Analysis of Typical Inclusion Evolution and Formation Mechanism in the Smelting Process of W350 Non-Oriented Silicon Steel
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
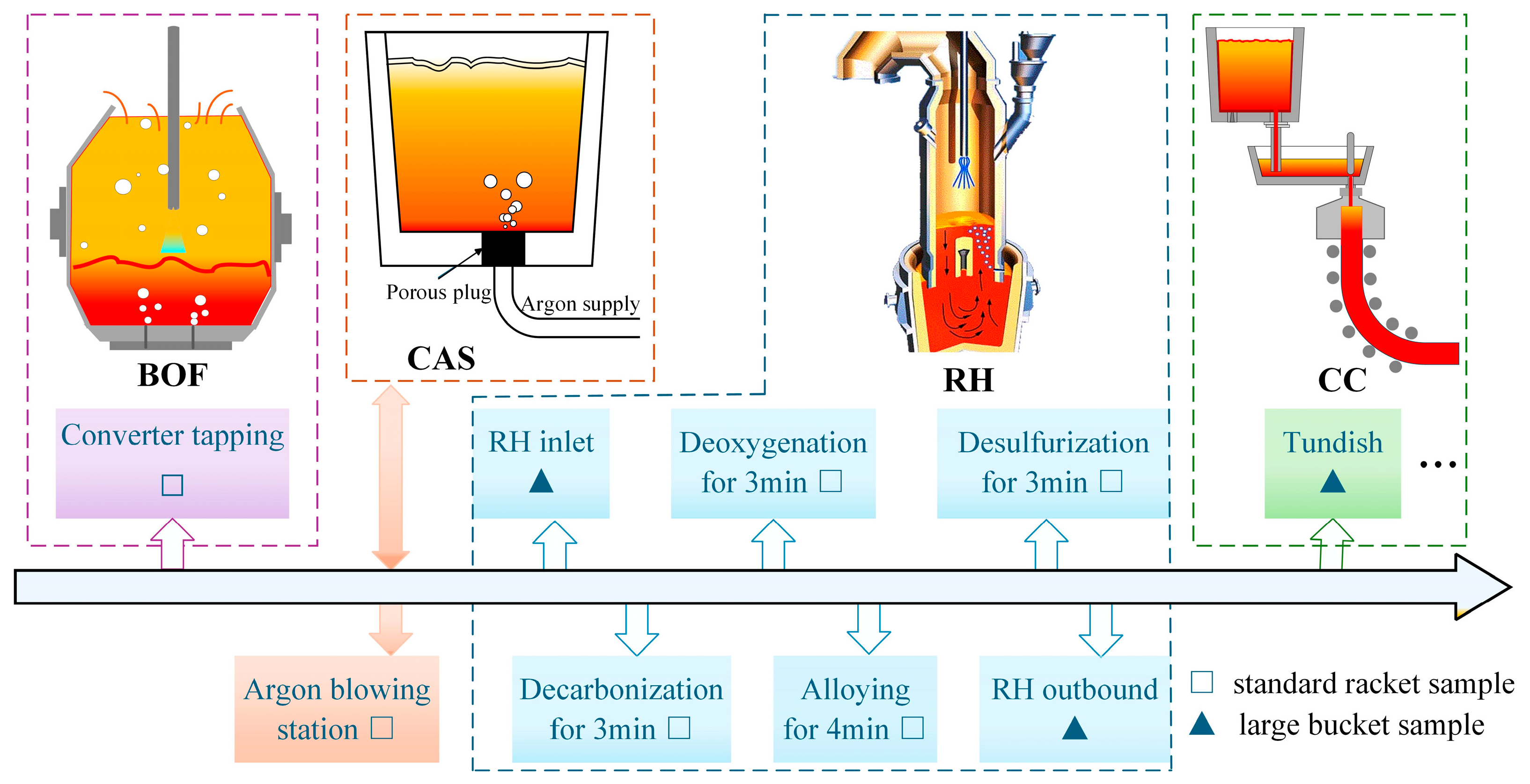
2.2. Sampling and Analysis Method
3. Results
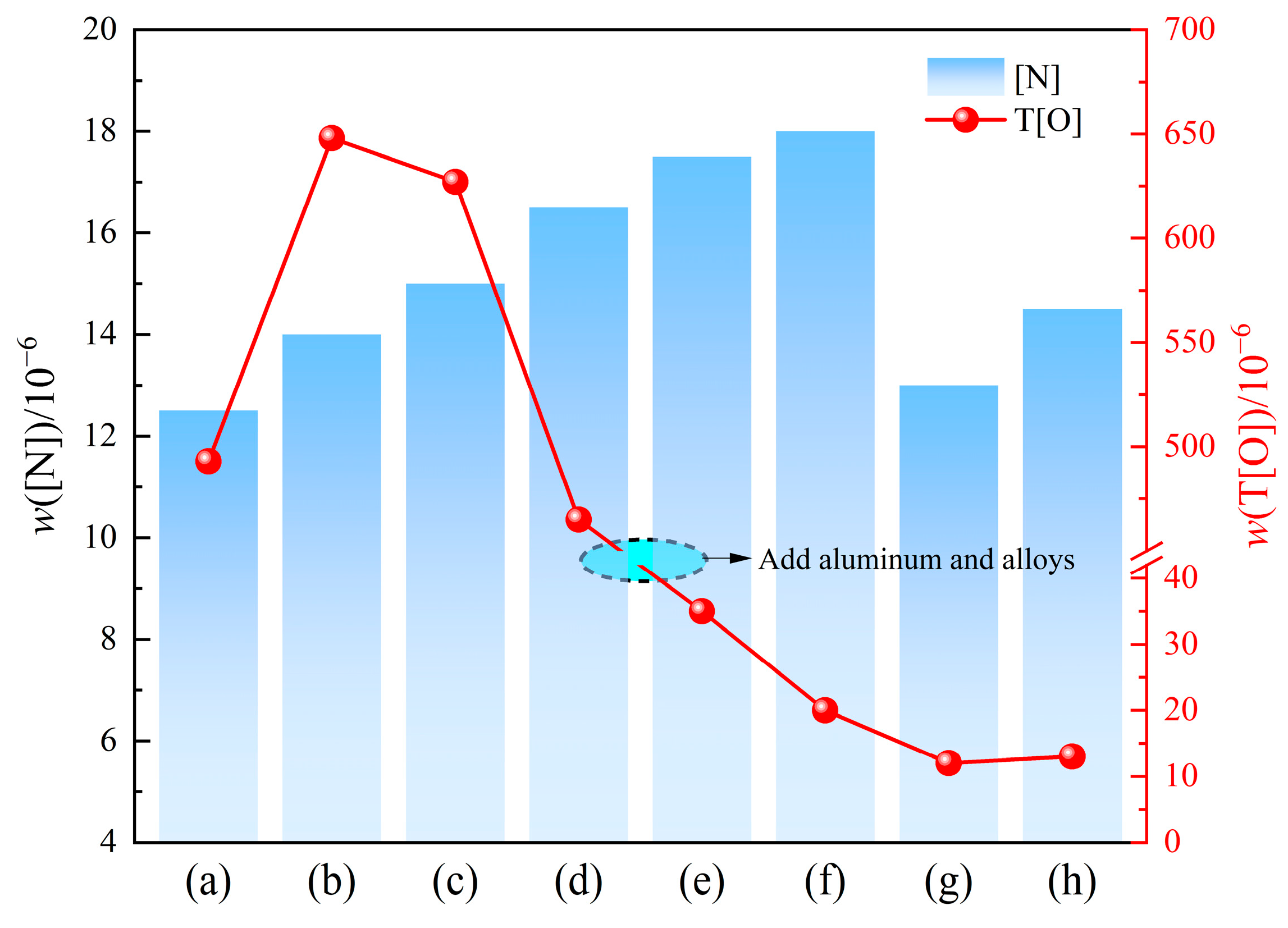
3.1. Changes in w(T[O]) and w([N]) in Molten Steel
3.2. Changes in Typical Inclusion Characteristics
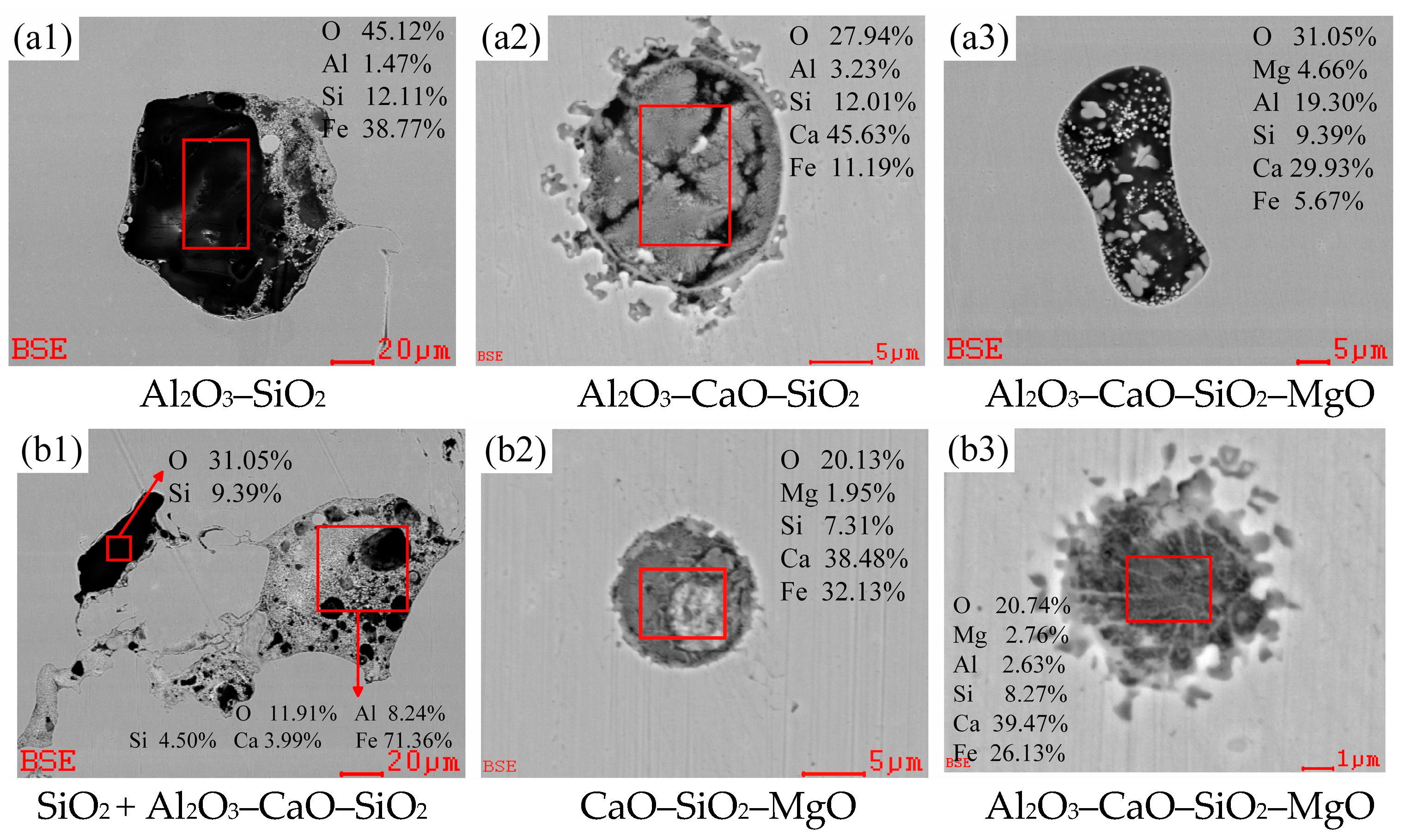
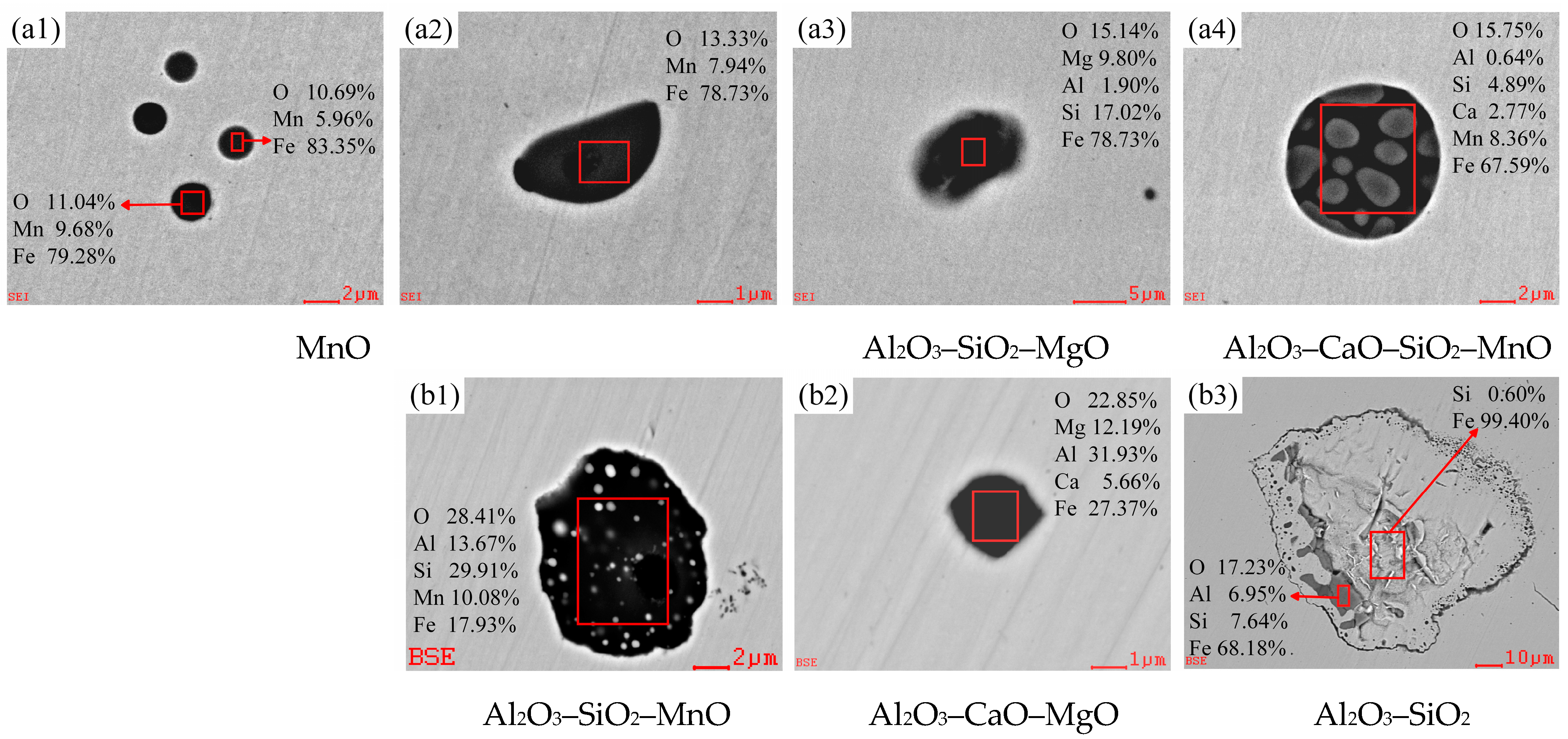
3.2.1. Evolution of Morphology and Chemical Composition of Typical Inclusions
3.2.2. Changes of Composition and Type in Oxide Inclusions
3.2.3. Changes of Size and Quantity in Oxide Inclusions
3.3. Analysis of Formation Mechanism of Typical Inclusions
3.3.1. Thermodynamic Analysis of Al2O3–MgO Formation
3.3.2. Thermodynamic Analysis of the Second-Phase Precipitation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, Z.Z.; Zhao, Y.; Luo, H.W. Electrical Steel; Metallurgical Industry Press: Beijing, China, 2012. [Google Scholar]
- Karami, R.; Butler, D.; Tamimi, S. Manufacturing of non-grain-oriented electrical steels: Review. Int. J. Adv. Manuf. Technol. 2024, 133, 1083–1109. [Google Scholar] [CrossRef]
- Oda, Y.; Okubo, T.; Takata, M. Recent Development of Non-Oriented Electrical Steel in JFE Steel; JFE Technical Report; JFE: Tokyo, Japan, 2016; pp. 7–13. [Google Scholar]
- Chen, Z. Research on the development of electrical steel and 20-high rolling millin China in the decade of the new era. Electr. Steel 2023, 5, 28–32. [Google Scholar]
- Zhu, C.; Liu, Y.; Xiao, Y.; Yan, W.; Li, G. A New Review on Inclusion and Precipitate Control in Grain-Oriented Silicon Steels. JOM 2022, 74. [Google Scholar] [CrossRef]
- Lv, X.J.; Zhang, F.; Wang, B.; Chengyi, Z.; Guangqiang, L. Effects of Inclusions on Magnetic Properties of Non-Oriented Silicon Steel. Spec. Steel 2012, 33, 22–25. [Google Scholar]
- Saxena, A.; Chaudhuri, S.K. Correlating the Aluminum Content with Ferrite Grain Size and Core Loss in Non-oriented Electrical Steel. ISIJ Int. 2007, 44, 1273–1275. [Google Scholar] [CrossRef][Green Version]
- Shimanaka, H.; Ito, Y.; Matsumara, K.; Fukuda, B. Recent development of non-oriented electrical steel sheets. J. Magn. Magn. Mater. 1982, 26, 57–64. [Google Scholar] [CrossRef]
- Peng, Q.C.; Qiu, L.; Zou, J.; Peng, M.Y.; Zhang, L.Z.; Zhou, J.F. Evolution of inclusions in non-oriented silicon steel W800 during production process. Iron Steel 2015, 50, 34–38. [Google Scholar]
- Luo, Y.; Yang, W.; Ren, Q.; Hu, Z.; Li, M.; Zhang, L. Evolution of Non-metallic Inclusions and Precipitates in Oriented Silicon Steel. Metall. Mater. Trans. B 2018, 49, 926–932. [Google Scholar] [CrossRef]
- Yuan, Y.B.; Yang, L.B.; Zhao, J.X.; Wang, C.Y. Evolution of Inclusions in 20CrMnTiH gear steel during BOF-LF-VD-CC. China Metall. 2024, 34, 36–43. [Google Scholar]
- Yadav, N.; Roy, G.G. Evolution of Inclusions and Their Statistics in Low-Carbon Aluminum-Killed (LCAK) Steel Treated with Mischmetal. Metall. Mater. Trans. Part B 2024, 55. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Jiang, M.; Wang, X.H. Formation and evolution of inclusions in Q345Dsteel during secondary refining process. Iron Steel 2022, 57, 63–72. [Google Scholar]
- Hu, Z.Y.; Ren, Q.; Zhang, L.F. Evolution of oxide inclusions in W800 non oriented electrical steel. J. Iron Steel Res. 2018, 30, 282–287. [Google Scholar]
- Cao, J.Q.; Chen, C.; Xue, L.Q.; Li, Z.; Zhao, J.; Lin, W.M. Analysis of inclusions in whole smelting process of non-oriented silicon steel DG47A. Iron Steel 2023, 58, 61–71. [Google Scholar]
- Sun, Y.-H.; Zeng, Y.-N.; Xu, R.; Cai, K.-K. Formation mechanism and control of MgO·Al2O3 inclusions in non-oriented silicon steel. Int. J. Miner. Metall. Mater. 2014, 21, 1068–1076. [Google Scholar] [CrossRef]
- Li, Z.; Liu, C.-J.; Sun, Q.; Jiang, M.-F. Effect of Deoxidation Process on Distribution Characteristics of Inclusions in Silicon Steel Slabs. J. Iron Steel Res. Int. 2015, 22, 104–110. [Google Scholar] [CrossRef]
- Zeng, Y.X. Cleanness control of sulfur bearing gear steel 20CrMnTiH. Iron Steel 2021, 56, 76–81. [Google Scholar]
- Wu, S.J.; Wan, Y.; Chen, W.Q.; Yu, Y. Examination and Study on Cleanliness of Non-Oriented Silicon SteelXG800WR in 210t BOF-RH-CC Steelmaking Process. Spec. Steel 2011, 32, 56–59. [Google Scholar]
- Lu, X.; Zhang, Z.; Lv, M.; Li, X.; Song, B.; Fang, M. Evolution of Non-Metallic Inclusions in 27SiMn Steel. Metals 2022, 12, 718. [Google Scholar] [CrossRef]
- Guo, F.H.; Qiu, S.T.; Qiao, J.L.; Liu, L. Controlling Oxygen in Steelmaking Process of Non-Oriented Electrical Steel. Spec. Steel 2019, 40, 38–42. [Google Scholar]
- Zinngrebe, E.; Šedá, P.; Van Hoek, C.; Van Arendonk, B. Analysis and Significance of Non-metallic Inclusions in Non Grain-oriented Electrical Steel. ISIJ Int. 2013, 53, 1913–1922. [Google Scholar] [CrossRef][Green Version]
- Li, F.; Li, H.; Huang, D.; Zheng, S.; You, J. Mechanism of MnS Precipitation on Al2O3–SiO2 Inclusions in Non-oriented Silicon Steel. Met. Mater. Int. 2018, 24, 1394–1402. [Google Scholar] [CrossRef]
- Zhang, Z.Z.; Lin, Y.; Shang, D.L.; Lei, S.; Dong, Q. Non-Metallic inclusions in RH Refining Process. Iron Steel 2014, 49, 32–35. [Google Scholar]
- Petrovic, D.S.; Arh, B.; Tehovnik, F.; Pirnat, M. Magnesium Non-metallic Inclusions in Non-oriented Electrical Steel Sheets. Trans. Iron Steel Inst. Jpn. 2011, 51, 2069–2075. [Google Scholar] [CrossRef]
- Kawakami, K.; Taniguchi, T.; Nakashima, K. Generation Mechanisms of Non-metallic Inclusions in High-cleanliness Steel. J. Iron Steel Inst. Jpn. 2007, 93, 743. [Google Scholar] [CrossRef]
- Wan, Y.; Chen, W. Effect of boron content on the microstructure and magnetic properties of non-oriented electrical steels. J. Wuhan Univ. Technol. -Mater 2015, 6. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, C.; Huang, L.; Chen, X.; Li, G. Influence of Inclusions on Magnetic Properties of Al-Killed Non-oriented Silicon Steels. JOM 2022, 74, 2645–2655. [Google Scholar] [CrossRef]
- Zhang, B.W.; Li, X.Y.; Yu, H.; Wang, Q.; Xu, H.Q.; Shi, Z.P.; Feng, J. The Rmodynamic Calculation and Analysis of Solidified Phase Transformation and Precipitation Phase of Non-oriented Silicon Steel. Hebei Metall. 2023, 20–27. [Google Scholar]
- Li, X.; Wang, M.; Bao, Y.; Gong, J.; Wang, X.; Pang, W. Precipitation Behavior of AlN in High-Magnetic-Induction Grain-Oriented Silicon Steel Slab. JOM 2019, 71, 3135–3141. [Google Scholar] [CrossRef]
- Deng, Z.; Zhu, M. Evolution Mechanism of Non-metallic Inclusions in Al-Killed Alloyed Steel during Secondary Refining Process. ISIJ Int. 2013, 53, 450–458. [Google Scholar] [CrossRef]
- Mitsutaka, H.; Kimihisa, I. Thermodynamic Data for Steelmaking; Tohoku University Press: Sendai, Japan, 2010. [Google Scholar]
- Fujii, K.; Nagasaka, T.; Hino, M. Activities of the Constituents in Spinel Solid Solution and Free Energies of Formation of MgO, MgO·Al2O3. Trans. Iron Steel Inst. Jpn. 2000, 40, 1059–1066. [Google Scholar] [CrossRef]
- Chen, Q.; Engström, A.; Höglund, L.; Strandlund, H.; Sundman, B. Thermo-Calc Program Interface and Their Applications-Direct Insertion of Thermodynamic and Kinetic Data into Modelling of Materials Processing, Structure and Property. Mater. Sci. Forum 2005, 475–479, 3145–3148. [Google Scholar] [CrossRef]
- Zang, X.; Liu, C.; Qiu, J.; Wang, Y. Effect of Al content on the agglomeration behavior of inclusions in high Al steel. J. Mater. Res. Technol. 2023, 25, 2251–2260. [Google Scholar] [CrossRef]
- Qiao, J.L.; Guo, F.H.; Fu, B.; Hu, J.W.; Xiang, L.; Qiu, S.T. Precipitation Mechanism of Sulfide in Non-oriented Silicon Steel. Mater. Rep. 2021, 35, 20106–20112. [Google Scholar]
- Paltanea, V.M.; Paltanea, G.; Gavrila, H. Non-oriented silicon iron alloys—state of the art and challenges. Rev. Romaine Sci. Tech.-Ser. Électrotech. Énerg. 2014, 59, 371–380. [Google Scholar]

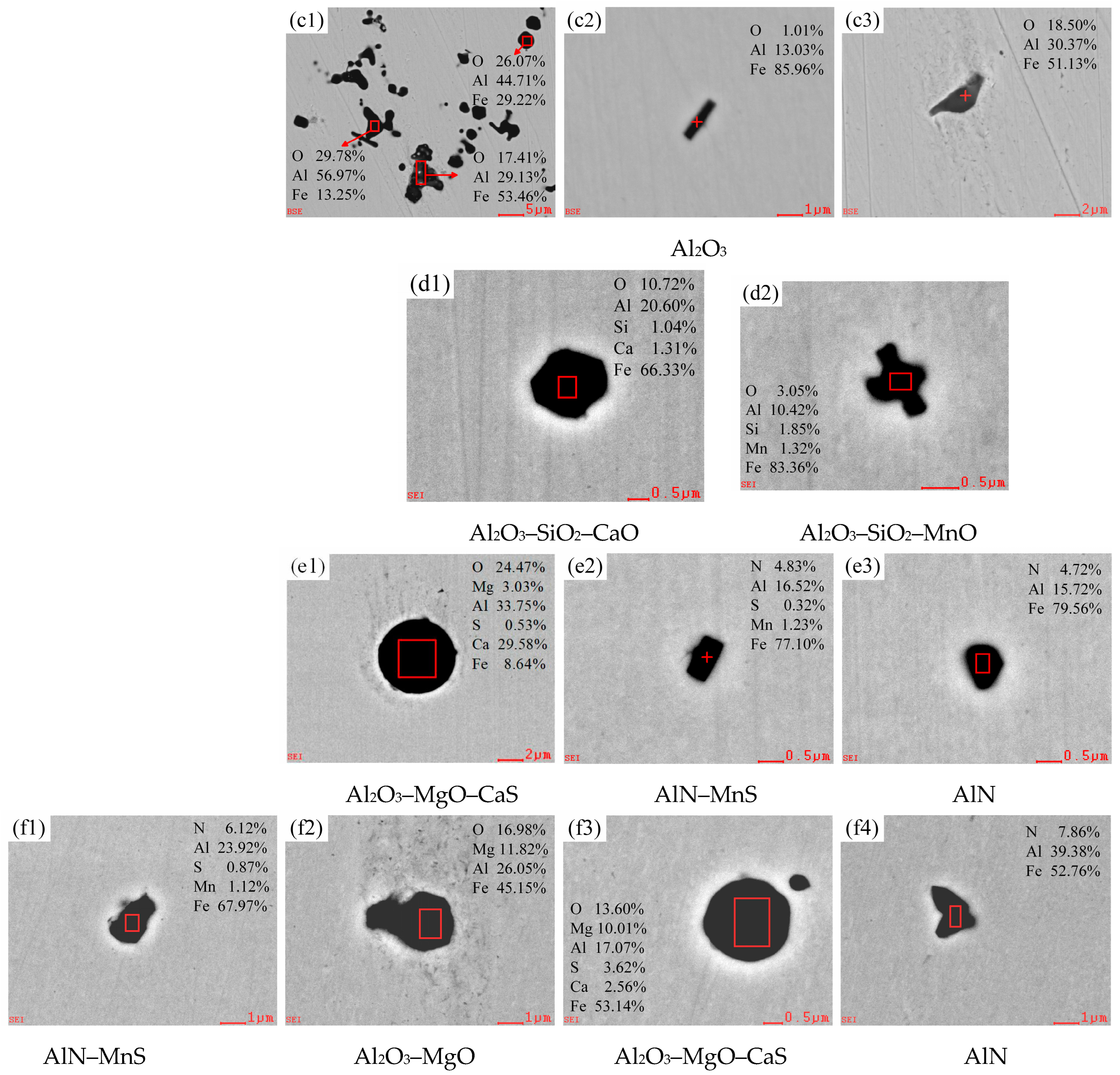
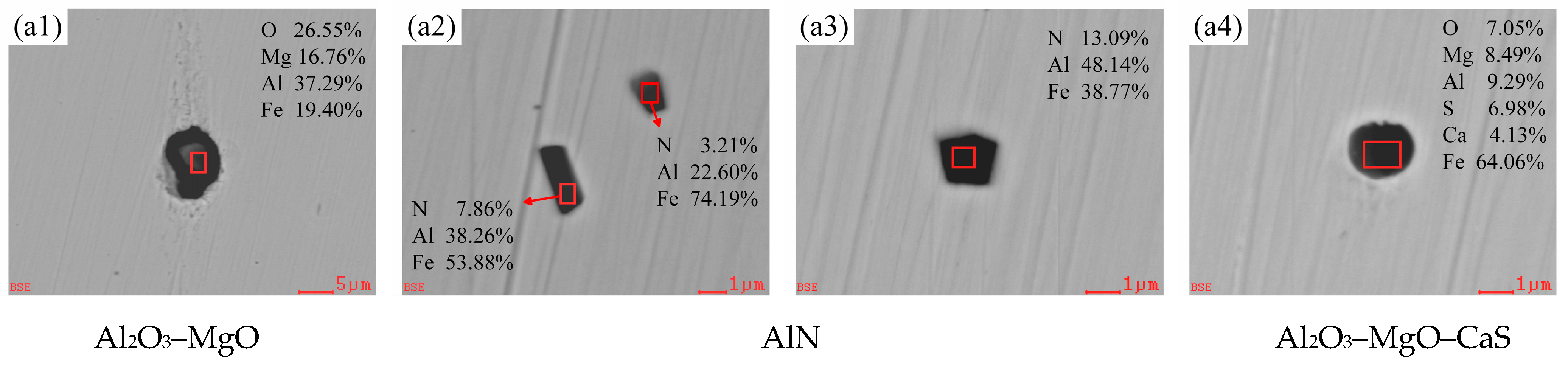
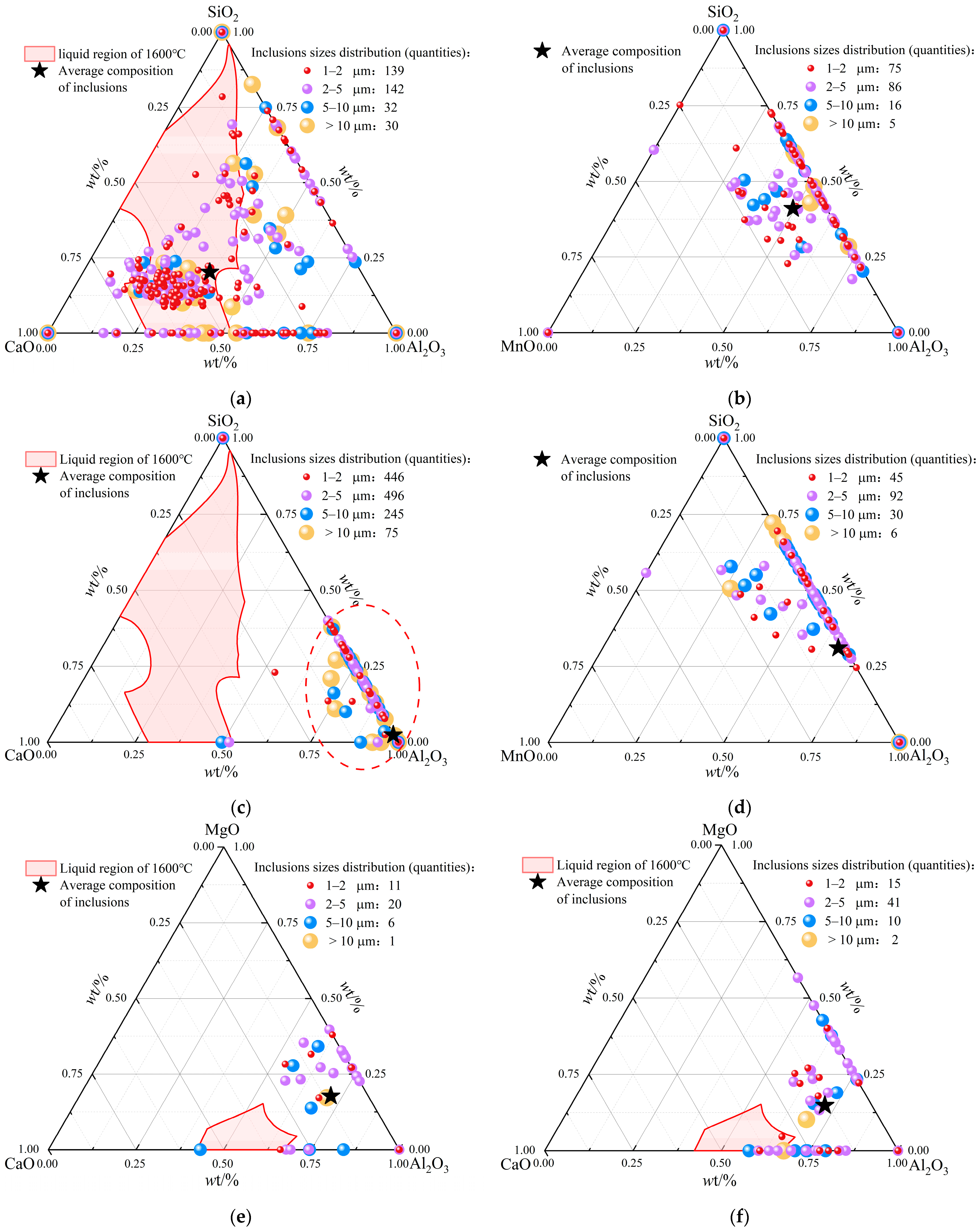


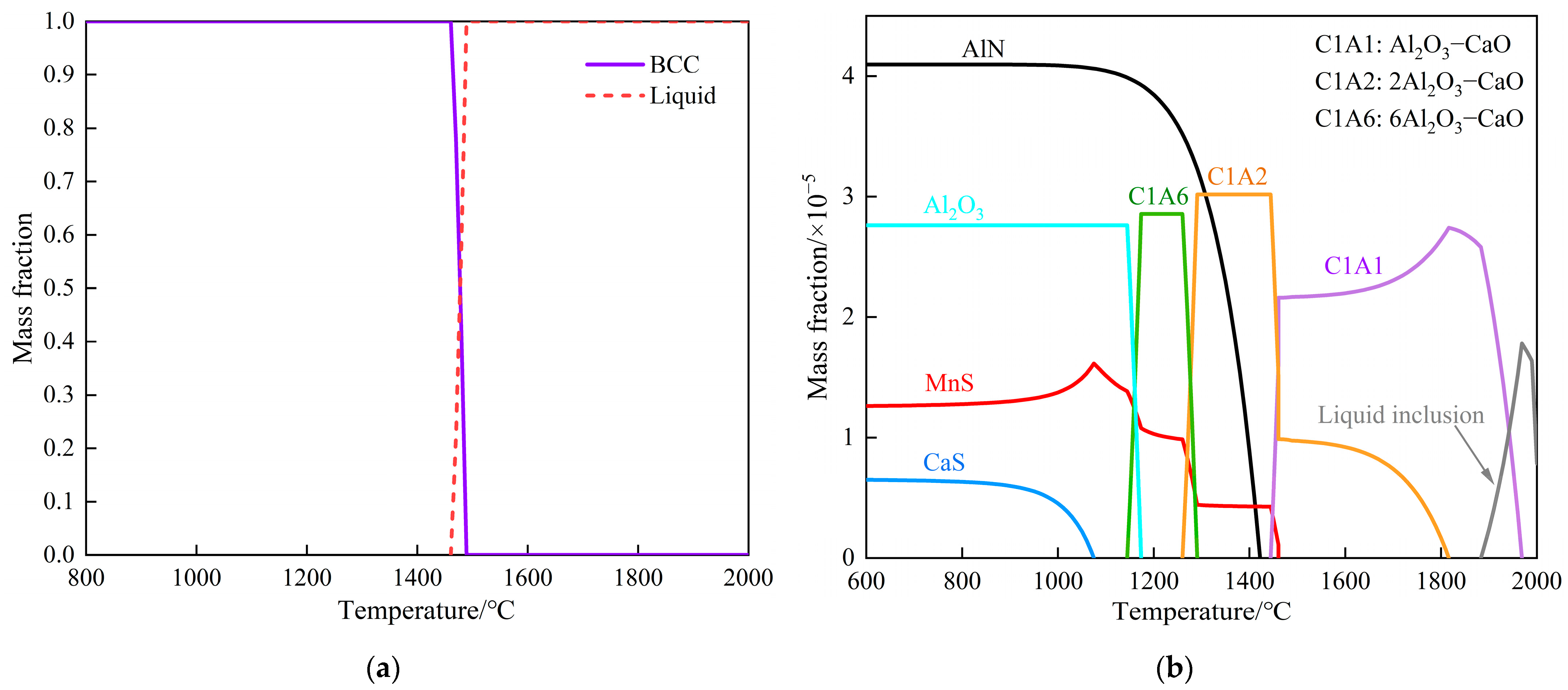
| C | Si | Mn | P | S | Als | Ti | N |
|---|---|---|---|---|---|---|---|
| ≤0.0020 | 3.30~3.40 | 0.45~0.55 | ≤0.020 | ≤0.0010 | 0.60~0.70 | ≤0.0015 | ≤0.0010 |
| Process Nodes | C | Si | Mn | P | S | Als | Ca | N | O |
|---|---|---|---|---|---|---|---|---|---|
| RH outbound | 0.0018 | 3.3138 | 0.4940 | 0.0137 | 0.0010 | 0.6515 | 0.0010 | 0.0012 | 0.0012 |
| Tundish | 0.0019 | 3.3181 | 0.4992 | 0.0138 | 0.0008 | 0.6481 | 0.0007 | 0.0014 | 0.0013 |
| Process Nodes | Main Inclusions | Partial Inclusions |
|---|---|---|
| The end-point of converter blowing | Al2O3–CaO–SiO2, Al2O3–CaO–SiO2–MgO | Al2O3–CaO |
| Argon blowing station | Al2O3–CaO–SiO2 | SiO2, CaO–SiO2–MgO |
| RH inlet | MnO, Al2O3–SiO2–MgO | Al2O3–CaO–SiO2–MnO |
| After RH decarbonization | Al2O3–SiO2–MnO | Al2O3–CaO–MgO |
| After RH deoxygenation | Al2O3 | Al2O3–CaO–SiO2 |
| After RH alloying | Al2O3–SiO2–MnO | Al2O3–CaO–SiO2 |
| After RH desulfurization | Al2O3–MgO–CaS | AlN–MnS, AlN |
| RH outbound | AlN, Al2O3–MgO | Al2O3–MgO–CaS, AlN–MnS |
| Tundish | Al2O3–MgO, AlN | Al2O3–MgO–CaS |
| Reaction Equation | Log K |
|---|---|
| 2[Al] + 3[O] = Al2O3 | −20.57 + 64,000/T |
| [Mg] + [O] = MgO | 4.28 + 4700/T |
| MgO + Al2O3 = MgO•Al2O3 | 0.32 + 980/T |
| C | Als | Si | Mn | P | S | Ca | N | O | |
|---|---|---|---|---|---|---|---|---|---|
| Al | 0.091 | 0.045 | 0.0056 | −0.004 | 0.033 | 0.030 | −0.047 | −0.058 | −6.60 |
| Mg | −0.240 | −0.270 | −0.096 | — | — | −1.380 | — | — | −460 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, J.; Yang, L.; Peng, B.; Wei, G.; Yuan, Y. Analysis of Typical Inclusion Evolution and Formation Mechanism in the Smelting Process of W350 Non-Oriented Silicon Steel. Materials 2025, 18, 1188. https://doi.org/10.3390/ma18061188
Shi J, Yang L, Peng B, Wei G, Yuan Y. Analysis of Typical Inclusion Evolution and Formation Mechanism in the Smelting Process of W350 Non-Oriented Silicon Steel. Materials. 2025; 18(6):1188. https://doi.org/10.3390/ma18061188
Chicago/Turabian StyleShi, Jiagui, Libin Yang, Bowen Peng, Guoqiang Wei, and Yibo Yuan. 2025. "Analysis of Typical Inclusion Evolution and Formation Mechanism in the Smelting Process of W350 Non-Oriented Silicon Steel" Materials 18, no. 6: 1188. https://doi.org/10.3390/ma18061188
APA StyleShi, J., Yang, L., Peng, B., Wei, G., & Yuan, Y. (2025). Analysis of Typical Inclusion Evolution and Formation Mechanism in the Smelting Process of W350 Non-Oriented Silicon Steel. Materials, 18(6), 1188. https://doi.org/10.3390/ma18061188






