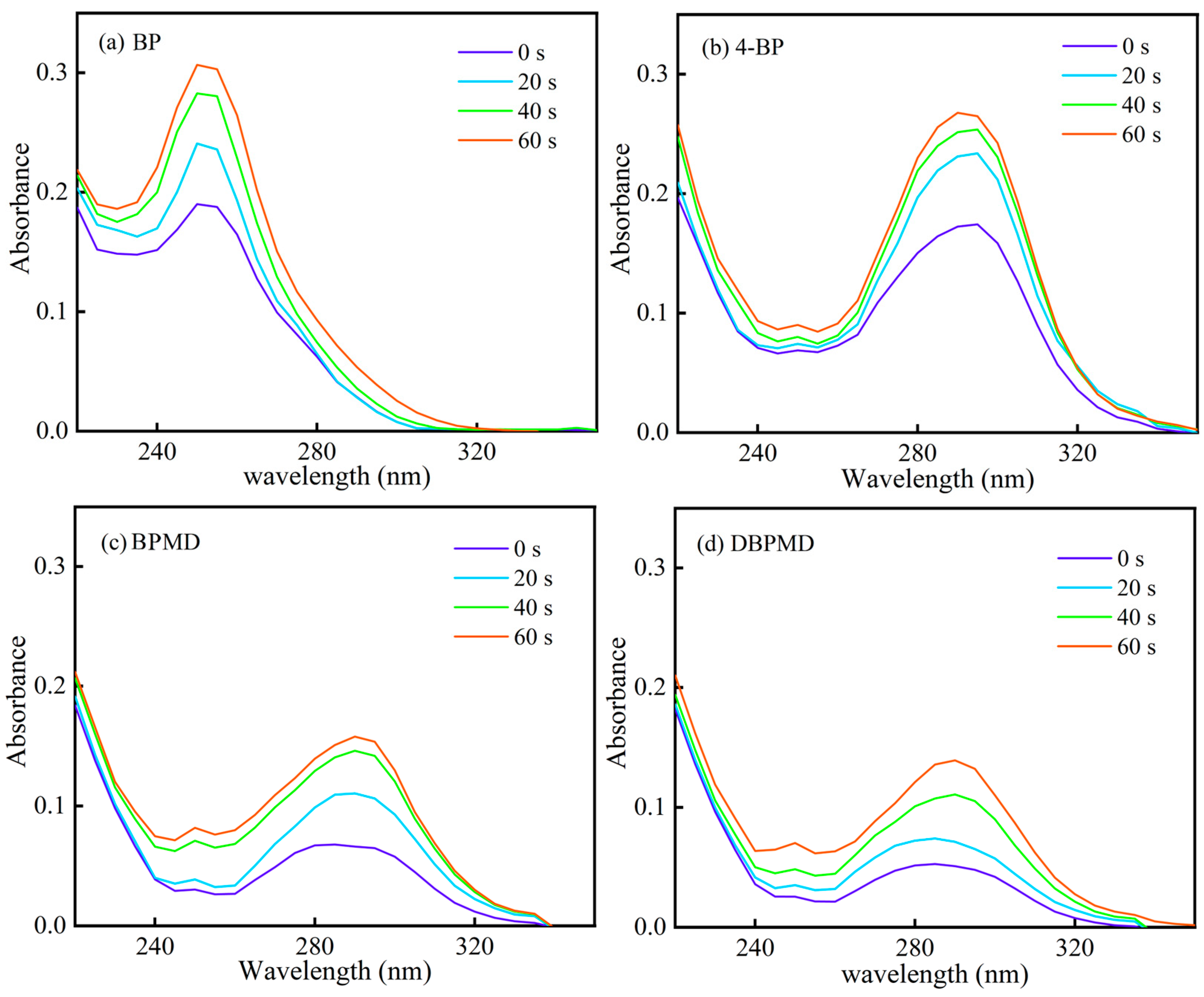
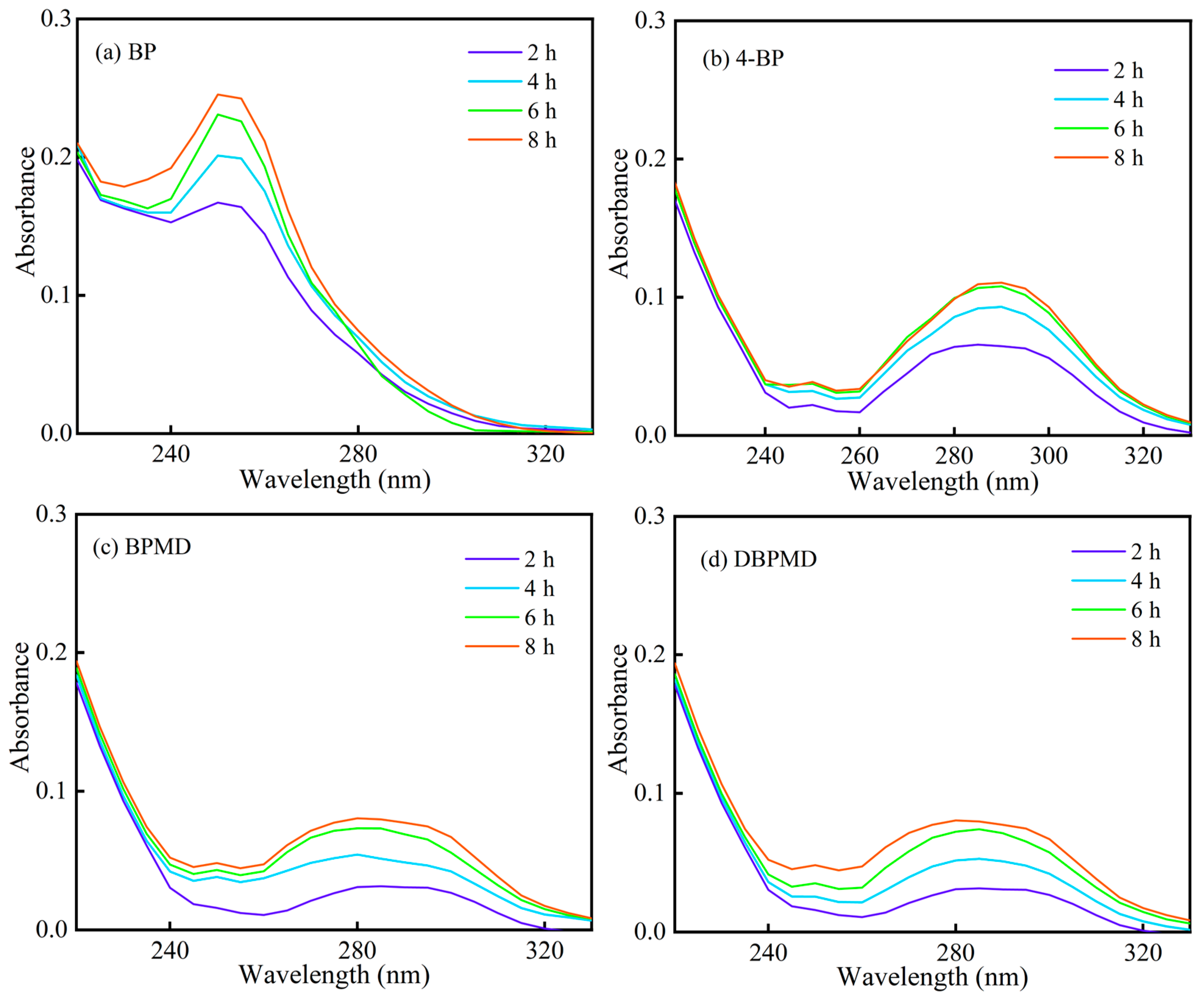
2.10. UV Photolysis Behavior
2.11. Study of Migration Performance and Extraction Rate
2.12. Initiation Activity
2.13. Optical Crosslinking Dynamics
2.14. Crystallinity and Mechanical Properties Testing
3. Results and Discussion
3.1. Characterization of Infrared Absorption Spectroscopy
3.2. NMR Hydrogen Spectroscopic Characterization
3.2.1. NMR Hydrogen Spectroscopic Characterization of BPMD
3.2.2. NMR Hydrogen Spectroscopic Characterization of DBPMD
3.3. Mass Spectrometry Characterization
3.4. Elemental Analysis Characterization
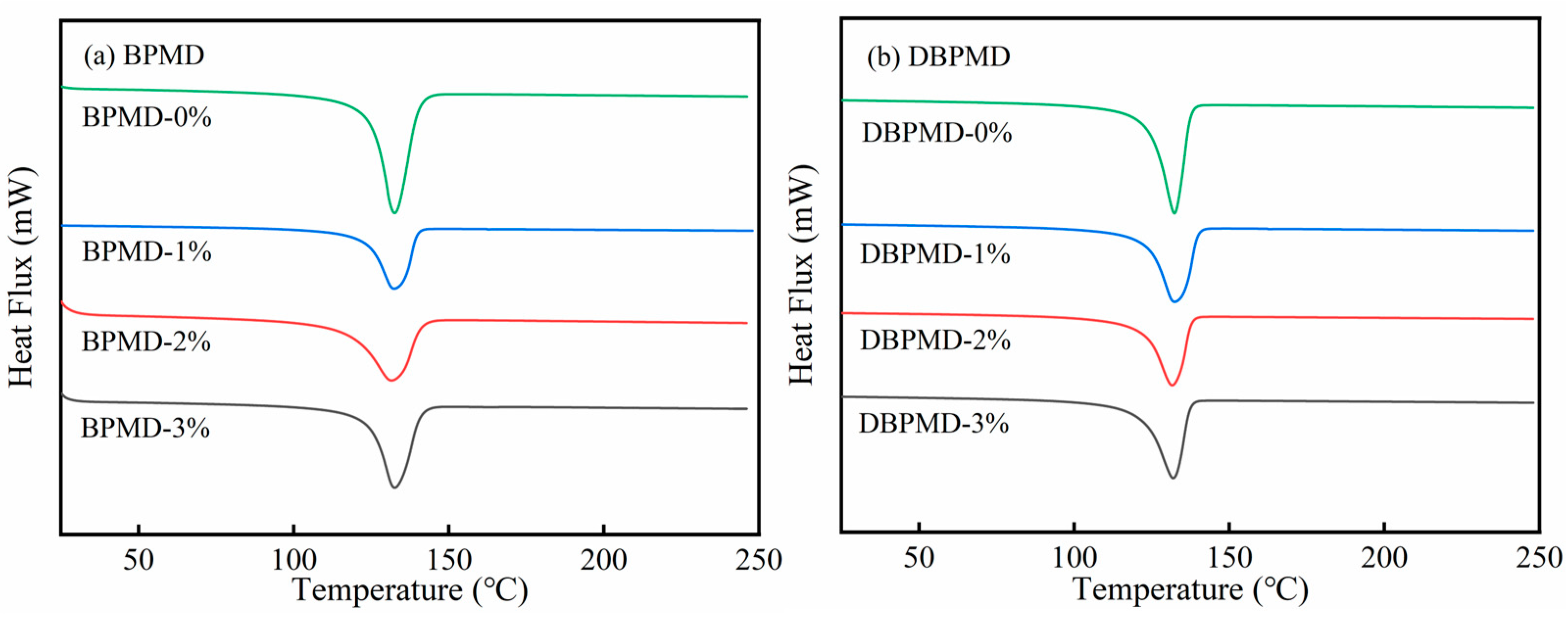
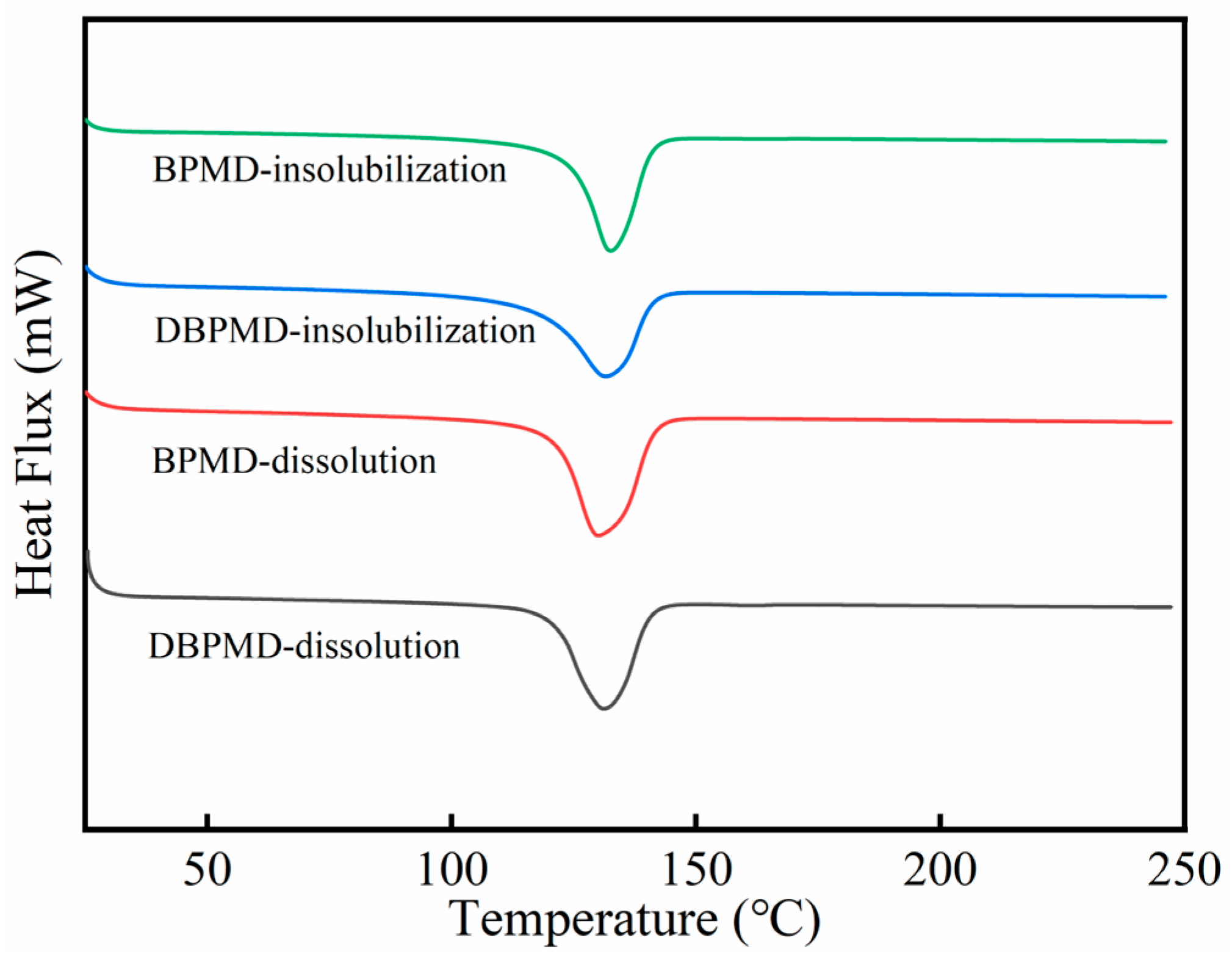
3.5. DSC Characterization
3.6. UV Absorption Spectroscopy
3.7. UV Photolysis
3.8. Migration Performance
3.9. Determination of Extraction Amount
3.10. Eliciting Activity Contrasts
3.11. Optical Cross-Linking Dynamics Studies
3.11.1. Effect of Photoinitiator Concentration on Photocrosslinking Kinetics
3.11.2. Effect of Crosslinker Type on Optical Crosslinking Kinetics
3.11.3. Effect of Light Distance on Optical Crosslinking Dynamics
3.12. Crystallinity and Mechanical Properties
3.12.1. Crystallinity Studies
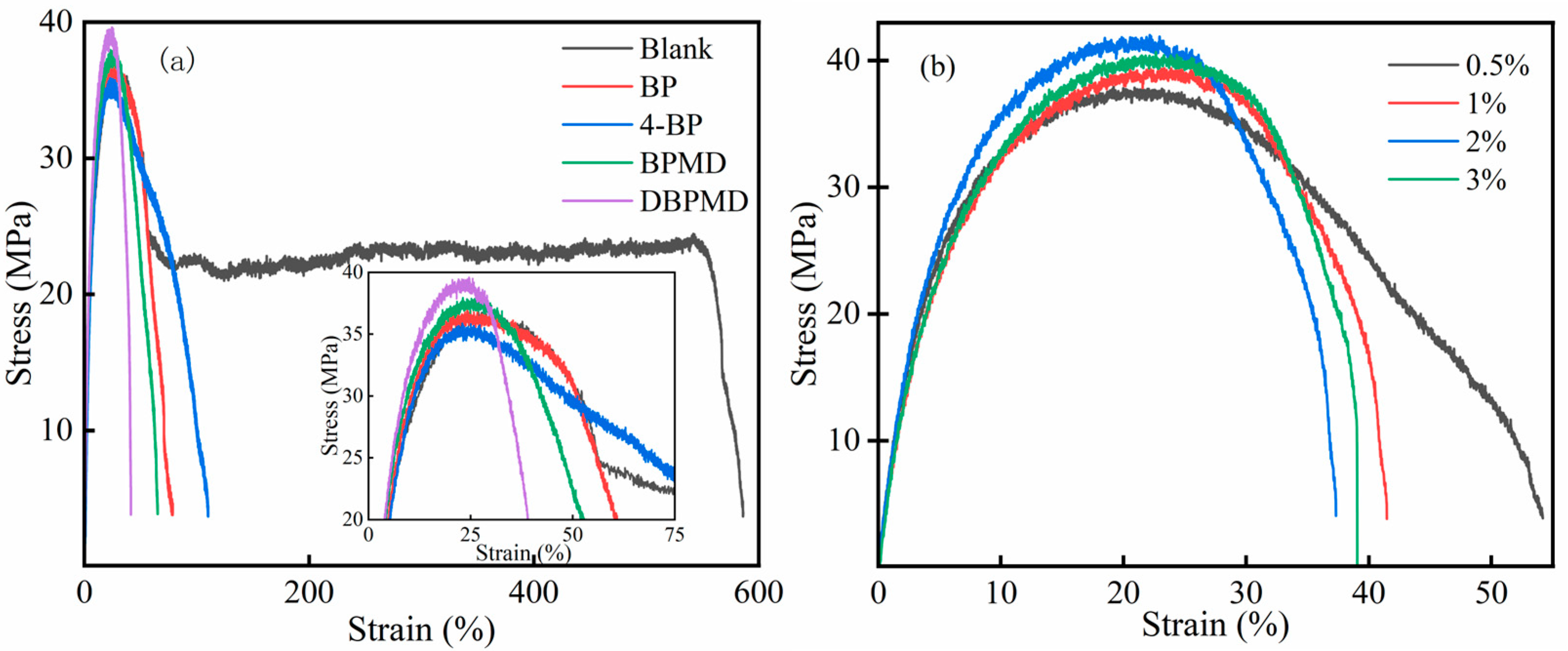
3.12.2. Tensile Properties Study
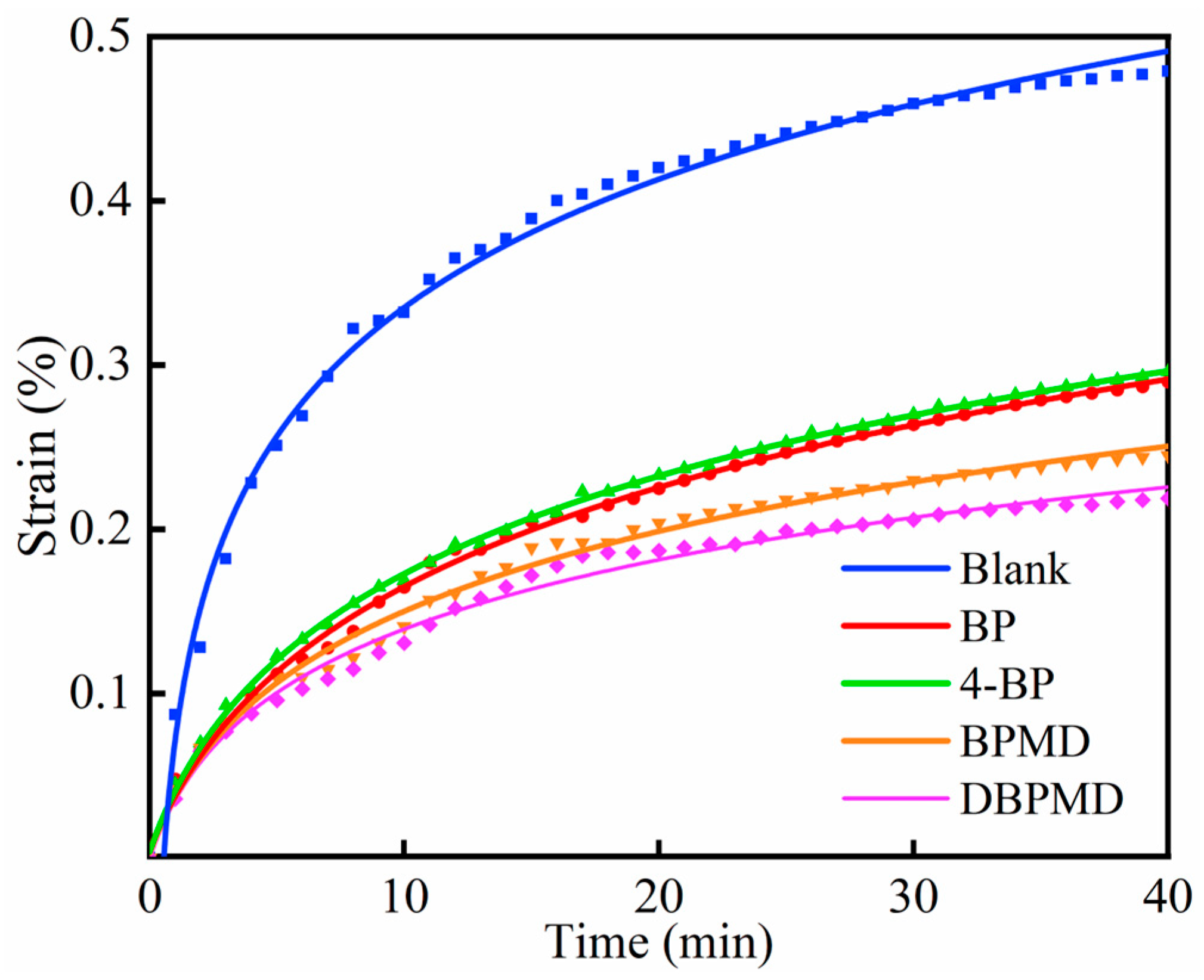
3.12.3. Creep Performance Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Selvin, M.; Shah, S.; Maria, H.J.; Thomas, S.; Tuladhar, R.; Jacob, M. Review on recycling of cross-linked polyethylene. Ind. Eng. Chem. Res. 2024, 63, 1200–1214. [Google Scholar] [CrossRef]
- Kouhi, M.; Butan, S.; Li, Y.; Shakour, E.; Banu, M. Role of chemically functionalization of bamboo fibers on polyethylene-based composite performance: A solution for recycling. Polymers 2021, 13, 2564. [Google Scholar] [CrossRef] [PubMed]
- Pearson, A.; Duncan, M.; Hammami, A.; Naguib, H.E. Interfacial adhesion and thermal stability of high-density polyethylene glass fiber composites. Compos. Sci. Technol. 2022, 227, 109570. [Google Scholar] [CrossRef]
- Mao, Q.; Su, B.; Ma, R.; Li, Z. Investigation of Tensile Creep Behavior for High-Density Polyethylene (HDPE) via Experiments and Mathematical Model. Materials 2021, 14, 6188. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Yu, J.; Liu, L.; Xue, P.; Chen, K. Influence of high-density polyethylene content on the rheology, crystal structure, and mechanical properties of melt spun ultra-high-molecular weight polyethylene/high-density polyethylene blend fibers. J. Ind. Text. 2023, 53, 15280837221150198. [Google Scholar] [CrossRef]
- Zha, S.; Lan, H.-Q.; Huang, H. Review on lifetime predictions of polyethylene pipes: Limitations and trends. Int. J. Press. Vessel. Pip. 2022, 198, 104663. [Google Scholar] [CrossRef]
- Wang, H.; Xu, L.; Li, R.; Hu, J.; Wang, M.; Wu, G. Improving the creep resistance and tensile property of UHMWPE sheet by radiation cross-linking and annealing. Radiat. Phys. Chem. 2016, 125, 41–49. [Google Scholar] [CrossRef]
- Luo, Y.; Zhou, T.; Jin, T.; Wang, C.; Ma, Y.; Song, H.; Zhang, H. Study on the low-temperature performance of chemically cross-linked polyethylene composite modified asphalt binder. Chem. Eng. Sci. 2025, 302, 120827. [Google Scholar] [CrossRef]
- Zhang, J.; Li, J.; Li, H.; Yang, M.; Li, Y.; Qi, Y.; Xu, Y.; Hu, W.; Liu, B. Recent Overview and Future Research Prospects of Cross-linked Polyethylene Materials: Cross-linking Methods and Applications. Preprints 2024, 2024091601. [Google Scholar] [CrossRef]
- Chodák, I. High modulus polyethylene fibres: Preparation, properties and modification by crosslinking. Prog. Polym. Sci. 1998, 23, 1409–1442. [Google Scholar] [CrossRef]
- Yen, S.C.; Ni, J.S.; Chen, Y.C. Synthesis of One-Component Type II Visible-Light-Absorbing Chalcones Containing Fused Aromatic Rings and Investigation of Free Radical Photopolymerization Properties. Macromol. Chem. Phys. 2024, 225, 2300428. [Google Scholar] [CrossRef]
- Dietliker, K.; Hüsler, R.; Birbaum, J.-L.; Ilg, S.; Villeneuve, S.; Studer, K.; Jung, T.; Benkhoff, J.; Kura, H.; Matsumoto, A. Advancements in photoinitiators—Opening up new applications for radiation curing. Prog. Org. Coat. 2007, 58, 146–157. [Google Scholar] [CrossRef]
- Zhang, H.; Shang, Y.; Li, M.; Zhao, H.; Wang, X.; Han, B. Theoretical study on the reaction mechanism in the UV radiation cross-linking process of polyethylene. RSC Adv. 2016, 6, 110831–110839. [Google Scholar] [CrossRef]
- Wang, X.; Li, W.; Nie, J.; Zhu, X. A deep-curing UV-LED light photoinitiator based on diphenylpropanetrione. J. Photochem. Photobiol. A Chem. 2024, 454, 115674. [Google Scholar] [CrossRef]
- Duan, K.; Chen, X.; Ni, J.; Mei, Z.; Zhou, C.; Ye, X.; Zhu, H.; Yu, D. Preparation and properties of ultraviolet crosslinked high-density polyethylene materials. China Plast. 2024, 38, 24. Available online: https://www.plaschina.com.cn/EN/Y2024/V38/I5/24 (accessed on 1 May 2024).
- Dong, T.; Niu, F.; Qiang, Z.; Wang, X.; Guo, J.; Wang, Y.; He, Y. Structure and properties evolution of UV crosslinked ultra-high molecular weight polyethylene fiber during processing. J. Polym. Sci. 2024, 62, 2385–2398. [Google Scholar] [CrossRef]
- Cınar, S.A.; Guven, M.N.; Eren, T.N.; Cesur, B.; Aleksanyan, M.; Dedeoglu, B.; Okte, N.; Aviyente, V.; Morlet-Savary, F.; Lalevée, J. Structure-reactivity relationships of novel monomeric photoinitiators. J. Photochem. Photobiol. A Chem. 2016, 329, 77–87. [Google Scholar] [CrossRef]
- Cesur, B.; Karahan, O.; Agopcan, S.; Eren, T.N.; Okte, N.; Avci, D. Difunctional monomeric and polymeric photoinitiators: Synthesis and photoinitiating behaviors. Prog. Org. Coat. 2015, 86, 71–78. [Google Scholar] [CrossRef]
- Li, W.; Nie, J.; Zhao, Y.; Zhu, X. Photoinitiators with low migration capability based on benzophenone. Eur. Polym. J. 2024, 202, 112591. [Google Scholar] [CrossRef]
- Zhou, J.; Allonas, X.; Ibrahim, A.; Liu, X. Progress in the development of polymeric and multifunctional photoinitiators. Prog. Polym. Sci. 2019, 99, 101165. [Google Scholar] [CrossRef]
- Taschner, R.; Gauss, P.; Knaack, P.; Liska, R. Biocompatible photoinitiators based on poly-α-ketoesters. J. Polym. Sci. 2020, 58, 242–253. [Google Scholar] [CrossRef]
- Davidson, R.S.; Dias, A.A.; Illsley, D. A new series of type II (benzophenone) polymeric photoinitiators. J. Photochem. Photobiol. A Chem. 1995, 89, 75–87. [Google Scholar] [CrossRef]
- Huang, T.-L.; Chen, Y.-C. Synthesis and free radical photopolymerization of one-component type II photoinitiator based on benzophenone segment. J. Photochem. Photobiol. A Chem. 2022, 429, 113900. [Google Scholar] [CrossRef]
- Li, W.; Nie, J.; Zhao, Y.; Zhu, X. A low mobility UV-LED benzophenone photoinitiator. J. Photochem. Photobiol. A Chem. 2024, 455, 115785. [Google Scholar] [CrossRef]
- Deng, L.; Tang, L.; Qu, J. Synthesis and photopolymerization of novel UV-curable macro-photoinitiators. Prog. Org. Coat. 2020, 141, 105546. [Google Scholar] [CrossRef]
- Huang, T.-L.; Chen, Y.-C. Ketone number and substitution effect of benzophenone derivatives on the free radical photopolymerization of visible-light type-II photoinitiators. Polymers 2021, 13, 1801. [Google Scholar] [CrossRef]
- Paradowska-Stolarz, A.; Malysa, A.; Mikulewicz, M. Comparison of the compression and tensile modulus of two chosen resins used in dentistry for 3D printing. Materials 2022, 15, 8956. [Google Scholar] [CrossRef]
- Jasem, M.H.; Abbod, E.A.; Jassim, T.A.; Hussien, Z.Y.; Omaraa, E. Steady-State Creep Behaviour of Functionally Graded Silicone Rubber with Cellulose Addition. J. Eng. Sustain. Dev. 2025, 29, 236–241. [Google Scholar] [CrossRef]
- Cheng, L.; Shi, W. Synthesis and photoinitiating behavior of benzophenone-based polymeric photoinitiators used for UV curing coatings. Prog. Org. Coat. 2011, 71, 355–361. [Google Scholar] [CrossRef]
- Feng, C.; Wang, Q.L.; Liu, F.; Zhang, B. Synthesis and application of new benzophenone photoinitiators. ChemistrySelect 2023, 8, e202302572. [Google Scholar] [CrossRef]
- Dumur, F. Recent advances on visible light Triphenylamine-based photoinitiators of polymerization. Eur. Polym. J. 2022, 166, 111036. [Google Scholar] [CrossRef]
- Davidson, R.S.; Dias, A.A.; Illsley, D.R. Type II polymeric photoinitiators (polyetherimides) with built-in amine synergist. J. Photochem. Photobiol. A Chem. 1995, 91, 153–163. [Google Scholar] [CrossRef]
- Deka, S.; Kakati, D. Benzoin-terminated polyurethane as macrophotoinitiator for synthesis of polyurethane–polymethyl methacrylate block copolymers. J. Appl. Polym. Sci. 2009, 111, 3089–3093. [Google Scholar] [CrossRef]
- Liang, Q.; Wang, Z.; Du, W.; Liu, W.; Cao, J.; Ren, J.; Lian, W.; Lu, H.; Li, H. Determination of 18 photoinitiators in food paper packaging materials by FastPrep-based extraction combined with GC–MS. Food Chem. 2022, 377, 131980. [Google Scholar] [CrossRef] [PubMed]
- Sanai, Y.; Ninomiya, T.; Arimitsu, K. Improvements in the physical properties of UV-curable coating by utilizing type II photoinitiator. Prog. Org. Coat. 2021, 151, 106038. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhu, G.-R.; Xiao, X.-X.; Liu, Q.-S.; Jiang, M.; Guo, D.-M.; Zhao, H.-B.; Li, W.-D.; Chen, L.; Liu, B.-W. Controllable micro cross-linking towards multifunctional flame-retardant aliphatic polyamide. Chem. Eng. J. 2023, 472, 144983. [Google Scholar] [CrossRef]
- Cho, H.-K.; Kim, H. Preparation of de-crosslinked polyethylene from waste crosslinked high-density polyethylene using supercritical twin-screw extrusion. Korean J. Chem. Eng. 2024, 41, 1767–1773. [Google Scholar] [CrossRef]
- Bobovitch, A.; Gutman, E.M.; Henning, S.; Michler, G.H. Morphology and stress-relaxation of biaxially oriented cross-linked polyethylene films. Mater. Lett. 2003, 57, 2597–2601. [Google Scholar] [CrossRef]
- Datta, S.; Stocek, R.; Naskar, K. Influence of ultraviolet radiation on mechanical properties of a photoinitiator compounded high vinyl styrene–butadiene–styrene block copolymer. Polymers 2021, 13, 1287. [Google Scholar] [CrossRef]
- Montoya-Ospina, M.C.; Verhoogt, H.; Ordner, M.; Tan, X.; Osswald, T.A. Effect of cross-linking on the mechanical properties, degree of crystallinity and thermal stability of polyethylene vitrimers. Polym. Eng. Sci. 2022, 62, 4203–4213. [Google Scholar] [CrossRef]
- Mao, H.-D.; Zhang, T.-T.; Guo, Z.-Y.; Bai, D.-Y.; Wang, J.; Xiu, H.; Fu, Q. A cross-linked polyethylene with recyclability and mechanical robustness enabled by establishment of multiple hydrogen bonds network via reactive melt blending. Chin. J. Polym. Sci. 2023, 41, 1104–1114. [Google Scholar] [CrossRef]

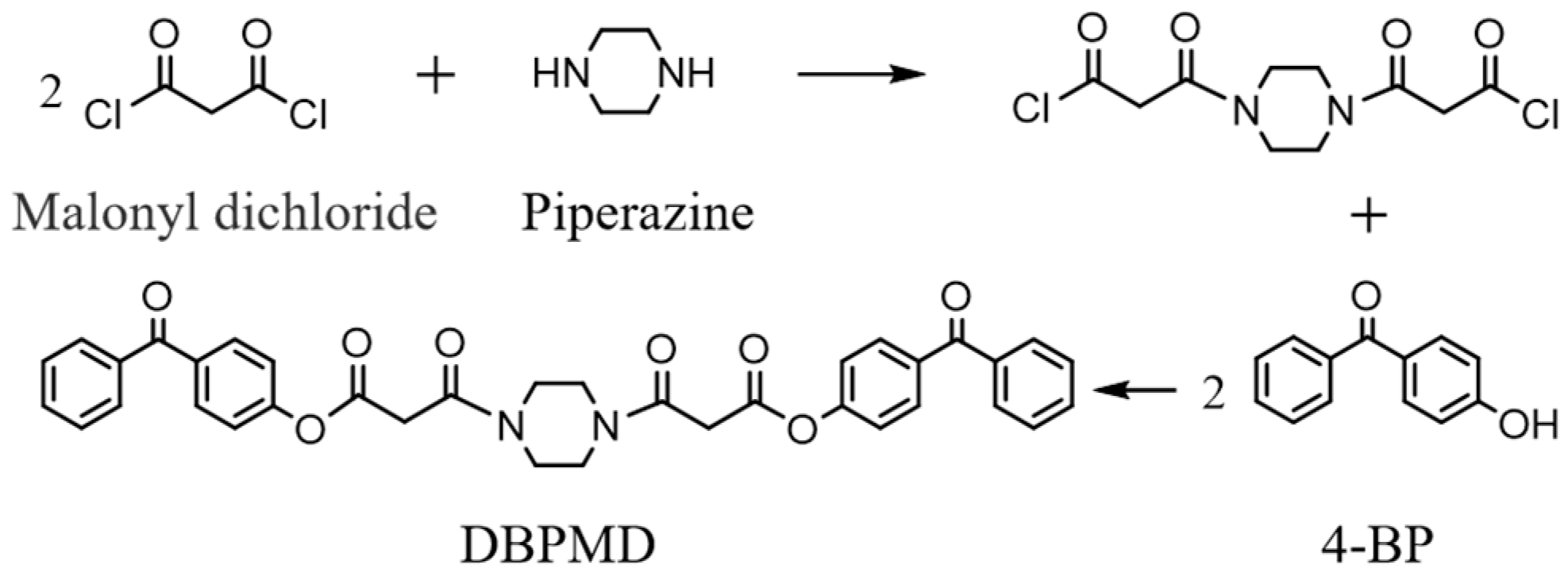


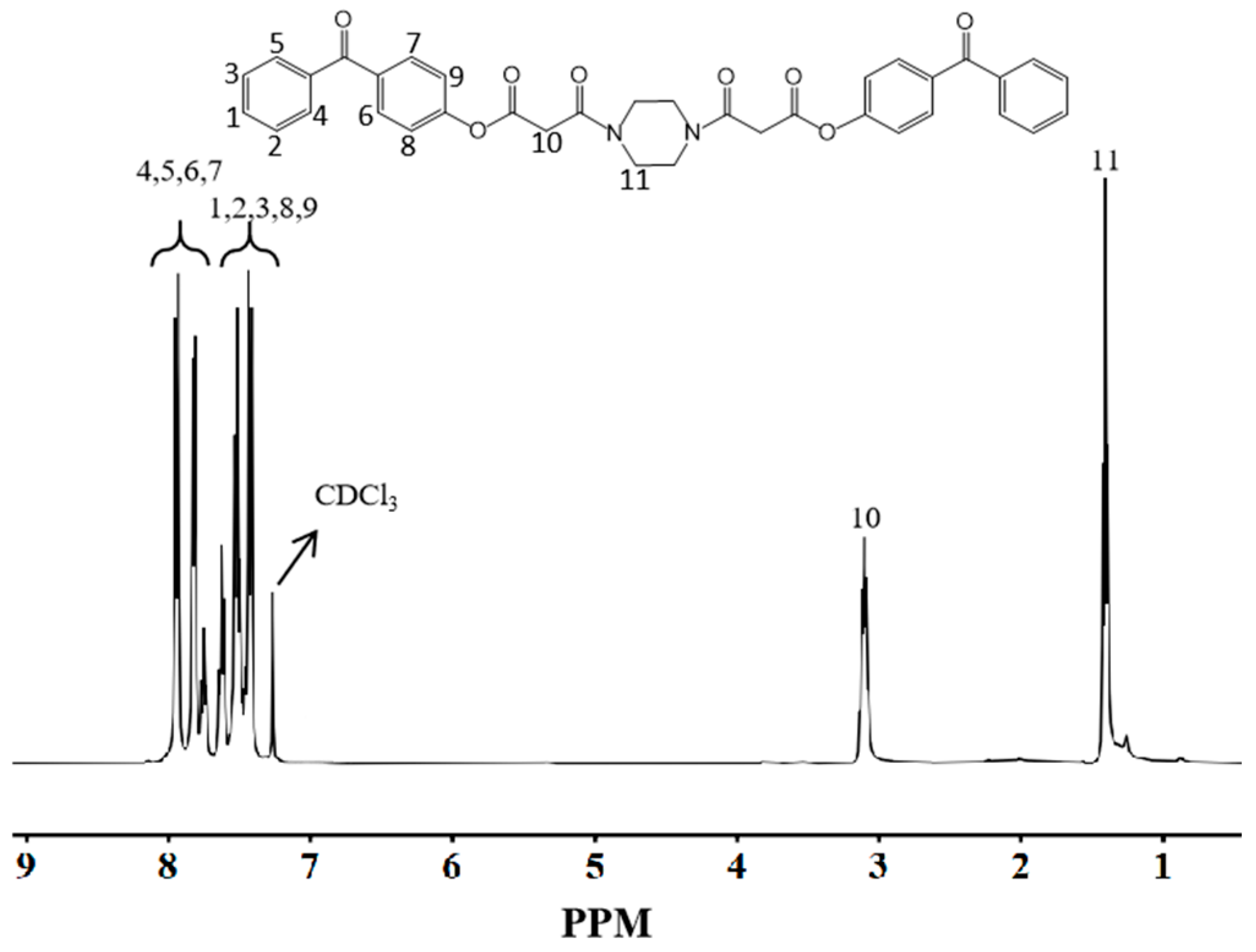
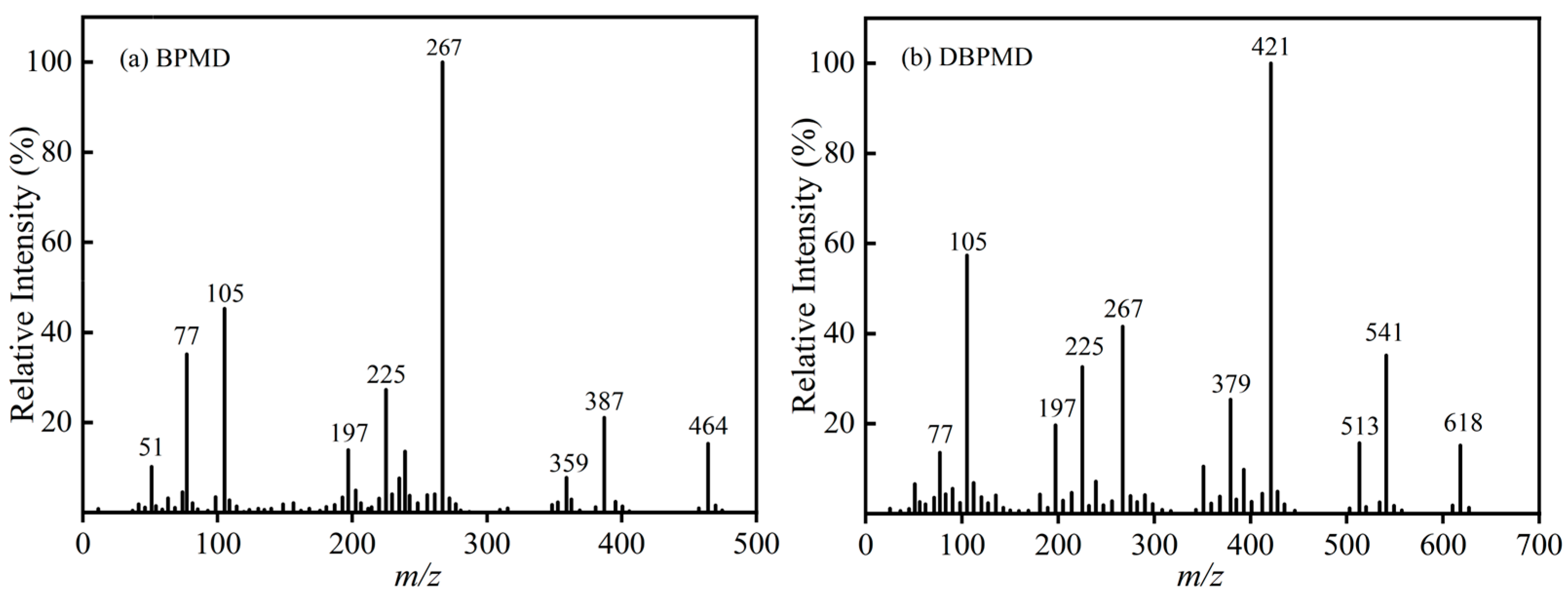
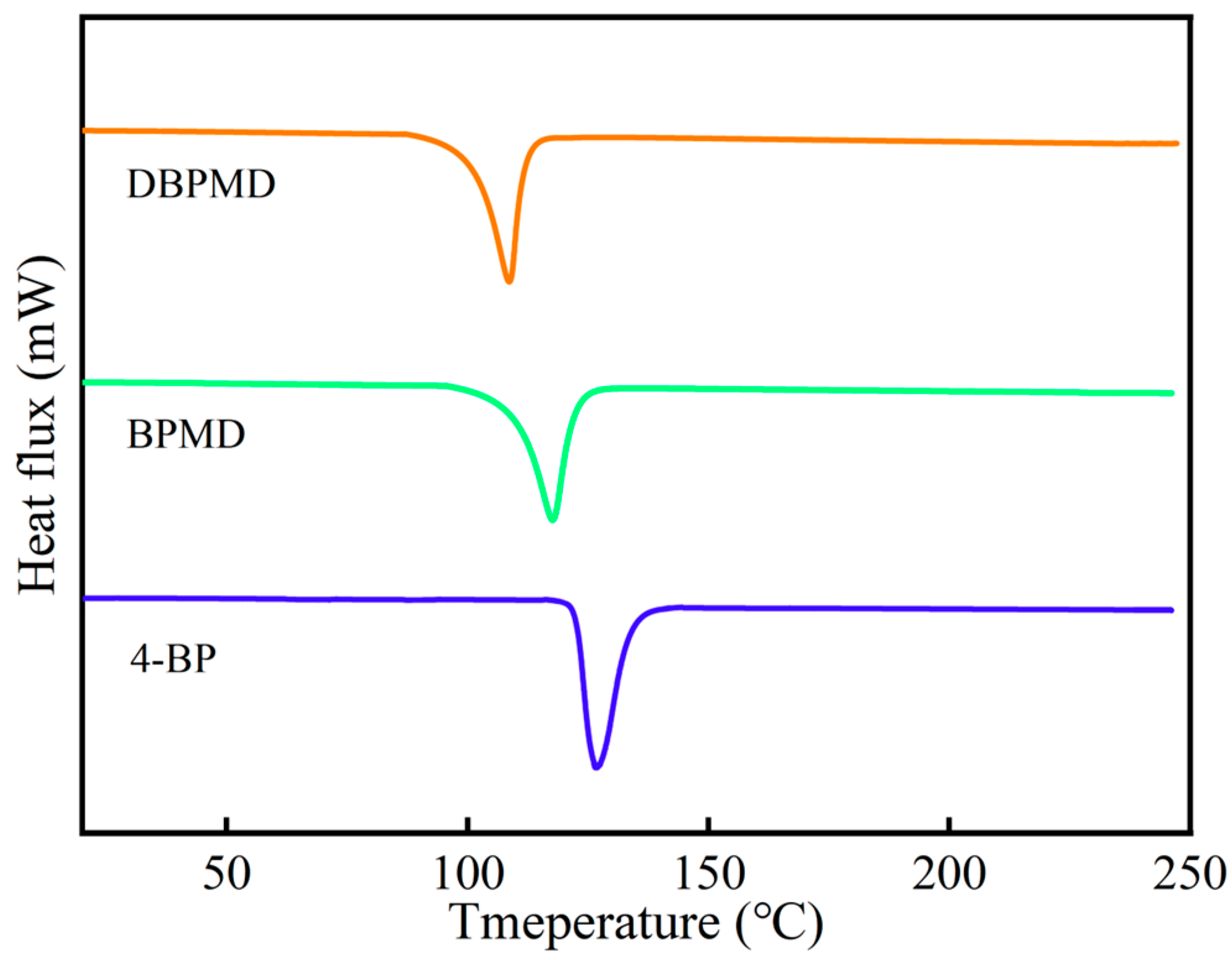

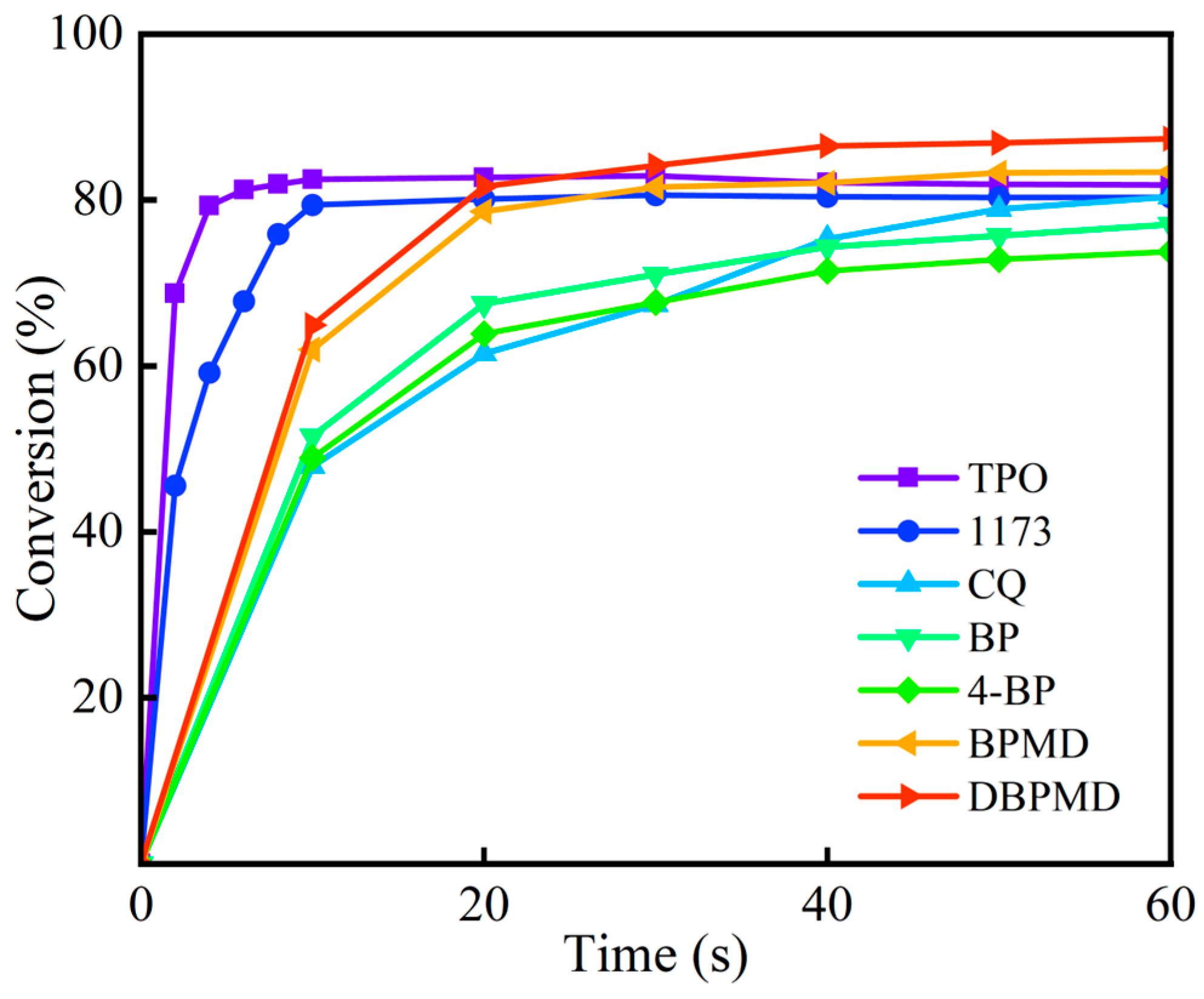
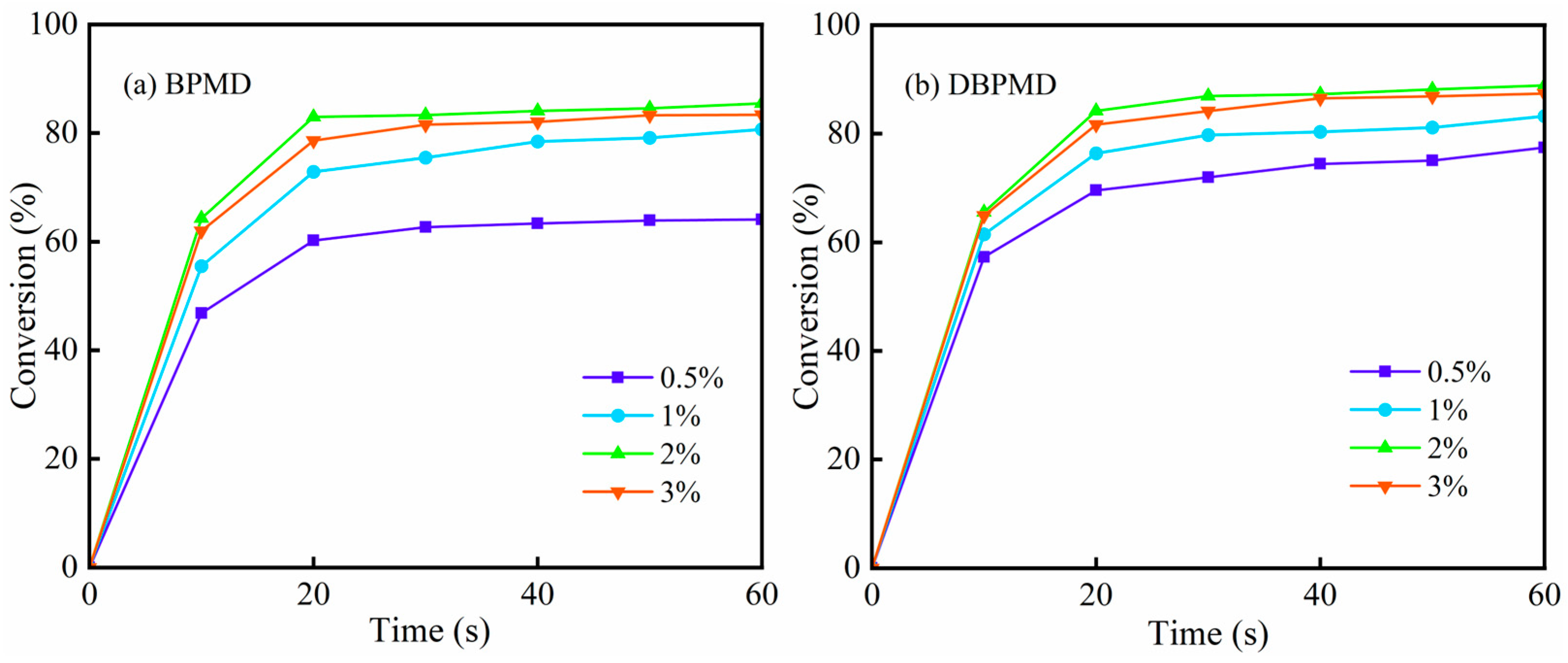
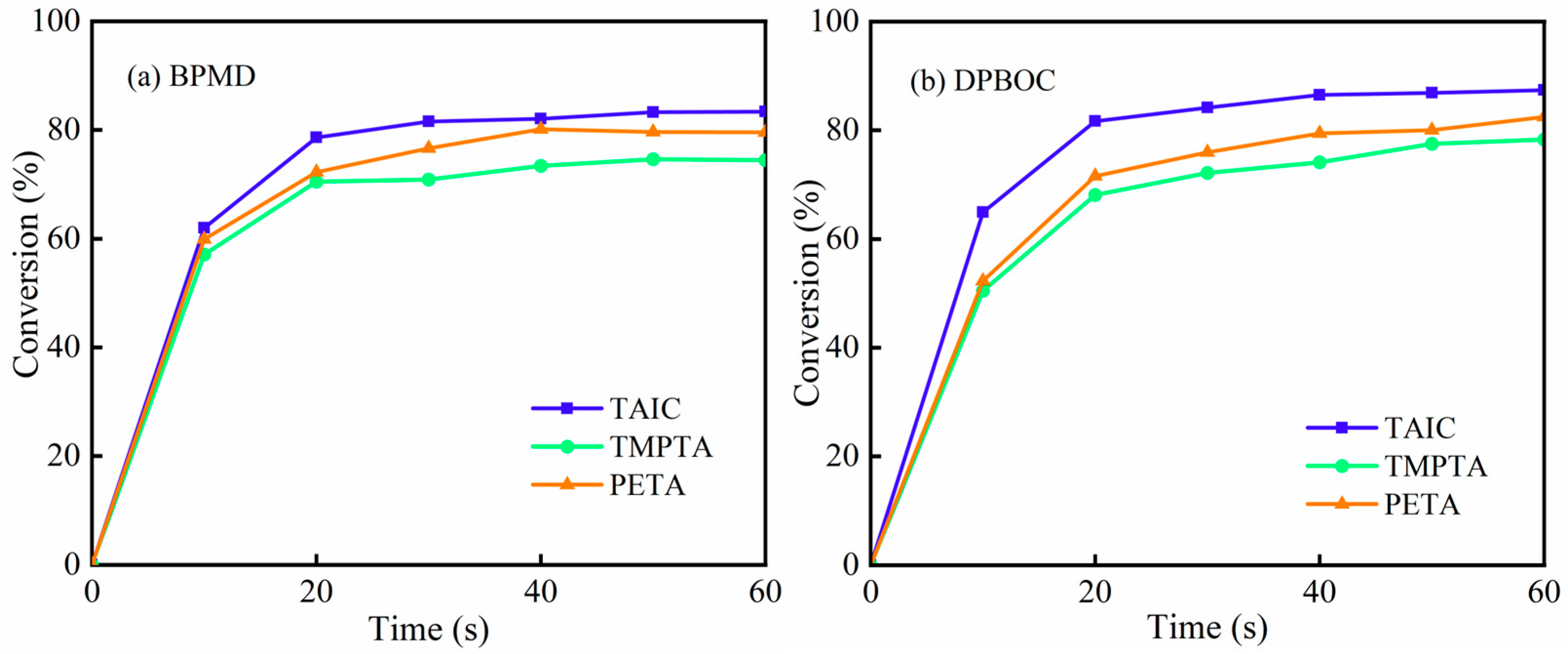
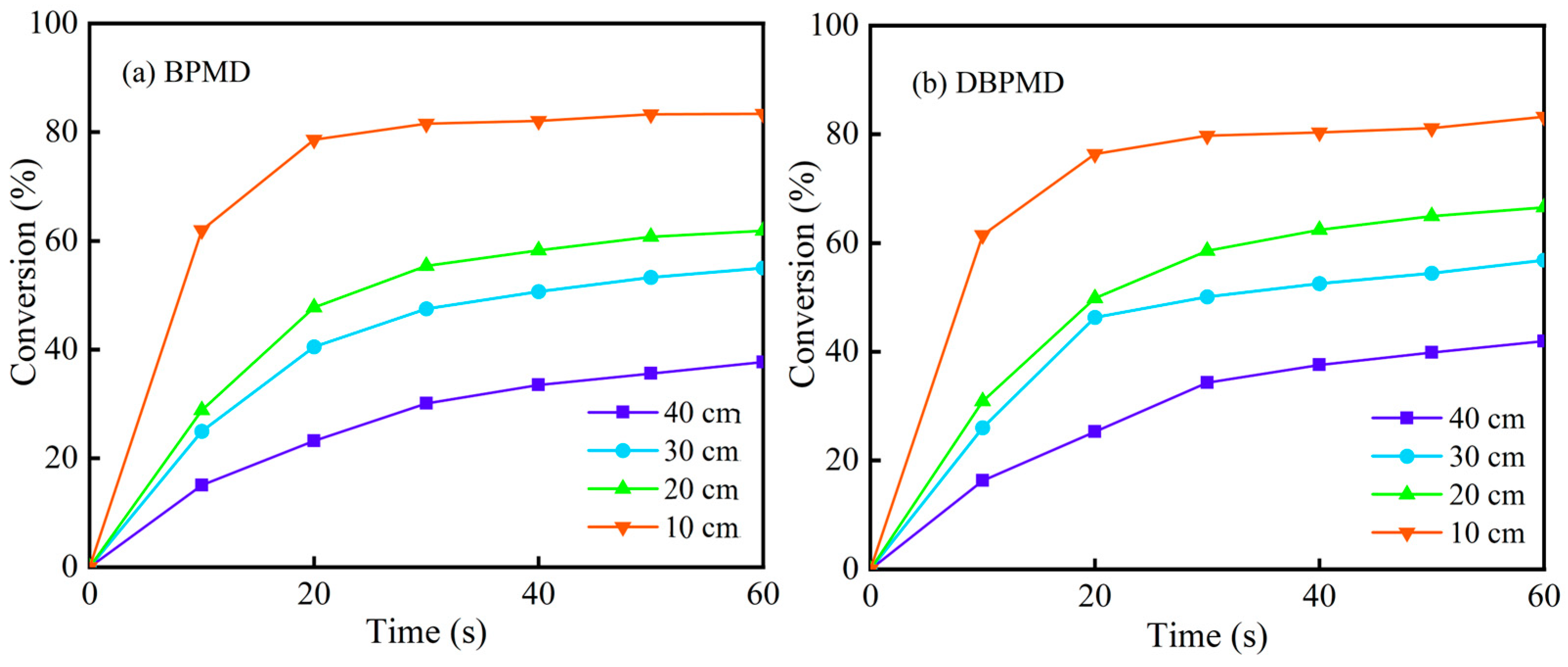
| BP | 4-BP | BPMD | DBPMD | TAIC | HDPE | |
|---|---|---|---|---|---|---|
| a | 1 | 1 | 98 | |||
| b | 1 | 1 | 98 | |||
| c | 1 | 1 | 98 | |||
| d | 1 | 1 | 98 |
| Photoinitiators | Element | Measured Value (%) | Theoretical Value (J/g) | Deviation (%) |
|---|---|---|---|---|
| BPMD | C | 73.69 | 74.99 | −1.30 |
| H | 4.15 | 4.34 | −0.19 | |
| N | 0.00 | 0.00 | 0.00 | |
| O | 22.16 | 20.67 | +1.49 | |
| DBPMD | C | 68.32 | 69.89 | −1.57 |
| H | 4.63 | 4.89 | −0.26 | |
| N | 4.39 | 4.53 | −0.14 | |
| O | 22.66 | 20.69 | +1.97 |
| Photoinitiators | λmax/nm | εmax/(L·mol−1·cm−1) |
|---|---|---|
| BP | 252 | 16,912 |
| BP | 283 | 1510 |
| 4-BP | 283 | 12,292 |
| BPMD | 283 | 20,568 |
| DBPMD | 283 | 30,528 |
| Photoinitiators | Amax | Extraction Quality 10−4 (g) | Extraction Rate (%) |
|---|---|---|---|
| BP | 0.6734 | 1.45 | 14.5 |
| 4-BP | 0.5248 | 1.69 | 16.9 |
| BPMD | 0.2298 | 1.01 | 10.1 |
| DBPMD | 0.2244 | 0.87 | 8.7 |
| Photoinitiators | Quality Score (w%) | Melting Point (°C) | Enthalpy of Fusion (J/g) | Crystallinity (%) |
|---|---|---|---|---|
| BPMD | 0 | 132.62 | 224.88 | 76.75 |
| 1 | 132.03 | 204.37 | 69.78 | |
| 2 | 131.46 | 206.33 | 70.42 | |
| 3 | 131.13 | 208.70 | 71.23 | |
| DBPMD | 0 | 132.62 | 224.88 | 76.75 |
| 1 | 131.98 | 205.45 | 70.12 | |
| 2 | 131.35 | 207.71 | 70.89 | |
| 3 | 131.05 | 209.79 | 71.56 |
| Photoinitiators | Quality Score (w%) | Tensile Strength (MPa) | Elongation at Break (%) |
|---|---|---|---|
| - | - | 36.62 | 586 |
| BP | 1 | 35.81 | 79 |
| 4-BP | 1 | 35.31 | 110 |
| BPMD | 1 | 37.77 | 65 |
| DBPMD | 1 | 39.34 | 41 |
| DBPMD | 0.5 | 37.61 | 51 |
| DBPMD | 2 | 40.82 | 37 |
| DBPMD | 3 | 39.59 | 39 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, F.; Jing, Z.; Wang, Y.; Jiang, G. A Novel Type II Photoinitiator with Self-Supplied Hydrogen for Anti-Creep Crosslinking Polyethylene Film. Materials 2025, 18, 1313. https://doi.org/10.3390/ma18061313
Yang F, Jing Z, Wang Y, Jiang G. A Novel Type II Photoinitiator with Self-Supplied Hydrogen for Anti-Creep Crosslinking Polyethylene Film. Materials. 2025; 18(6):1313. https://doi.org/10.3390/ma18061313
Chicago/Turabian StyleYang, Fei, Zhaoyuan Jing, Yingqiu Wang, and Guodong Jiang. 2025. "A Novel Type II Photoinitiator with Self-Supplied Hydrogen for Anti-Creep Crosslinking Polyethylene Film" Materials 18, no. 6: 1313. https://doi.org/10.3390/ma18061313
APA StyleYang, F., Jing, Z., Wang, Y., & Jiang, G. (2025). A Novel Type II Photoinitiator with Self-Supplied Hydrogen for Anti-Creep Crosslinking Polyethylene Film. Materials, 18(6), 1313. https://doi.org/10.3390/ma18061313





