A Comparative In Vitro Study on Heat Generation with Static Guided and Conventional Implant Bed Preparation Using Stainless Steel Twist Drills and a Standardized Bovine Model
Abstract
1. Introduction
2. Materials and Methods
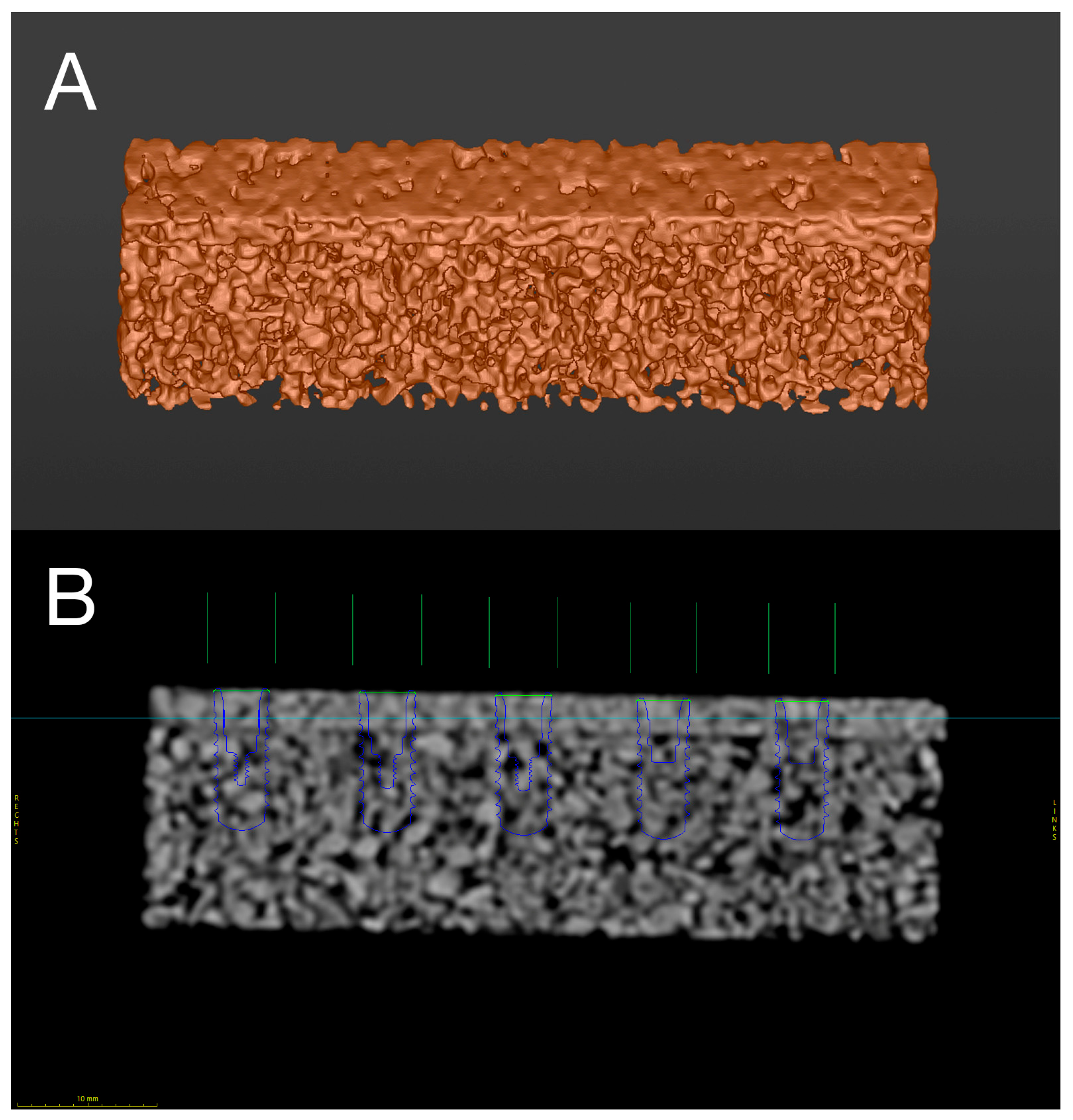
2.1. Digital Preplanning and Manufacturing
2.2. Automated Surgical Simulator
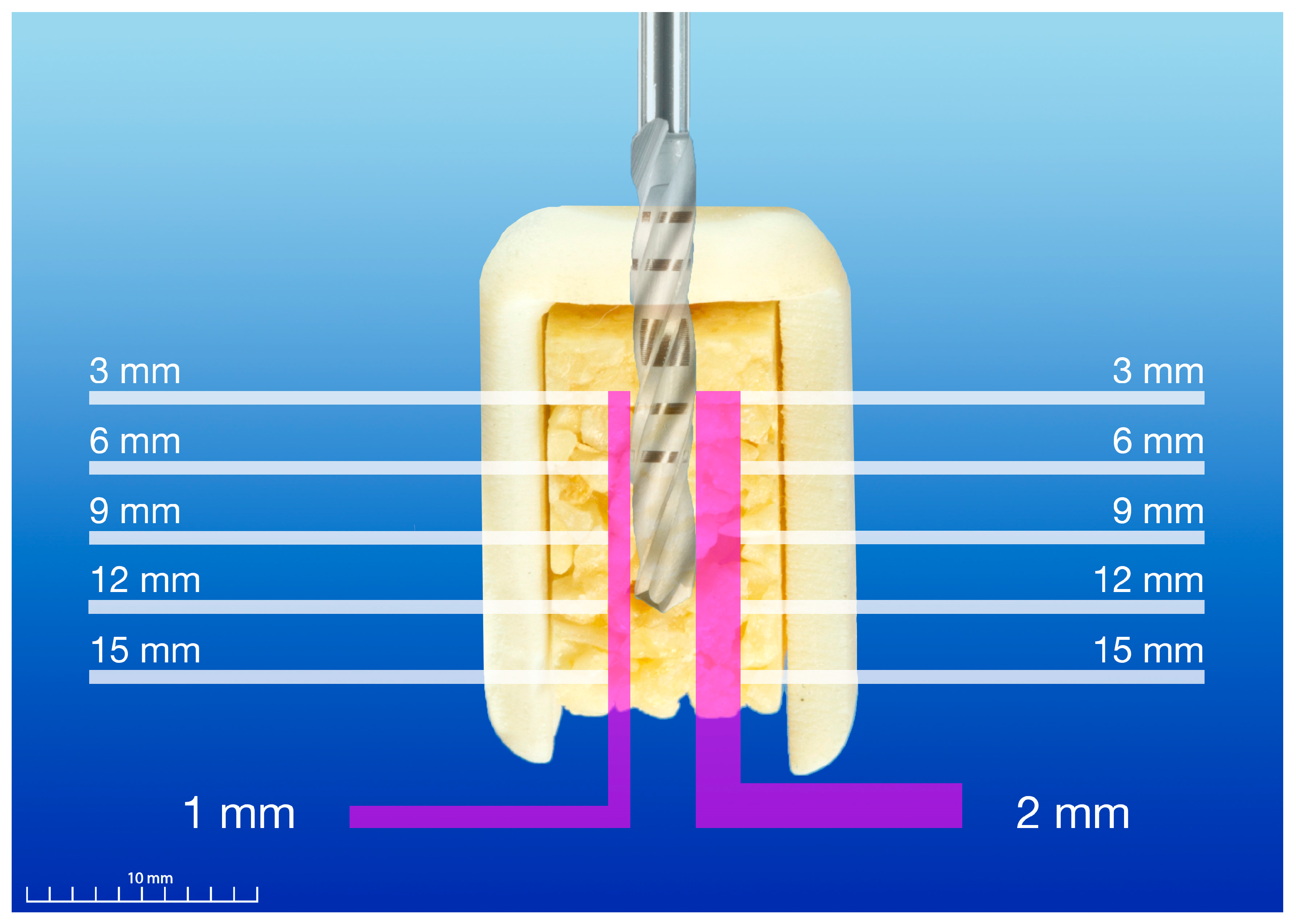
2.3. Temperature Measurement
2.4. Experimental Protocol
2.5. Statistical Analysis
3. Results
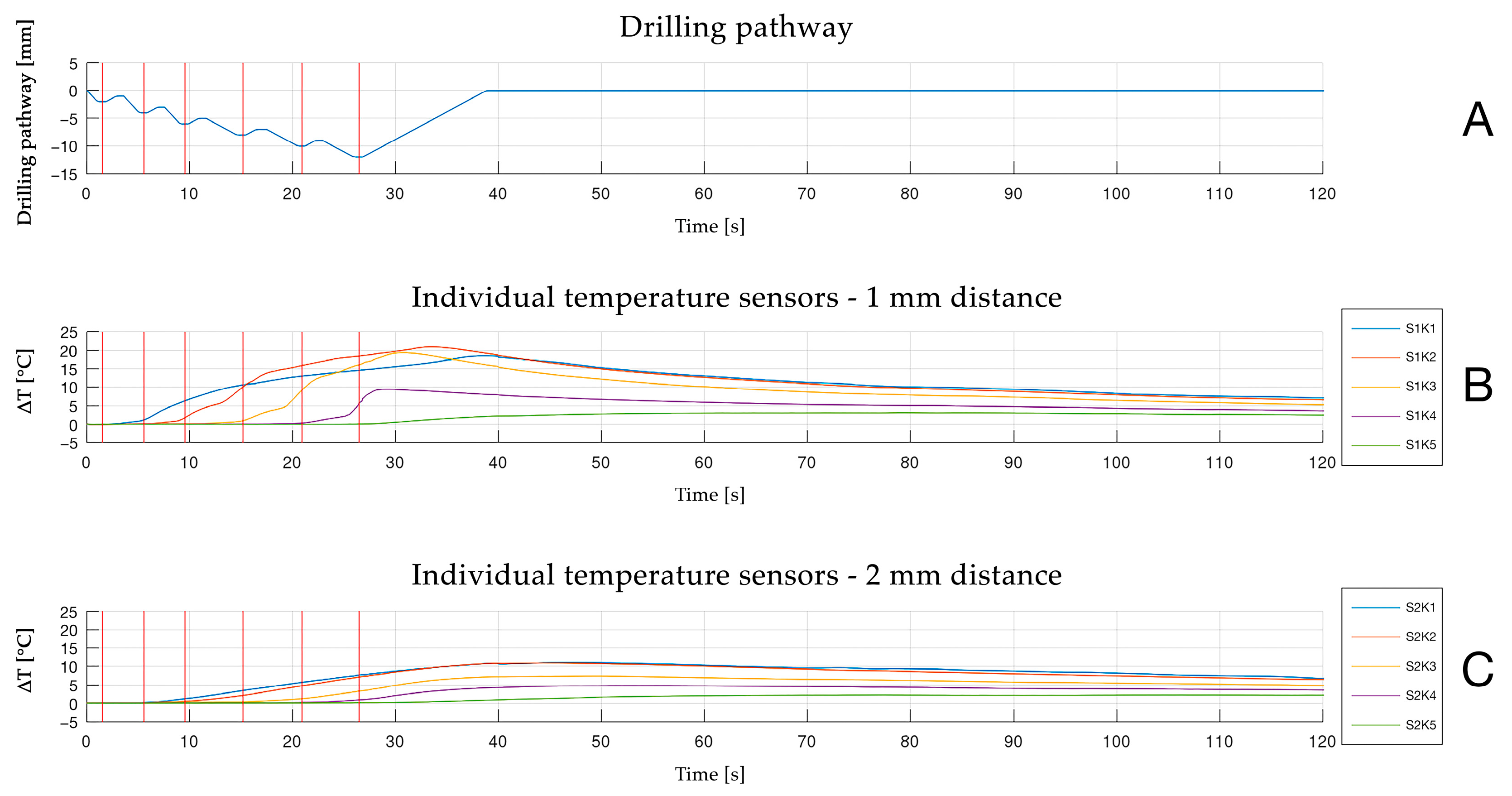
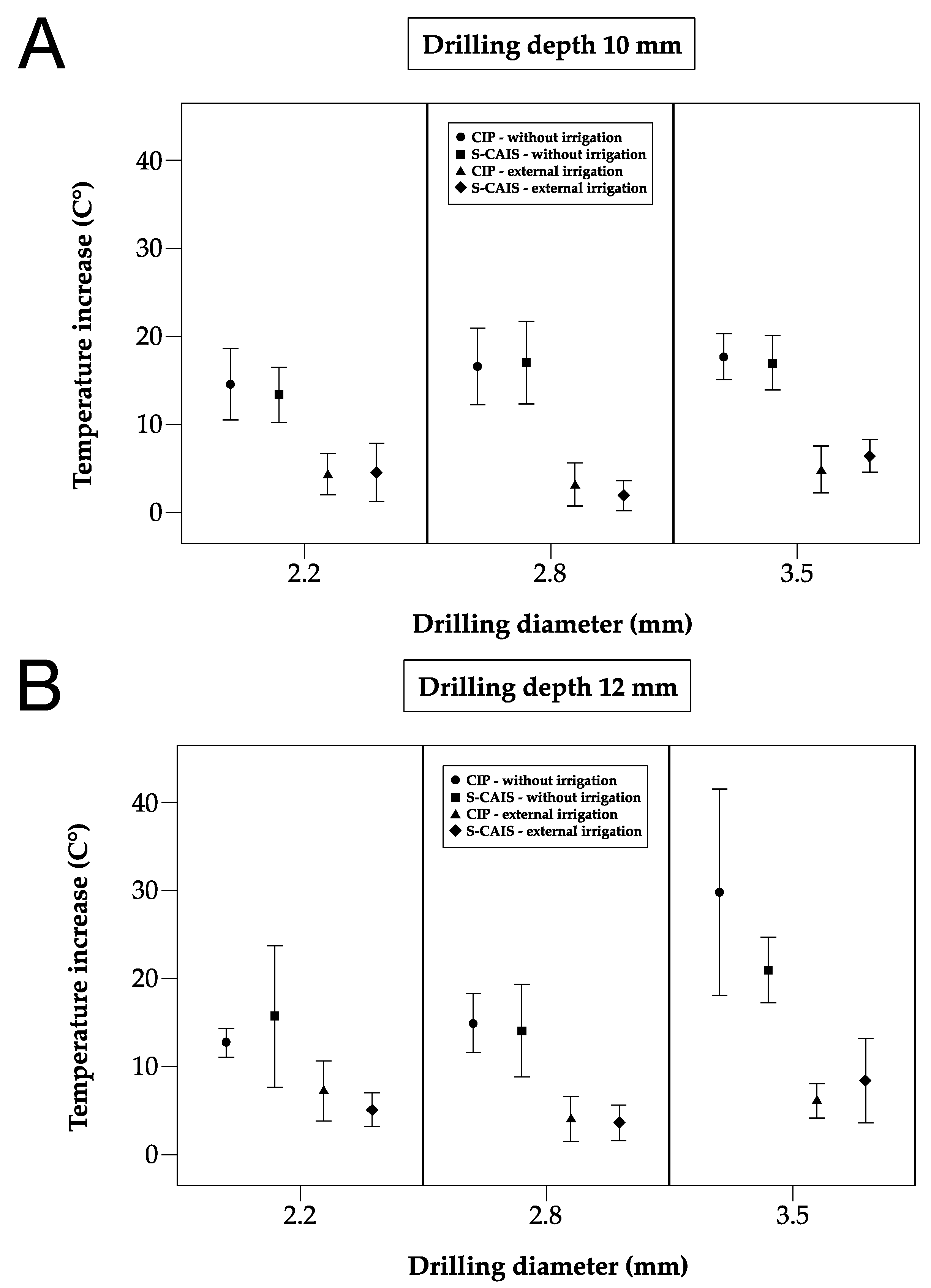
3.1. Maximum Temperature Increase
3.2. Temperature Increase and Surgical Technique
3.3. Temperature Increase and Sensor Location/Corresponding Depth
3.3.1. Drill Diameter 2.2 mm
3.3.2. Drill Diameter 2.8 mm
3.3.3. Drill Diameter 3.5 mm
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 3D | three-dimensional |
| CIP | conventional implant preparation |
| DICOM | Digital Imaging and Communications in Medicine |
| MSCT | multi-slice computed tomography |
| RCT | randomized controlled trial |
| S-CAIS | static computer-assisted implant surgery |
| SD | standard deviations |
| STL | segmented stereolithography |
References
- Brånemark, P.I.; Adell, R.; Breine, U.; Hansson, B.O.; Lindström, J.; Ohlsson, A. Intra-Osseous Anchorage of Dental Prostheses. I. Experimental Studies. Scand. J. Plast. Reconstr. Surg. 1969, 3, 81–100. [Google Scholar] [CrossRef]
- Brånemark, P.I.; Hansson, B.O.; Adell, R.; Breine, U.; Lindström, J.; Hallén, O.; Ohman, A. Osseointegrated Implants in the Treatment of the Edentulous Jaw. Experience from a 10-Year Period. Scand. J. Plast. Reconstr. Surg. Suppl. 1977, 16, 1–132. [Google Scholar]
- Hartmann, A.; Hildebrandt, H.; Younan, Z.; Al-Nawas, B.; Kämmerer, P.W. Long-term Results in Three-dimensional, Complex Bone Augmentation Procedures with Customized Titanium Meshes. Clin. Oral Implant. Res. 2022, 33, 1171–1181. [Google Scholar] [CrossRef]
- Maló, P.; de Araújo Nobre, M.; Lopes, A.; Francischone, C.; Rigolizzo, M. “All-on-4” Immediate-Function Concept for Completely Edentulous Maxillae: A Clinical Report on the Medium (3 Years) and Long-Term (5 Years) Outcomes. Clin. Implant. Dent. Relat. Res. 2012, 14 (Suppl. S1), e139–e150. [Google Scholar] [CrossRef] [PubMed]
- Heimes, D.; Becker, P.; Pabst, A.; Smeets, R.; Kraus, A.; Hartmann, A.; Sagheb, K.; Kämmerer, P.W. How Does Dental Implant Macrogeometry Affect Primary Implant Stability? A Narrative Review. Int. J. Implant. Dent. 2023, 9, 20. [Google Scholar] [CrossRef] [PubMed]
- Parvini, P.; Trimpou, G.; Begic, A.; Cafferata, E.A.; Petsos, H.; Müller, K.M.; Schwarz, F.; Eickholz, P.; Obreja, K. Esthetic and Clinical Outcomes after Immediate Placement and Restoration: Comparison of Two Implant Systems in the Anterior Maxilla-A Cross-Sectional Study. Clin. Implant. Dent. Relat. Res. 2023, 25, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Wittneben, J.-G.; Molinero-Mourelle, P.; Hamilton, A.; Alnasser, M.; Obermaier, B.; Morton, D.; Gallucci, G.O.; Wismeijer, D. Clinical Performance of Immediately Placed and Immediately Loaded Single Implants in the Esthetic Zone: A Systematic Review and Meta-Analysis. Clin. Oral Implant. Res. 2023, 34 (Suppl. S26), 266–303. [Google Scholar] [CrossRef]
- Zuiderveld, E.G.; Meijer, H.J.A.; Gareb, B.; Vissink, A.; Raghoebar, G.M. Single Immediate Implant Placement in the Maxillary Aesthetic Zone with and without Connective Tissue Grafting: Results of a 5-Year Randomized Controlled Trial. J. Clin. Periodontol. 2024, 51, 487–498. [Google Scholar] [CrossRef]
- Ellis, R.; Chen, S.; Davies, H.; Fitzgerald, W.; Xu, J.; Darby, I. Primary Stability and Healing Outcomes of Apically Tapered and Straight Implants Placed into Fresh Extraction Sockets. A Pre-clinical in Vivo Study. Clin. Oral Implant. Res. 2020, 31, 705–714. [Google Scholar] [CrossRef]
- Wei, S.-M.; Shi, J.-Y.; Qiao, S.-C.; Zhang, X.; Lai, H.-C.; Zhang, X.-M. Accuracy and Primary Stability of Tapered or Straight Implants Placed into Fresh Extraction Socket Using Dynamic Navigation: A Randomized Controlled Clinical Trial. Clin. Oral Investig. 2022, 26, 2733–2741. [Google Scholar] [CrossRef]
- Buser, D.; Janner, S.F.M.; Wittneben, J.; Brägger, U.; Ramseier, C.A.; Salvi, G.E. 10-Year Survival and Success Rates of 511 Titanium Implants with a Sandblasted and Acid-Etched Surface: A Retrospective Study in 303 Partially Edentulous Patients. Clin. Implant. Dent. Relat. Res. 2012, 14, 839–851. [Google Scholar] [CrossRef] [PubMed]
- Howe, M.-S.; Keys, W.; Richards, D. Long-Term (10-Year) Dental Implant Survival: A Systematic Review and Sensitivity Meta-Analysis. J. Dent. 2019, 84, 9–21. [Google Scholar] [CrossRef]
- Mura, P. Immediate Placement of Tapered Implants with a Moderately Rough Anodized Surface and Smooth Collar in Fresh Extraction Sockets: A Retrospective Analysis with 10-year Follow-up. Clin. Implant. Dent. Relat. Res. 2019, 21, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Rasmusson, L.; Roos, J.; Bystedt, H. A 10-Year Follow-up Study of Titanium Dioxide-Blasted Implants. Clin. Implant. Dent. Relat. Res. 2005, 7, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Buser, D.; Martin, W.; Belser, U.C. Optimizing Esthetics for Implant Restorations in the Anterior Maxilla: Anatomic and Surgical Considerations. Int. J. Oral Maxillofac. Implant. 2004, 19, 43–61. [Google Scholar]
- Amorfini, L.; Pesce, P.; Migliorati, M.; Drago, S.; Storelli, S.; Romeo, E.; Menini, M. Implant Rehabilitation of the Esthetic Area: A Five-year Retrospective Study Comparing Conventional and Fully Guided Surgery. Clin. Implant. Dent. Relat. Res. 2023, 25, 438–446. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, X.; Wang, M.; Jiang, Q.; Mo, A. Accuracy of Full-Guided and Half-Guided Surgical Templates in Anterior Immediate and Delayed Implantation: A Retrospective Study. Materials 2020, 14, 26. [Google Scholar] [CrossRef]
- Esposito, M.; Hirsch, J.M.; Lekholm, U.; Thomsen, P. Biological Factors Contributing to Failures of Osseointegrated Oral Implants. (I). Success Criteria and Epidemiology. Eur. J. Oral Sci. 1998, 106, 527–551. [Google Scholar] [CrossRef]
- Esposito, M.; Hirsch, J.M.; Lekholm, U.; Thomsen, P. Biological Factors Contributing to Failures of Osseointegrated Oral Implants. (II). Etiopathogenesis. Eur. J. Oral Sci. 1998, 106, 721–764. [Google Scholar] [CrossRef]
- Augustin, G.; Zigman, T.; Davila, S.; Udilljak, T.; Staroveski, T.; Brezak, D.; Babic, S. Cortical Bone Drilling and Thermal Osteonecrosis. Clin. Biomech. 2012, 27, 313–325. [Google Scholar] [CrossRef]
- Eriksson, R.A.; Albrektsson, T. The Effect of Heat on Bone Regeneration: An Experimental Study in the Rabbit Using the Bone Growth Chamber. J. Oral Maxillofac. Surg. 1984, 42, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Ochsner, P.E.; Baumgart, F.; Kohler, G. Heat-Induced Segmental Necrosis after Reaming of One Humeral and Two Tibial Fractures with a Narrow Medullary Canal. Injury 1998, 29 (Suppl. S2), B1–B10. [Google Scholar] [CrossRef]
- Tur, D.; Giannis, K.; Unger, E.; Mittlböck, M.; Rausch-Fan, X.; Strbac, G.D. Thermal Effects of Various Drill Materials during Implant Site Preparation-Ceramic vs. Stainless Steel Drills: A Comparative in Vitro Study in a Standardised Bovine Bone Model. Clin. Oral Implant. Res. 2021, 32, 154–166. [Google Scholar] [CrossRef]
- Oh, H.J.; Wikesjö, U.M.; Kang, H.-S.; Ku, Y.; Eom, T.-G.; Koo, K.-T. Effect of Implant Drill Characteristics on Heat Generation in Osteotomy Sites: A Pilot Study. Clin. Oral Implant. Res. 2011, 22, 722–726. [Google Scholar] [CrossRef] [PubMed]
- Lajolo, C.; Valente, N.A.; Romandini, W.G.; Petruzzi, M.; Verdugo, F.; D’Addona, A. Bone Heat Generated Using Conventional Implant Drills versus Piezosurgery Unit during Apical Cortical Plate Perforation. J. Periodontol. 2018, 89, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Lucchiari, N.; Frigo, A.C.; Stellini, E.; Coppe, M.; Berengo, M.; Bacci, C. In Vitro Assessment with the Infrared Thermometer of Temperature Differences Generated During Implant Site Preparation: The Traditional Technique Versus the Single-Drill Technique. Clin. Implant. Dent. Relat. Res. 2016, 18, 182–191. [Google Scholar] [CrossRef]
- Rashad, A.; Kaiser, A.; Prochnow, N.; Schmitz, I.; Hoffmann, E.; Maurer, P. Heat Production during Different Ultrasonic and Conventional Osteotomy Preparations for Dental Implants. Clin. Oral Implant. Res. 2011, 22, 1361–1365. [Google Scholar] [CrossRef]
- Strbac, G.D.; Giannis, K.; Unger, E.; Mittlböck, M.; Watzek, G.; Zechner, W. A Novel Standardized Bone Model for Thermal Evaluation of Bone Osteotomies with Various Irrigation Methods. Clin. Oral Implant. Res. 2014, 25, 622–631. [Google Scholar] [CrossRef]
- Strbac, G.D.; Unger, E.; Donner, R.; Bijak, M.; Watzek, G.; Zechner, W. Thermal Effects of a Combined Irrigation Method during Implant Site Drilling. A Standardized in Vitro Study Using a Bovine Rib Model. Clin. Oral Implant. Res. 2014, 25, 665–674. [Google Scholar] [CrossRef]
- Karaca, F.; Aksakal, B.; Kom, M. Influence of Orthopaedic Drilling Parameters on Temperature and Histopathology of Bovine Tibia: An in Vitro Study. Med. Eng. Phys. 2011, 33, 1221–1227. [Google Scholar] [CrossRef]
- Dioguardi, M.; Spirito, F.; Quarta, C.; Sovereto, D.; Basile, E.; Ballini, A.; Caloro, G.A.; Troiano, G.; Lo Muzio, L.; Mastrangelo, F. Guided Dental Implant Surgery: Systematic Review. J. Clin. Med. 2023, 12, 1490. [Google Scholar] [CrossRef]
- Frösch, L.; Mukaddam, K.; Filippi, A.; Zitzmann, N.U.; Kühl, S. Comparison of Heat Generation between Guided and Conventional Implant Surgery for Single and Sequential Drilling Protocols—An in Vitro Study. Clin. Oral Implant. Res. 2019, 30, 121–130. [Google Scholar] [CrossRef]
- Markovic, A.; Lazic, Z.; Misic, T.; Scepanovic, M.; Todorovic, A.; Thakare, K.; Janjic, B.; Vlahovic, Z.; Glisic, M. Effect of Surgical Drill Guide and Irrigans Temperature on Thermal Bone Changes during Drilling Implant Sites—Thermographic Analysis on Bovine Ribs. Vojnosanit Pregl. 2016, 73, 744–750. [Google Scholar] [CrossRef] [PubMed]
- Parvizi, S.; Cameron, A.; Tadakamadla, S.; Figueredo, C.M.S.; Reher, P. A Novel Irrigation System to Reduce Heat Generation during Guided Implantology: An In Vitro Study. J. Clin. Med. 2023, 12, 3944. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Brintouch, I.; Romanos, G.; Delgado-Ruiz, R. Cooling Efficiency of Sleeveless 3D-Printed Surgical Guides with Different Cylinder Designs. Medicina 2024, 60, 239. [Google Scholar] [CrossRef] [PubMed]
- Barrak, I.; Joób-Fancsaly, Á.; Braunitzer, G.; Varga, E.; Boa, K.; Piffkó, J. Intraosseous Heat Generation During Osteotomy Performed Freehand and Through Template with an Integrated Metal Guide Sleeve: An In Vitro Study. Implant. Dent. 2018, 27, 342–350. [Google Scholar] [CrossRef]
- Abboud, M.; Delgado-Ruiz, R.A.; Kucine, A.; Rugova, S.; Balanta, J.; Calvo-Guirado, J.L. Multistepped Drill Design for Single-Stage Implant Site Preparation: Experimental Study in Type 2 Bone. Clin. Implant. Dent. Relat. Res. 2015, 17 (Suppl. S2), e472–e485. [Google Scholar] [CrossRef]
- Delgado-Ruiz, R.A.; Sacks, D.; Palermo, A.; Calvo-Guirado, J.L.; Perez-Albacete, C.; Romanos, G.E. Temperature and Time Variations during Osteotomies Performed with Different Piezosurgical Devices: An in Vitro Study. Clin. Oral Implant. Res. 2016, 27, 1137–1143. [Google Scholar] [CrossRef]
- Delgado-Ruiz, R.A.; Velasco Ortega, E.; Romanos, G.E.; Gerhke, S.; Newen, I.; Calvo-Guirado, J.L. Slow Drilling Speeds for Single-Drill Implant Bed Preparation. Experimental in Vitro Study. Clin. Oral Investig. 2018, 22, 349–359. [Google Scholar] [CrossRef]
- Strbac, G.D.; Giannis, K.; Unger, E.; Mittlböck, M.; Vasak, C.; Watzek, G.; Zechner, W. Drilling- and Withdrawing-Related Thermal Changes during Implant Site Osteotomies. Clin. Implant. Dent. Relat. Res. 2015, 17, 32–43. [Google Scholar] [CrossRef]
- Tur, D.; Giannis, K.; Unger, E.; Mittlböck, M.; Rausch-Fan, X.; Strbac, G.D. Drilling- and Withdrawing-Related Thermal Effects of Implant Site Preparation for Ceramic and Stainless Steel Twist Drills in Standardized Bovine Bone. Clin. Implant. Dent. Relat. Res. 2023, 25, 152–165. [Google Scholar] [CrossRef]
- Lekholm, U.; Zarb, G.A. Patient Selection and Preparation. In Tissue-Integrated Prostheses: Osseointegration in Clinical Dentistry; Branemark, P.-I., Zarb, G.A., Albrektsson, T., Eds.; Quintessenz: Vienna, Austria, 1985; pp. 199–210. [Google Scholar]
- Pell, D.J.; Soshi, M. Analysis and Optimization of Bone Machining for Robotic Orthopedic Surgeries. Int. J. Med. Robot. 2018, 14, e1910. [Google Scholar] [CrossRef] [PubMed]
- Davidson, S.R.; James, D.F. Drilling in Bone: Modeling Heat Generation and Temperature Distribution. J. Biomech. Eng. 2003, 125, 305–314. [Google Scholar] [CrossRef] [PubMed]
- D’haese, J.; Ackhurst, J.; Wismeijer, D.; De Bruyn, H.; Tahmaseb, A. Current State of the Art of Computer-Guided Implant Surgery. Periodontol 2000 2017, 73, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, N.; Alaejos-Algarra, F.; Mareque-Bueno, J.; Ferrés-Padró, E.; Hernández-Alfaro, F. Thermal Changes and Drill Wear in Bovine Bone during Implant Site Preparation. A Comparative in Vitro Study: Twisted Stainless Steel and Ceramic Drills: Thermal Changes and Drill Wear in Bovine Bone. Clin. Oral Implants. Res. 2012, 23, 963–969. [Google Scholar] [CrossRef]
- Gaspar, J.; Borrecho, G.; Oliveira, P.; Salvado, F.; Martins dos Santos, J. Osteotomy at Low-Speed Drilling without Irrigation versus High-Speed Drilling with Irrigation: An Experimental Study. Acta Med. Port. 2013, 26, 231–236. [Google Scholar]
- Koopaie, M.; Kolahdouz, S.; Kolahdouz, E.M. Comparison of Wear and Temperature of Zirconia and Tungsten Carbide Tools in Drilling Bone: In Vitro and Finite Element Analysis. Br. J. Oral Maxillofac. Surg. 2019, 57, 557–565. [Google Scholar] [CrossRef]
- Scarano, A.; Lorusso, F.; Noumbissi, S. Infrared Thermographic Evaluation of Temperature Modifications Induced during Implant Site Preparation with Steel vs. Zirconia Implant Drill. J. Clin. Med. 2020, 9, 148. [Google Scholar] [CrossRef]
- Calvo-Guirado, J.L.; Delgado-Peña, J.; Maté-Sánchez, J.E.; Mareque Bueno, J.; Delgado-Ruiz, R.A.; Romanos, G.E. Novel Hybrid Drilling Protocol: Evaluation for the Implant Healing—Thermal Changes, Crestal Bone Loss, and Bone-to-implant Contact. Clin. Oral Implant. Res. 2015, 26, 753–760. [Google Scholar] [CrossRef]
- Soldatos, N.; Nelson-Rabe, L.; Palanker, N.; Angelov, N.; Romanos, G.; Weltman, R. Temperature Changes during Implant Osteotomy Preparations in Fresh Human Cadaver Tibiae, Comparing Straight with Tapered Drills. Materials 2022, 15, 2369. [Google Scholar] [CrossRef]
- Lanis, A.; Peña-Cardelles, J.F.; Negreiros, W.M.; Hamilton, A.; Gallucci, G.O. Impact of Digital Technologies on Implant Surgery in Fully Edentulous Patients: A Scoping Review. Clin. Oral Implant. Res. 2024, 35, 1000–1010. [Google Scholar] [CrossRef]
- Sarhan, M.M.; Ibrahim, E.A.; Ezzelarab, S.; Marei, M.K. Navigating the Future of Guided Dental Implantology: A Scoping Review. Int. J. Med. Robot. 2024, 20, e2627. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, P.L.; Queiroz, T.P.; Margonar, R.; de Souza Carvalho, A.C.G.; Betoni, W.; Rezende, R.R.R.; dos Santos, P.H.; Garcia, I.R. Evaluation of Bone Heating, Drill Deformation, and Drill Roughness after Implant Osteotomy: Guided Surgery and Classic Drilling Procedure. Int. J. Oral Maxillofac. Implant. 2014, 29, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Migliorati, M.; Amorfini, L.; Signori, A.; Barberis, F.; Silvestrini Biavati, A.; Benedicenti, S. Internal Bone Temperature Change During Guided Surgery Preparations for Dental Implants: An In Vitro Study. Int. J. Oral Maxillofac. Implant. 2013, 28, 1464–1469. [Google Scholar] [CrossRef]
- Misir, A.F.; Sumer, M.; Yenisey, M.; Ergioglu, E. Effect of Surgical Drill Guide on Heat Generated from Implant Drilling. J. Oral Maxillofac. Surg. 2009, 67, 2663–2668. [Google Scholar] [CrossRef] [PubMed]
- Gargallo-Albiol, J.; Barootchi, S.; Marqués-Guasch, J.; Wang, H.-L. Fully Guided Versus Half-Guided and Freehand Implant Placement: Systematic Review and Meta-Analysis. Int. J. Oral Maxillofac. Implant. 2020, 35, 1159–1169. [Google Scholar] [CrossRef]
- Turkyilmaz, I.; Benli, M.; Schoenbaum, T.R. Clinical Performance of 11,646 Dental Implants Using Surgical Guides and Two Different Surgical Approaches: A Systematic Review and Meta-Analysis. Int. J. Oral Maxillofac. Implant. 2023, 38, 16–29. [Google Scholar] [CrossRef]
- Milberg, J.; Fuchsberger, A. Possibilities of reducing thermal damage to bone when using spiral drills in osteosynthesis. Biomed. Tech. 1984, 29, 309–317. [Google Scholar] [CrossRef]
- Pupulin, F.; Oresta, G.; Sunar, T.; Parenti, P. On the Thermal Impact during Drilling Operations in Guided Dental Surgery: An Experimental and Numerical Investigation. J. Mech. Behav. Biomed. Mater. 2024, 150, 106327. [Google Scholar] [CrossRef]
- Kuster, M.; Mukaddam, K.; Zitzmann, N.; Filippi, A.; Kühl, S. Influence of a Novel Drill Design on Heat Generation During Conventional and Guided Implant Osteotomy. Int. J. Oral Maxillofac. Implant. 2021, 36, e31–e41. [Google Scholar] [CrossRef]
- Cordioli, G.; Majzoub, Z. Heat Generation during Implant Site Preparation: An in Vitro Study. Int. J. Oral Maxillofac. Implant. 1997, 12, 186–193. [Google Scholar]
- Ashry, A.; Elattar, M.S.; Elsamni, O.A.; Soliman, I.S. Effect of Guiding Sleeve Design on Intraosseous Heat Generation During Implant Site Preparation (In Vitro Study). J. Prosthodont. 2022, 31, 147–154. [Google Scholar] [CrossRef]
- Gargallo-Albiol, J.; Salomó-Coll, O.; Lozano-Carrascal, N.; Wang, H.; Hernández-Alfaro, F. Intra-osseous Heat Generation during Implant Bed Preparation with Static Navigation: Multi-factor in Vitro Study. Clin. Oral Implant. Res. 2021, 32, 590–597. [Google Scholar] [CrossRef]
- Sannino, G.; Gherlone, E.F. Thermal Changes During Guided Flapless Implant Site Preparation: A Comparative Study. Int. J. Oral Maxillofac. Implant. 2018, 33, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Waltenberger, L.; Wied, S.; Wolfart, S.; Tuna, T. Effect of Different Dental Implant Drilling Template Designs on Heat Generation during Osteotomy—An in Vitro Study. Clin. Oral Implant. Res. 2022, 33, 53–64. [Google Scholar] [CrossRef]
- dos Santos, P.L.; Queiroz, T.P.; Margonar, R.; Gomes de Souza Carvalho, A.C.; Okamoto, R.; de Souza Faloni, A.P.; Garcia, I.R. Guided Implant Surgery: What Is the Influence of This New Technique on Bone Cell Viability? J. Oral Maxillofac. Surg. 2013, 71, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Aparicio, C.; Manresa, C.; Francisco, K.; Claros, P.; Alández, J.; González-Martín, O.; Albrektsson, T. Zygomatic Implants: Indications, Techniques and Outcomes, and the Zygomatic Success Code. Periodontol 2000 2014, 66, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Esposito, M.; Davó, R.; Marti-Pages, C.; Ferrer-Fuertes, A.; Barausse, C.; Pistilli, R.; Ippolito, D.R.; Felice, P. Immediately Loaded Zygomatic Implants vs Conventional Dental Implants in Augmented Atrophic Maxillae: 4 Months Post-Loading Results from a Multicentre Randomised Controlled Trial. Eur. J. Oral Implantol. 2018, 11, 11–28. [Google Scholar]
- Stocchero, M.; Sivolella, S.; Brunello, G.; Zoppello, A.; Cavallin, F.; Biasetto, L. Bone Temperature Variation Using a 3D-Printed Surgical Guide with Internal Irrigation. Appl. Sci. 2021, 11, 2588. [Google Scholar] [CrossRef]
- Orgev, A.; Gonzaga, L.; Martin, W.; Morton, D.; Lin, W.-S. Addition of an Irrigation Channel to a Surgical Template to Facilitate Cooling during Implant Osteotomy. J. Prosthet. Dent. 2021, 126, 164–166. [Google Scholar] [CrossRef]
- Alevizakos, V.; Mitov, G.; Von See, C. Guided Implant Placement Using an Internally Cooling Surgical Template: A Technical Note. J. Oral Implantol. 2020, 46, 533–535. [Google Scholar] [CrossRef]
- Jeong, S.-M.; Yoo, J.-H.; Fang, Y.; Choi, B.-H.; Son, J.-S.; Oh, J.-H. The Effect of Guided Flapless Implant Procedure on Heat Generation from Implant Drilling. J. Cranio-Maxillofac. Surg. 2014, 42, 725–729. [Google Scholar] [CrossRef] [PubMed]
- Boa, K.; Barrak, I.; Varga, E.; Joob-Fancsaly, A.; Varga, E.; Piffko, J. Intraosseous Generation of Heat during Guided Surgical Drilling: An Ex Vivo Study of the Effect of the Temperature of the Irrigating Fluid. Br. J. Oral Maxillofac. Surg. 2016, 54, 904–908. [Google Scholar] [CrossRef] [PubMed]
- Barausse, C.; Pistilli, R.; Bonifazi, L.; Tayeb, S.; Pellegrino, G.; Ravidà, A.; Felice, P. Four-Mm-Short Implants in the Rehabilitation of Posterior Atrophic Jaws: A Retrospective Study on 212 Patients with a Mean Follow-up of 8.02 Years. Clin. Oral Implant. Res. 2024, 35, 1607–1615. [Google Scholar] [CrossRef]
- Ravidà, A.; Serroni, M.; Borgnakke, W.S.; Romandini, M.; Wang, I.-C.I.; Arena, C.; Annunziata, M.; Cecoro, G.; Saleh, M.H.A. Short (≤6 Mm) Compared with ≥10-Mm Dental Implants in Different Clinical Scenarios: A Systematic Review of Randomized Clinical Trials with Meta-Analysis, Trial Sequential Analysis and Quality of Evidence Grading. J. Clin. Periodontol. 2024, 51, 936–965. [Google Scholar] [CrossRef]
- Lee, J.; Ozdoganlar, O.B.; Rabin, Y. An Experimental Investigation on Thermal Exposure during Bone Drilling. Med. Eng. Phys. 2012, 34, 1510–1520. [Google Scholar] [CrossRef] [PubMed]
- Benington, I.C.; Biagioni, P.A.; Crossey, P.J.; Hussey, D.L.; Sheridan, S.; Lamey, P.-J. Temperature Changes in Bovine Mandibular Bone during Implant Site Preparation: An Assessment Using Infra-Red Thermography. J. Dent. 1996, 24, 263–267. [Google Scholar] [CrossRef]
- Tehemar, S.H. Factors Affecting Heat Generation during Implant Site Preparation: A Review of Biologic Observations and Future Considerations. Int. J. Oral Maxillofac. Implant. 1999, 14, 127–136. [Google Scholar]


| Diameter | Technique/p-Value | Drilling Depth 10 mm | Drilling Depth 12 mm | ||
|---|---|---|---|---|---|
| Without Irrigation | External Irrigation | Without Irrigation | External Irrigation | ||
| 2.2 mm | S-CAIS | 13.06 (2.03) | 3.78 (1.86) | 15.75 (4.91) | 5.29 (1.05) |
| CIP | 13.62 (2.93) | 3.68 (1.38) | 12.86 (1.15) | 5.93 (2.08) | |
| p-value | 0.6228 | 0.8934 | 0.1004 | 0.3942 | |
| 2.8 mm | S-CAIS | 16.70 (3.04) | 2.14 (1.12) | 13.82 (4.22) | 3.39 (1.50) |
| CIP | 16.54 (3.01) | 2.44 (1.39) | 15.08 (2.20) | 4.13 (1.69) | |
| p-value | 0.9051 | 0.6005 | 0.4156 | 0.3172 | |
| 3.5 mm | S-CAIS | 15.05 (1.90) | 6.36 (1.15) | 20.80 (2.66) | 9.14 (3.00) |
| CIP | 17.36 (1.76) | 5.25 (1.90) | 27.10 (7.27) | 6.00 (1.27) | |
| p-value | 0.0115 * | 0.1342 | 0.0253 * | 0.0101 * | |
| Diameter | Technique | Distance | Drilling Depth 10 mm | Drilling Depth 12 mm | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Without Irrigation | External Irrigation | Without Irrigation | External Irrigation | |||||||||||
| Med | Min | Max | Med | Min | Max | Med | Min | Max | Med | Min | Max | |||
| 2.2 mm | S-CAIS | 1 mm | 6 | 3 | 6 | 9 | 6 | 9 | 6 | 6 | 6 | 12 | 6 | 12 |
| 2 mm | 3 | 3 | 6 | 9 | 6 | 9 | 6 | 3 | 6 | 9 | 6 | 12 | ||
| CIP | 1 mm | 6 | 3 | 9 | 9 | 6 | 9 | 6 | 6 | 9 | 12 | 9 | 12 | |
| 2 mm | 3 | 3 | 6 | 6 | 6 | 9 | 4.5 | 3 | 9 | 12 | 6 | 15 | ||
| 2.8 mm | S-CAIS | 1 mm | 6 | 3 | 6 | 7.5 | 3 | 12 | 6 | 3 | 9 | 11 | 6 | 12 |
| 2 mm | 3 | 3 | 6 | 7.5 | 3 | 15 | 6 | 3 | 6 | 9 | 6 | 12 | ||
| CIP | 1 mm | 6 | 3 | 9 | 9 | 9 | 12 | 6 | 3 | 9 | 12 | 6 | 12 | |
| 2 mm | 4.5 | 3 | 6 | 9 | 6 | 15 | 3 | 3 | 6 | 12 | 6 | 12 | ||
| 3.5 mm | S-CAIS | 1 mm | 3 | 3 | 6 | 9 | 3 | 9 | 6 | 3 | 6 | 9 | 9 | 12 |
| 2 mm | 3 | 3 | 6 | 6 | 6 | 9 | 6 | 3 | 6 | 7.5 | 6 | 12 | ||
| CIP | 1 mm | 3 | 3 | 6 | 9 | 6 | 9 | 6 | 3 | 6 | 9 | 6 | 12 | |
| 2 mm | 6 | 3 | 6 | 6 | 6 | 9 | 3 | 3 | 6 | 9 | 6 | 12 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tur, D.; Tian, Z.; Giannis, K.; Unger, E.; Mittlboeck, M.; Rausch-Fan, X.; Strbac, G.D. A Comparative In Vitro Study on Heat Generation with Static Guided and Conventional Implant Bed Preparation Using Stainless Steel Twist Drills and a Standardized Bovine Model. Materials 2025, 18, 1277. https://doi.org/10.3390/ma18061277
Tur D, Tian Z, Giannis K, Unger E, Mittlboeck M, Rausch-Fan X, Strbac GD. A Comparative In Vitro Study on Heat Generation with Static Guided and Conventional Implant Bed Preparation Using Stainless Steel Twist Drills and a Standardized Bovine Model. Materials. 2025; 18(6):1277. https://doi.org/10.3390/ma18061277
Chicago/Turabian StyleTur, Dino, Zhiwei Tian, Katharina Giannis, Ewald Unger, Martina Mittlboeck, Xiaohui Rausch-Fan, and Georg D. Strbac. 2025. "A Comparative In Vitro Study on Heat Generation with Static Guided and Conventional Implant Bed Preparation Using Stainless Steel Twist Drills and a Standardized Bovine Model" Materials 18, no. 6: 1277. https://doi.org/10.3390/ma18061277
APA StyleTur, D., Tian, Z., Giannis, K., Unger, E., Mittlboeck, M., Rausch-Fan, X., & Strbac, G. D. (2025). A Comparative In Vitro Study on Heat Generation with Static Guided and Conventional Implant Bed Preparation Using Stainless Steel Twist Drills and a Standardized Bovine Model. Materials, 18(6), 1277. https://doi.org/10.3390/ma18061277






