Preparation of Wood Fiber–Polyurethane Plastic Composite with Water Resistance and High Strength
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Wood Fiber Pretreatment and Preparation of Polyurethane Prepolymer
2.3. Reaction of Wood Fibers with Prepolymer
2.4. Preparation of Prepolymers–ADF Composite
2.5. Characterization and Testing
2.5.1. Scanning Electron Microscopy (SEM)
2.5.2. Attenuated Total Reflectance Infrared Spectroscopy (ATR-IR)
2.5.3. Thermal Gravimetric Analysis (TGA)
2.5.4. Ultraviolet–Visible–Near Infrared Spectroscopy (UV–Vis-NIR)
2.5.5. Elemental Analysis
2.5.6. Water Stability Tests
2.5.7. Tensile Test
3. Results and Discussion
3.1. Pretreatment of Wood Fibers for Modification
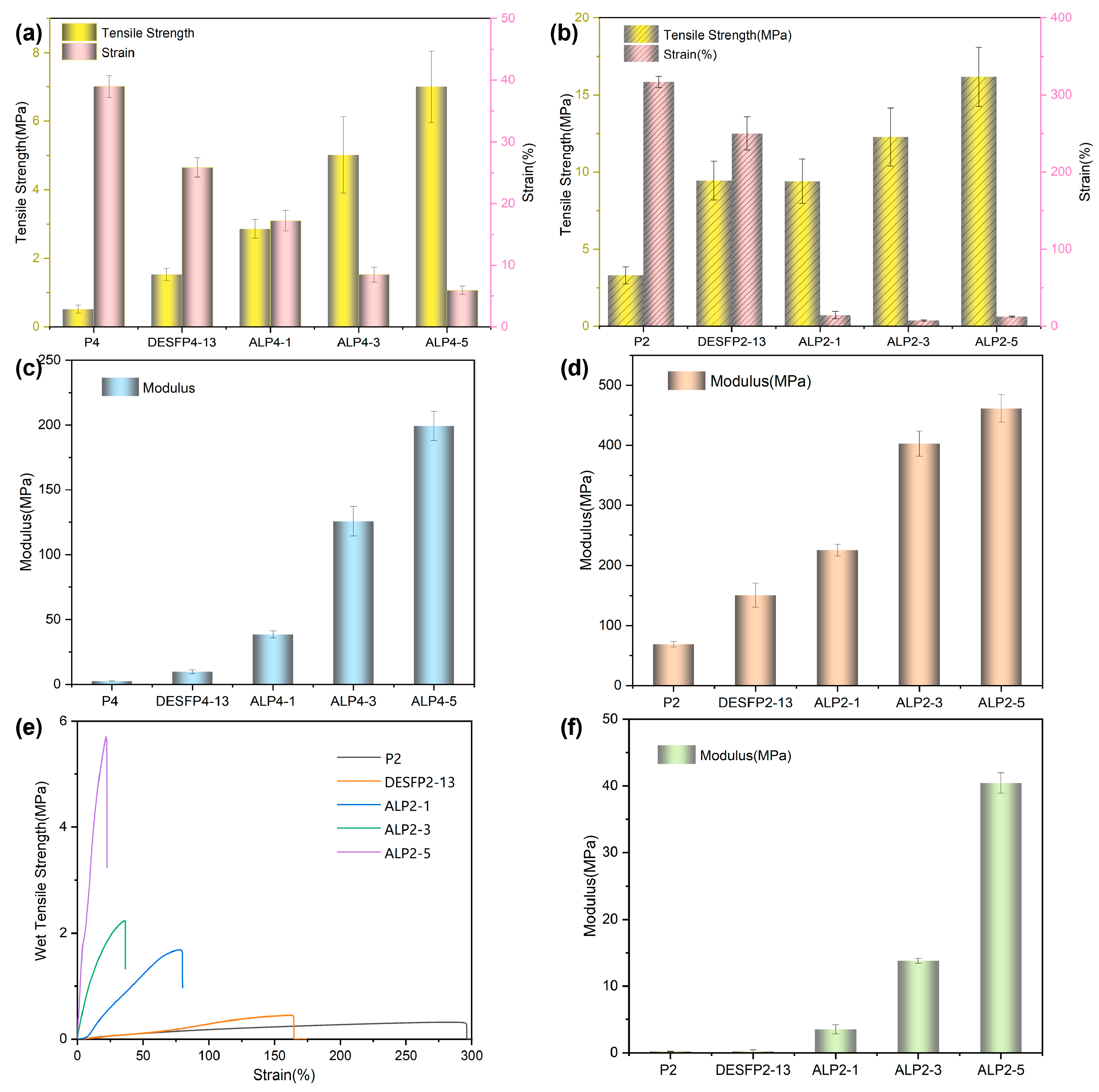
3.2. Mechanical Properties of Composite Films
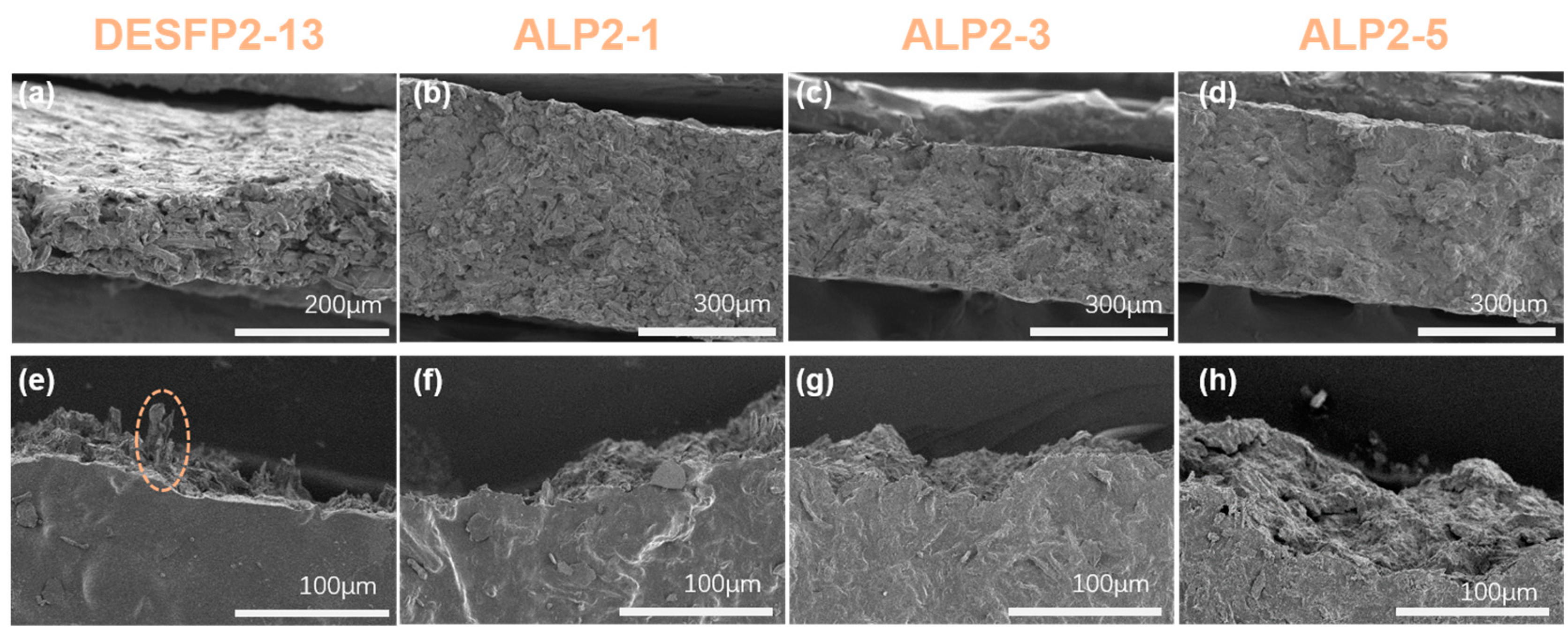
3.3. Fracture Morphology of Composite Films
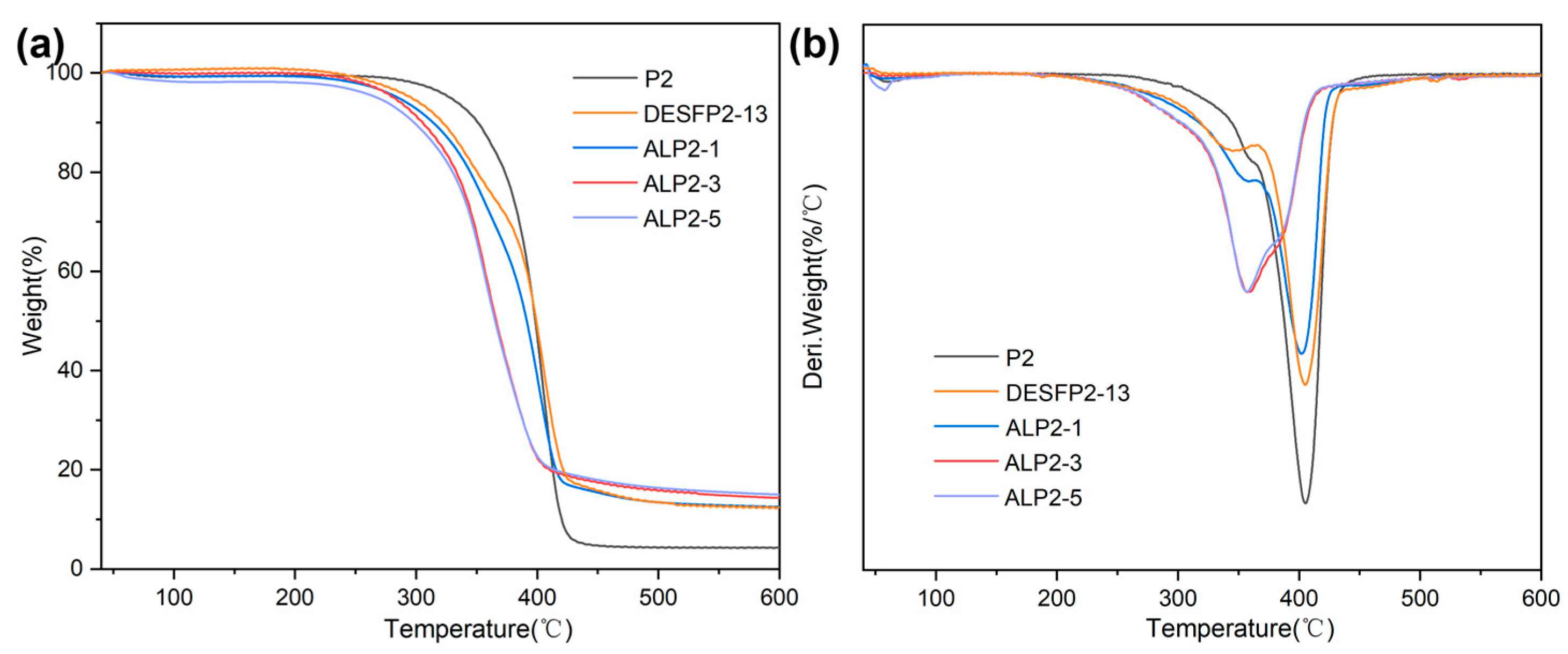
3.4. Thermal Properties of Composite Films
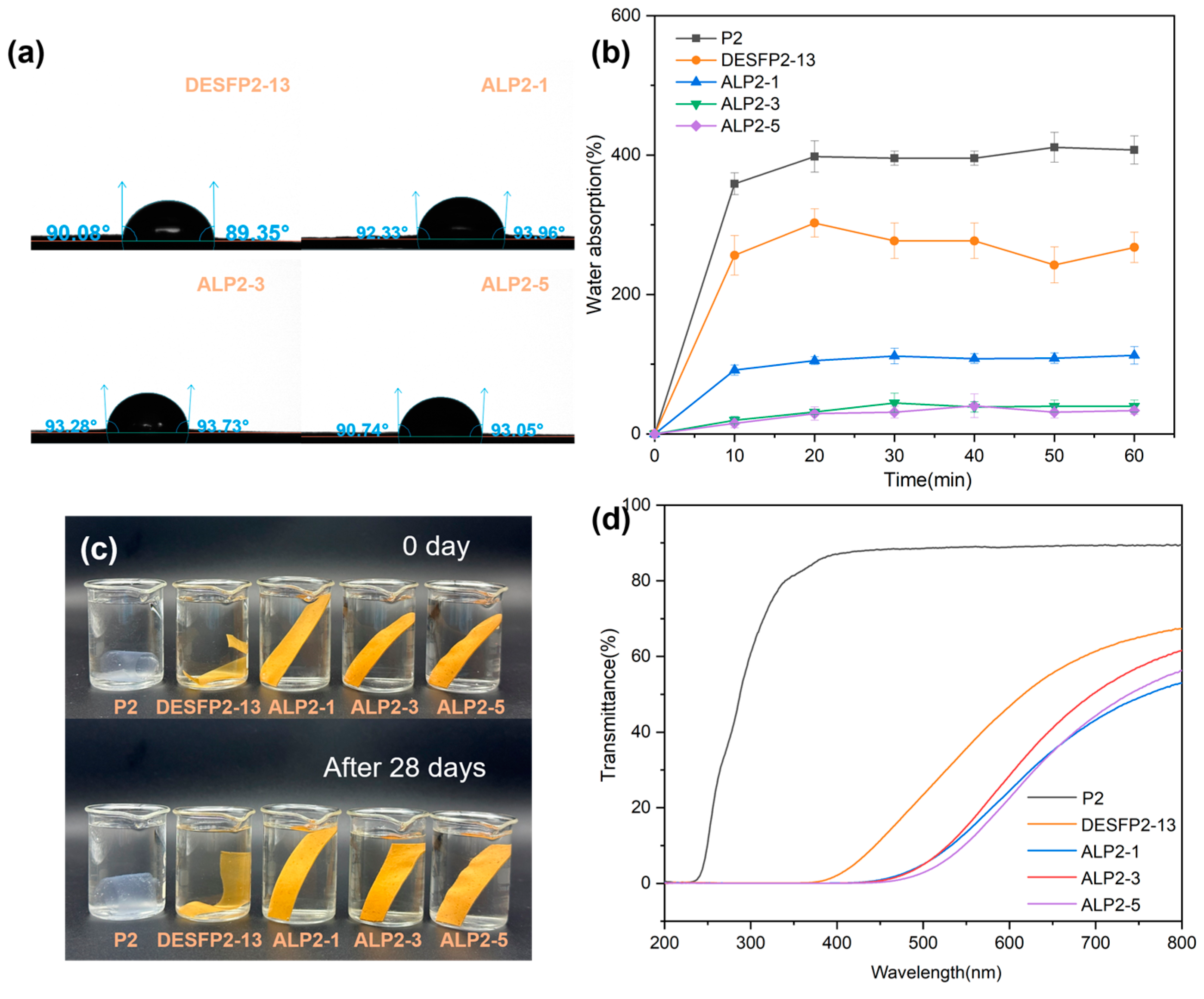
3.5. Water Resistance of Composite Films
3.6. UV Resistance of Composite Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rosenboom, J.-G.; Langer, R.; Traverso, G. Bioplastics for a circular economy. Nat. Rev. Mater. 2022, 7, 117–137. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Cao, G.; Zhao, L.; Liu, B.; Ren, N. Alkali/urea pretreatment of rice straw at low temperature for enhanced biological hydrogen production. Bioresour. Technol. 2018, 267, 71–76. [Google Scholar] [CrossRef]
- Jagannathan, P.; Muthukumaran, C.; Tamilarasan, K. A sequential pretreatment of lignocelluloses in bamboo biomass to fermentable sugars by acid/enzymatic hydrolysis. 3 Biotech 2017, 7, 260. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Chen, C.; Yao, Y.; Li, J.; He, S.; Zhou, Y.; Li, T.; Pan, X.; Yao, Y.; Hu, L. A strong, biodegradable and recyclable lignocellulosic bioplastic. Nat. Sustain. 2021, 4, 627–635. [Google Scholar] [CrossRef]
- Li, P.; Yang, C.; Jiang, Z.; Jin, Y.; Wu, W. Lignocellulose pretreatment by deep eutectic solvents and related technologies: A review. J. Bioresour. Bioprod. 2023, 8, 33–44. [Google Scholar] [CrossRef]
- Sreekala, M.S.; Kumaran, M.G.; Joseph, S.; Jacob, M.; Thomas, S. Oil palm fibre reinforced phenol formaldehyde composites: Influence of fibre surface modifications on the mechanical performance. Appl. Compos. Mater. 2000, 7, 295–329. [Google Scholar] [CrossRef]
- Nassar, M.M.A.; Alzebdeh, K.I.; Pervez, T.; Al-Hinai, N.; Munam, A. Progress and challenges in sustainability, compatibility, and production of eco-composites: A state-of-art review. J. Appl. Polym. Sci. 2021, 138, 51284. [Google Scholar] [CrossRef]
- Xu, J.; Xu, J.; Zhang, S.; Xia, J.; Liu, X.; Chu, X.; Duan, J.; Li, X. Synergistic effects of metal salt and ionic liquid on the pretreatment of sugarcane bagasse for enhanced enzymatic hydrolysis. Bioresour. Technol. 2018, 249, 1058–1061. [Google Scholar] [CrossRef]
- Alvarez-Vasco, C.; Ma, R.; Quintero, M.; Guo, M.; Geleynse, S.; Ramasamy, K.K.; Wolcott, M.; Zhang, X. Unique low-molecular-weight lignin with high purity extracted from wood by deep eutectic solvents (DES): A source of lignin for valorization. Green Chem. 2016, 18, 5133–5141. [Google Scholar] [CrossRef]
- Fan, Z.; Sun, H.; Zhang, L.; Zhao, X.; Hu, Y. Lightweight, High-Strength Wood Prepared by Deep Eutectic Solvent Treatment as a Green Structural Material. ACS Sustain. Chem. Eng. 2022, 10, 9600–9611. [Google Scholar] [CrossRef]
- Sánchez-Badillo, J.A.; Gallo, M.; Rutiaga-Quiñones, J.G.; Garza, J.; López-Albarrán, P. Insights on the cellulose pretreatment at room temperature by choline-chloride-based deep eutectic solvents: An atomistic study. Cellulose 2022, 29, 6517–6548. [Google Scholar] [CrossRef]
- Zhou, H.; Guan, Y.; Yan, X.; Pan, Z.; Xu, J.; Dai, L.; Zhang, M.; Si, C. All-lignocellulose-based hard bioplastic. Ind. Crops Prod. 2023, 193, 116164. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, X.; Cheng, Y.; Zhang, J.; Mi, Q.; Yin, C.; Wu, J.; Zhang, J. Super-rapid and highly-efficient esterification of cellulose to achieve an accurate chromatographic analysis of its molecular weight. Carbohydr. Polym. 2022, 286, 119301. [Google Scholar] [CrossRef]
- Duan, L.; Liu, R.; Duan, Y.; Li, Z.; Li, Q. A simultaneous strategy for the preparation of acetylation modified cellulose nanofiber/polypropylene composites. Carbohydr. Polym. 2022, 277, 118744. [Google Scholar] [CrossRef] [PubMed]
- Lei, W.; Fang, C.; Zhou, X.; Li, Y.; Pu, M. Polyurethane elastomer composites reinforced with waste natural cellulosic fibers from office paper in thermal properties. Carbohydr. Polym. 2018, 197, 385–394. [Google Scholar] [CrossRef]
- Yuan, T.; Du, W.; Bai, K.; Huang, D.; Nguyen, T.T.; Li, J.; Ji, X. Preparation of an environment-friendly fiberboard with high mechanical strength using delignified wood fiber. Vacuum 2022, 196, 110753. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, L.; Zhou, J.; Yang, F.; Huang, Q.; Cai, Y. Softened Wood Treated by Deep Eutectic Solvents. ACS Omega 2020, 5, 22163–22170. [Google Scholar] [CrossRef] [PubMed]
- Collard, F.-X.; Blin, J. A review on pyrolysis of biomass constituents: Mechanisms and composition of the products obtained from the conversion of cellulose, hemicelluloses and lignin. Renew. Sustain. Energy Rev. 2014, 38, 594–608. [Google Scholar] [CrossRef]
- Özsin, G.; Pütün, A.E. Insights into pyrolysis and co-pyrolysis of biomass and polystyrene: Thermochemical behaviors, kinetics and evolved gas analysis. Energy Convers. Manag. 2017, 149, 675–685. [Google Scholar] [CrossRef]
- Song, J.; Chen, C.; Zhu, S.; Zhu, M.; Dai, J.; Ray, U.; Li, Y.; Kuang, Y.; Li, Y.; Quispe, N.; et al. Processing bulk natural wood into a high-performance structural material. Nature 2018, 554, 224–228. [Google Scholar] [CrossRef]
- Zia, F.; Zia, K.M.; Nazli, Z.-H.; Tabasum, S.; Khosa, M.K.; Zuber, M. Preparation of hydroxyethyl cellulose/halloysite nanotubes graft polylactic acid-based polyurethane bionanocomposites. Int. J. Biol. Macromol. 2020, 153, 591–599. [Google Scholar] [CrossRef]
- Zuber, M.; Shah, S.A.A.; Jamil, T.; Asghar, M.I. Performance behavior of modified cellulosic fabrics using polyurethane acrylate copolymer. Int. J. Biol. Macromol. 2014, 67, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Hou, D.-F.; Liu, Z.-Y.; Zhou, L.; Tan, H.; Yang, W.; Yang, M.-B. A facile strategy towards heterogeneous preparation of thermoplastic cellulose grafted polyurethane from amorphous regenerated cellulose paste. Int. J. Biol. Macromol. 2020, 161, 177–186. [Google Scholar] [CrossRef]
- Yao, X.; Qi, X.; He, Y.; Tan, D.; Chen, F.; Fu, Q. Simultaneous Reinforcing and Toughening of Polyurethane via Grafting on the Surface of Microfibrillated Cellulose. ACS Appl. Mater. Interfaces 2014, 6, 2497–2507. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, X.; Li, M.-C.; Dong, J.; Lee, S.; Cheng, H.N.; Lei, T.; Wu, Q. Cellulose nanocrystal driven microphase separated nanocomposites: Enhanced mechanical performance and nanostructured morphology. Int. J. Biol. Macromol. 2019, 130, 685–694. [Google Scholar] [CrossRef]
- Çetin, N.S.; Özmen, N.; Birinci, E. Acetylation of Wood with Various Catalysts. J. Wood Chem. Technol. 2011, 31, 142–153. [Google Scholar] [CrossRef]
- Tserki, V.; Zafeiropoulos, N.E.; Simon, F.; Panayiotou, C. A study of the effect of acetylation and propionylation surface treatments on natural fibres. Compos. Part A Appl. Sci. Manuf. 2005, 36, 1110–1118. [Google Scholar] [CrossRef]
- Hill, C.A.S.; Khalil, H.P.S.A.; Hale, M.D. A study of the potential of acetylation to improve the properties of plant fibres. Ind. Crops Prod. 1998, 8, 53–63. [Google Scholar] [CrossRef]
- Zafeiropoulos, N.E.; Williams, D.R.; Baillie, C.A.; Matthews, F.L. Engineering and characterisation of the interface in flax fibre/polypropylene composite materials. Part I. Development and investigation of surface treatments. Compos. Part A Appl. Sci. Manuf. 2002, 33, 1083–1093. [Google Scholar] [CrossRef]
- Colom, X.; Carrasco, F.; Pagès, P.; Cañavate, J. Effects of different treatments on the interface of HDPE/lignocellulosic fiber composites. Compos. Sci. Technol. 2003, 63, 161–169. [Google Scholar] [CrossRef]
- Lepetit, A.; Drolet, R.; Tolnai, B.; Zerrouki, R.; Montplaisir, D. Effect of acetylation on the properties of microfibrillated cellulose-LDPE composites. J. Appl. Polym. Sci. 2017, 134, 44933. [Google Scholar] [CrossRef]
- de Souza, F.M.; Kahol, P.K.; Gupta, R.K. Introduction to Polyurethane Chemistry. In Polyurethane Chemistry: Renewable Polyols and Isocyanates; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2021; Volume 1380, pp. 1–24. ISBN 978-0-8412-9840-8. [Google Scholar]
- Liu, N.; Zhao, Y.; Kang, M.; Wang, J.; Wang, X.; Feng, Y.; Yin, N.; Li, Q. The effects of the molecular weight and structure of polycarbonatediols on the properties of waterborne polyurethanes. Prog. Org. Coat. 2015, 82, 46–56. [Google Scholar] [CrossRef]
- Cakić, S.M.; Ristić, I.S.; Marinović-Cincović, M.; Špírková, M. The effects of the structure and molecular weight of the macrodiol on the properties polyurethane anionic adhesives. Int. J. Adhes. Adhes. 2013, 41, 132–139. [Google Scholar] [CrossRef]
- Banerjee, S.S.; Aher, N.; Patil, R.; Khandare, J. Poly(ethylene glycol)-Prodrug Conjugates: Concept, Design, and Applications. J. Drug Deliv. 2012, 2012, 103973. [Google Scholar] [CrossRef]
- Rhodes, A.; Sandhu, S.S.; Onis, S.J. 2—Surface modification of biomaterials by covalent binding of poly(ethylene glycol) (PEG). In Surface Modification of Biomaterials; Williams, R., Ed.; Woodhead Publishing Series in Biomaterials; Woodhead Publishing: Cambridge, UK, 2011; pp. 39–55. ISBN 978-1-84569-640-5. [Google Scholar]
- Nogales, A.; Mitchell, G.R.; Vaughan, A.S. Anisotropic crystallization in polypropylene induced by deformation of a nucleating agent network. Macromolecules 2003, 36, 4898–4906. [Google Scholar] [CrossRef]
- Bartczak, Z.; Lezak, E. Evolution of lamellar orientation and crystalline texture of various polyethylenes and ethylene-based copolymers in plane-strain compression. Polymer 2005, 46, 6050–6063. [Google Scholar] [CrossRef]
- Olszewski, A.; Kosmela, P.; Piszczyk, Ł. Towards sustainable catalyst-free biomass-based polyurethane-wood composites (PU-WC): From valorization and liquefaction to future generation of biocomposites. J. Clean. Prod. 2024, 468, 143046. [Google Scholar] [CrossRef]
- Srivabut, C. Multi-objective optimization of turning process parameters and wood sawdust contents using response surface methodology for the minimized surface roughness of recycled plastic/wood sawdust composites. Compos. Part C Open Access 2024, 14, 100477. [Google Scholar] [CrossRef]
- Yang, A.; Zhang, R.; Xu, Z.; Liu, T.; Fang, Y.; Wang, W.; Xu, M.; Song, Y.; Wang, Q. Preparation of situ microfiber-reinforced co-extruded high-filled wood-plastic composite with excellent mechanical, creep resistance, and water resistance properties. Constr. Build. Mater. 2024, 415, 135002. [Google Scholar] [CrossRef]
- Zhang, J.; Xia, L.; Fu, Z.; Sun, X.; Zhou, S.; Liu, X.; Zhang, C.; Xu, W. Fabrication of polyurethane porous composite films using biomass-based Juncus effususus fibers for oil removal from water. Ind. Crops Prod. 2022, 176, 114290. [Google Scholar] [CrossRef]
- Gosselink, R.J.A.; Snijder, M.H.B.; Kranenbarg, A.; Keijsers, E.R.P.; de Jong, E.; Stigsson, L.L. Characterisation and application of NovaFiber lignin. Ind. Crops Prod. 2004, 20, 191–203. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Wang, Y.; Zhu, W.; Lan, D.; Song, Y.-M. Flexible poly(butylene adipate-co-butylene terephthalate) enabled high-performance polylactide/wood fiber biocomposite foam. Ind. Crops Prod. 2023, 204, 117381. [Google Scholar] [CrossRef]
- Tai, N.L.; Adhikari, R.; Shanks, R.; Adhikari, B. Starch-polyurethane films synthesized using polyethylene glycol-isocyanate (PEG-iso): Effects of molecular weight, crystallinity, and composition of PEG-iso on physiochemical characteristics and hydrophobicity of the films. Food Packag. Shelf Life 2017, 14, 116–127. [Google Scholar] [CrossRef]
- Olszewski, A.; Kosmela, P.; Piszczyk, Ł. A novel approach in wood waste utilization for manufacturing of catalyst-free polyurethane-wood composites (PU-WC). Sustain. Mater. Technol. 2023, 36, e00619. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, W.; Li, Z.; Xin, R.; He, Y.; Yang, J.; Li, S.; Chen, M. Bamboo-based cellulose nanofibers as reinforcement for polyurethane imitation wood. Ind. Crops Prod. 2024, 210, 118177. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Li, M.; Wyman, C.E.; Cai, C.M.; Ragauskas, A.J. Fast Fractionation of Technical Lignins by Organic Cosolvents. ACS Sustain. Chem. Eng. 2018, 6, 6064–6072. [Google Scholar] [CrossRef]
- Fanta, G.F.; Felker, F.C.; Hay, W.T.; Selling, G.W. Increased water resistance of paper treated with amylose-fatty ammonium salt inclusion complexes. Ind. Crops Prod. 2017, 105, 231–237. [Google Scholar] [CrossRef]
- D7490; Standard Test Method for Measurement of the Surface Tension of Solid Coatings, Substrates and Pigments Using Contact Angle Measurements. ASTM Standard: West Conshohocken, PA, USA, 2022. Available online: https://www.astm.org/d7490-13r22.html (accessed on 25 August 2024).
- Iyer, K.A.; Flores, A.M.; Torkelson, J.M. Comparison of polyolefin biocomposites prepared with waste cardboard, microcrystalline cellulose, and cellulose nanocrystals via solid-state shear pulverization. Polymer 2015, 75, 78–87. [Google Scholar] [CrossRef]
- Prambauer, M.; Paulik, C.; Burgstaller, C. The influence of paper type on the properties of structural paper—Polypropylene composites. Compos. Part A Appl. Sci. Manuf. 2015, 74, 107–113. [Google Scholar] [CrossRef]
- Masoodi, R.; Pillai, K.M. A study on moisture absorption and swelling in bio-based jute-epoxy composites. J. Reinf. Plast. Compos. 2012, 31, 285–294. [Google Scholar] [CrossRef]
- Gao, J.; Wang, X.; Xu, Q.; Kuai, B.; Wang, Z.; Cai, L.; Ge, S.; Zhang, Y.L.; Li, G. Efficient preparation and properties of wood fiber transparent materials with powdered wood. Ind. Crops Prod. 2023, 193, 116291. [Google Scholar] [CrossRef]
- Tran, M.H.; Phan, D.-P.; Lee, E.Y. Review on lignin modifications toward natural UV protection ingredient for lignin-based sunscreens. Green Chem. 2021, 23, 4633–4646. [Google Scholar] [CrossRef]
- Liu, T.; Liu, Z.; Zhou, Z.; Shi, S.Q.; Aladejana, J.T.; Gong, S.; Fang, Z.; Li, J. A novel sol-gel strategy for constructing wood fibers and aramid nanofiber nanocomposite with strong, tough and recyclable properties. Compos. Sci. Technol. 2023, 238, 110026. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, X.; Kang, S.; Liu, Y.; Bushra, R.; Guo, J.; Zhu, W.; Khan, M.R.; Jin, Y.; Song, J. Preparation of biodegradable recycled fiber composite film using lignin-based polyurethane emulsion as strength agent. Ind. Crops Prod. 2023, 197, 116512. [Google Scholar] [CrossRef]
- Ning, Y.; Deng, H.-H.; Li, Q.-M.; Mi, Q.-H.; Han, B.; Ai, X.-Z. Effects of red and blue light quality on the metabolites and key enzyme activities of carbon-nitrogen metabolism in celery. Zhiwu Shengli Xuebao Plant Physiol. J. 2015, 51, 112–118. [Google Scholar] [CrossRef]
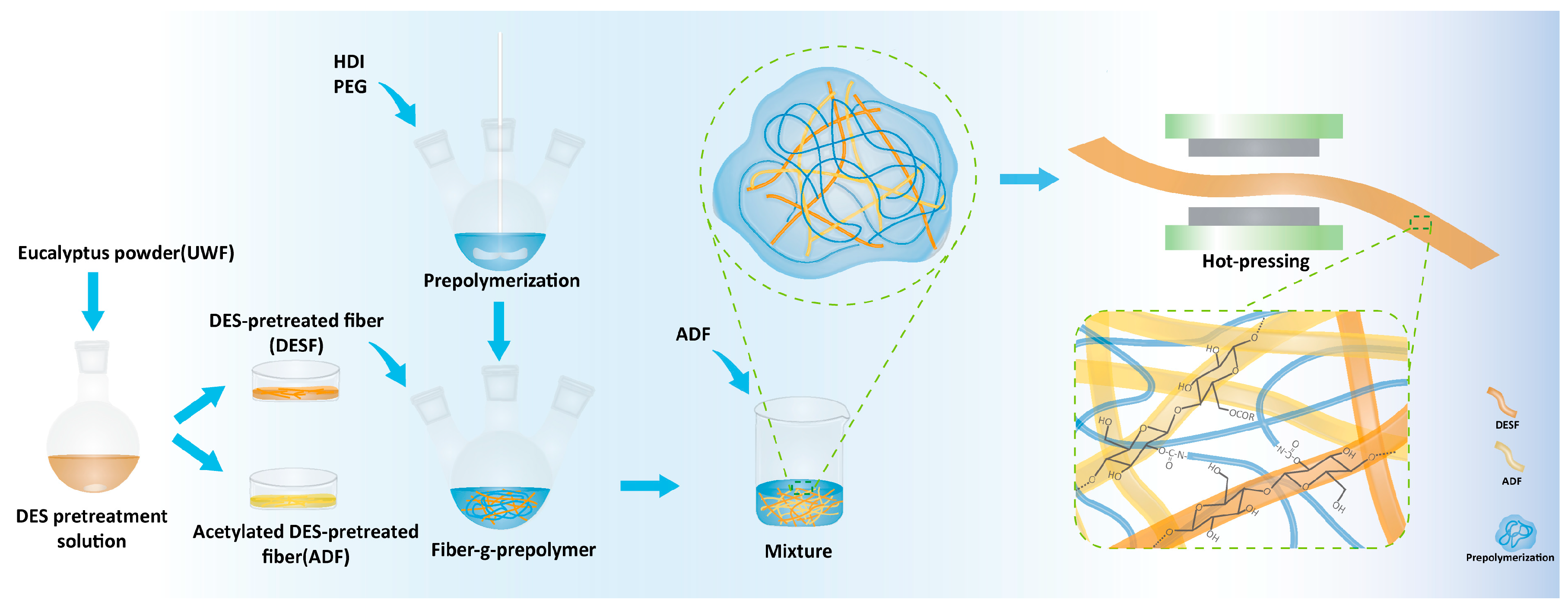

| PEG in Prepolymer | ADF and Prepolymer-g-Fiber | Composites |
|---|---|---|
| PEG 2000 | Without ADF and DESF | P2 |
| PEG 2000 | 1:4 | ALP2-1 |
| PEG 2000 | 3:4 | ALP2-3 |
| PEG 2000 | 5:4 | ALP2-5 |
| PEG 400 | Without ADF and DESF | P4 |
| PEG 400 | 1:4 | ALP4-1 |
| PEG 400 | 3:4 | ALP4-3 |
| PEG 400 | 5:4 | ALP4-5 |
| Scheme | N (%) | C (%) | H (%) |
|---|---|---|---|
| DESF | 0.03 | 49.06 | 5.738 |
| DESFP2-13 | 0.30 | 49.13 | 6.543 |
| DESFP2-15 | 0.51 | 49.14 | 6.682 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, X.; Fu, S.; Liu, H. Preparation of Wood Fiber–Polyurethane Plastic Composite with Water Resistance and High Strength. Materials 2025, 18, 1314. https://doi.org/10.3390/ma18061314
Yuan X, Fu S, Liu H. Preparation of Wood Fiber–Polyurethane Plastic Composite with Water Resistance and High Strength. Materials. 2025; 18(6):1314. https://doi.org/10.3390/ma18061314
Chicago/Turabian StyleYuan, Xi, Shiyu Fu, and Hao Liu. 2025. "Preparation of Wood Fiber–Polyurethane Plastic Composite with Water Resistance and High Strength" Materials 18, no. 6: 1314. https://doi.org/10.3390/ma18061314
APA StyleYuan, X., Fu, S., & Liu, H. (2025). Preparation of Wood Fiber–Polyurethane Plastic Composite with Water Resistance and High Strength. Materials, 18(6), 1314. https://doi.org/10.3390/ma18061314






