Effect of Mo Addition on the Susceptibility of Advanced High Strength Steels to Liquid Metal Embrittlement
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Mechanical Testing
3.2. Resistance Spot Welding
4. Discussion
5. Conclusions
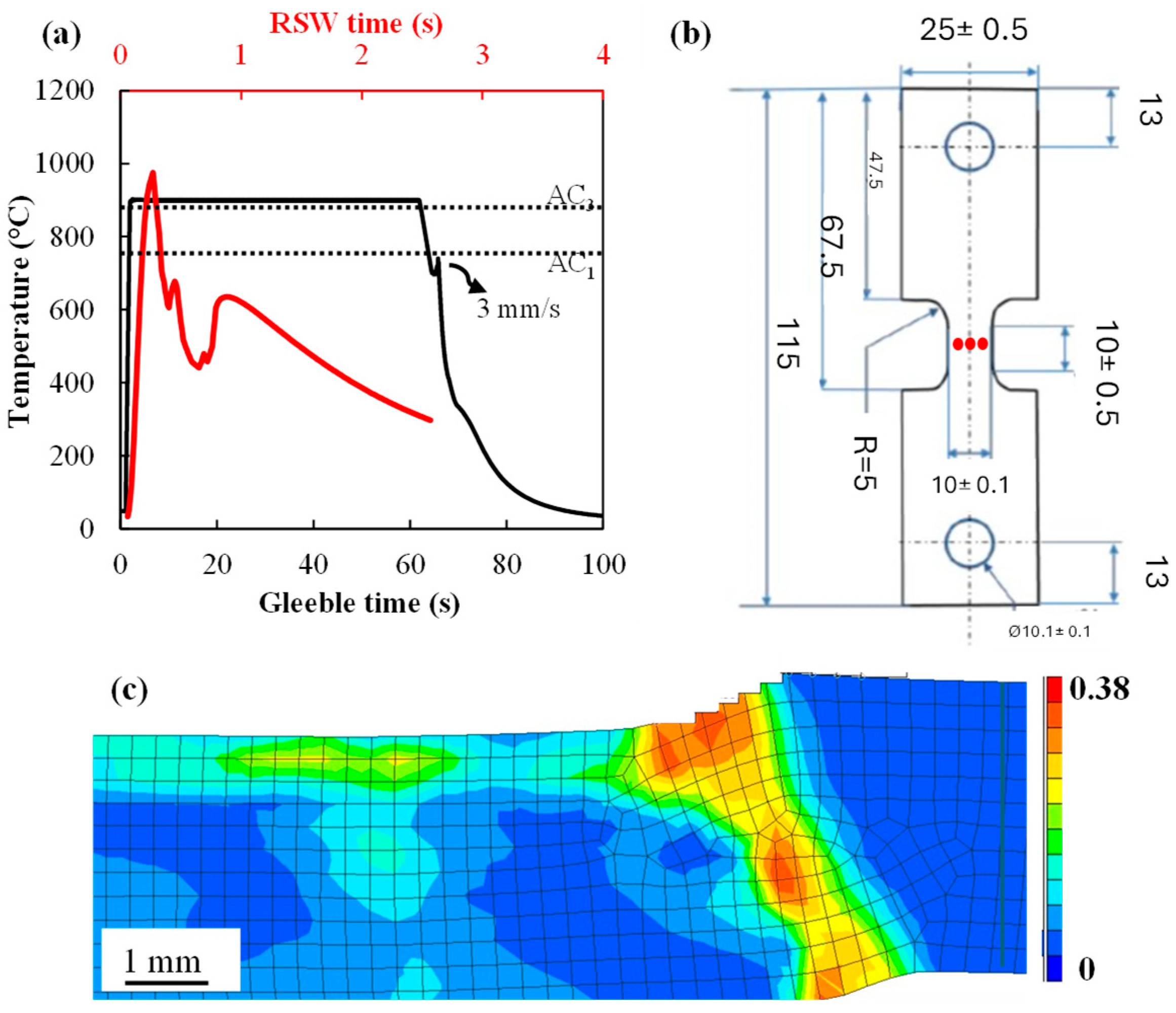
- RSW simulations of the shoulder region showed that the sample experienced temperatures higher than Ac3 during the welding. Comparing the RSW and Gleeble samples revealed that, in both cases, LME cracks propagated along PAGBs and their microhardness values were comparable (~545 ± 8 Hv). Thus, the austenization of the steel during RSW was implied.
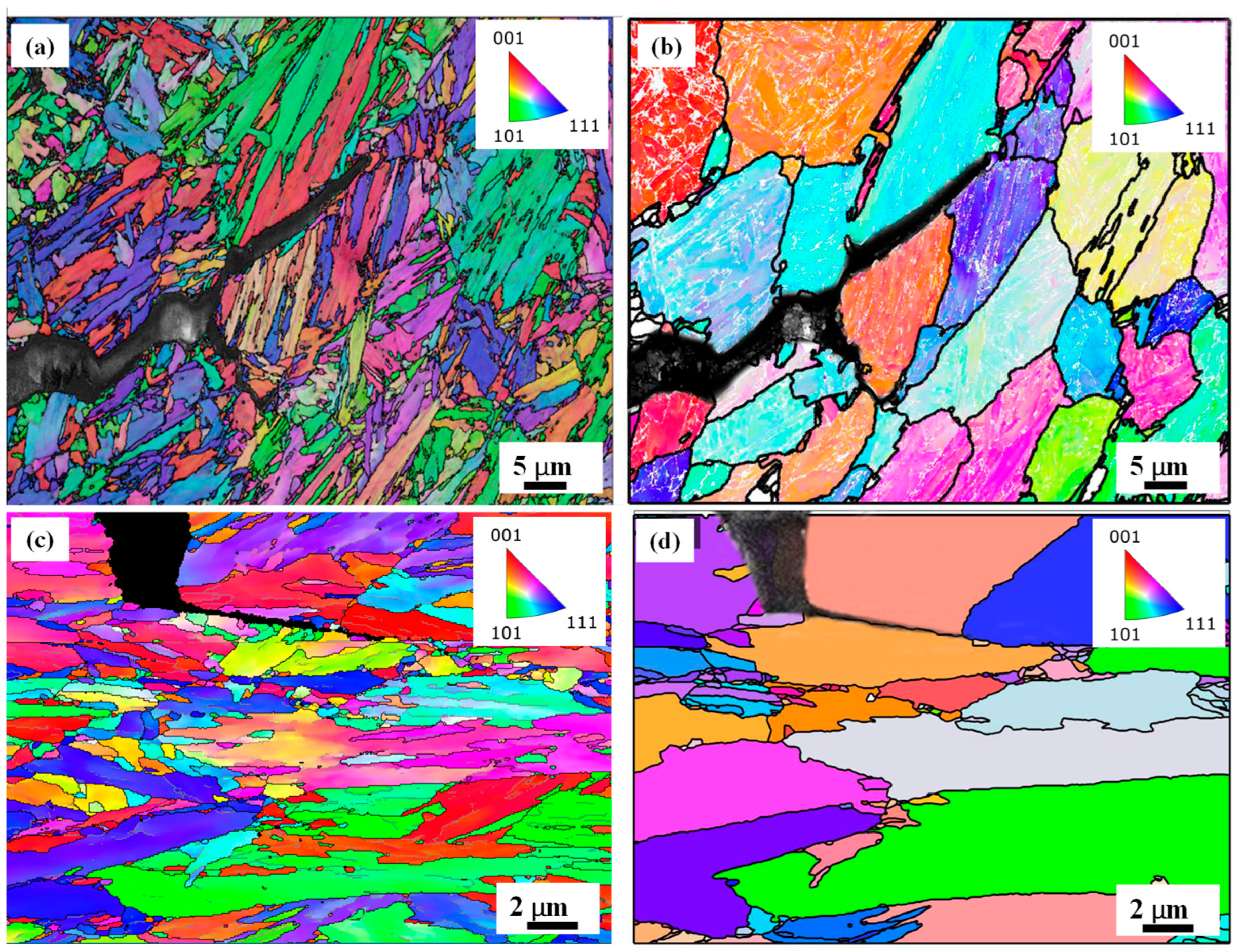
- The larger force and stroke observed for the 0.2 Mo sample during hot tensile testing were accompanied by less LME cracking in the cross section of this sample in comparison to the 0 Mo alloy, without any significant difference in their grain sizes.
- SEM images showed that longer cracks, with more crack openings, were observed in the cross-section of the resistance spot-welded 0 Mo sample. This resulted in a higher crack severity index for the 0 Mo sample (0.61) in comparison to the 0.2 Mo sample (0.45).
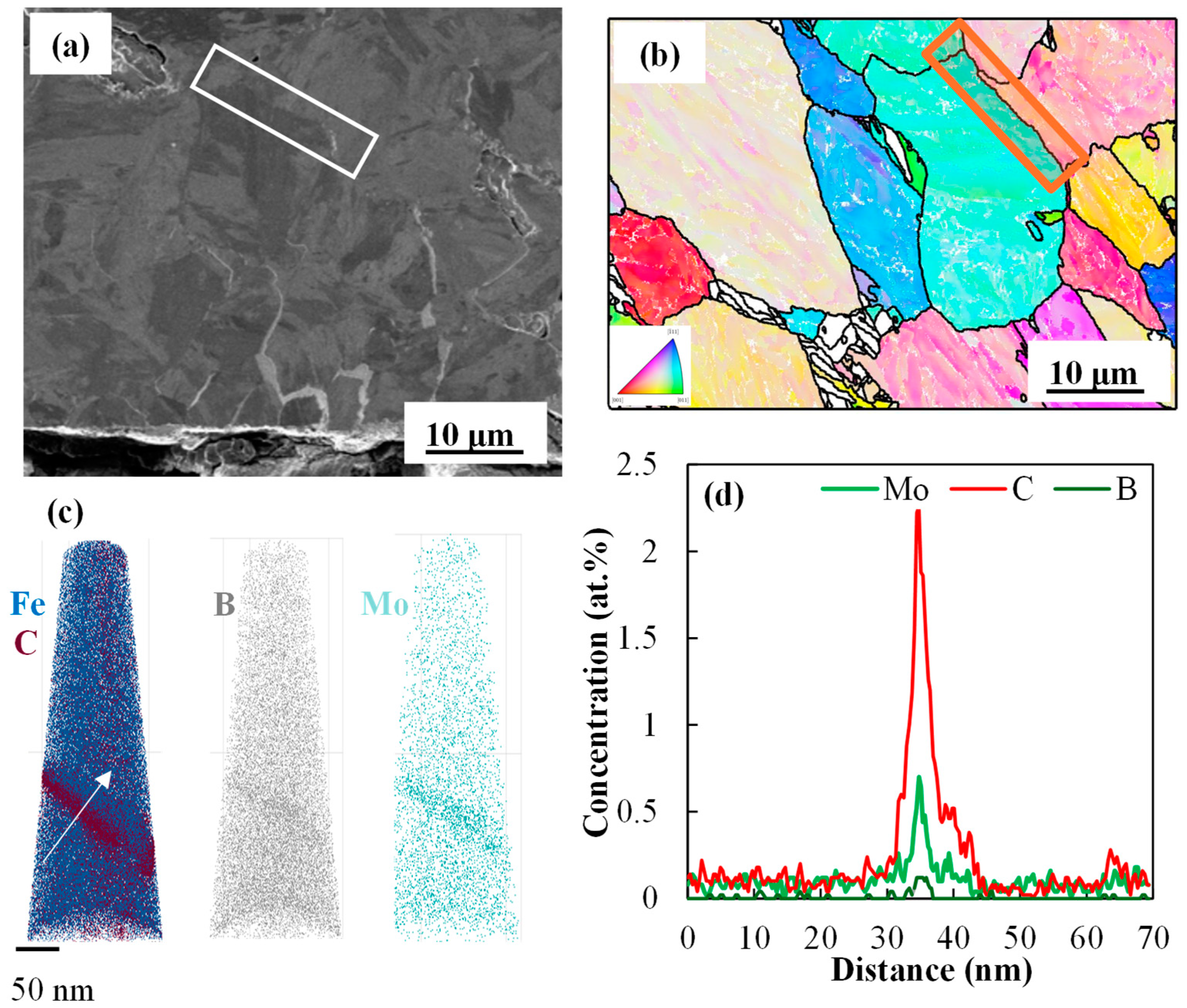
- APT results shed light on the enhanced segregation of B on PAGBs in the presence of Mo, leading to increased cohesion of these grain boundaries and delayed LME cracking.
- STEM-EDS results showed a more gradual drop in Zn profile for the 0.2 Mo sample, as a result of more diffusion of Zn in the substrate, consuming the Zn reservoir for further feeding the liquid Zn inside the LME crack.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PAGB | Prior Austenite Grain Boundary |
| GB | Grain Boundary |
| RSW | Resistance Spot Welding |
| LME | Liquid Metal Embrittlement |
| AHSS | Advanced High Strength Steel |
Appendix A
Appendix A.1. Finite Element Simulation Model
Appendix A.2. Mesh and Boundary Conditions
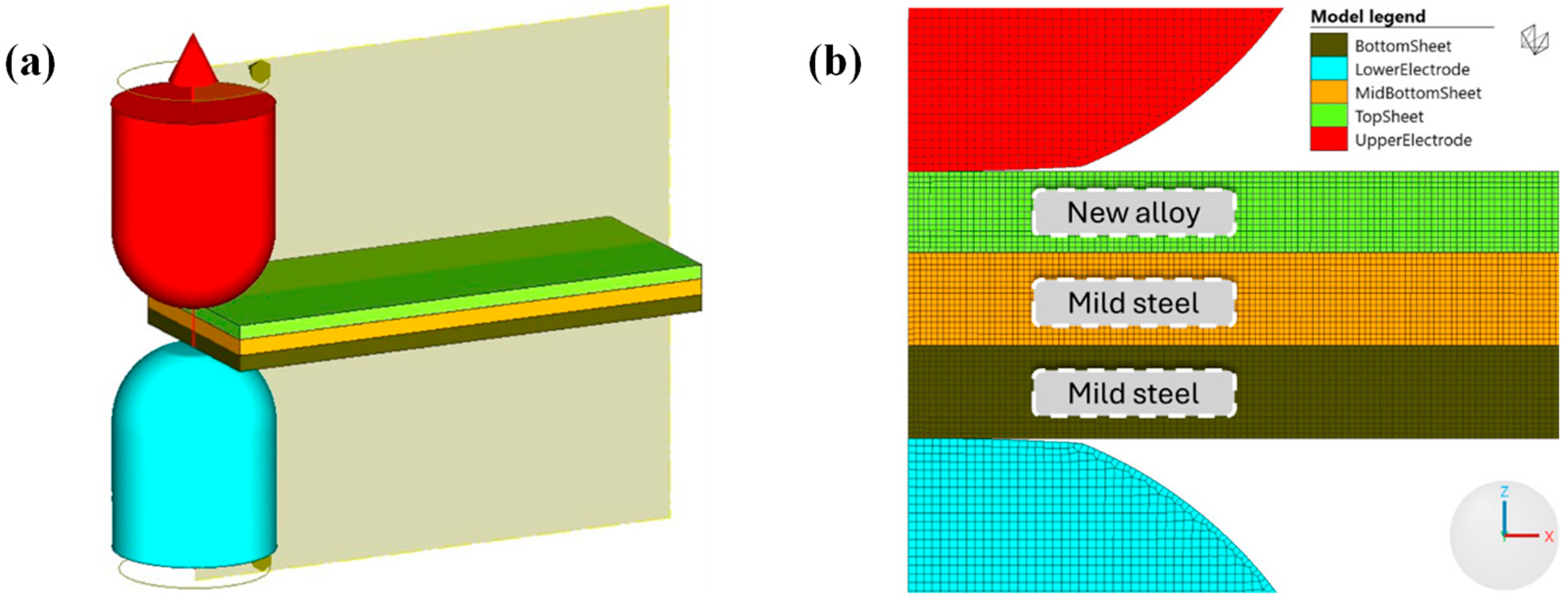
Appendix A.3. Material Properties
Appendix A.4. Contact Interactions
References
- Jung, G.; Woo, I.S.; Suh, D.W.; Kim, S.J. Liquid Zn assisted embrittlement of advanced high strength steels with different microstructures. Met. Mater. Int. 2016, 22, 187–195. [Google Scholar] [CrossRef]
- Oh, M.S.; Kim, S.H.; Kim, J.S.; Lee, J.W.; Shon, J.H.; Jin, Y.S. Surface and cut-edge corrosion behavior of Zn-Mg-Al alloy-coated steel sheets as a function of the alloy coating microstructure. Met. Mater. Int. 2016, 22, 26–33. [Google Scholar] [CrossRef]
- Lee, C.W.; Fan, D.W.; Sohn, I.R.; Lee, S.J.; De Cooman, B.C. Liquid-metal-induced embrittlement of Zn-coated hot stamping steel. Metall. Mater. Trans. A 2012, 43, 5122–5127. [Google Scholar] [CrossRef]
- Soomro, I.A.; Pedapati, S.R.; Awang, M. A review of advances in resistance spot welding of automotive sheet steels: Emerging methods to improve joint mechanical performance. Int. J. Adv. Manuf. Technol. 2022, 118, 1335–1366. [Google Scholar] [CrossRef]
- Choi, D.Y.; Uhm, S.H.; Enloe, C.M.; Lee, H.; Kim, G.; Horvath, C. Liquid metal embrittlement of resistance spot welded 1180TRIP steel-effects of crack geometry on weld mechanical performance. Mater. Sci. Technol 2017, 2017, 454–462. [Google Scholar]
- Siar, O.; Benlatreche, Y.; Dupuy, T.; Dancette, S.; Fabrègue, D. Effect of severe welding conditions on liquid metal embrittlement of a 3rd-generation advanced high-strength steel. Metals 2020, 10, 1166. [Google Scholar] [CrossRef]
- Perka, A.K.; John, M.; Kuruveri, U.B.; Menezes, P.L. Advanced high-strength steels for automotive applications: Arc and laser welding process, properties, and challenges. Metals 2022, 12, 1051. [Google Scholar] [CrossRef]
- Mintz, B.; Qaban, A.; Kang, S.E. The influence of small additions of alloying elements on the hot ductility of AHSS steels: A critical review part 2. Metals 2023, 13, 406. [Google Scholar] [CrossRef]
- Uranga-Zuaznabar, P.; Shang, C.J.; Senuma, T.; Yang, J.R.; Guo, A.M.; Mohrbacher, H. Molybdenum alloying in high-performance flat-rolled steel grades. Adv. Manuf. 2020, 8, 15–34. [Google Scholar] [CrossRef]
- Niu, M.C.; Chen, C.J.; Li, W.; Yang, K.; Luan, J.H.; Wang, W.; Jiao, Z.B. Atomic-scale understanding of solute interaction effects on grain boundary segregation, precipitation, and fracture of ultrahigh-strength maraging steels. Acta Mater. 2023, 253, 118972. [Google Scholar] [CrossRef]
- Výrostková, A.; Kroupa, A.; Janovec, J.; Svoboda, M. Carbide reactions and phase equilibria in low alloy Cr–Mo–V steels tempered at 773–993 K. Part I: Experimental measurements. Acta Mater. 1998, 46, 31–38. [Google Scholar] [CrossRef]
- Yamaguchi, M. First-principles study on the grain boundary embrittlement of metals by solute segregation: Part I. Iron (Fe)-solute (B, C, P, and S) systems. Metall. Mater. Trans. A 2011, 42, 319–329. [Google Scholar] [CrossRef]
- Yu, J.; McMahon, C.J. The effects of composition and carbide precipitation on temper embrittlement of 2.25 Cr-1 Mo steel: Part II. Effects of Mn and Si. Metall. Trans. A 1980, 11, 291–300. [Google Scholar] [CrossRef]
- Yoo, J.; Jo, M.C.; Jo, M.C.; Kim, S.; Kim, S.H.; Oh, J.; Sohn, S.S.; Lee, S. Effects of solid solution and grain-boundary segregation of Mo on hydrogen embrittlement in 32MnB5 hot-stamping steels. Acta Mater. 2021, 207, 116661. [Google Scholar] [CrossRef]
- Lee, J.; Lee, T.; Mun, D.J.; Bae, C.M.; Lee, C.S. Comparative study on the effects of Cr, V, and Mo carbides for hydrogen-embrittlement resistance of tempered martensitic steel. Sci. Rep. 2019, 9, 5219. [Google Scholar] [CrossRef]
- Welding Simulation Simufact Welding—Simufact Software Solutions. Available online: https://www.simufact.com/simufactwelding-welding-simulation.html (accessed on 10 January 2021).
- Kaczynski, C.; Dupuy, T.; Perlade, A. Critical LME elongation: A criterion measured with Gleeble correlated to LME sensitivity during spot welding. Metall. Res. Technol. 2024, 121, 513. [Google Scholar] [CrossRef]
- MathWorks, Inc. Optimization Toolbox Version: 9.4 (R2022b). 2022. Available online: https://www.mathworks.com (accessed on 1 January 2023).
- Razmpoosh, M.H.; Biro, E.; Chen, D.L.; Goodwin, F.; Zhou, Y. Liquid metal embrittlement in laser lap joining of TWIP and medium-manganese TRIP steel: The role of stress and grain boundaries. Mater. Charact. 2018, 145, 627–633. [Google Scholar] [CrossRef]
- Razmpoosh, M.H.; Langelier, B.; Marzbanrad, E.; Zurob, H.S.; Zhou, N.; Biro, E. Atomic-scale investigation of liquid-metal-embrittlement crack-path: Revealing mechanism and role of grain boundary chemistry. Acta Mater. 2021, 204, 116519. [Google Scholar] [CrossRef]
- Nathans, M.W. A Survey of Metal Solubilities in Liquid Zinc (No. ANL-5753); Argonne National Lab.: Lemont, IL, USA, 1957. [Google Scholar]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Jo, M.C.; Yoo, J.; Kim, S.; Kim, S.; Oh, J.; Bian, J.; Sohn, S.S.; Lee, S. Effects of Nb and Mo alloying on resistance to hydrogen embrittlement in 1.9 GPa-grade hot-stamping steels. Mater. Sci. Eng. A 2020, 789, 139656. [Google Scholar] [CrossRef]
- Park, C.; Kang, N.; Liu, S. Effect of grain size on the resistance to hydrogen embrittlement of API 2W Grade 60 steels using in situ slow-strain-rate testing. Corros. Sci. 2017, 128, 33–41. [Google Scholar] [CrossRef]
- Akbari, E.; Kürnsteiner, P.; Steineder, K.; Gruber, M.; Arndt, M.; Letofsky-Papst, I.; Sierlinger, R.; Groiss, H. Insight into grain boundaries with reduced liquid metal embrittlement susceptibility in a boron-added 3rd generation advanced high strength steel. Mater. Des. 2024, 237, 112584. [Google Scholar] [CrossRef]
- Razmpoosh, M.H.; Macwan, A.; Goodwin, F.; Biro, E.; Zhou, Y. Crystallographic study of liquid-metal-embrittlement crack path. Mater. Lett. 2020, 267, 127511. [Google Scholar] [CrossRef]
- Lin, H.; Pope, D.P. The influence of grain boundary geometry on intergranular crack propagation in Ni3Al. Acta Metall. Et Mater. 1993, 41, 553–562. [Google Scholar] [CrossRef]
- Bhattacharya, D.; Cho, L.; Colburn, J.; Smith, D.; Marshall, D.; Van Der Aa, E.; Pichler, A.; Ghassemi-Armaki, H.; Pottore, N.; Findley, K.O.; et al. Influence of selected alloying variations on liquid metal embrittlement susceptibility of quenched and partitioned steels. Mater. Des. 2022, 224, 111356. [Google Scholar] [CrossRef]
- Song, S.H.; Zhao, Y.; Cui, Y.; Sun, J.; Si, H.; Li, J.Q. Effect of grain boundary character distribution and grain boundary phosphorus segregation on the brittleness of an interstitial-free steel. Mater. Lett. 2016, 182, 328–331. [Google Scholar] [CrossRef]
- Li, Y.J.; Ponge, D.; Choi, P.; Raabe, D. Segregation of boron at prior austenite grain boundaries in a quenched martensitic steel studied by atom probe tomography. Scr. Mater. 2015, 96, 13–16. [Google Scholar] [CrossRef]
- Dumbill, S.; Boothby, R.M.; Williams, T.M. Grain boundary segregation in Nimonic PE16. Mater. Sci. Technol. 1991, 7, 385–390. [Google Scholar] [CrossRef]
- Karlsson, L. Non-equilibrium grain boundary segregation of boron in austenitic stainless steel-III. Computer simulations. Acta Met. 1988, 36, 25–34. [Google Scholar] [CrossRef]
- Mei, H.; Cheng, L.; Chen, L.; Yang, S.; Wang, F.; Li, J.; Kong, L. Effect of alloying elements on zinc-induced liquid metal embrittlement in steels: A first-principles study. Comput. Mater. Sci. 2024, 242, 113104. [Google Scholar] [CrossRef]
- Straumal, B.; Baretzky, B. Grain boundary phase transitions and their influence on properties of polycrystals. Interface Sci. 2004, 12, 147–155. [Google Scholar] [CrossRef]
- Murugan, S.P.; Jeon, J.B.; Ji, C.; Park, Y.D. Liquid zinc penetration induced intergranular brittle cracking in resistance spot welding of galvannealed advanced high strength steel. Weld. World 2020, 64, 1957–1969. [Google Scholar] [CrossRef]
- Bhattacharya, D.; Cho, L.; Colburn, J.; van der Aa, E.; Pichler, A.; Pottore, N.; Ghassemi-Armaki, H.; Findley, K.O.; Speer, J.G. Silicon Effect on Retardation of Fe-Zn Alloying Behavior: Towards an Explanation of Liquid Zinc Embrittlement Susceptibility of Third Generation Advanced High Strength Steels. Corros. Sci. 2024, 235, 112161. [Google Scholar] [CrossRef]
- Gupta, L.; Maji, B.C.; Neogy, S.; Singh, R.N.; Krishnan, M. Precipitation behaviour of 20MnMoNi55 RPV steel in the temperature range of 630–670 °C. Mater. Today Commun. 2022, 30, 103096. [Google Scholar] [CrossRef]
- Ishida, K.; Nishizawa, T. Ferrite/austenite stabilizing parameter of alloying elements in steel at 200~500 °C. Trans. Jpn. Inst. Met. 1974, 15, 217–224. [Google Scholar] [CrossRef]
- Rathore, S.S.; Salve, M.M.; Dabhade, V.V. Effect of molybdenum addition on the mechanical properties of sinter-forged Fe–Cu–C alloys. J. Alloys Compd. 2015, 649, 988–995. [Google Scholar] [CrossRef]
- Pavanati, H.C.; Lourenço, J.M.; Maliska, A.M.; Klein, A.N.; Muzart, J.L.R. Ferrite stabilization induced by molybdenum enrichment in the surface of unalloyed iron sintered in an abnormal glow discharge. Appl. Surf. Sci. 2007, 253, 9105–9111. [Google Scholar] [CrossRef]
- Heilmann, S.; Mathiszik, C.; Merx, M.; Müller, J.; Zschetzsche, J.; Ihlenfeldt, S.; Füssel, U. Numerical simulation strategies and test setup for resistance spot welding process with motion overlay. Weld. World 2017, 61, 35–46. [Google Scholar] [CrossRef]
- Prabitz, K.M. Multiphysics finite element simulation of resistance spot welding to evaluate liquid metal embrittlement in advanced high strength steels. In Mathematical Modelling of Weld Phenomena 12; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Prabitz, K.; Pichler, M.; Antretter, T.; Schubert, H.; Hilpert, B.; Gruber, M.; Sierlinger, R.; Ecker, W. Validated multi-physical finite element modelling of the spot welding process of the advanced high strength steel dp1200HD. Materials 2021, 14, 5411. [Google Scholar] [CrossRef]
- Biegler, M.; Böhne, C.; Seitz, G.; Meschut, G.; Rethmeier, M. Investigation of liquid metal embrittlemment avoidance strategies for dual phase steels via electro-thermomechanical finite element simulation. In Proceedings of the 6th International Conference on Steels in Cars and Trucks, Milan, Italy, 19–23 June 2022. [Google Scholar]
- Li, Y.; Lin, Z.; Shen, Q.; Lai, X. Numerical analysis of transport phenomena in resistance spot welding process. J. Manuf. Sci. Eng. 2011, 133, 031019. [Google Scholar] [CrossRef]
- Li, Y.B.; Lin, Z.Q.; Hu, S.J.; Chen, G.L. Numerical analysis of magnetic fluid dynamics behaviors during resistance spot welding. J. Appl. Phys. 2007, 101, 053506. [Google Scholar] [CrossRef]
- MSC Software Corporation. Marc Volume B: Element Library; Simufact Welding; MSC Software Corporation: Newport Beach, CA, USA, 2022; Volume B, p. 949. [Google Scholar]
- Kaars, J.; Mayr, P.; Koppe, K. Generalized dynamic transition resistance in spot welding of aluminized 22MnB5. Mater. Des. 2016, 106, 139–145. [Google Scholar] [CrossRef]


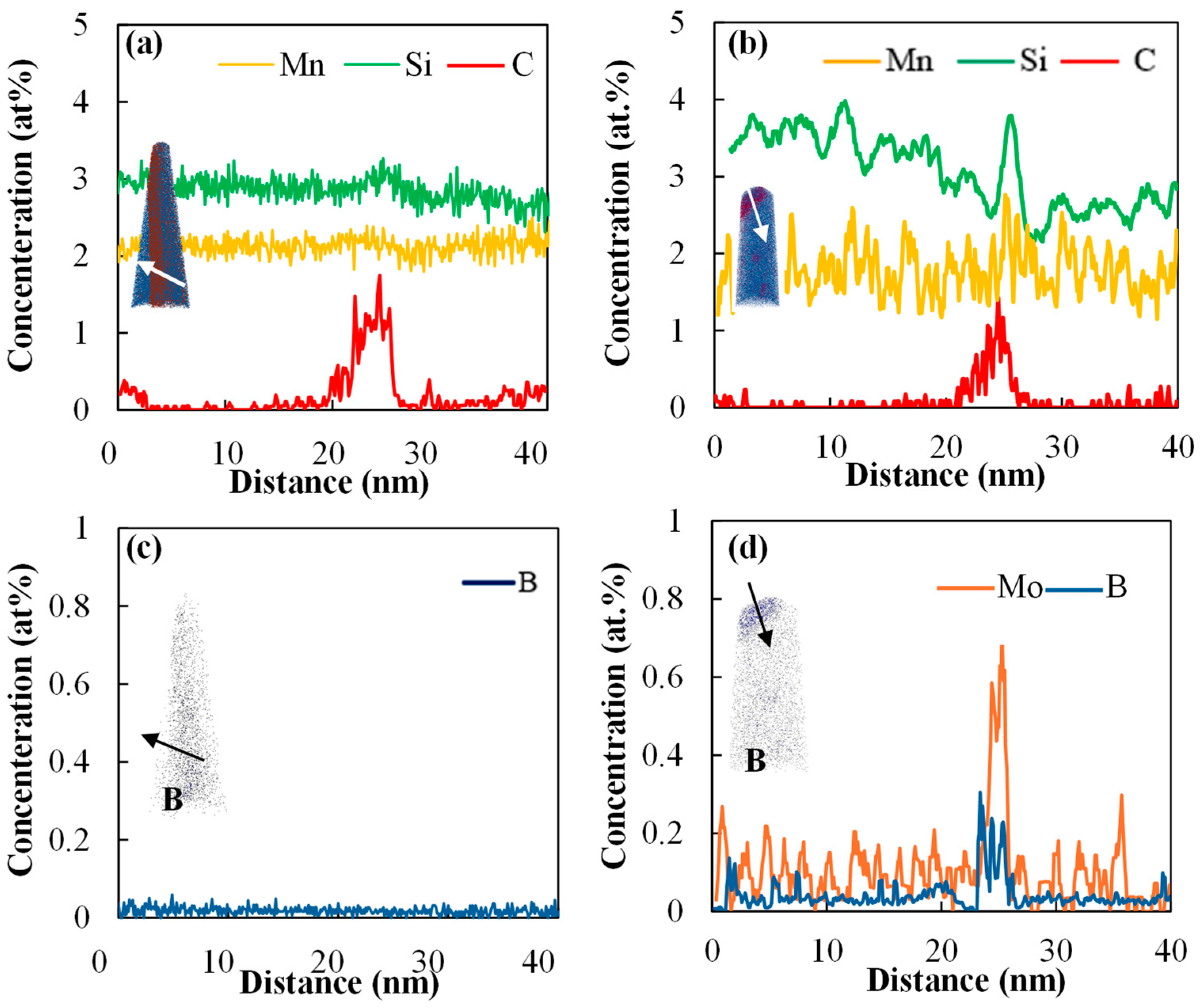
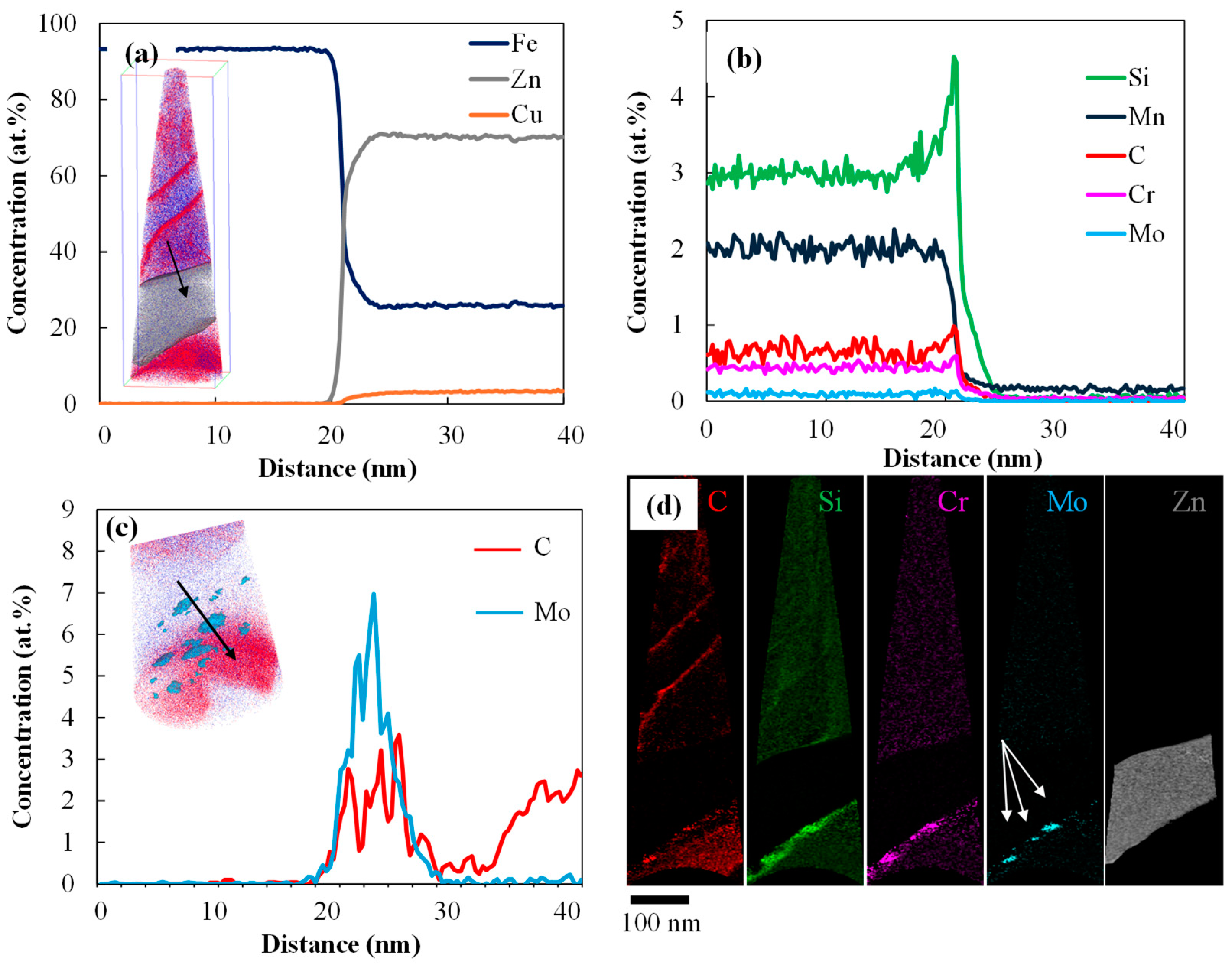

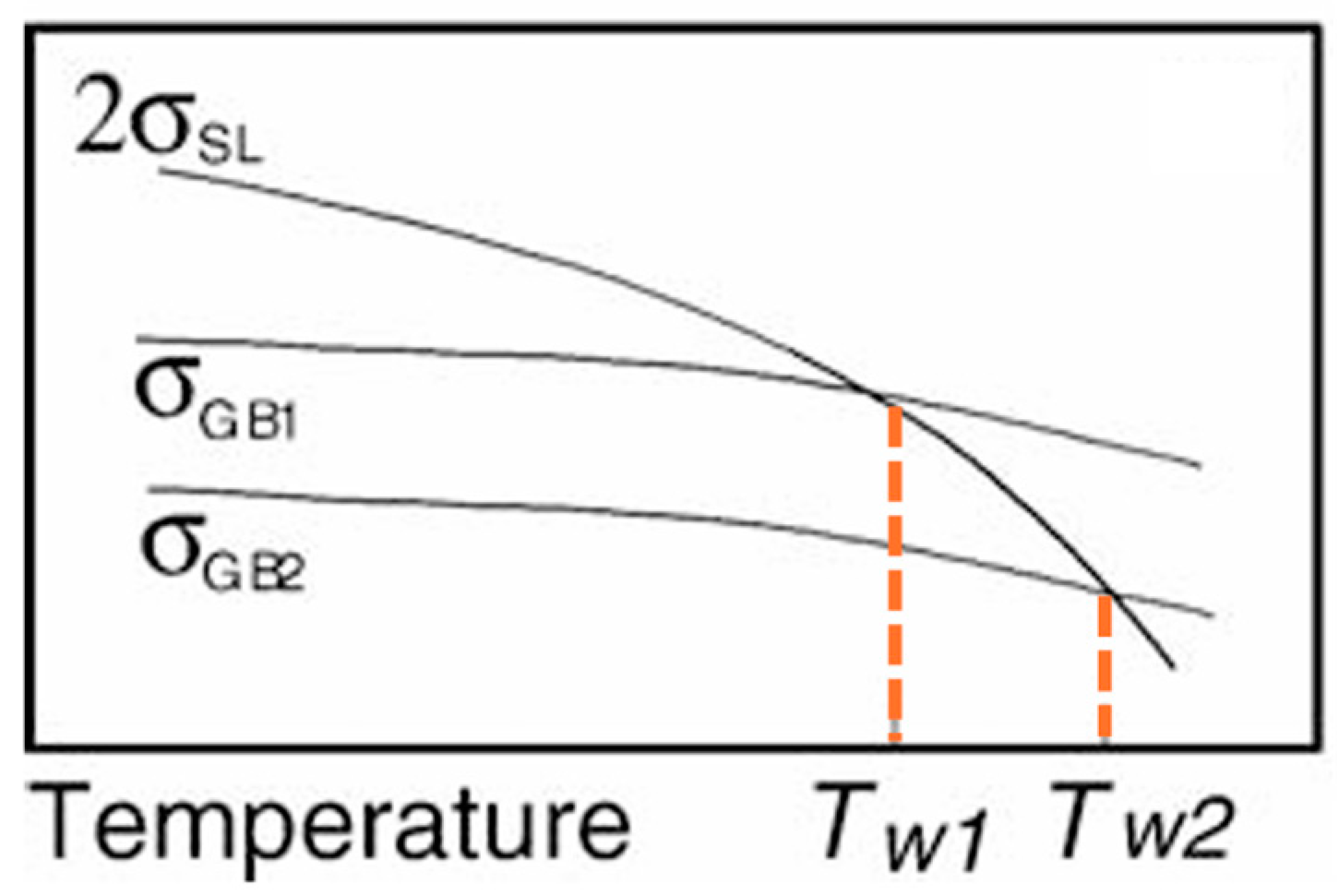
| Steel | C | Si | Mn | Mo | Cr | B |
|---|---|---|---|---|---|---|
| 0 Mo | 0.2 | 1.5 | 2 | 0 | 0.41 | 0.0003 |
| 0.2 Mo | 0.2 | 1.5 | 2 | 0.2 | 0.41 | 0.0003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdiyan, F.; McDermid, J.R.; Okigami, F.; Pourbahari, B.; Macwan, A.; Saenz de Miera, M.; Langelier, B.; Arcuri, G.A.; Zurob, H.S. Effect of Mo Addition on the Susceptibility of Advanced High Strength Steels to Liquid Metal Embrittlement. Materials 2025, 18, 1291. https://doi.org/10.3390/ma18061291
Abdiyan F, McDermid JR, Okigami F, Pourbahari B, Macwan A, Saenz de Miera M, Langelier B, Arcuri GA, Zurob HS. Effect of Mo Addition on the Susceptibility of Advanced High Strength Steels to Liquid Metal Embrittlement. Materials. 2025; 18(6):1291. https://doi.org/10.3390/ma18061291
Chicago/Turabian StyleAbdiyan, Fateme, Joseph R. McDermid, Fernando Okigami, Bita Pourbahari, Andrew Macwan, Mirnaly Saenz de Miera, Brian Langelier, Gabriel A. Arcuri, and Hatem S. Zurob. 2025. "Effect of Mo Addition on the Susceptibility of Advanced High Strength Steels to Liquid Metal Embrittlement" Materials 18, no. 6: 1291. https://doi.org/10.3390/ma18061291
APA StyleAbdiyan, F., McDermid, J. R., Okigami, F., Pourbahari, B., Macwan, A., Saenz de Miera, M., Langelier, B., Arcuri, G. A., & Zurob, H. S. (2025). Effect of Mo Addition on the Susceptibility of Advanced High Strength Steels to Liquid Metal Embrittlement. Materials, 18(6), 1291. https://doi.org/10.3390/ma18061291









