Abstract
Enhancing the limited utilization and overall yield of Pt-based catalysts is essential for advancing proton exchange membrane fuel cell technology. Herein, we report a facile one-pot method that utilizes TEG as both a solvent and a reductant to efficiently synthesize a Pd@Pt core-shell icosahedron. By controlling the surface energy between Pd and Pt precursors, we achieved the formation of Pd@Pt core-shell icosahedra, resulting in a fourfold reduction in reaction time and an eightfold increase in yield. Moreover, the core-shell structures exhibited a significant enhancement in electrocatalytic activity, stability, and Pt utilization efficiency. In comparison to commercial Pt/C, the Pd@Pt core-shell icosahedron exhibited efficient mass activity (MA, 1.54 A mg−1Pt) and specific activity (SA, 2.24 mA cm−2Pt) at 0.9 V (vs. RHE), while demonstrating excellent stability with minimal loss of activity even after 10,000 potential cycles. The Pd@Pt icosahedra configuration integrates the advantages of multiply twinned nanostructures, leading to rich electrochemical active surface sites and fast charge transport, thereby improving its catalytic performance and long-term stability during electrocatalytic reactions.
1. Introduction
Proton exchange membrane fuel cells (PEMFCs) face a significant challenge, primarily due to the requirement to incorporate substantial amounts of Pt-based catalysts into the cathode in order to accelerate the slow kinetics of the oxygen reduction reaction (ORR) [1,2,3,4,5]. However, the limited availability of precious metals such as Pt, along with rising costs and sustainability concerns, presents a substantial barrier to the large-scale commercialization of PEMECs technology. It is a significant challenge to reduce the amount of Pt while maintaining the catalyst’s performance. An effective approach to addressing this challenge involves improving the Pt utilization efficiency through the reduction of Pt-based catalysts’ particle size and morphology, which in turn improves the dispersion of Pt atoms [6,7,8,9,10]. Consequently, commercial ORR catalysts typically consist of Pt particles with dimensions in the range of 2 to 5 nm. Although these small Pt particles are extensively utilized, optimizing their specific activity remains a significant challenge [11,12,13,14].
Recent years have seen the investigation of several alternative strategies aimed at enhancing Pt utilization efficiency, by simultaneously improving the structure of Pt-based catalysts and increasing their specific activity [15,16,17,18,19,20,21,22,23]. One such strategy involves surface structure engineering through facile-controlled synthesis, aimed at optimizing Pt utilization and minimizing the quantity of Pt required. Alloy catalysts have garnered widespread attention from researchers due to their excellent ORR performance, such as PtAg/3DMGS [24], a Pt-Ni octahedron [25], and Pd-Pt Alloy hollow nanostructure [26]. However, alloy-based catalysts may experience alloying effects that reduce the density of active sites or disrupt their electronic structure, resulting in decreased catalytic performance and stability. In contrast, the deposition of Pt atoms as a conformal shell onto nanoparticles composed of less costly and/or more abundant metals represents a key strategy [27,28,29,30,31,32,33]. To achieve this, both chemical and electrochemical methods were developed to form Pt conformal shells, each just a few atomic layers thick on Pd-based nanoparticles [34,35]. The resulting bimetallic nanoparticles exhibit enhanced specific activity toward the ORR. Despite these promising advancements, the conventional strategy for constructing such core-shell structures relies on a hard template approach and epitaxial growth strategy. This synthesis method is not only complex in terms of procedure but also inefficient in yield. Consequently, the production of high-quality nanocrystals as catalysts while maintaining rapid synthesis and enhancing yield remains a considerable challenge [36].
Herein, we have innovatively developed a one-pot method that utilizes TEG as both a solvent and a reductant to rapidly produce a Pd@Pt core-shell icosahedron. Interestingly, controlling the surface energy between Pd and Pt precursors to form a Pd@Pt core-shell icosahedron resulted in a fourfold reduction in reaction time and an eightfold increase in yield, along with a substantially enhanced electrocatalytic performance. The Pd@Pt core-shell icosahedron showed the highest mass activity (MA) of 1.54 A mg−1Pt and a specific activity (SA) of 2.24 mA cm−2Pt at 0.9 V versus the reversible hydrogen electrode (vs. RHE), which is 6.2 and 7.0 times higher than that of commercial Pt/C (0.24 A mg−1Pt and 0.32 mA cm−2Pt, respectively). Impressively, the Pd@Pt exhibited superior electrocatalyst stability, with only a 2.6 mV shift in ORR polarization curves (maintaining 90.3% MA) over 10,000 potential cycles.
2. Materials and Methods
2.1. Materials
Platinum (II) acetylacetonate (Pt(acac)2, Aladdin, Shanghai, China, 97%), perchloric acid (HClO4, Aladdin, Shanghai, China, 70.0~72.0%), potassium hydroxide (KOH, Aladdin, Shanghai, China, ≥85%), Ethanol (C2H6O, Aladdin, Shanghai, China, ≥99.7%), aqueous hydrazine solution (N2H4, Aladdin, Shanghai, China, 35 wt %), methanol (CH4O, Aladdin, Shanghai, China, 99.5%), triethylene glycol (TEG, Aladdin, Shanghai, China, ≥99.0%), acetone (C3H6O, Aladdin, Shanghai, China, ≥99.5%), Polyvinylpyrrolidone (PVP, Aladdin, Shanghai, China, Mw = 58,000), Sodium tetrachloropalladate (II) (Na2PdCl4, Macklin, Shanghai, China, 98%), Nafion (Macklin, Shanghai, China, 5 wt%), Commercial Pt/C catalyst (Sinero technology Co., Ltd., Suzhou, China, 20 wt%). Deionized water (Ulupure, Chengdu, China, 18.2 MΩ cm−1) was utilized in all experiments.
2.2. Methods
Preparation of Pd@Pt Core-Shell Icosahedron
Typically, 50 mg of PVP were dissolved in 7 mL of TEG within a glass vial, and the resulting solution was then heated to 160 °C in an oil bath, while magnetic stirring was continued for 10 min. In a separate glass vial, 36 mg of Na2PdCl4 and 12 mg of Pt(acac)2 were combined with 3 mL of TEG. This mixture was stirred for 30 min to ensure thorough dissolution of the metal precursors. Once the metal precursor solution was prepared, it was rapidly added to the vial containing the PVP-TEG solution. The combined solution was then subjected to a reaction at 160 °C for 90 min, with continuous stirring under the same conditions. After this reaction time, the process was terminated by quenching the mixture in an ice-water bath to halt any further chemical reaction. The product formed during the reaction was isolated through centrifugation, followed by washing once with acetone and twice with ethanol to remove any remaining impurities to obtain the Pd@Pt nanostructures.
2.3. Characterization
X-ray diffraction (XRD) characterization was performed on a D8 X-ray diffractometer (Bruker, Karlsruhe, Germany) with Cu-Kα radiation (λ = 1.54056 Å) operated at 40 kV and 40 mA. Transmission electron microscopy (TEM) was generated utilizing a JEOL JEM-1400 Plus microscope (Beijing, China). High-resolution transmission electron microscopy (HRTEM) were obtained on a Talos F200S-type field emission transmission electron microscope (Hillsboro, OR, USA). The actual Pt loading was quantified using inductively coupled plasma-optical emission spectrometry (ICP-OES) on a prodigy instrument (Hubson, NH, USA).
2.4. Electrochemical Measurements
All electrochemical measurements were conducted using a CS310H electrochemical workstation equipped with a three-electrode system and a rotating disk electrode (RDE) system. A graphite rod served as the counter-electrode, while Ag/AgCl was used as the reference electrodes. The glassy carbon electrode (Ø = 5 mm, S = 0.196 cm−2) functioned as the working electrode. The glassy carbon electrode was first polished by alumina powder with diameters of 1.5, 0.5, and 0.05 µm for 15 min and subsequently rinsed by sonicating in isopropyl and deionized water. Then, 0.98 mg of carbon-loaded catalyst was dispersed in 20 µL of Nafion ionomer (5wt%, Macklin) and 980 µL of ethanol solution (AR, ≥99.7%) to form the catalyst ink, which was then applied onto the surface of the glassy carbon electrode by dispensing 5 µL of the ink with a micropipette after 30 min of sonication in an ice bath. The catalyst ink was dried to form a high-quality catalyst thin film for electrochemical measurements, which were conducted at a rotation rate of 20 rpm for no less than 15 min at room temperature. The electrochemical tests for the Pd@Pt and Pt/C (20 wt%, JM) catalysts were measured under the same experimental conditions. The Pt loading for all the catalysts was maintained at 6.5 µg cm−2, with all loading masses normalized relative to the geometric electrode area of 0.196 cm−2.
The catalysts on the working electrodes were subjected to sonication and subsequently dispersed in ethanol to examine the morphological changes after the durability tests. Cyclic voltammograms (CVs) were recorded by scanning between 0.03 and 1.1 V versus a reversible hydrogen electrode (RHE) at a scan rate of 50 mV s−1 in N2-saturated 0.1 M HClO4 electrolyte solution. Linear scanning voltammetry (LSV) was performed from 0.2 to 1.1 V versus RHE at a sweep rate of 10 mV s−1 in O2-saturated 0.1 M HClO4 electrolyte solution with a rotation rate of 1600 rpm. Electrochemical impedance spectroscopy (EIS) was performed on various samples across the frequency range of 100 kHz to 0.1 Hz, employing an alternating voltage with an amplitude of 10 mV. ORR accelerated durability tests (ADTs) were performed in an aqueous solution of O2-saturated 0.1 M HClO4 for potential cycles at a sweep rate of 50 mV s−1.
The potential recorded by the Ag/AgCl electrode could be converted to the RHE potential using the following equation [37]:
E (vs. RHE) = E (vs. Ag/AgCl) + 0.197 + 0.0592PH
The specific electrochemical active surface area (ECSA) was calculated using the following equation [38,39]:
where QH represents the charge associated with the adsorption of Hupd, m is the Pt loading on the working electrode, and C (210 µC cm−2) is the charge corresponding to the monolayer adsorption of hydrogen on the Pt surface.
ECSA = QH/m × C
3. Results
Figure 1 schematically illustrates the synthetic strategy used to fabricate the Pd@Pt core-shell icosahedron enclosed. Specifically, we demonstrated the configuration of the Pd@Pt core-shell icosahedron by the solvothermal reduction of Na2PdCl4 and Pt(acac)2 in a mixture of TEG and PVP at 160 °C. In this system, TEG served dual roles, acting both as the solvent and the reducing agent, and PVP acted as a stabilizing surfactant and co-reducing agent, ensuring proper control over the particle dispersion uniformity. We speculate that PdCl42− was reduced at a much faster rate than Pt(acac)2 under the same conditions. Due to the lower surface energy of Pd compared to Pt, PdCl42− was preferentially reduced in the presence of TEG (RnOH + M2+ + H2O → Rn-1COOH + M0 +2H+), resulting in the aggregation of larger clusters, which later crystallized into a highly uniform and well-defined Pd icosahedron. Following this, Pt2+ in Pt(acac)2 was reduced to Pt atoms, which were then uniformly deposited onto the surfaces of the Pd icosahedron, leading to the formation of a homogeneous Pt shell. The notable difference in surface energy between Pd and Pt effectively impeded the migration of Pt atoms into the Pd core, thereby ensuring the establishment of a distinct core-shell structure [40,41].
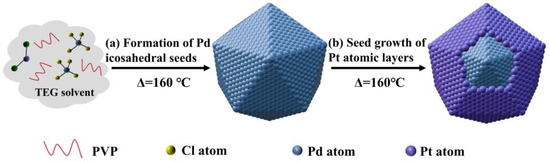
Figure 1.
Schematic illustration of the growth process of the Pd@Pt icosahedron.
To better understand the structural evolution of a one-pot synthesis of a Pd@Pt icosahedron, the morphology and composition of the intermediate Pd@Pt icosahedron was extracted from the solution at various reaction times and analyzed using transmission electron microscopy (TEM) and scanning transmission electron microscopy-energy dispersive X-ray spectroscopy (STEM-EDS) mapping. The TEM images showed different stages of the Pd2+-Pd and Pt2+-Pt reduction between Na2PdCl4 and Pt(acac)2 (Figure 2). The solution immediately turned black when mixing Na2PdCl4 and Pt(acac)2 at 160 °C, implying a fast increase in the generation of nanocrystals. As shown in Figure 2a, nanoparticles formed at t = 30 s. After an additional 1 min, the nanoparticles transformed into an icosahedral shape, which subsequently agglomerated into larger clusters and eventually crystallized into well-defined icosahedral structures under the influence of TEG (Figure 2b). As the reaction time was prolonged to 2 min, the icosahedral shape tended to stay and grow (Figure 2c). In 5 min, the sample size increased to 12 nm, with only a few Pt depositions observed, indicating that Pd2+ primarily participated in the reduction process during this period (Figure 2d and Figure S1). As the reaction progressed, no notable changes in the sample size were observed (Figure 2e). At t = 90 min, the Pt deposition reaction was completed. When the reaction proceeded to 90 min, Pt atoms were uniformly deposited on the surface of Pd, leading to the formation of a high-quality Pd@Pt core-shell icosahedron (Figure 2f–h). It can be concluded that the Pd@Pt icosahedral structures were formed through rapid nucleation of Pd atoms during the reduction and deposition growth of Pt atoms (Figure 1). Compared to previous reports, the yield of the one-pot method increased nearly eight times and the reaction time was reduced almost fourfold [34].
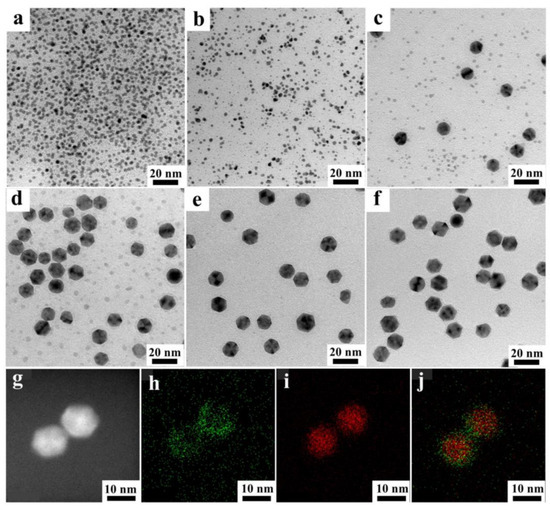
Figure 2.
TEM images of Pd@Pt core-shell icosahedron with different reaction times at 90 °C: (a) 0.5 min, (b) 1 min, (c) 2 min, (d) 5 min, (e) 60 min and (f) 90 min. (g) STEM-EDS images and (h–j) EDS elemental mapping images of Pd@Pt core-shell icosahedron.
From the perspective of reaction kinetics, the effects of temperature and PVP concentration on the Pd@Pt core-shell icosahedral structures were investigated. The experimental results at various temperatures are presented in Figure S2. At a reaction temperature of 100 °C, the product exhibited a small particle size of only 2 nm (Figure S2a), which can be attributed to the insufficient driving force or energy to effectively promote the formation of the icosahedral structure. As the reaction temperature increased to 160 °C, a more uniform icosahedral structure was observed (Figure S2b). As the temperature increased to 220 °C, additional morphologies such as tetrahedra and octahedra emerged at the edges of the icosahedral structure (Figure S2c), maybe resulting from excessively rapid reaction kinetics [42]. Similarly, the PVP concentration significantly influenced the morphology of the icosahedra. At a low PVP concentration (10 mg), insufficient surface stability resulted in the aggregation of the sample (Figure S3a). When the PVP concentration was increased to 50 mg, the dispersibility of the product improved significantly, leading to the formation of regular tetrahedral structures (Figure S3b). However, when the PVP concentration was further increased to 100 mg, small particles appeared around the samples (Figure S3c), likely resulting from the weak reducing properties of PVP’s hydroxyl groups, which promoted the aggregation of undeposited Pt atoms and the formation of Pt particles [43]. Therefore, precise control of the temperature and PVP concentration is crucial for the preparation of a high-purity Pd@Pt core-shell icosahedron.
We further conducted analysis of the samples using scanning electron microscopy (SEM), TEM at various magnifications, and high-resolution transmission electron microscopy (HRTEM). TEM images clearly indicate that the majority of the samples exhibit a well-defined hexagonal morphology, typical of the Pd@Pt core-shell icosahedron. Importantly, the Pd@Pt core-shell structures maintained excellent dispersion throughout the sample, as evidenced by the uniform distribution of particles without noticeable agglomeration (Figure 3a–c and Figure S4). The size of the icosahedral nanoparticles was measured to be 11.5 ± 0.5 nm (Figure S5), which indicates that the synthetic approach employed effectively controlled both the particle size and distribution, contributing to the stability and even dispersion of the nanostructures.
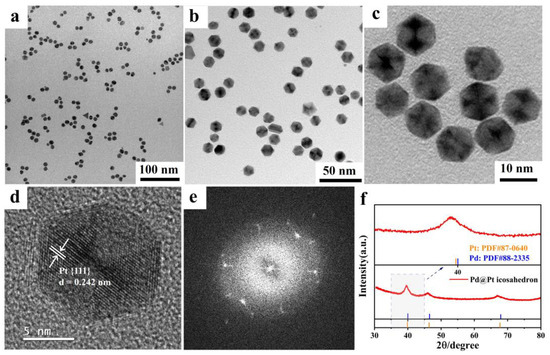
Figure 3.
TEM images of Pd@Pt core-shell icosahedron with different magnifications. (a) 100 nm, (b) 50 nm, (c) 10 nm. HRTEM images of (d) Pd@Pt core-shell icosahedron. (e) FFT pattern of the image shown in (d). (f) XRD patterns of Pd@Pt core-shell icosahedron.
HRTEM micrographs showed the characteristic {111} planes within the tetrahedral crystal domains, and the lattice constant was determined to be 0.242 nm (Figure 3d). The TEM image (Figure S6) clearly shows the fivefold axis of symmetry of the Pd@Pt core-shell icosahedron [44,45,46,47]. The fast Fourier transform (FFT) image provided further evidence supporting the presence of the twin crystal structure (Figure 3e). The phase compositions of the Pd@Pt core-shell icosahedron was further explored by the powder X-ray diffraction (XRD) patterns. As shown in Figure 3f, no distinct XRD peaks for Pt were observed, likely due to their overlap with Pd peaks [48]. Notably, three prominent peaks of the Pd@Pt icosahedron were observed at 66.9°, 46.1°, and 39.6°. Compared to the (220), (200), and (111) planes of Pd face-centered cubic (fcc) (JCPDS 88-2335), these peaks exhibit slight negative shifts, indicating that the Pd@Pt core-shell icosahedron may have undergone tensile strain effects. These observations further underscore the successful formation of the Pd@Pt core-shell icosahedral structure, which possesses distinct crystallographic characteristics that may influence its catalytic properties.
We then deposited the Pd@Pt core-shell icosahedron sample onto carbon to prepare the Pd@Pt core-shell icosahedron electrocatalysis and evaluated its ORR performance using the rotating disk electrode (RDE) method. The electrocatalytic performance of the Pd@Pt core-shell icosahedron was evaluated and compared with that of commercial Pt/C. Figure 4a illustrates the cyclic voltammetry (CV) curves of the samples, which were recorded in a N2-saturated 0.1 M HClO4 solution. The measurements were performed at a scan rate of 50 mV s−1 over a potential range of 0.2–1.1 V versus the RHE. The electrochemical active surface area (ECSA) of the Pd@Pt core-shell icosahedron was calculated to be 65.2 m2g−1Pt, which was considerably smaller than that of the commercial Pt/C, with an ECSA of 74.7 m2g−1Pt. Figure 4b presents the ORR polarization curves of the samples, recorded in an O2-saturated 0.1 M HClO4 aqueous solution using a glass carbon RDE at room temperature. The half-wave potential (E1/2) of the Pd@Pt core-shell icosahedron (E1/2 = 0.908 V) shows a significant positive shift compared with commercial Pt/C (E1/2 = 0.852 V), demonstrating the enhanced ORR catalyst activities of the Pd@Pt core-shell icosahedron. As shown in Figure 4c, the Tafel plots for specific activity display slopes of 55.9, 77.1 mV dec−1 for the Pd@Pt core-shell icosahedron and Pt/C electrocatalysts, respectively. The notably smaller Tafel slope observed for the Pd@Pt core-shell icosahedron indicates a significant enhancement in ORR kinetics [49,50]. The kinetic currents derived from the ORR polarization curves were determined using the Koutecky-Levich equation and subsequently normalized with respect to the ECSA and Pt mass to determine the SA and MA, respectively. At 0.9 V, the SA of the Pd@Pt core-shell icosahedron was 1.54 mA cm−2Pt, approximately 7.3 times higher than that of the Pt/C (0.21 mA cm−2Pt). As a pivotal indicator of the commercialization potential of a Pt-based catalysts, it is worth noting that the MA (1.86 A mg−1Pt) of the Pd@Pt core-shell icosahedron was 6.2 times higher than that of the Pt/C catalyst (0.30 A mg−1Pt) at 0.9 V versus RHE (Figure 4d). Electrochemical impedance spectroscopy (EIS) measurements reveal that the charge transfer resistance of Pd@Pt is higher than that of Pt/C (Figure S7). Consequently, we can infer that Pd@Pt demonstrates superior electron transfer efficiency compared to Pt/C [51]. The ORR activity of the Pd@Pt core-shell icosahedron was compared to the performance of Pd-Pt catalysts reported in recent years (Table S1); this reveals that the Pd@Pt core-−shell catalyst is among the highest-performing Pd-Pt catalysts.
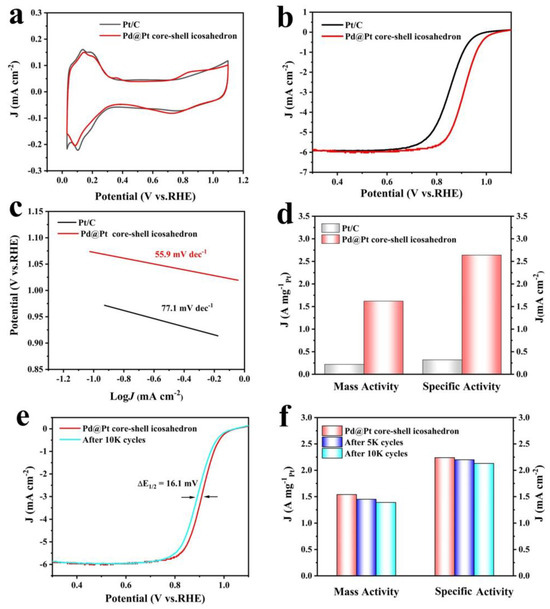
Figure 4.
Electrocatalytic performance of Pd@Pt core-shell icosahedron and commercial Pt/C catalyst for ORR. (a) CV curves. (b) ORR polarization curves. (c) Tafel plots. (d) MA and SA. (e) ORR polarization curve evolutions for the Pd@Pt core-shell icosahedron before and after 10,000 potential cycles. (f) MA and SA for the Pd@Pt core-shell icosahedron before and after 10,000 potential cycles.
The electrocatalytic durability of all the catalysts was evaluated by performing linear potential sweeps between 0.2 and 1.1 V versus RHE at a scan rate of 50 mV/s in O2-saturated 0.1 M HClO4 solutions. As shown in Figure 4e and Figure S8, the evolution of the CV and ORR polarization curves of the Pd@Pt core-shell icosahedron before and after potential cycling demonstrates that, after 10,000 potential cycles, the half-wave potential decreased by 16.1 mV when compared to the fresh sample. The SA and MA of the Pd@Pt core-shell icosahedron decreased by only 9.7% and 4.9% (Figure 4f), respectively. The TEM image revealed that the structural characteristics and sizes of the Pd@Pt core-shell icosahedron exhibited minimal changes after 10,000 cycles (Figure S9). In comparison, the commercial Pt/C exhibited a significantly larger negative shift (~71.2 mV) in the ORR polarization curves, accompanied by a 61.9% decrease in MA, a 46.4% reduction in SA, and pronounced carbon corrosion after 10,000 cycles (Figure S10).
4. Conclusions
In summary, we developed a general and robust approach for the one-pot method that leverages TEG as both a solvent and a reductant to efficiently synthesize a Pd@Pt core-shell icosahedron. By precisely controlling the surface energy between Pd and Pt precursors, we were able to facilitate the formation of these core-shell structures, leading to notable improvements, such as a fourfold reduction in reaction time and an eightfold increase in yield. The Pd@Pt core-shell icosahedra demonstrated significantly enhanced ORR activity and stability compared to state-of-the-art commercial Pt/C. Specifically, the as-prepared Pd@Pt core-shell icosahedra exhibited a MA of 1.54 A mg−1Pt and a SA of 2.24 mA cm−2Pt at 0.9 V versus RHE, which are approximately 6.2 and 7.0 times greater than those of commercial Pt/C, respectively. Impressively, the Pd@Pt core-shell icosahedron exhibits exceptional stability for ORR, with negligible activity decay and structural degradation over 10,000 cycles. This study demonstrates the engineering of core-shell bimetallic nanocrystals, utilizing surface energy differences between metals to enhance performance for ORR and other reactions. In addition, it provides valuable insights for the future development of electrocatalysts, with potential applications in renewable energy devices and beyond.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ma18061279/s1, Figure S1. (a) HAADF-STEM images and (b–d) EDS elemental mapping images of the reaction to Pd@Pt core-shell icosahedron for 5 min; Figure S2. TEM images of Pd@Pt core-shell icosahedron with different reaction temperature. (a) 100 °C, (b) 160 °C, (c) 220 °C; Figure S3. TEM images of Pd@Pt core-shell icosahedron with different PVP content at 180 °C; (a) 10 mg, (b) 50 mg, (c) 100 mg; Figure S4. SEM images of Pd@Pt core-shell icosahedron; Figure S5. The size distribution histogram of Pd@Pt core-shell icosahedron; Figure S6. TEM image of an individual Pd@Pt core-shell icosahedron; Figure S7. EIS curves of Pt/C and Pd@Pt core-shell icosahedron; Figure S8. CV curve evolutions for Pd@Pt core-shell icosahedron before and after 10,000 potential cycles; Figure S9. TEM images of Pd@Pt core-shell icosahedron (a) before and (b) after 10,000 cycles’ durability test. TEM images showed negligible change in overall morphology or size of the Pd@Pt core-shell icosahedron after 10,000 cycles; Figure S10. The durability testing results for the Pt/C. (a) CV evolution before and after 10,000 potential cycles. (b) ORR polarization curve evolution before and after 10,000 potential cycles. (c) Mass activity and specific activity before and after different potential cycles. Table S1. Comparison of the ORR activity in our work with that of other catalysts reported in recent years. References [52,53,54,55,56,57,58,59,60,61,62] have been cited in Supplementary Materials.
Author Contributions
Y.L. conceived the idea and led the material synthesis design. Z.T., C.L., Y.J., M.L. and Q.Z. performed the experiments, conducted sample testing, and gathered and analyzed the data. Z.T., D.Z., X.W. and S.R. participated in data interpretation and performed formal analyses. Z.T. and Y.L. were involved in writing, reviewing, and editing the manuscript. All authors discussed the results and commented on the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the National Natural Science Foundation of China (52072281) and the National Innovation and Entrepreneurship Training Program for College Students (No. S202210497011).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Pan, L.J.; Ott, S.; Dionigi, F.; Strasser, P. Current challenges related to the deployment of shape-controlled Pt alloy oxygen reduction reaction nanocatalysts into low Pt-loaded cathode layers of proton exchange membrane fuel cells. Curr. Opin. Electrochem. 2019, 18, 61–71. [Google Scholar] [CrossRef]
- Riemer, M.; Duval-Dachary, S.; Bachmann, T.M. Environmental implications of reducing the platinum group metal loading in fuel cells and electrolysers: Anion exchange membrane versus proton exchange membrane cells. Sustain. Energy Technol. Assess. 2023, 56, 10. [Google Scholar] [CrossRef]
- Zhao, D.; Chen, Z.; Yang, W.J.; Liu, S.J.; Zhang, X.; Yu, Y.; Cheong, W.C.; Zheng, L.R.; Ren, F.Q.; Ying, G.B.; et al. MXene (Ti3C2) Vacancy-Confined Single-Atom Catalyst for Efficient Functionalization of CO2. J. Am. Chem. Soc. 2019, 141, 4086–4093. [Google Scholar] [CrossRef]
- Fan, L.H.; Deng, H.; Zhang, Y.G.; Du, Q.; Leung, D.Y.C.; Wang, Y.; Jiao, K. Towards ultralow platinum loading proton exchange membrane fuel cells. Energy Environ. Sci. 2023, 16, 1466–1479. [Google Scholar] [CrossRef]
- Xiao, F.; Wang, Q.; Xu, G.L.; Qin, X.P.; Hwang, I.H.; Sun, C.J.; Liu, M.; Hua, W.; Wu, H.W.; Zhu, S.Q.; et al. Atomically dispersed Pt and Fe sites and Pt-Fe nanoparticles for durable proton exchange membrane fuel cells. Nat. Catal. 2022, 5, 503–512. [Google Scholar] [CrossRef]
- Ma, Z.; Cano, Z.P.; Yu, A.P.; Chen, Z.W.; Jiang, G.P.; Fu, X.G.; Yang, L.; Wu, T.N.; Bai, Z.Y.; Lu, J. Enhancing Oxygen Reduction Activity of Pt-based Electrocatalysts: From Theoretical Mechanisms to Practical Methods. Angew. Chem. Int. Edit. 2020, 59, 18334–18348. [Google Scholar] [CrossRef]
- Zhang, H.G.; Hwang, S.; Wang, M.Y.; Feng, Z.X.; Karakalos, S.; Luo, L.L.; Qiao, Z.; Xie, X.H.; Wang, C.M.; Su, D.; et al. Single Atomic Iron Catalysts for Oxygen Reduction in Acidic Media: Particle Size Control and Thermal Activation. J. Am. Chem. Soc. 2017, 139, 14143–14149. [Google Scholar] [CrossRef]
- Han, B.H.; Carlton, C.E.; Kongkanand, A.; Kukreja, R.S.; Theobald, B.R.; Gan, L.; O’Malley, R.; Strasser, P.; Wagner, F.T.; Shao-Horn, Y. Record activity and stability of dealloyed bimetallic catalysts for proton exchange membrane fuel cells. Energy Environ. Sci. 2015, 8, 258–266. [Google Scholar] [CrossRef]
- Jung, J.I.; Risch, M.; Park, S.; Kim, M.G.; Nam, G.; Jeong, H.Y.; Shao-Horn, Y.; Cho, J. Optimizing nanoparticle perovskite for bifunctional oxygen electrocatalysis. Energy Environ. Sci. 2016, 9, 176–183. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, S.; Fang, Z.C.; Ding, J.; Sang, W.; Wang, Y.C.; Zhao, J.; Peng, Z.M.; Zeng, J. Octahedral Pd@Pt1.8Ni Core-Shell Nanocrystals with Ultrathin PtNi Alloy Shells as Active Catalysts for Oxygen Reduction Reaction. J. Am. Chem. Soc. 2015, 137, 2804–2807. [Google Scholar] [CrossRef]
- Yang, Z.X.; Li, Y.; Wang, X.T.; Li, J.M.; Wang, J.Q.; Zhang, G.K. Facet-dependent activation of oxalic acid over hematite nanocrystals under the irradiation of visible light for efficient degradation of pollutants. J. Environ. Sci. 2024, 142, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Meier, J.C.; Galeano, C.; Katsounaros, I.; Witte, J.; Bongard, H.J.; Topalov, A.A.; Baldizzone, C.; Mezzavilla, S.; Schüth, F.; Mayrhofer, K.J.J. Design criteria for stable Pt/C fuel cell catalysts. Beilstein J. Nanotechnol. 2014, 5, 44–67. [Google Scholar] [CrossRef] [PubMed]
- Araya, S.S.; Zhou, F.; Liso, V.; Sahlin, S.L.; Vang, J.R.; Thomas, S.; Gao, X.; Jeppesen, C.; Kaer, S.K. A comprehensive review of PBI-based high temperature PEM fuel cells. Int. J. Hydrog. Energy 2016, 41, 21310–21344. [Google Scholar] [CrossRef]
- Eom, K.; Kim, G.; Cho, E.; Jang, J.H.; Kim, H.J.; Yoo, S.J.; Kim, S.K.; Hong, B.K. Effects of Pt loading in the anode on the durability of a membrane-electrode assembly for polymer electrolyte membrane fuel cells during startup/shutdown cycling. Int. J. Hydrog. Energy 2012, 37, 18455–18462. [Google Scholar] [CrossRef]
- Qiao, Z.; Wang, C.Y.; Li, C.Z.; Zeng, Y.C.; Hwang, S.; Li, B.Y.; Karakalos, S.; Park, J.; Kropf, A.J.; Wegener, E.C.; et al. Atomically dispersed single iron sites for promoting Pt and Pt3Co fuel cell catalysts: Performance and durability improvements. Energy Environ. Sci. 2021, 14, 4948–4960. [Google Scholar] [CrossRef]
- Lao, M.M.; Rui, K.; Zhao, G.Q.; Cui, P.X.; Zheng, X.S.; Dou, S.X.; Sun, W.P. Platinum/Nickel Bicarbonate Heterostructures towards Accelerated Hydrogen Evolution under Alkaline Conditions. Angew. Chem. Int. Edit. 2019, 58, 5432–5437. [Google Scholar] [CrossRef] [PubMed]
- Di, K.; Zhang, V.L.; Lim, H.S.; Ng, S.C.; Kuok, M.H.; Yu, J.W.; Yoon, J.B.; Qiu, X.P.; Yang, H.S. Direct Observation of the Dzyaloshinskii-Moriya Interaction in a Pt/Co/Ni Film. Phys. Rev. Lett. 2015, 114, 5. [Google Scholar] [CrossRef]
- Fang, X.Z.; Shang, Q.C.; Wang, Y.; Jiao, L.; Yao, T.; Li, Y.F.; Zhang, Q.; Luo, Y.; Jiang, H.L. Single Pt Atoms Confined into a Metal-Organic Framework for Efficient Photocatalysis. Adv. Mater. 2018, 30, 7. [Google Scholar] [CrossRef]
- Chen, Y.J.; Ji, S.F.; Sun, W.M.; Chen, W.X.; Dong, J.C.; Wen, J.F.; Zhang, J.; Li, Z.; Zheng, L.R.; Chen, C.; et al. Discovering Partially Charged Single-Atom Pt for Enhanced Anti-Markovnikov Alkene Hydrosilylation. J. Am. Chem. Soc. 2018, 140, 7407–7410. [Google Scholar] [CrossRef]
- Jeong, H.; Kwon, O.; Kim, B.S.; Bae, J.; Shin, S.; Kim, H.E.; Kim, J.; Lee, H.J. Highly durable metal ensemble catalysts with full dispersion for automotive applications beyond single-atom catalysts. Nat. Catal. 2020, 3, 368–375. [Google Scholar] [CrossRef]
- Sun, Q.; Li, X.H.; Wang, K.X.; Ye, T.N.; Chen, J.S. Inorganic non-carbon supported Pt catalysts and synergetic effects for oxygen reduction reaction. Energy Environ. Sci. 2023, 16, 1838–1869. [Google Scholar] [CrossRef]
- Peng, R.S.; Li, S.J.; Sun, X.B.; Ren, Q.M.; Chen, L.M.; Fu, M.L.; Wu, J.L.; Ye, D.Q. Size effect of Pt nanoparticles on the catalytic oxidation of toluene over Pt/CeO2 catalysts. Appl. Catal. B Environ. 2018, 220, 462–470. [Google Scholar] [CrossRef]
- Wang, H.; Liu, J.X.; Allard, L.F.; Lee, S.; Liu, J.L.; Li, H.; Wang, J.Q.; Wang, J.; Oh, S.; Li, W.; et al. Surpassing the single-atom catalytic activity limit through paired Pt-O-Pt ensemble built from isolated Pt1 atoms. Nat. Commun. 2019, 10, 12. [Google Scholar] [CrossRef]
- Li, Z.S.; Li, Y.Y.; He, C.Y.; Shen, P.K. Bimetallic PtAg alloyed nanoparticles and 3-D mesoporous graphene nanosheet hybrid architectures for advanced oxygen reduction reaction electrocatalysts. J. Mater. Chem. A 2017, 5, 23158–23169. [Google Scholar] [CrossRef]
- Xie, M.H.; Shen, M.; Chen, R.H.; Xia, Y.A. Development of Highly-Active Catalysts toward Oxygen Reduction by Controlling the Shape and Composition of Pt-Ni Nanocrystals. ACS Appl. Mater. Interfaces 2023, 15, 49146–49153. [Google Scholar] [CrossRef]
- Hong, J.W.; Kang, S.W.; Choi, B.S.; Kim, D.; Lee, S.B.; Han, S.W. Controlled Synthesis of Pd-Pt Alloy Hollow Nanostructures with Enhanced Catalytic Activities for Oxygen Reduction. ACS Nano 2012, 6, 2410–2419. [Google Scholar] [CrossRef]
- Zhao, X.R.; Sasaki, K. Advanced Pt-Based Core-Shell Electrocatalysts for Fuel Cell Cathodes. Accounts Chem. Res. 2022, 55, 1226–1236. [Google Scholar] [CrossRef]
- Bu, L.Z.; Zhang, N.; Guo, S.J.; Zhang, X.; Li, J.; Yao, J.L.; Wu, T.; Lu, G.; Ma, J.Y.; Su, D.; et al. Biaxially strained PtPb/Pt core/shell nanoplate boosts oxygen reduction catalysis. Science 2016, 354, 1410–1414. [Google Scholar] [CrossRef]
- Wang, X.; Vara, M.; Luo, M.; Huang, H.W.; Ruditskiy, A.; Park, J.; Bao, S.X.; Liu, J.Y.; Howe, J.; Chi, M.F.; et al. Pd@Pt Core-Shell Concave Decahedra: A Class of Catalysts for the Oxygen Reduction Reaction with Enhanced Activity and Durability. J. Am. Chem. Soc. 2015, 137, 15036–15042. [Google Scholar] [CrossRef]
- Tao, L.; Wang, K.; Lv, F.; Mi, H.T.; Lin, F.X.; Luo, H.; Guo, H.Y.; Zhang, Q.H.; Gu, L.; Luo, M.C.; et al. Precise synthetic control of exclusive ligand effect boosts oxygen reduction catalysis. Nat. Commun. 2023, 14, 9. [Google Scholar] [CrossRef]
- Guo, Y.; Hou, B.; Cui, X.Z.; Liu, X.C.; Tong, X.L.; Yang, N.J. Pt Atomic Layers Boosted Hydrogen Evolution Reaction in Nonacidic Media. Adv. Energy Mater. 2022, 12, 11. [Google Scholar] [CrossRef]
- Weber, P.; Weber, D.J.; Dosche, C.; Oezaslan, M. Highly Durable Pt-Based Core-Shell Catalysts with Metallic and Oxidized Co Species for Boosting the Oxygen Reduction Reaction. ACS Catal. 2022, 12, 6394–6408. [Google Scholar] [CrossRef]
- Ji, S.D.; Zhang, C.; Guo, R.Y.; Jiang, Y.J.; He, T.N.; Zhan, Q.; Li, R.; Zheng, Y.Z.; Li, Y.N.; Dai, S.; et al. Effect of Interfacial Interaction on Electrocatalytic Activity and Durability of Pt-Based Core-Shell Nanocatalysts. ACS Catal. 2024, 14, 11721–11732. [Google Scholar] [CrossRef]
- Wang, X.; Choi, S.I.; Roling, L.T.; Luo, M.; Ma, C.; Zhang, L.; Chi, M.F.; Liu, J.Y.; Xie, Z.X.; Herron, J.A.; et al. Palladium-platinum core-shell icosahedra with substantially enhanced activity and durability towards oxygen reduction. Nat. Commun. 2015, 6, 8. [Google Scholar] [CrossRef]
- Choi, S.I.; Shao, M.H.; Lu, N.; Ruditskiy, A.; Peng, H.C.; Park, J.; Guerrero, S.; Wang, J.G.; Kim, M.J.; Xia, Y.N. Synthesis and Characterization of Pd@Pt-Ni Core-Shell Octahedra with High Activity toward Oxygen Reduction. ACS Nano 2014, 8, 10363–10371. [Google Scholar] [CrossRef]
- Luo, L.X.; Fu, C.H.; Wu, A.M.; Zhuang, Z.C.; Zhu, F.J.; Jiang, F.L.; Shen, S.Y.; Cai, X.Y.; Kang, Q.; Zheng, Z.F.; et al. Hydrogen-assisted scalable preparation of ultrathin Pt shells onto surfactant-free and uniform Pd nanoparticles for highly efficient oxygen reduction reaction in practical fuel cells. Nano Res. 2022, 15, 1892–1900. [Google Scholar] [CrossRef]
- Jin, H.; Xu, Z.W.; Hu, Z.Y.; Yin, Z.W.; Wang, Z.; Deng, Z.; Wei, P.; Feng, S.H.; Dong, S.H.; Liu, J.F.; et al. Mesoporous Pt@Pt-skin Pt3Ni core-shell framework nanowire electrocatalyst for efficient oxygen reduction. Nat. Commun. 2023, 14, 10. [Google Scholar] [CrossRef]
- Luo, L.X.; Fu, C.H.; Shen, S.Y.; Zhu, F.J.; Zhang, J.L. Probing structure-designed Cu-Pd nanospheres and their Pt-monolayer-shell derivatives as high-performance electrocatalysts for alkaline and acidic oxygen reduction reactions. J. Mater. Chem. A 2020, 8, 22389–22400. [Google Scholar] [CrossRef]
- Luo, L.X.; Zhu, F.J.; Tian, R.X.; Li, L.; Shen, S.Y.; Yan, X.H.; Zhang, J.L. Composition-Graded PdxNi1-x Nanospheres with Pt Monolayer Shells as High-Performance Electrocatalysts for Oxygen Reduction Reaction. ACS Catal. 2017, 7, 5420–5430. [Google Scholar] [CrossRef]
- Zhou, S.Y.; Figueras-Valls, M.; Shi, Y.F.; Ding, Y.; Mavrikakis, M.; Xia, Y.A. Fast and Non-equilibrium Uptake of Hydrogen by Pd Icosahedral Nanocrystals. Angew. Chem. Int. Edit. 2023, 62, 10. [Google Scholar] [CrossRef]
- Broge, N.L.N.; Sondergaard-Pedersen, F.; Sommer, S.; Iversen, B.B. Formation Mechanism of Epitaxial Palladium-Platinum Core-Shell Nanocatalysts in a One-Step Supercritical Synthesis. Adv. Funct. Mater. 2019, 29, 9. [Google Scholar] [CrossRef]
- Wang, Y.; Peng, H.C.; Liu, J.Y.; Huang, C.Z.; Xia, Y.N. Use of Reduction Rate as a Quantitative Knob for Controlling the Twin Structure and Shape of Palladium Nanocrystals. Nano Lett. 2015, 15, 1445–1450. [Google Scholar] [CrossRef]
- Huang, H.W.; Wang, Y.; Ruditskiy, A.; Peng, H.C.; Zhao, X.; Zhang, L.; Liu, J.Y.; Ye, Z.Z.; Xia, Y.N. Polyol Syntheses of Palladium Decahedra and Icosahedra as Pure Samples by Maneuvering the Reaction Kinetics with Additives. ACS Nano 2014, 8, 7041–7050. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.B.; Qi, L.; You, H.J.; Gross, A.; Li, J.; Yang, H. Icosahedral Platinum Alloy Nanocrystals with Enhanced Electrocatalytic Activities. J. Am. Chem. Soc. 2012, 134, 11880–11883. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.K.; Zhou, S.Y.; Figueras-Valls, M.; Ding, Y.; Lyu, Z.; Mavrikakis, M.; Xia, Y.A. Compressively Strained and Interconnected Platinum Cones with Greatly Enhanced Activity and Durability toward Oxygen Reduction. Adv. Funct. Mater. 2024, 34, 8. [Google Scholar] [CrossRef]
- Li, Z.S.; Li, B.L.; Yu, C.L.; Wang, H.Q.; Li, Q.Y. Recent Progress of Hollow Carbon Nanocages: General Design Fundamentals and Diversified Electrochemical Applications. Adv. Sci. 2023, 10, 53. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Liu, Y.R.; Chen, S.; Zheng, Y.; Fu, X.G.; Zhang, Y.; Wang, W.L. Strain engineering of Pt-based electrocatalysts for oxygen reaction reduction. Front. Energy 2024, 18, 241–262. [Google Scholar] [CrossRef]
- Liu, G.G.; Zhou, W.; Ji, Y.R.; Chen, B.; Fu, G.T.; Yun, Q.B.; Chen, S.M.; Lin, Y.X.; Yin, P.F.; Cui, X.Y.; et al. Hydrogen-Intercalation-Induced Lattice Expansion of Pd@Pt Core-Shell Nanoparticles for Highly Efficient Electrocatalytic Alcohol Oxidation. J. Am. Chem. Soc. 2021, 143, 11262–11270. [Google Scholar] [CrossRef]
- Chen, J.B.; Ying, J.; Xiao, Y.X.; Dong, Y.; Ozoemena, K.I.; Lenaerts, S.; Yang, X.Y. Stoichiometry design in hierarchical CoNiFe phosphide for highly efficient water oxidation. Sci. China Mater. 2022, 65, 2685–2693. [Google Scholar] [CrossRef]
- Chen, J.B.; Ying, J.; Tian, Y.; Xiao, Y.X.; Yang, X.Y. Electrocatalysis under Magnetic Fields. Adv. Funct. Mater. 2025, 35, 19. [Google Scholar] [CrossRef]
- Chen, J.B.; Ying, J.; Xiao, Y.X.; Tian, G.; Dong, Y.; Shen, L.; de Torresi, S.I.C.; Symes, M.D.; Janiak, C.; Yang, X.Y. Directed Mass and Electron Transfer Promoted by Hierarchical Porous Co-P-O Leads to Enhancement of the Overall Water Splitting Efficiency. ACS Catal. 2023, 13, 14802–14812. [Google Scholar] [CrossRef]
- Liu, M.K.; Lyu, Z.H.; Zhang, Y.; Chen, R.H.; Xie, M.H.; Xia, Y.N. Twin-Directed Deposition of Pt on Pd Icosahedral Nanocrystals for Catalysts with Enhanced Activity and Durability toward Oxygen Reduction. Nano Lett. 2021, 21, 2248–2254. [Google Scholar] [CrossRef]
- Wu, R.F.; Tsiakaras, P.; Shen, P.K. Facile synthesis of bimetallic Pt-Pd symmetry-broken concave nanocubes and their enhanced activity toward oxygen reduction reaction. Appl. Catal. B-Environ. 2019, 251, 49–56. [Google Scholar] [CrossRef]
- Lee, S.R.; Park, J.; Gilroy, K.D.; Yang, X.; Figueroa-Cosme, L.; Ding, Y.; Xia, Y.N. Palladium@Platinum Concave Nanocubes with Enhanced Catalytic Activity toward Oxygen Reduction. ChemCatChem. 2016, 8, 3082–3088. [Google Scholar] [CrossRef]
- Li, H.H.; Ma, S.Y.; Fu, Q.Q.; Liu, X.J.; Wu, L.; Yu, S.H. Scalable Bromide-Triggered Synthesis of Pd@Pt Core-Shell Ultrathin Nanowires with Enhanced Electrocatalytic Performance toward Oxygen Reduction Reaction. J. Am. Chem. Soc. 2015, 137, 7862–7868. [Google Scholar] [CrossRef]
- Choi, R.; Choi, S.I.; Choi, C.H.; Nam, K.M.; Woo, S.I.; Park, J.T.; Han, S.W. Designed Synthesis of Well-Defined Pd@Pt Core-Shell Nanoparticles with Controlled Shell Thickness as Efficient Oxygen Reduction Electrocatalysts. Chem.-Eur. J. 2013, 19, 8190–8198. [Google Scholar] [CrossRef]
- Ma, Y.X.; Yin, L.S.; Cao, G.J.; Huang, Q.L.; He, M.S.; Wei, W.X.; Zhao, H.; Zhang, D.G.; Wang, M.Y.; Yang, T. Pt-Pd Bimetal Popcorn Nanocrystals: Enhancing the Catalytic Performance by Combination Effect of Stable Multipetals Nanostructure and Highly Accessible Active Sites. Small 2018, 14, 10. [Google Scholar] [CrossRef]
- Cho, K.Y.; Yeom, Y.S.; Seo, H.Y.; Lee, A.S.; Do, X.H.; Hong, J.P.; Jeong, H.K.; Baek, K.Y.; Yoon, H.G. Fine-sized Pt nanoparticles dispersed on PdPt bimetallic nanocrystals with non-covalently functionalized graphene toward synergistic effects on the oxygen reduction reaction. Electrochim. Acta. 2017, 257, 412–422. [Google Scholar] [CrossRef]
- Wang, X.; Park, J.; Zhang, L.; Xia, Y.N. Atomic layer-by-layer deposition of platinum on palladium octahedra for enhanced catalysts toward the oxygen reduction reaction. Abstr. Pap. Am. Chem. Soc. 2015, 250, 1. [Google Scholar]
- Xiong, X.L.; Chen, W.H.; Wang, W.; Li, J.; Chen, S.L. Pt-Pd nanodendrites as oxygen reduction catalyst in polymer-electrolyte-membrane fuel cell. Int. J. Hydrog. Energy 2017, 42, 25234–25243. [Google Scholar] [CrossRef]
- He, D.S.; He, D.P.; Wang, J.; Lin, Y.; Yin, P.Q.; Hong, X.; Wu, Y.; Li, Y.D. Ultrathin Icosahedral Pt-Enriched Nanocage with Excellent Oxygen Reduction Reaction Activity. J. Am. Chem. Soc. 2016, 138, 1494–1497. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, J.W.; Zhu, G.L.; Han, H.Y. Pd@Pt Core-Shell Nanodots Arrays for Efficient Electrocatalytic Oxygen Reduction. ACS Appl. Nano Mater. 2019, 2, 3695–3700. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).