Effect of Annealing on LiCoO2 Thin Film Deposited by RF Magnetron Sputtering
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Deposition
2.2. Material Characterization
3. Results and Discussion
3.1. Morphological, Structural, and Chemical Aspects of LiCoO2
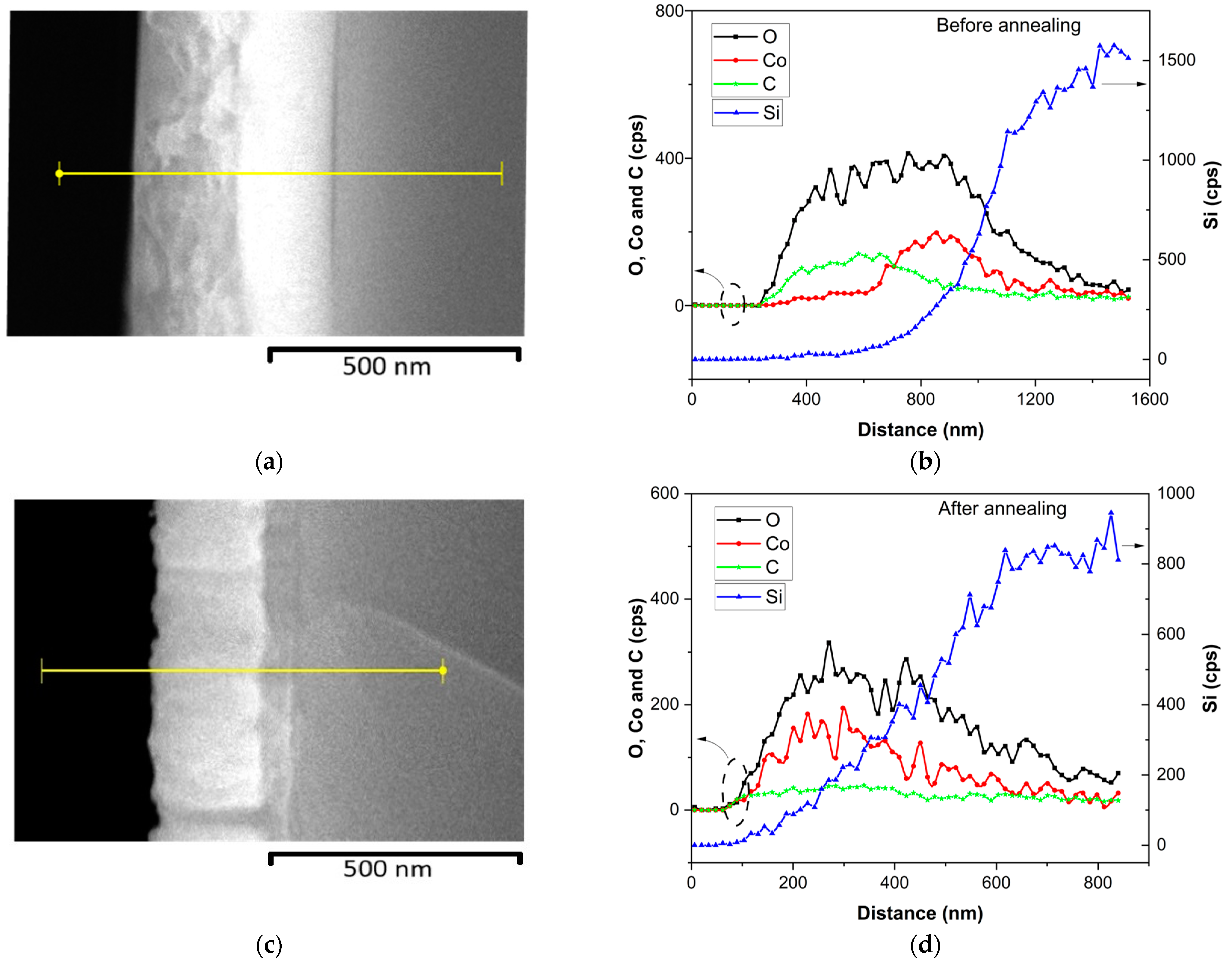
3.1.1. SEM and EDS Analyses
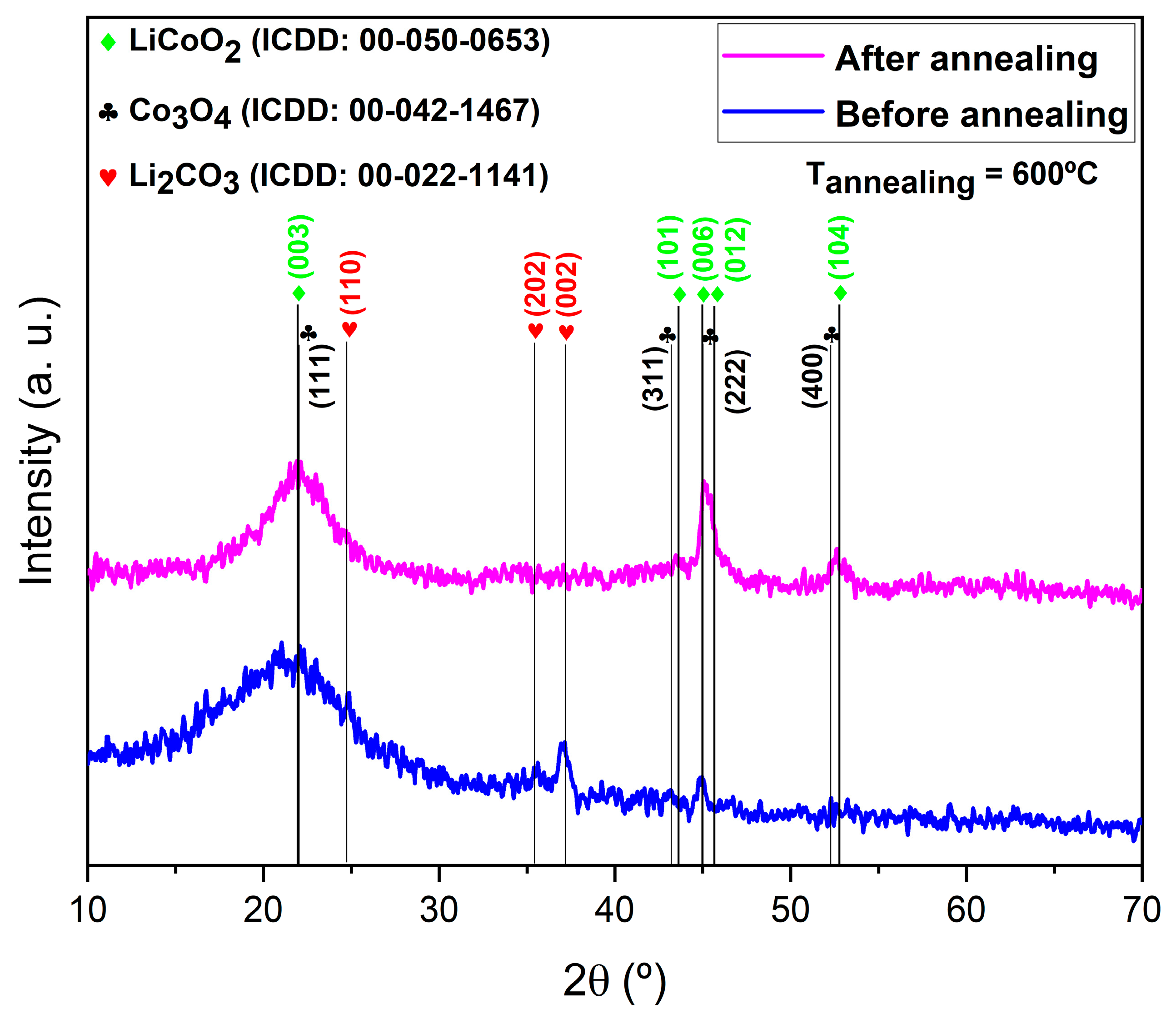
3.1.2. XRD Analysis
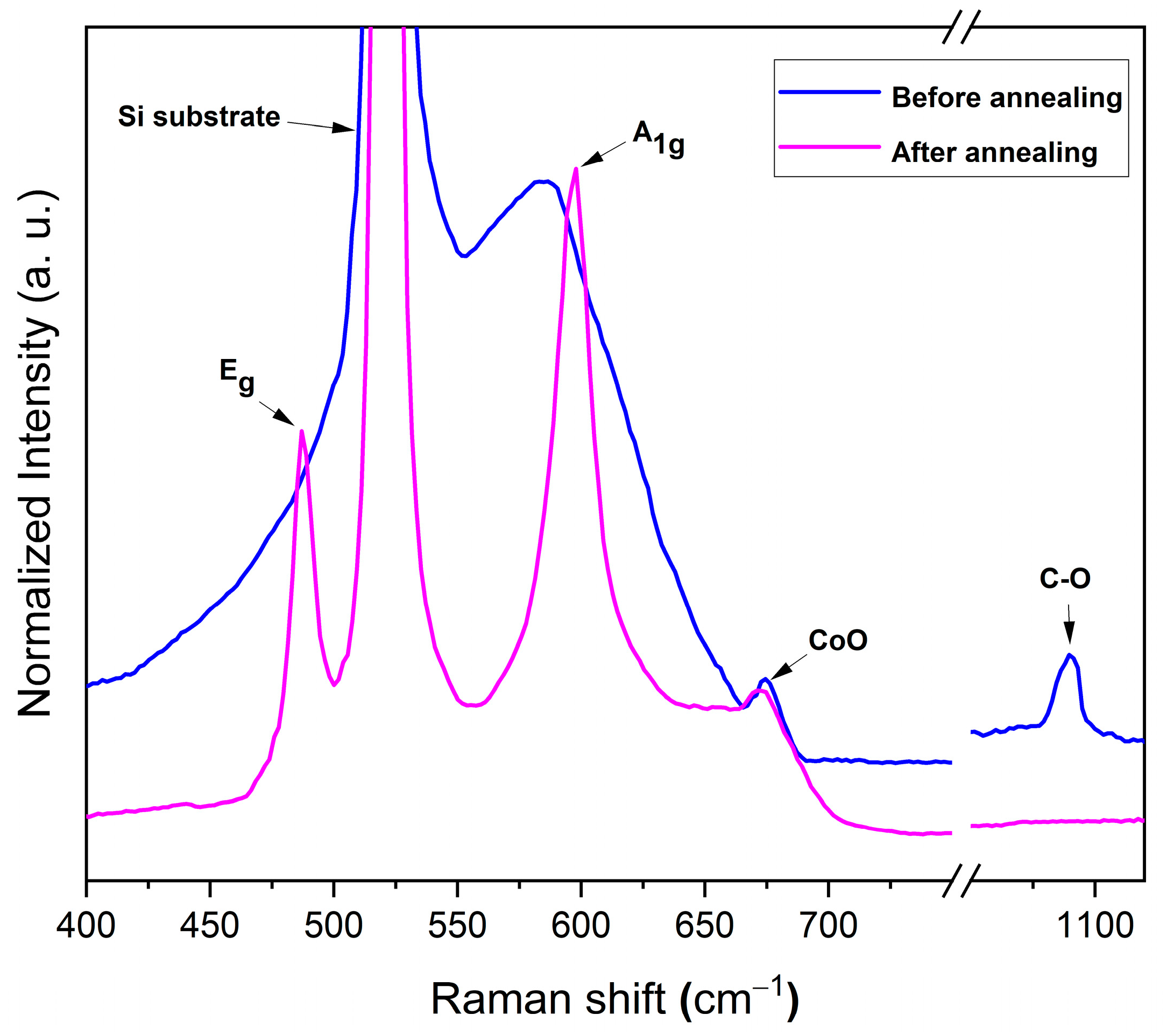
3.1.3. Raman Analysis
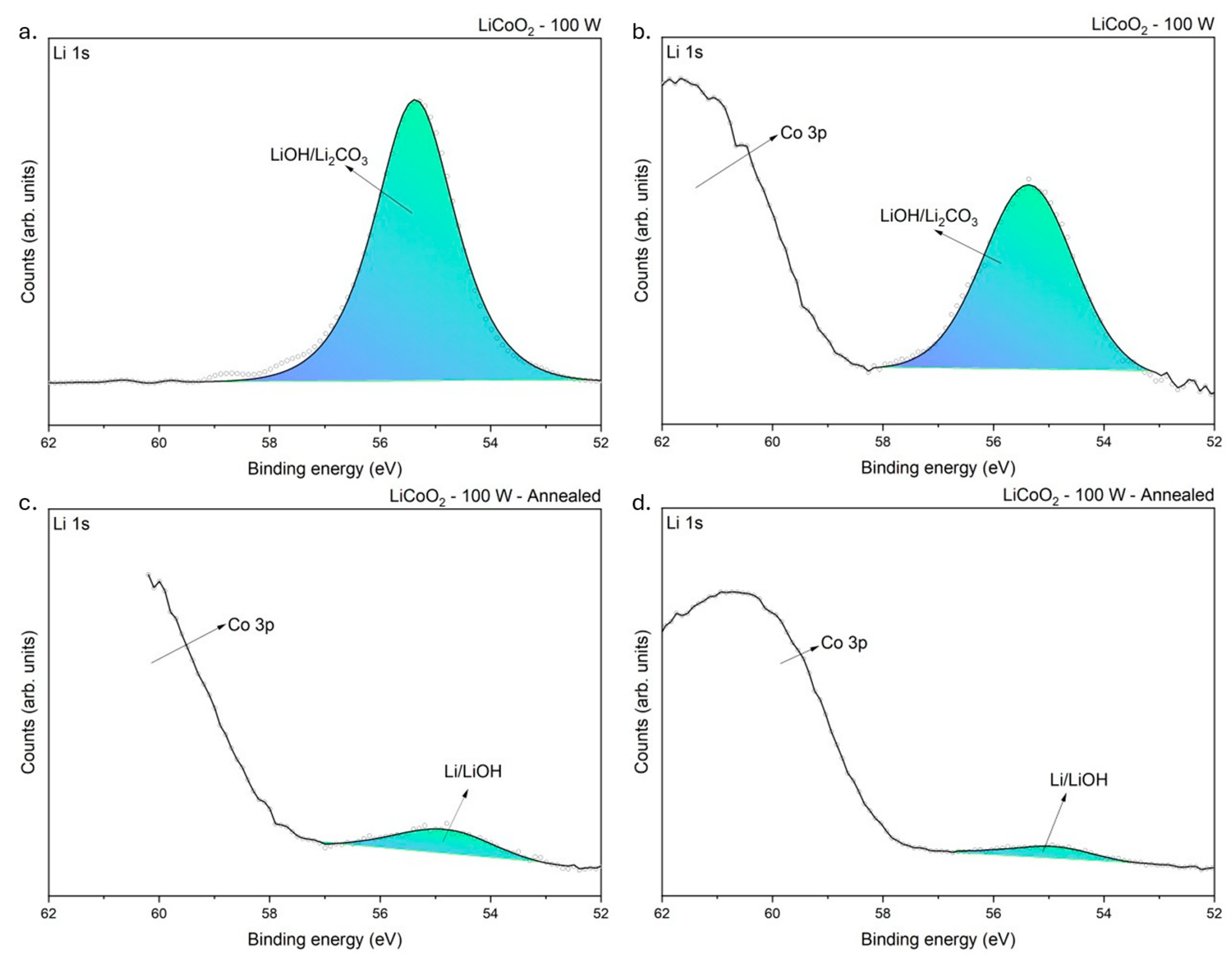
3.1.4. XPS Analysis
3.2. Electrochemical Features of LiCoO2
3.2.1. SEM Analysis
3.2.2. CV and GCD Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khejonrak, A.; Chanlek, N.; Sukkha, U.; Triamnak, N.; Chirawatkul, P.; Kidkhunthod, P.; Suttapun, M.; Vittayakorn, N.; Manyum, P.; Rujirawat, S.; et al. Effect of Thermal Annealing on the Structure of LiCoO2 Powders Prepared by Co-Precipitation Method. Radiat. Phys. Chem. 2021, 189, 109766. [Google Scholar] [CrossRef]
- Khan, F.M.N.U.; Rasul, M.G.; Sayem, A.S.M.; Mandal, N.K. Design and Optimization of Lithium-Ion Battery as an Efficient Energy Storage Device for Electric Vehicles: A Comprehensive Review. J. Energy Storage 2023, 71, 108033. [Google Scholar] [CrossRef]
- Mizushima, K.; Jones, P.C.; Wiseman, P.J.; Goodenough, J.B. LixCoO2 (0<x<-1): A New Cathode Material for Batteries of High Energy Density. Mater. Res. Bull. 1980, 15, 783–789. [Google Scholar] [CrossRef]
- Wang, K.; Wan, J.; Xiang, Y.; Zhu, J.; Leng, Q.; Wang, M.; Xu, L.; Yang, Y. Recent Advances and Historical Developments of High Voltage Lithium Cobalt Oxide Materials for Rechargeable Li-Ion Batteries. J. Power Sources 2020, 460, 228062. [Google Scholar] [CrossRef]
- Bekzhanov, A.; Uzakbaiuly, B.; Mukanova, A.; Bakenov, Z. Annealing Optimization of Lithium Cobalt Oxide Thin Film for Use as a Cathode in Lithium-Ion Microbatteries. Nanomaterials 2022, 12, 2188. [Google Scholar] [CrossRef]
- Pan, H.; Yang, Y. Effects of Radio-Frequency Sputtering Powers on the Microstructures and Electrochemical Properties of LiCoO2 Thin Film Electrodes. J. Power Sources 2009, 189, 633–637. [Google Scholar] [CrossRef]
- Nagendra, A.; Biswas, A.; Kanrar, B.; Shinde, D.; Vishwanadh, B.; Sarkar, S.K.; Bhattacharyya, D. Multilayer and Graded Thin-Film Electrodes: Paving the Way for High-Capacity and Long-Lasting Li-Ion Batteries. ACS Appl. Electron. Mater. 2024, 6, 2213–2224. [Google Scholar] [CrossRef]
- Zheng, X.; Cai, Z.; Sun, J.; He, J.; Rao, W.; Wang, J.; Zhang, Y.; Gao, Q.; Han, B.; Xia, K.; et al. Nickel-Rich Layered Oxide Cathodes for Lithium-Ion Batteries: Failure Mechanisms and Modification Strategies. J. Energy Storage 2023, 58, 106405. [Google Scholar] [CrossRef]
- Julien, C.M.; Mauger, A. Pulsed Laser Deposited Films for Microbatteries. Coatings 2019, 9, 386. [Google Scholar] [CrossRef]
- Yellareswara Rao, K.; Narasimham, S.; Narayan, K.; Mohan Rao, G. Investigations on Sputter Deposited Lithium Nickel Manganese Oxide Thin Film Cathodes for Micro Battery Applications. Mater. Today Proc. 2021, 40, S28–S34. [Google Scholar] [CrossRef]
- Noh, J.; Cho, G.; Jung, K.; Kang, W.; Ha, C.; Ahn, H.; Ahn, J.-H.; Nam, T.; Kim, K. Fabrication of LiCoO2 Thin Film Cathodes by DC Magnetron Sputtering Method. Mater. Res. Bull. 2012, 47, 2823–2826. [Google Scholar] [CrossRef]
- Julien, C.M.; Mauger, A.; Hussain, O.M. Sputtered LiCoO2 Cathode Materials for All-Solid-State Thin-Film Lithium Microbatteries. Materials 2019, 12, 2687. [Google Scholar] [CrossRef] [PubMed]
- Kelly, P.; Arnell, R. Magnetron Sputtering: A Review of Recent Developments and Applications. Vacuum 2000, 56, 159–172. [Google Scholar] [CrossRef]
- Jan, D.-J.; Lee, C.-C.; Yu, Y.-J.; Chiang, H.-W. Evaluation of Lithium Cobalt Oxide Films Deposited by Radio Frequency Magnetron Sputtering as Thin-Film Battery Cathodes. Jpn. J. Appl. Phys. 2019, 58, 085501. [Google Scholar] [CrossRef]
- Liao, C.-L.; Lee, Y.-H.; Fung, K.-Z. The Film Growth and Electrochemical Properties of Rf-Sputtered LiCoO2 Thin Films. J. Alloys Compd. 2007, 436, 303–308. [Google Scholar] [CrossRef]
- Alkan, E.; Singil, M.; Gungor, H.; Guler, A.; Kuruahmet, D.; Guler, O.; Akbulut, H. Investigation of LiCoO2 Thin Film Battery Properties with Changing Ar/O2 Gas Atmosphere. Therm. Sci. 2023, 27, 3071–3080. [Google Scholar] [CrossRef]
- Kim, W.-S. Characteristics of LiCoO2 Thin Film Cathodes According to the Annealing Ambient for the Post-Annealing Process. J. Power Sources 2004, 134, 103–109. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, M.; Yan, Y.; Wei, Y.; Liu, W.; Zhang, X.; Li, J.; Fu, Z.; Li, J.; Zhang, X. Annealing of LiCoO 2 Films on Flexible Stainless Steel for Thin Film Lithium Batteries. J. Mater. Res. 2020, 35, 31–41. [Google Scholar] [CrossRef]
- Jeon, S.-W.; Lim, J.-K.; Lim, S.-H.; Lee, S.-M. As-Deposited LiCoO2 Thin Film Cathodes Prepared by Rf Magnetron Sputtering. Electrochim. Acta 2005, 51, 268–273. [Google Scholar] [CrossRef]
- Peltzer, D.; Múnera, J.; Cornaglia, L. Operando Raman Spectroscopic Studies of Lithium Zirconates during CO 2 Capture at High Temperature. RSC Adv. 2016, 6, 8222–8231. [Google Scholar] [CrossRef]
- Thornton, J.A. Influence of Apparatus Geometry and Deposition Conditions on the Structure and Topography of Thick Sputtered Coatings. J. Vac. Sci. Technol. 1974, 11, 666–670. [Google Scholar] [CrossRef]
- Trask, J.; Anapolsky, A.; Cardozo, B.; Januar, E.; Kumar, K.; Miller, M.; Brown, R.; Bhardwaj, R. Optimization of 10-Μm, Sputtered, LiCoO 2 Cathodes to Enable Higher Energy Density Solid State Batteries. J. Power Sources 2017, 350, 56–64. [Google Scholar] [CrossRef]
- Matsushita, T.; Dokko, K.; Kanamura, K. Comparison of Electrochemical Behavior of LiCoO[Sub 2] Thin Films Prepared by Sol-Gel and Sputtering Processes. J. Electrochem. Soc. 2005, 152, A2229. [Google Scholar] [CrossRef]
- Mendoza, L.; Baddour-Hadjean, R.; Cassir, M.; Pereira-Ramos, J.P. Raman Evidence of the Formation of LT-LiCoO2 Thin Layers on NiO in Molten Carbonate at 650 °C. Appl. Surf. Sci. 2004, 225, 356–361. [Google Scholar] [CrossRef]
- Inaba, M.; Todzuka, Y.; Yoshida, H.; Grincourt, Y.; Tasaka, A.; Tomida, Y.; Ogumi, Z. Raman Spectra of LiCo1−yNiyO2. Chem. Lett. 1995, 24, 889–890. [Google Scholar] [CrossRef]
- Huang, W.; Frech, R. Vibrational Spectroscopic and Electrochemical Studies of the Low and High Temperature Phases of LiCo1−x MxO2 (M = Ni or Ti). Solid State Ion. 1996, 86–88, 395–400. [Google Scholar] [CrossRef]
- Tang, C.-W.; Wang, C.-B.; Chien, S.-H. Characterization of Cobalt Oxides Studied by FT-IR, Raman, TPR and TG-MS. Thermochim. Acta 2008, 473, 68–73. [Google Scholar] [CrossRef]
- Kumar, P.J.; Babu, K.J.; Hussain, O.M. Characteristics of HT-LiCoO2 Cathode Films Synthesized by Rf Magnetron Sputtering. AIP Conf. Proc. 2012, 1447, 779–780. [Google Scholar]
- Moulder, J.F. Handbook of X Ray Photoelectron Spectroscopy; Physical Electronics, Ed.; Chanhassen, MN, USA, 1995; ISBN 978-0964812413. [Google Scholar]
- Ghorbanzade, P.; López-Aranguren, P.; López del Amo, J.M. A Vision for LLZO Carbonate Formation: Perspectives on Surface Treatment Approaches and Characterization Techniques. ChemElectroChem 2024, 11, e202400136. [Google Scholar] [CrossRef]
- Castro, J.D.; Lima, M.J.; Carvalho, S. Wetting and Corrosion Properties of CuxOy Films Deposited by Magnetron Sputtering for Maritime Applications. Appl. Surf. Sci. 2022, 584, 152582. [Google Scholar] [CrossRef]
- Castro, J.D.; Lima, M.J.; Carvalho, I.; Henriques, M.; Carvalho, S. Cu Oxidation Mechanism on Cu-Zr(O)N Coatings: Role on Functional Properties. Appl. Surf. Sci. 2021, 555, 149704. [Google Scholar] [CrossRef]
- Castro, J.D.; Carvalho, I.; Sánchez-López, J.C.; Rojas, T.C.; Escobar-Galindo, R.; Carvalho, S. Unleashing the Antibiofouling Potential of Nano-Structured ZrN-Cu Coating through Electricity. Surf. Coat. Technol. 2024, 494, 131503. [Google Scholar] [CrossRef]
- Haber, J.; Stoch, J.; Ungier, L. X-Ray Photoelectron Spectra of Oxygen in Oxides of Co, Ni, Fe and Zn. J. Electron. Spectros. Relat. Phenom. 1976, 9, 459–467. [Google Scholar] [CrossRef]
- NIST Chemistry Webbook. Available online: https://webbook.nist.gov/chemistry/ (accessed on 10 January 2025).
- Oku, M.; Hirokawa, K. X-Ray Photoelectron Spectroscopy of Co3O4, Fe3O4, Mn3O4, and Related Compounds. J. Electron. Spectros. Relat. Phenom. 1976, 8, 475–481. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving Surface Chemical States in XPS Analysis of First Row Transition Metals, Oxides and Hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- McIntyre, N.S.; Cook, M.G. X-Ray Photoelectron Studies on Some Oxides and Hydroxides of Cobalt, Nickel, and Copper. Anal. Chem. 1975, 47, 2208–2213. [Google Scholar] [CrossRef]
- Yang, Z.; Xing, G.; Yang, J.; Mao, C.; Du, J. Effect of Annealing Temperature on Structure and Electrochemical Properties of LiCoO2 Cathode Thin Films. Rare Metals 2006, 25, 189–192. [Google Scholar] [CrossRef]
- Tan, H.; Takeuchi, S.; Bharathi, K.K.; Takeuchi, I.; Bendersky, L.A. Microscopy Study of Structural Evolution in Epitaxial LiCoO 2 Positive Electrode Films during Electrochemical Cycling. ACS Appl. Mater. Interfaces 2016, 8, 6727–6735. [Google Scholar] [CrossRef]
- Kim, D.H.; Jeong, E.D.; Kim, S.P.; Shim, Y.B. Effect of PH on the Synthesis of LiCoO2 with Malonic Acid and Its Charge/Discharge Behavior for a Lithium Secondary Battery. Bull. Korean Chem. Soc. 2000, 21, 1125–1132. [Google Scholar]
- Julien, C.; Camacho-Lopez, M.A.; Escobar-Alarcon, L.; Haro-Poniatowski, E. Fabrication of LiCoO2 Thin-Film Cathodes for Rechargeable Lithium Microbatteries. Mater. Chem. Phys. 2001, 68, 210–216. [Google Scholar] [CrossRef]
- Abdul Aziz, N.A.; Abdullah, T.K.; Mohamad, A.A. Synthesis of LiCoO2 via Sol-Gel Method for Aqueous Rechargeable Lithium Batteries. Ionics 2018, 24, 403–412. [Google Scholar] [CrossRef]
- Polo da Fonseca, C.N.; Davalos, J.; Kleinke, M.; Fantini, M.C.A.; Gorenstein, A. Studies of LiCoOx Thin Film Cathodes Produced by r.f. Sputtering. J. Power Sources 1999, 81–82, 575–580. [Google Scholar] [CrossRef]










| Phase/Compound | Peak | Binding Energy—Eb (eV) | FWHM (eV) |
|---|---|---|---|
| C−O−H | C 1s | 285.94 ± 0.00 | 2.68 ± 0.00 |
| C−O3 | C 1s | 289.83 ± 0.36 | 2.28 ± 0.40 |
| O | O 1s | 529.67 ± 0.27 | 1.50 ± 0.46 |
| CoO | O 1s | 530.26 ± 0.00 | 1.57 ± 0.00 |
| CO3/OH | O 1s | 531.73 ± 0.10 | 1.99 ± 0.42 |
| Li/LiOH | Li 1s | 54.72 ± 0.02 | 1.88 ± 0.22 |
| LiOH/Li2CO3 | Li 1s | 55.37 ± 0.01 | 1.82 ± 0.12 |
| CoxOy | Co 2p | 780.44 ± 0.20 | 2.84 ± 0.57 |
| Co(OH)2 | Co 2p | 781.88 ± 0.32 | 3.50 ± 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benzarti, Z.; Castro, J.D.; Carneiro, E.; Pacheco, L.; Duarte, N.; Carvalho, S.; Serra, R.; Cavaleiro, A.; Alves, C.; Cruz, S. Effect of Annealing on LiCoO2 Thin Film Deposited by RF Magnetron Sputtering. Materials 2025, 18, 1217. https://doi.org/10.3390/ma18061217
Benzarti Z, Castro JD, Carneiro E, Pacheco L, Duarte N, Carvalho S, Serra R, Cavaleiro A, Alves C, Cruz S. Effect of Annealing on LiCoO2 Thin Film Deposited by RF Magnetron Sputtering. Materials. 2025; 18(6):1217. https://doi.org/10.3390/ma18061217
Chicago/Turabian StyleBenzarti, Zohra, José David Castro, Edgar Carneiro, Lara Pacheco, Nelson Duarte, Sandra Carvalho, Ricardo Serra, Albano Cavaleiro, Cristiana Alves, and Sandra Cruz. 2025. "Effect of Annealing on LiCoO2 Thin Film Deposited by RF Magnetron Sputtering" Materials 18, no. 6: 1217. https://doi.org/10.3390/ma18061217
APA StyleBenzarti, Z., Castro, J. D., Carneiro, E., Pacheco, L., Duarte, N., Carvalho, S., Serra, R., Cavaleiro, A., Alves, C., & Cruz, S. (2025). Effect of Annealing on LiCoO2 Thin Film Deposited by RF Magnetron Sputtering. Materials, 18(6), 1217. https://doi.org/10.3390/ma18061217









