Finite Element Analysis of a 3D-Printed Acetabular Prosthesis for an Acetabular Defect According to the Paprosky Classification
Abstract
1. Introduction
2. Materials and Methods
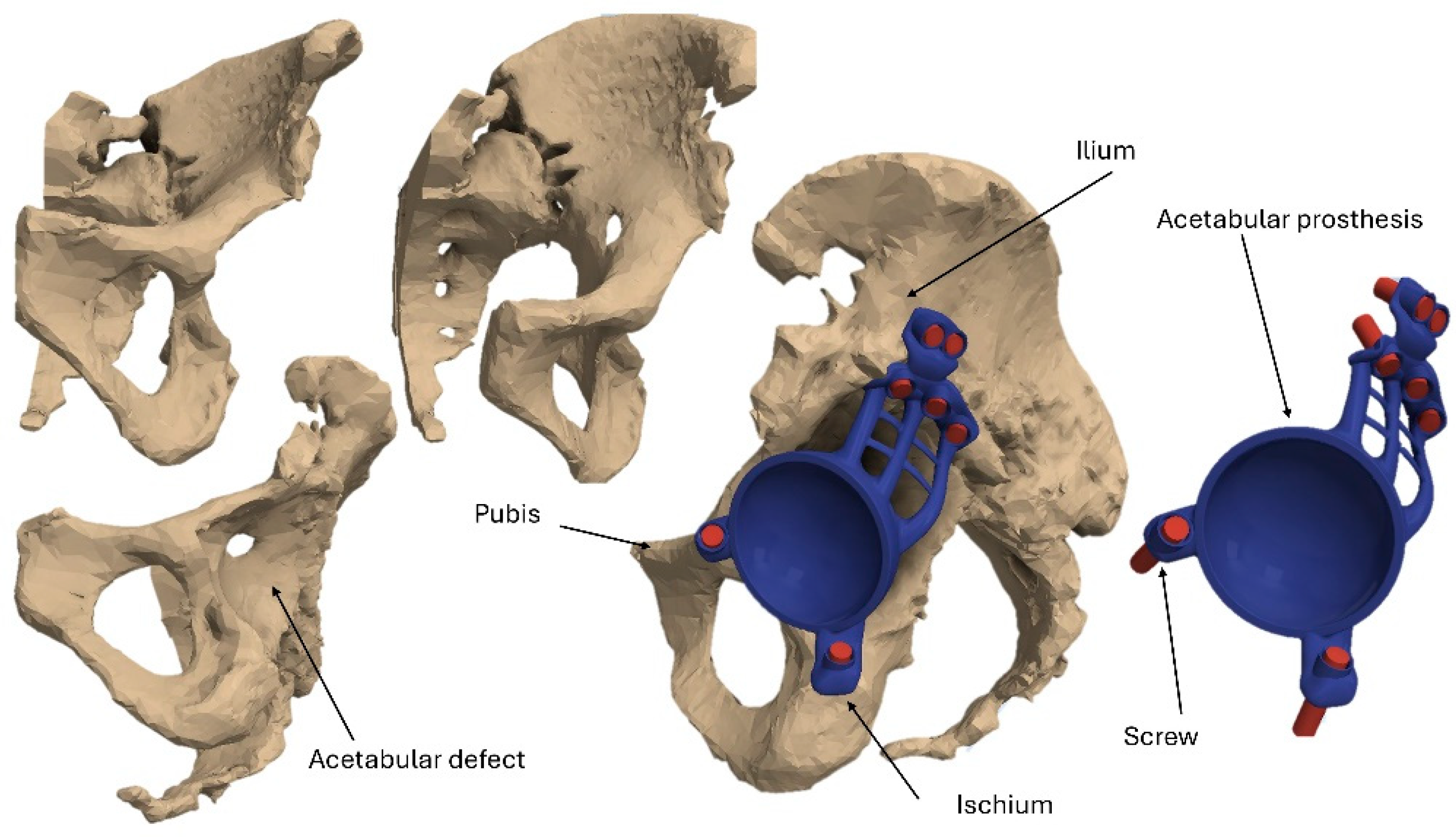
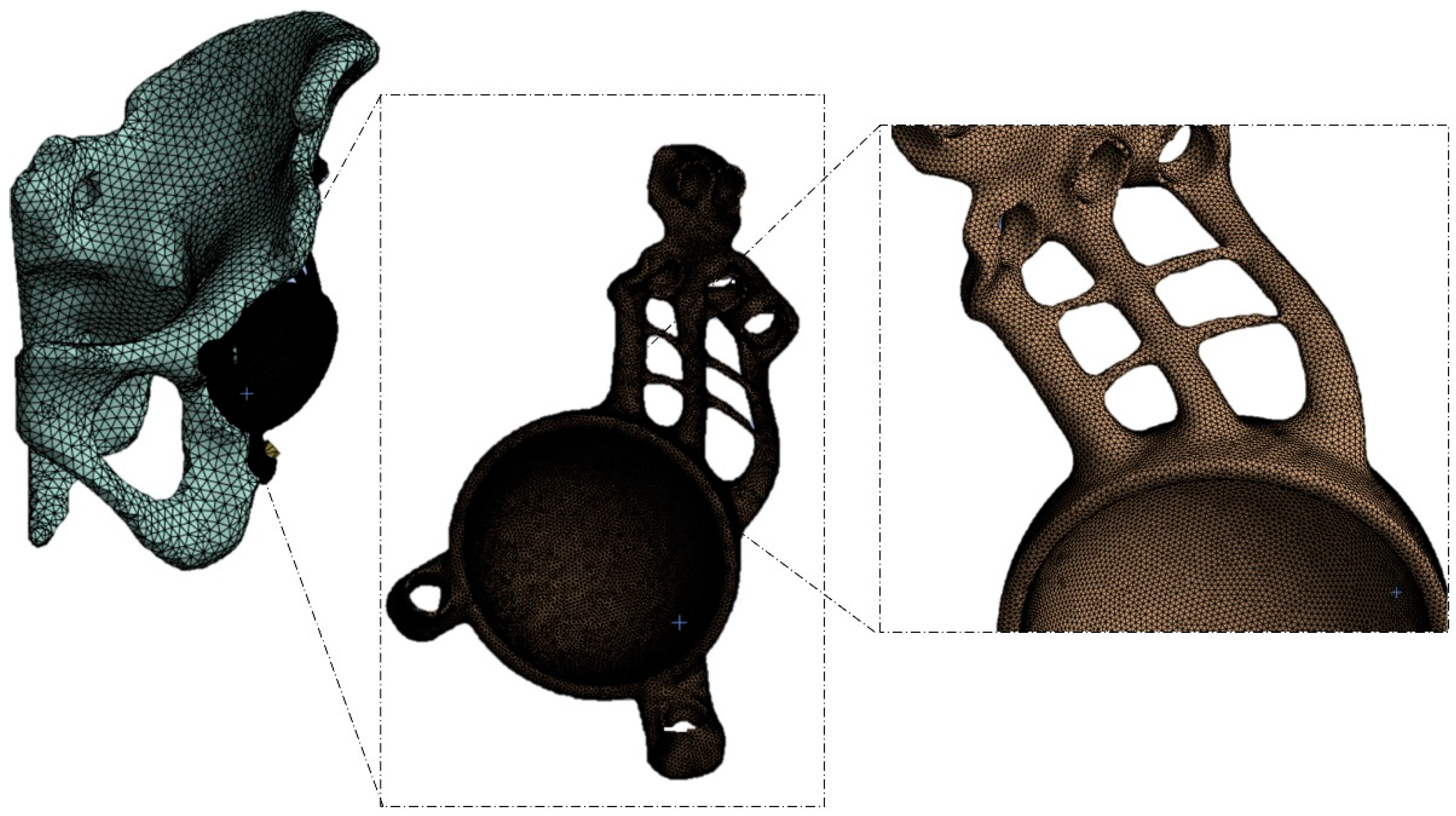
2.1. Modeling
2.2. Materials
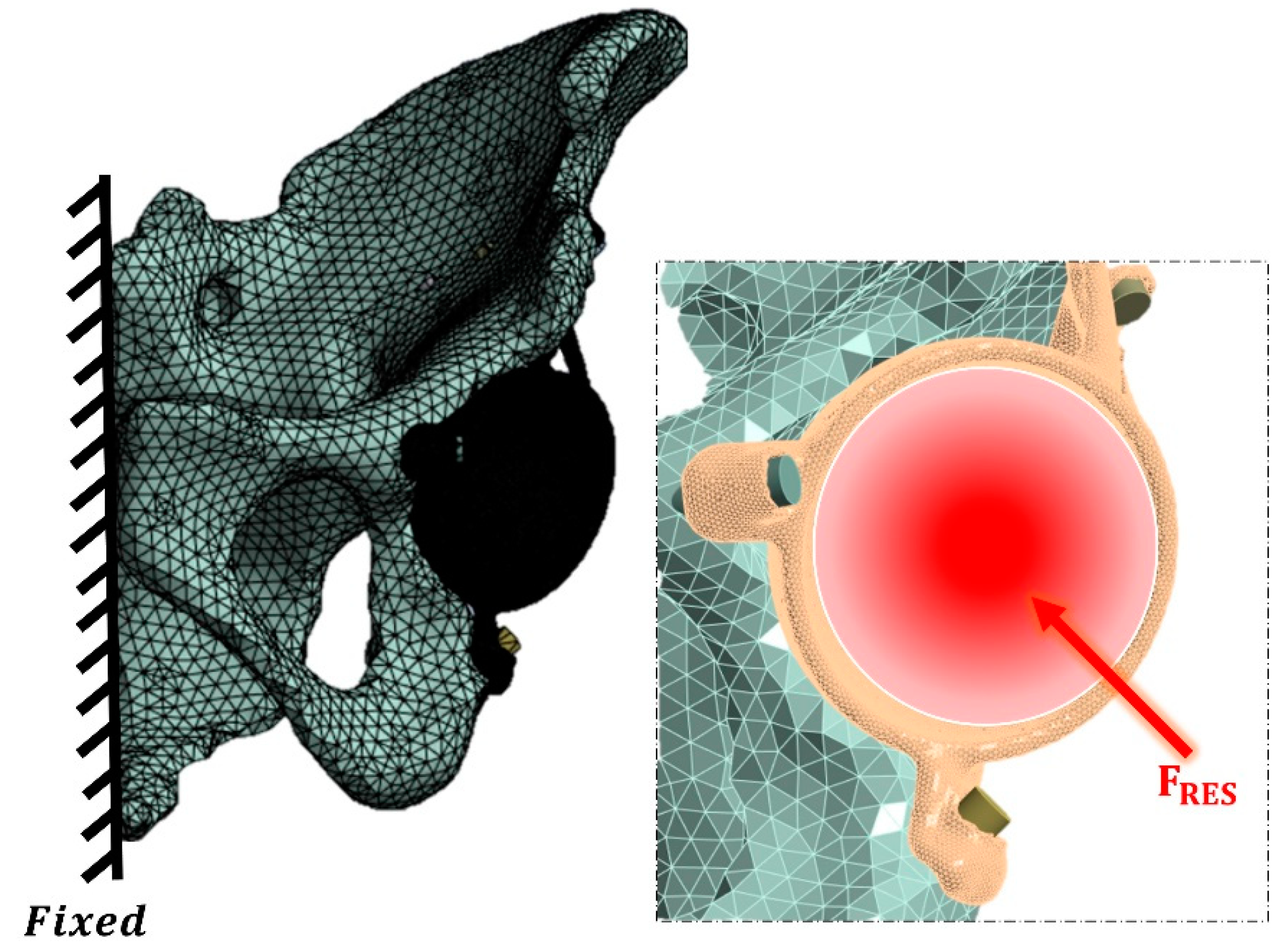
2.3. Loading and Boundary Conditions
- -
- <400 μm/m: Atrophy.
- -
- 400–3000 μm/m: Bone preservation and formation.
- -
- 3000–20,000 μm/m: Plastic deformation.
- -
- 20,000 μm/m: Fracture.
3. Results
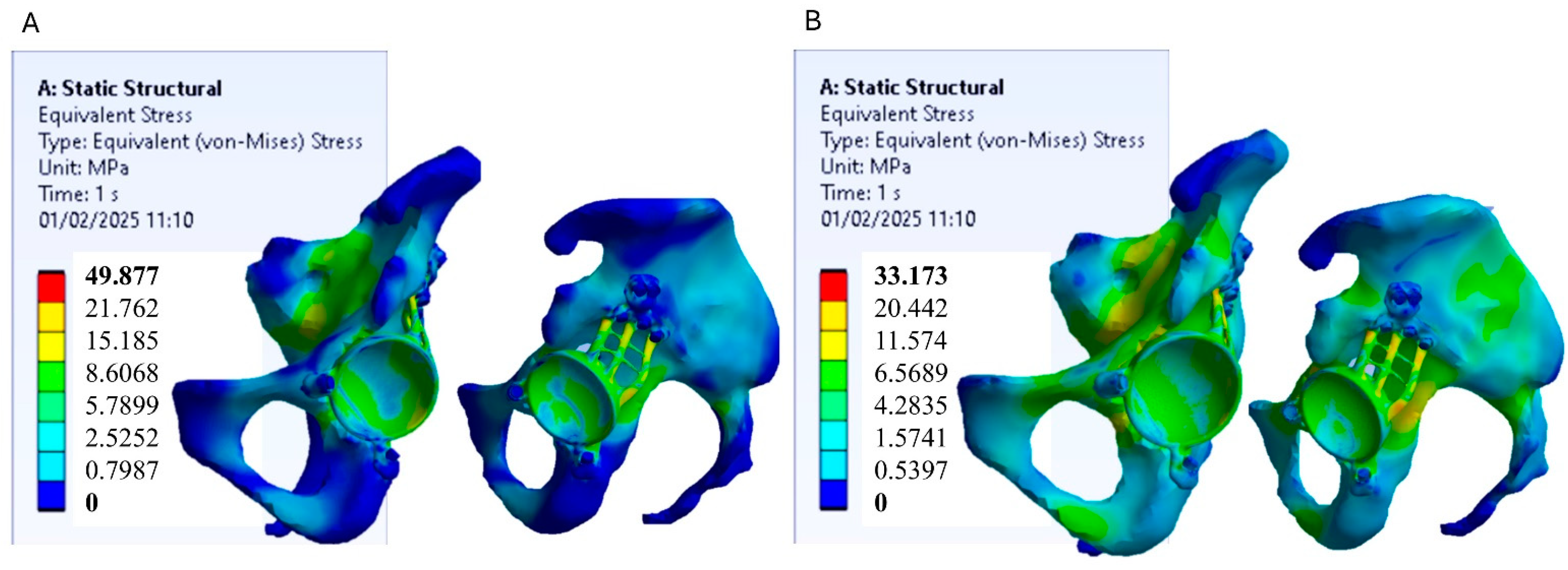
3.1. Stress Distribution
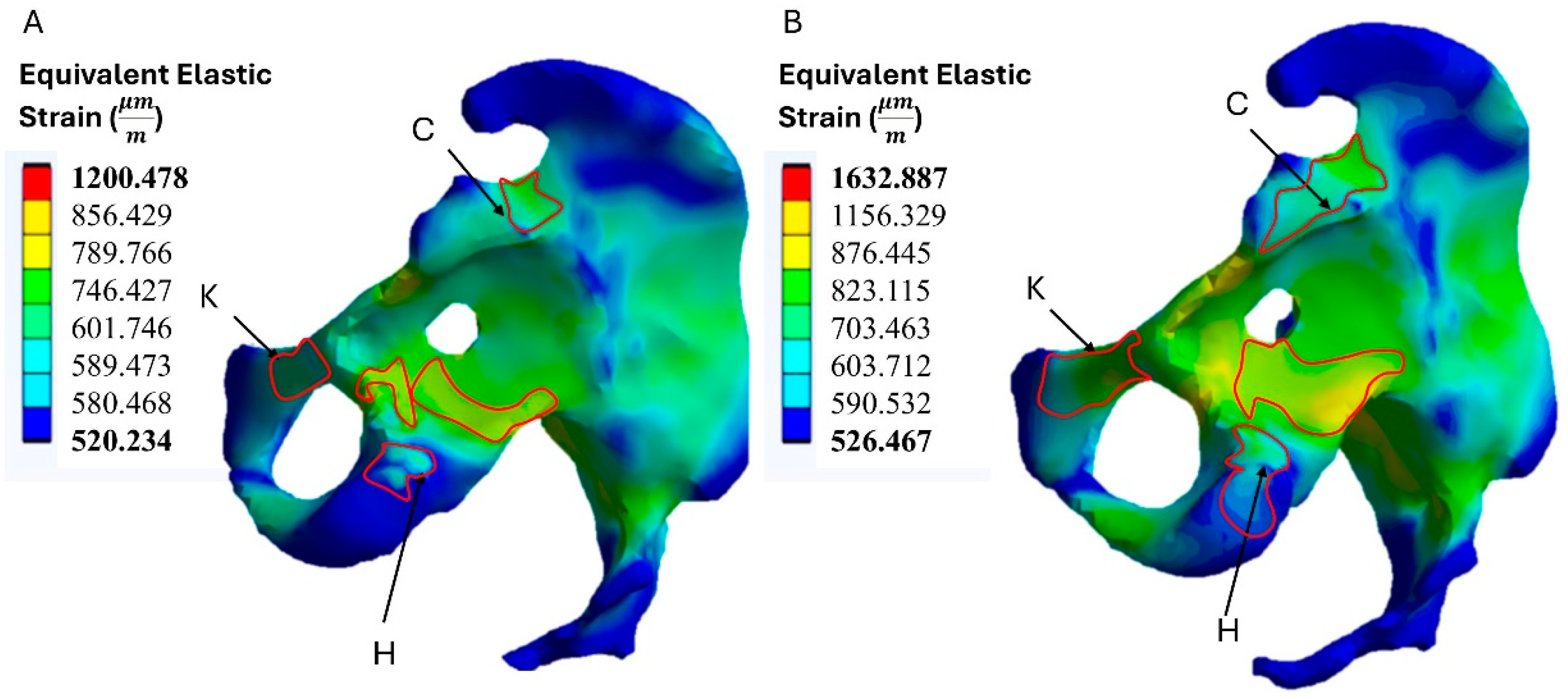
3.2. Strain Distribution on the Bone
4. Discussion
4.1. Using 3D Printing in THA
4.2. FEM Studies in THA
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eingartner, C. Current trends in total hip arthroplasty. Ortop. Traumatol. Rehabil. 2007, 9, 8–14. [Google Scholar] [PubMed]
- Otten, R.; van Roermund, P.M.; Picavet, H.S. Trends in the number of knee and hip arthroplasties: Considerably more knee and hip prostheses due to osteoarthritis in 2030. Ned. Tijdschr. Voor Geneeskd. 2010, 154, A1534. [Google Scholar]
- Manson, T.; Schmidt, A.H. Acetabular fractures in the elderly: A critical analysis review. JBJS Rev. 2016, 4, e1. [Google Scholar] [CrossRef]
- Sheth, N.P.; Nelson, C.L.; Springer, B.D.; Fehring, T.K.; Paprosky, W.G. Acetabular bone loss in revision total hip arthroplasty: Evaluation and management. JAAOS-J. Am. Acad. Orthop. Surg. 2013, 21, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Sanghavi, S.A.; Paprosky, W.G.; Sheth, N.P. Evaluation and Management of Acetabular Bone Loss in Revision Total Hip Arthroplasty: A 10-year Update. JAAOS-J. Am. Acad. Orthop. Surg. 2024, 32, e466–e475. [Google Scholar] [CrossRef]
- von Hertzberg-Boelch, S.P.; Wagenbrenner, M.; Arnholdt, J.; Frenzel, S.; Holzapfel, B.M.; Rudert, M. Custom made monoflange acetabular components for the treatment of Paprosky type III defects. J. Pers. Med. 2021, 11, 283. [Google Scholar] [CrossRef] [PubMed]
- St John, R.; Spicer, S.; Hadaya, M.; Brancaccio, H.; Park, S.; McMillan, S. Comparing functional outcomes between 3D printed acetabular cups and traditional prosthetic implants in hip arthroplasty: A systematic review and meta analysis. Arch. Orthop. Trauma Surg. 2025, 145, 1–11. [Google Scholar] [CrossRef]
- Cimatti, P.; Del Piccolo, N.; Dallari, B.; Mazzotta, A.; Dallari, D. Use of morselized bone allograft in revision hip arthroplasty for massive acetabular defect: A systematic review and meta-analysis. J. Exp. Orthop. 2024, 11, e70091. [Google Scholar] [CrossRef]
- Das, S.S.; Chakraborti, P. Formulation and selection of composite biomaterials for acetabular cup of hip prosthesis with wear analysis. Proc. Inst. Mech. Eng. Part. E J. Process Mech. Eng. 2024, 238, 2983–2993. [Google Scholar] [CrossRef]
- Sznajder, W.; Tański, W. Aseptic failure of total hip arthroplasty as an early complication requiring revision surgery: A comprehensive literature review. Med. Sci. Pulse 2024, 18, 96–105. [Google Scholar] [CrossRef]
- Esmaeili, S.; Shaker, F.; Ghaseminejad-Raeini, A.; Baghchi, M.; Sajadi, S.M.; Shafiei, S.H. Risk factors for acetabular fracture treatment failure: A systematic review and meta-analysis. BMC Musculoskelet. Disord. 2024, 25, 976. [Google Scholar] [CrossRef] [PubMed]
- Woisetschläger, M.; Booij, R.; Tesselaar, E.; Oei, E.H.; Schilcher, J. Improved visualization of the bone-implant interface and osseointegration in ex vivo acetabular cup implants using photon-counting detector CT. Eur. Radiol. Exp. 2023, 7, 19. [Google Scholar] [CrossRef]
- Malahias, M.A.; Sarantis, M.; Gkiatas, I.; Jang, S.J.; Gu, A.; Thorey, F.; Alexiades, M.M.; Nikolaou, V.S. The modern Burch-Schneider antiprotrusio cage for the treatment of acetabular defects: Is it still an option? A systematic review. Hip Int. 2023, 33, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Vohra, R.; Thorat, B.S. Revision of Acetabulum Using Rings and Cages. In Hip Arthroplasty: Current and Future Directions; Springer Nature: Singapore, 2024; pp. 629–647. [Google Scholar]
- Liu, Y.; Wang, F.; Ying, J.; Xu, M.; Wei, Y.; Li, J.; Xie, H.; Zhao, D.; Cheng, L. Biomechanical analysis and clinical observation of 3D-printed acetabular prosthesis for the acetabular reconstruction of total hip arthroplasty in Crowe III hip dysplasia. Front. Bioeng. Biotechnol. 2023, 11, 1219745. [Google Scholar] [CrossRef]
- Zhu, D.; Fu, J.; Wang, L.; Guo, Z.; Wang, Z.; Fan, H. Reconstruction with customized, 3D-printed prosthesis after resection of periacetabular Ewing’s sarcoma in children using “triradiate cartilage-based” surgical strategy: A technical note. J. Orthop. Transl. 2021, 28, 108–117. [Google Scholar]
- Hao, Y.; Luo, D.; Wu, J.; Wang, L.; Xie, K.; Yan, M.; Dai, K. A novel revision system for complex pelvic defects utilizing 3D-printed custom prosthesis. J. Orthop. Transl. 2021, 31, 102–109. [Google Scholar] [CrossRef]
- Citak, M.; Kochsiek, L.; Gehrke, T.; Haasper, C.; Suero, E.M.; Mau, H. Preliminary results of a 3D-printed acetabular component in the management of extensive defects. Hip Int. 2018, 28, 266–271. [Google Scholar] [CrossRef]
- Yao, A.; George, D.M.; Ranawat, V.; Wilson, C.J. 3D printed acetabular components for complex revision arthroplasty. Indian. J. Orthop. 2021, 55, 786–792. [Google Scholar] [CrossRef]
- Kostakos, T.A.; Nayar, S.K.; Alcock, H.; Savvidou, O.; Vlasis, K.; Papagelopoulos, P.J. Acetabular reconstruction in oncological surgery: A systematic review and meta-analysis of implant survivorship and patient outcomes. Surg. Oncol. 2021, 38, 101635. [Google Scholar] [CrossRef]
- Taunton, M.J.; Fehring, T.K.; Edwards, P.; Bernasek, T.; Holt, G.E.; Christie, M.J. Pelvic discontinuity treated with custom triflange component: A reliable option. Clin. Orthop. Relat. Res. 2012, 470, 428–434. [Google Scholar] [CrossRef]
- Berasi, C.C.; Berend, K.R.; Adams, J.B.; Ruh, E.L.; Lombardi, A.V. Are custom triflange acetabular components effective for reconstruction of catastrophic bone loss? Clin. Orthop. Relat. Res. 2015, 473, 528–535. [Google Scholar] [CrossRef]
- DeBoer, D.K.; Christie, M.J.; Brinson, M.F.; Morrison, J.C. Revision total hip arthroplasty for pelvic discontinuity. JBJS 2007, 89, 835–840. [Google Scholar] [CrossRef]
- Christie, M.J.; Barrington, S.A.; Brinson, M.F.; Ruhling, M.E.; DeBoer, D.K. Bridging massive acetabular defects with the triflange cup: 2-to 9-year results. Clin. Orthop. Relat. Res. 2001, 393, 216–227. [Google Scholar] [CrossRef] [PubMed]
- Goriainov, V.; King, L.J.; Oreffo, R.O.; Dunlop, D.G. Custom 3D-printed triflange implants for treatment of severe acetabular defects, with and without pelvic discontinuity: Early results of our first 19 consecutive cases. JBJS Open Access 2021, 6, e21. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Cai, H.; Kuong, E.; Chui, E.; Siu, Y.C.; Ji, T.; Drstvenšek, I. Surgical applications of three-dimensional printing in the pelvis and acetabulum: From models and tools to implants. Der Unfallchirurg 2019, 122, 278. [Google Scholar] [CrossRef]
- Wyatt, M.C. Custom 3D-printed acetabular implants in hip surgery–innovative breakthrough or expensive bespoke upgrade? Hip Int. 2015, 25, 375–379. [Google Scholar] [CrossRef]
- Magré, J.; Willemsen, K.; Kolken, H.M.A.; Zadpoor, A.A.; Vogely, H.C.; van der Wal, B.C.H.; Weinans, H. Deformable titanium for acetabular revision surgery: A proof of concept. 3D Print. Med. 2023, 9, 16. [Google Scholar] [CrossRef]
- Maeda, T.; Kuroda, Y.; Kamenaga, T.; Matsumoto, T.; Kuroda, R.; Hayashi, S. Impact of femoral stem alignment on periprosthetic bone density in THA: A study of the Avenir Complete stem. Arch. Orthop. Trauma Surg. 2024, 145, 74. [Google Scholar] [CrossRef]
- Tuninetti, V.; Fuentes, G.; Oñate, A.; Narayan, S.; Celentano, D.; García-Herrera, C.; Menacer, B.; Pincheira, G.; Garrido, C.; Valle, R. Computational Shape Design Optimization of Femoral Implants: Towards Efficient Forging Manufacturing. Appl. Sci. 2024, 14, 8289. [Google Scholar] [CrossRef]
- Ceddia, M.; Solarino, G.; Tucci, M.; Lamberti, L.; Trentadue, B. Stress analysis of tibial bone using three different materials for bone fixation plates. J. Compos. Sci. 2024, 8, 334. [Google Scholar] [CrossRef]
- Mi, Z.R.; Shuib, S.; Hassan, A.Y.; Shorki, A.A.; Ibrahim, M.M. Problem of stress shielding and improvement to the hip Implat designs: A review. J. Med. Sci. 2007, 7, 460–467. [Google Scholar]
- Chmielewska, A.; Dean, D. The role of stiffness-matching in avoiding stress shielding-induced bone loss and stress concentration-induced skeletal reconstruction device failure. Acta Biomater. 2024, 173, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Savio, D.; Bagno, A. When the total hip replacement fails: A review on the stress-shielding effect. Processes 2022, 10, 612. [Google Scholar] [CrossRef]
- Oladapo, B.I.; Zahedi, S.A.; Ismail, S.O. Mechanical performances of hip implant design and fabrication with PEEK composite. Polymer 2021, 227, 123865. [Google Scholar] [CrossRef]
- Sathishkumar, S.; Paulraj, J.; Chakraborti, P.; Chandradass, J.; Ghosh, S.K. Mechanical and tribological assessment of PEEK and PEEK based polymer composites for artificial hip joints. Int. J. Mater. Res. 2024, 115, 850–861. [Google Scholar] [CrossRef]
- Ceddia, M.; Solarino, G.; Dramisino, P.; De Giosa, G.; Rizzo, S.; Trentadue, B. Comparison of Stress between Three Different Functionally Graded Hip Stem Implants Made of Different Titanium Alloys and Composite Materials. J. Compos. Sci. 2024, 8, 449. [Google Scholar] [CrossRef]
- Eastlack, R.K.; Nunley, P.D.; Poelstra, K.A.; Vaccaro, A.R.; Stone, M.; Miller, L.E.; Legay, P.; Clin, J.; Agarwal, A. Finite element analysis comparing a PEEK posterior fixation device versus pedicle screws for lumbar fusion. J. Orthop. Surg. Res. 2023, 18, 855. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Mondal, S.; Ghosh, R. Biomechanical performance of the cemented acetabular cup with combined effects of bone quality, implant material combinations and bodyweight. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2022, 236, 1309–1327. [Google Scholar] [CrossRef]
- Warinsiriruk, E.; Thongchuea, N.; Pengrung, N.; Jarungvittayakon, C.; Sa-Ngasoongsong, P.; Chulsomlee, K. Investigation of mechanical behavior on the cement hip spacer geometry under finite element method and compression load test. J. Orthop. 2024, 47, 115–121. [Google Scholar] [CrossRef]
- Li, G.; Zhang, X.; Chen, M.; Luo, Z.; Ji, X.; Pan, C.; Li, H.; Shang, X.F. Bispherical metal augment improved biomechanical stability in severe acetabular deficiency reconstruction: A comparative finite element analysis. BMC Musculoskelet. Disord. 2024, 25, 691. [Google Scholar]
- Wang, T.; Zhao, B.; Yan, J.; Shao, B.; Mu, W. Finite element analysis of infra-acetabular screw fixation for the treatment of acetabular posterior column fracture. Int. Orthop. 2022, 46, 623–634. [Google Scholar] [CrossRef]
- Shaikh, N.; Shenoy, B.S.; Bhat, N.S.; Shetty, S.; Chethan, K.N. Wear estimation at the contact surfaces of oval shaped hip implants using finite element analysis. Cogent Eng. 2023, 10, 2222985. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, M.; Li, C.; Nakamura, Y.; Deng, L.; Yamako, G.; Chosa, E.; Pan, C. Biomechanical effect of metal augment and bone graft on cup stability for acetabular reconstruction of total hip arthroplasty in hip dysplasia: A finite element analysis. BMC Musculoskelet. Disord. 2022, 23, 277. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Kanagaraj, S. Finite Element Analysis of the Acetabular Cup in Total Hip Arthroplasty. In North-East Research Conclave; Springer Nature Singapore: Singapore, 2022; pp. 383–394. [Google Scholar]
- Soloviev, D.; Maslov, L.; Zhmaylo, M. Acetabular implant finite element simulation with customised estimate of bone properties. Materials 2023, 16, 398. [Google Scholar] [CrossRef]
- Pignolo, R.J.; Law, S.F.; Chandra, A. Bone aging, cellular senescence, and osteoporosis. J. Bone Miner. Res. Plus 2021, 5, e10488. [Google Scholar] [CrossRef] [PubMed]
- Vogel, D.; Klimek, M.; Saemann, M.; Bader, R. Influence of the Acetabular Cup Material on the Shell Deformation and Strain Distribution in the Adjacent Bone—A Finite Element Analysis. Materials 2020, 13, 1372. [Google Scholar] [CrossRef]
- Taddei, F.; Pancanti, A.; Viceconti, M. An improved method for the automatic mapping of computed tomography numbers onto finite element models. Med. Eng. Phys. 2004, 26, 61–69. [Google Scholar] [CrossRef]
- Sun, Y.; Fu, Y.; Liu, F.; Zhang, W.; Ma, H.; Li, Q.; Zhou, D.; Fu, B. Biomechanical tests and finite element analyses of pelvic stability using bilateral single iliac screws with different channels in lumbo-iliac fixation. Front. Surg. 2022, 9, 1035614. [Google Scholar] [CrossRef]
- Zhu, Y.; Babazadeh-Naseri, A.; Dunbar, N.J.; Brake, M.R.; Zandiyeh, P.; Li, G.; Leardini, A.; Spazzoli, B.; Fregly, B.J. Finite element analysis of screw fixation durability under multiple boundary and loading conditions for a custom pelvic implant. Med. Eng. Phys. 2023, 111, 103930. [Google Scholar] [CrossRef]
- Dong, E.; Wang, L.; Iqbal, T.; Li, D.; Liu, Y.; He, J.; Zhao, B.; Li, Y. Finite element analysis of the pelvis after customized prosthesis reconstruction. J. Bionic Eng. 2018, 15, 443–451. [Google Scholar] [CrossRef]
- Maslov, L.; Borovkov, A.; Maslova, I.; Soloviev, D.; Zhmaylo, M.; Tarasenko, F. Finite Element Analysis of Customized Acetabular Implant and Bone after Pelvic Tumour Resection throughout the Gait Cycle. Materials 2021, 14, 7066. [Google Scholar] [CrossRef] [PubMed]
- Sciammarella, C.A.; Singh, B.; Trentadue, B.; Sciammarella, F.M. Stress analysis of weldments by holographic moiré and the finite element method. Exp. Mech. 2000, 40, 15–21. [Google Scholar] [CrossRef]
- Frost, H.M. Bone’s mechanostat: A 2003 update. Anat. Rec. Part A Discov. Mol. Cell. Evol. Biol. 2003, 275, 1081–1101. [Google Scholar] [CrossRef]
- Paxton, E.W.; Cafri, G.; Nemes, S.; Lorimer, M.; Kärrholm, J.; Malchau, H.; Graves, S.E.; Namba, R.S.; Rolfson, O. An international comparison of THA patients, implants, techniques, and survivorship in Sweden, Australia, and the United States. Acta Orthop. 2019, 90, 148–152. [Google Scholar] [CrossRef]
- Lee, P.T.; Raz, G.; Safir, O.A.; Backstein, D.J.; Gross, A.E. Long-term results for minor column allografts in revision hip arthroplasty. Clin. Orthop. Relat. Res. 2010, 468, 3295–3303. [Google Scholar] [CrossRef]
- Rogers, B.A.; Whittingham-Jones, P.M.; Mitchell, P.A.; Safir, O.A.; Bircher, M.D.; Gross, A.E. The reconstruction of periprosthetic pelvic discontinuity. J. Arthroplast. 2012, 27, 1499–1506. [Google Scholar] [CrossRef] [PubMed]
- Amenabar, T.; Rahman, W.A.; Hetaimish, B.M.; Kuzyk, P.R.; Safir, O.A.; Gross, A.E. Promising mid-term results with a cup-cage construct for large acetabular defects and pelvic discontinuity. Clin. Orthop. Relat. Res. 2016, 474, 408–414. [Google Scholar] [CrossRef]
- Apostu, D.; Lucaciu, O.; Berce, C.; Lucaciu, D.; Cosma, D. Current methods of preventing aseptic loosening and improving osseointegration of titanium implants in cementless total hip arthroplasty: A review. J. Int. Med. Res. 2018, 46, 2104–2119. [Google Scholar] [CrossRef]
- Hoskins, W.; Rainbird, S.; Holder, C.; Graves, S.E.; Bingham, R. Revision for aseptic loosening of highly porous acetabular components in primary total hip arthroplasty: An analysis of 20,993 total hip replacements. J. Arthroplast. 2022, 37, 312–315. [Google Scholar] [CrossRef]
- Kolken, H.M.A.; De Jonge, C.P.; Van der Sloten, T.; Garcia, A.F.; Pouran, B.; Willemsen, K.; Weinans, H.; Zadpoor, A.A. Additively manufactured space-filling meta-implants. Acta Biomater. 2021, 125, 345–357. [Google Scholar] [CrossRef]
- Oyem, P.C.; Burke, Z.D.; Mesko, N.W.; Nystrom, L.M. Custom three-dimensional printed implants for reconstruction of oncologic pelvic defects. J. Surg. Oncol. 2024, 129, 424–435. [Google Scholar] [CrossRef] [PubMed]
- Snijders, T.E.; Schlösser, T.P.; van Stralen, M.; Castelein, R.M.; Stevenson, R.P.; Weinans, H.; de Gast, A. The effect of postural pelvic dynamics on the three-dimensional orientation of the acetabular cup in THA is patient specific. Clin. Orthop. Relat. Res. 2021, 479, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Broekhuis, D.; Tordoir, R.; Vallinga, Z.; Schoones, J.; Pijls, B.; Nelissen, R. Custom triflange acetabular components for large acetabular defect reconstruction in revision total hip arthroplasty: A systematic review and meta-analysis on 1218 patients. EFORT Open Rev. 2023, 8, 522–531. [Google Scholar] [CrossRef]
- Fu, J.; Ni, M.; Zhu, F.; Li, X.; Chai, W.; Hao, L.; Zhou, Y.; Zhang, G.; Chen, J. Reconstruction of Paprosky Type III Acetabular Defects by Three-Dimensional Printed Porous Augment: Techniques and Clinical Outcomes of 18 Consecutive Cases. Orthop. Surg. 2022, 14, 1004–1010. [Google Scholar] [CrossRef] [PubMed]
- Di Laura, A.; Henckel, J.; Hart, A. Custom 3D-printed implants for acetabular reconstruction: Intermediate-term functional and radiographic results. JBJS Open Access 2023, 8, e22. [Google Scholar] [CrossRef]
- Szymkiewicz, K. Numerical Optimization of Functionally Graded Ti-HAP Material for Tibial Bone Fixation System. Materials 2024, 17, 5187. [Google Scholar] [CrossRef]
- Zielinski, R.; Sowinski, J.; Piechaczek, M.; Okulski, J.; Kozakiewicz, M. Finite element analysis of subperiosteal implants in edentulism—On the basis of the MaI Implant® by Integra Implants®. Materials 2023, 16, 7466. [Google Scholar] [CrossRef]
- Comuzzi, L.; Ceddia, M.; Di Pietro, N.; Inchingolo, F.; Inchingolo, A.M.; Romasco, T.; Tumedei, M.; Specchiulli, A.; Piattelli, A.; Trentadue, B. Crestal and Subcrestal Placement of Morse Cone Implant–Abutment Connection Implants: An In Vitro Finite Element Analysis (FEA) Study. Biomedicines 2023, 11, 3077. [Google Scholar] [CrossRef]
- Callea, C.; Ceddia, M.; Piattelli, A.; Specchiulli, A.; Trentadue, B. Finite element analysis (FEA) for a different type of cono-in dental implant. Appl. Sci. 2023, 13, 5313. [Google Scholar] [CrossRef]
- Ceddia, M.; Lamberti, L.; Trentadue, B. FEA Comparison of the Mechanical Behavior of Three Dental Crown Materials: Enamel, Ceramic, and Zirconia. Maiterials 2024, 17, 673. [Google Scholar] [CrossRef]
- Alcántara-Arreola, E.A.; Silva-Garcés, K.N.; Mendoza-Martínez, J.; Cardoso-Palomares, M.A.; Torres-SanMiguel, C.R. Experimental analysis of stress shielding effects in screw spacers placed in porcine spinal tissue. J. Funct. Biomater. 2024, 15, 238. [Google Scholar] [CrossRef] [PubMed]
- Costăchel, B.C.; Bechir, A.; Târcolea, M.; Mihai, L.L.; Burcea, A.; Bechir, E.S. The Stresses and Deformations in the Abfraction Lesions of the Lower Premolars Studied by the Finite Element Analyses: Case Report and Review of Literature. Diagnostics 2024, 14, 788. [Google Scholar] [CrossRef] [PubMed]
- Kundakcioglu, A.; Gedik, B. Comparison of Screws with Different Diameters in Subperiosteal Implant Application with Finite Element Analysis. Int. J. Med. Sci. 2024, 21, 2595. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, M.R.; Goharian, A.; Abdul Kadir, M.R.; Wahit, M.U. Biomechanical and bioactivity concepts of polyetheretherketone composites for use in orthopedic implants—A review. J. Biomed. Mater. Res. Part A 2015, 103, 3689–3702. [Google Scholar] [CrossRef]
- Ceddia, M.; Lamberti, L.; Trentadue, B. A Finite Element Study of Simulated Fusion in an L4-L5 Model: Influence of the Combination of Materials in the Screw-and-Rod Fixation System on Reproducing Natural Bone Behavior. Biomimetics 2025, 10, 72. [Google Scholar] [CrossRef]

| Material | Young’s Modulus, GPa | Poisson’s Ratio | Yield Stress MPa | Fatigue |
|---|---|---|---|---|
| Cortical tissue | 20 | 0.3 | 80–150 | The same as the yield stress |
| Spongy tissue | 0.05 | 0.2 | 1.4–2.1 | |
| Ti-6Al-4V | 113.8 | 0.3 | 950 | 310–610 |
| PEEK | 23 | 0.4 | 120 | 80 |
| Position | Equivalent Elastic Strain () | |
|---|---|---|
| Model A | Model B | |
| C | 789.236 | 867.112 |
| K | 745.219 | 826.983 |
| H | 647.521 | 719.387 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ceddia, M.; Solarino, G.; Pulcrano, A.; Benedetto, A.; Trentadue, B. Finite Element Analysis of a 3D-Printed Acetabular Prosthesis for an Acetabular Defect According to the Paprosky Classification. Materials 2025, 18, 1295. https://doi.org/10.3390/ma18061295
Ceddia M, Solarino G, Pulcrano A, Benedetto A, Trentadue B. Finite Element Analysis of a 3D-Printed Acetabular Prosthesis for an Acetabular Defect According to the Paprosky Classification. Materials. 2025; 18(6):1295. https://doi.org/10.3390/ma18061295
Chicago/Turabian StyleCeddia, Mario, Giuseppe Solarino, Alessandro Pulcrano, Antonella Benedetto, and Bartolomeo Trentadue. 2025. "Finite Element Analysis of a 3D-Printed Acetabular Prosthesis for an Acetabular Defect According to the Paprosky Classification" Materials 18, no. 6: 1295. https://doi.org/10.3390/ma18061295
APA StyleCeddia, M., Solarino, G., Pulcrano, A., Benedetto, A., & Trentadue, B. (2025). Finite Element Analysis of a 3D-Printed Acetabular Prosthesis for an Acetabular Defect According to the Paprosky Classification. Materials, 18(6), 1295. https://doi.org/10.3390/ma18061295








