Porous Mortars Incorporating Active Biochar from Olive Stone Waste and Recycled Masonry Aggregate: Effects of Accelerated Carbonation Curing
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Mix Design
2.3. Characterisation and Testing Methods
3. Characterisation of Raw Materials
4. Results and Discussion
4.1. Physico-Mechanical Testing
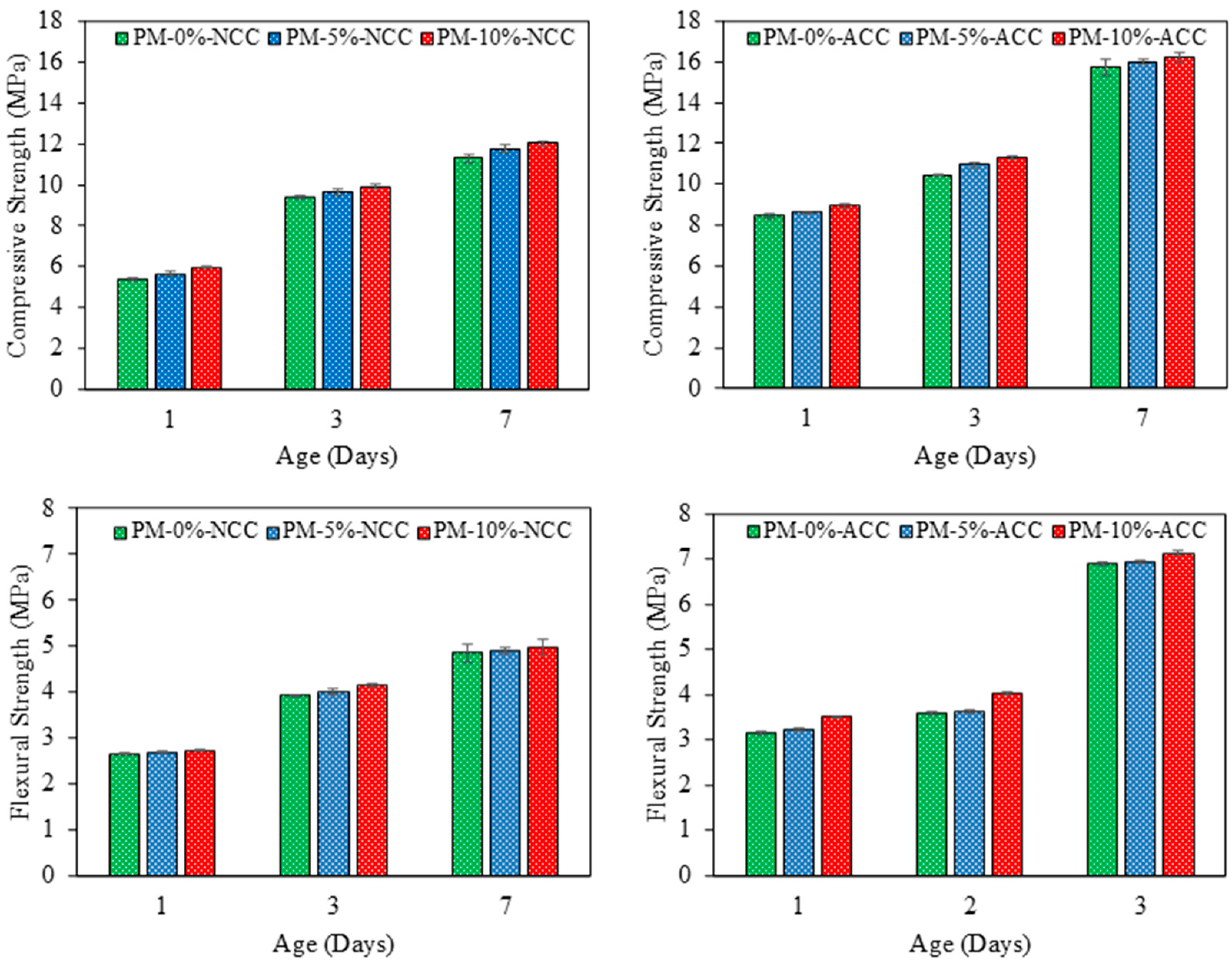
4.1.1. Compressive and Flexural Strengths
4.1.2. Dry Bulk Density, Water Absorption, and Accessible Porosity
4.2. Instrumental Testing
Heat of Hydration
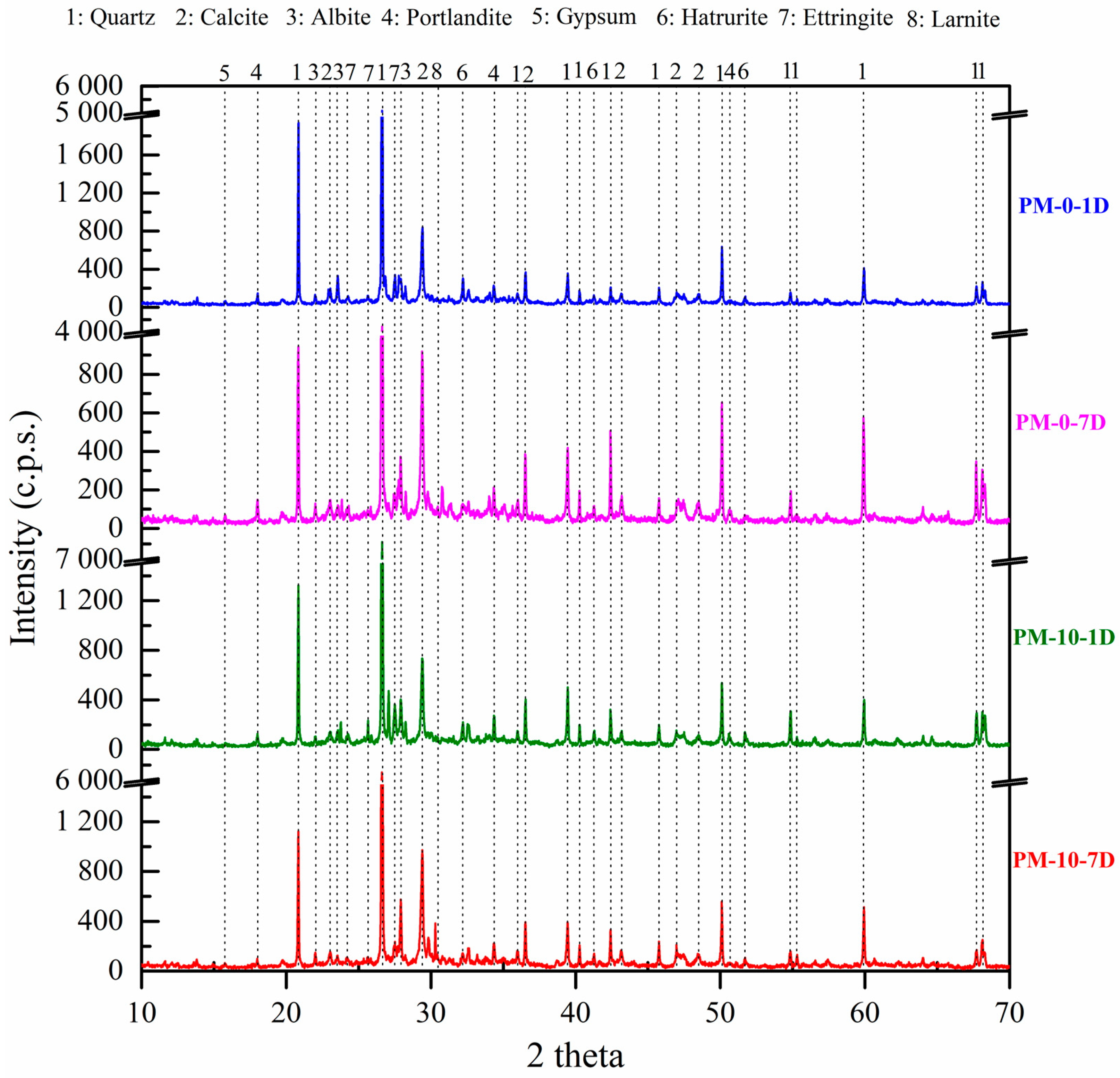
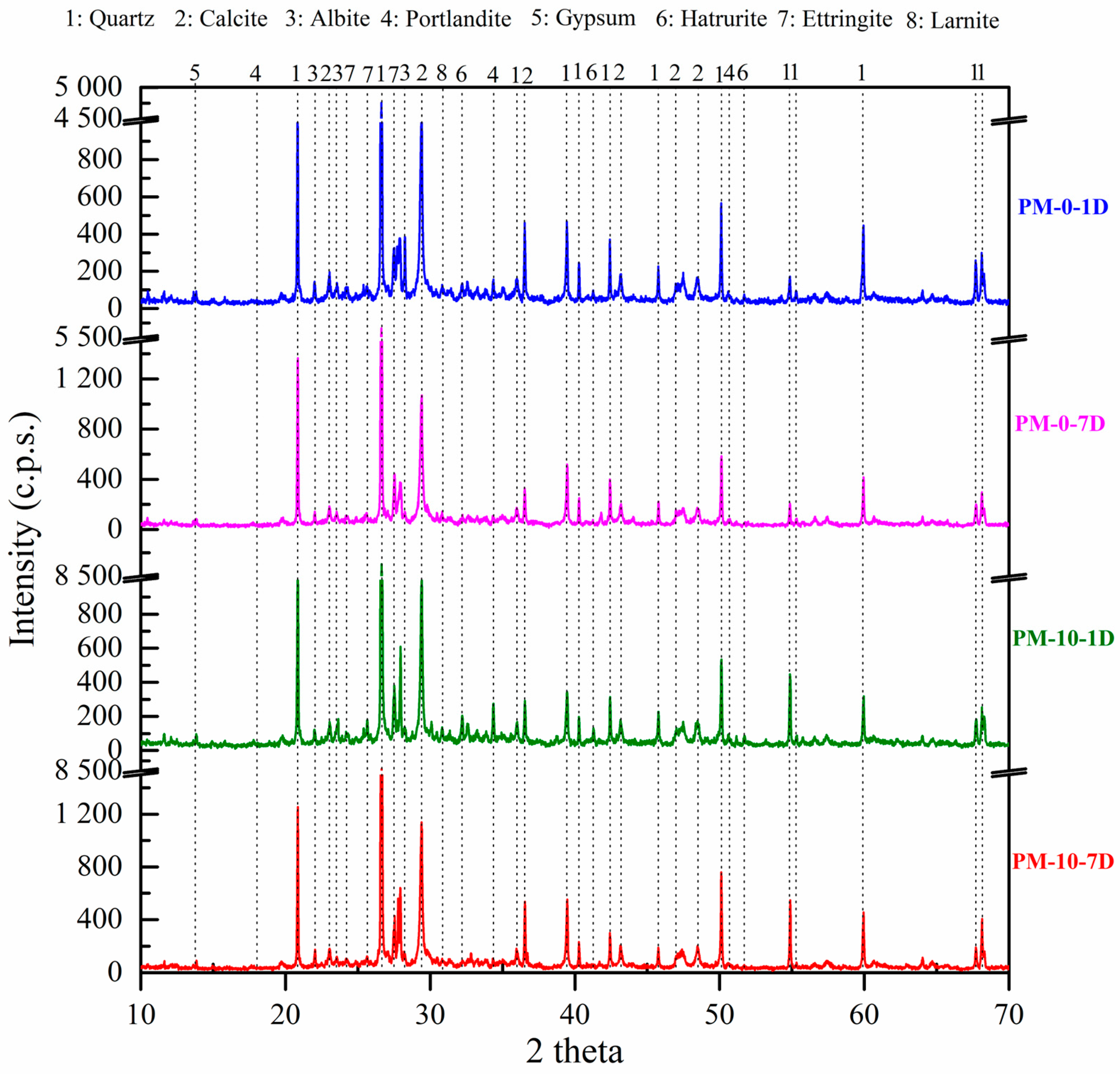
4.3. X-Ray Diffraction of Mortar
4.4. Carbon Capturing
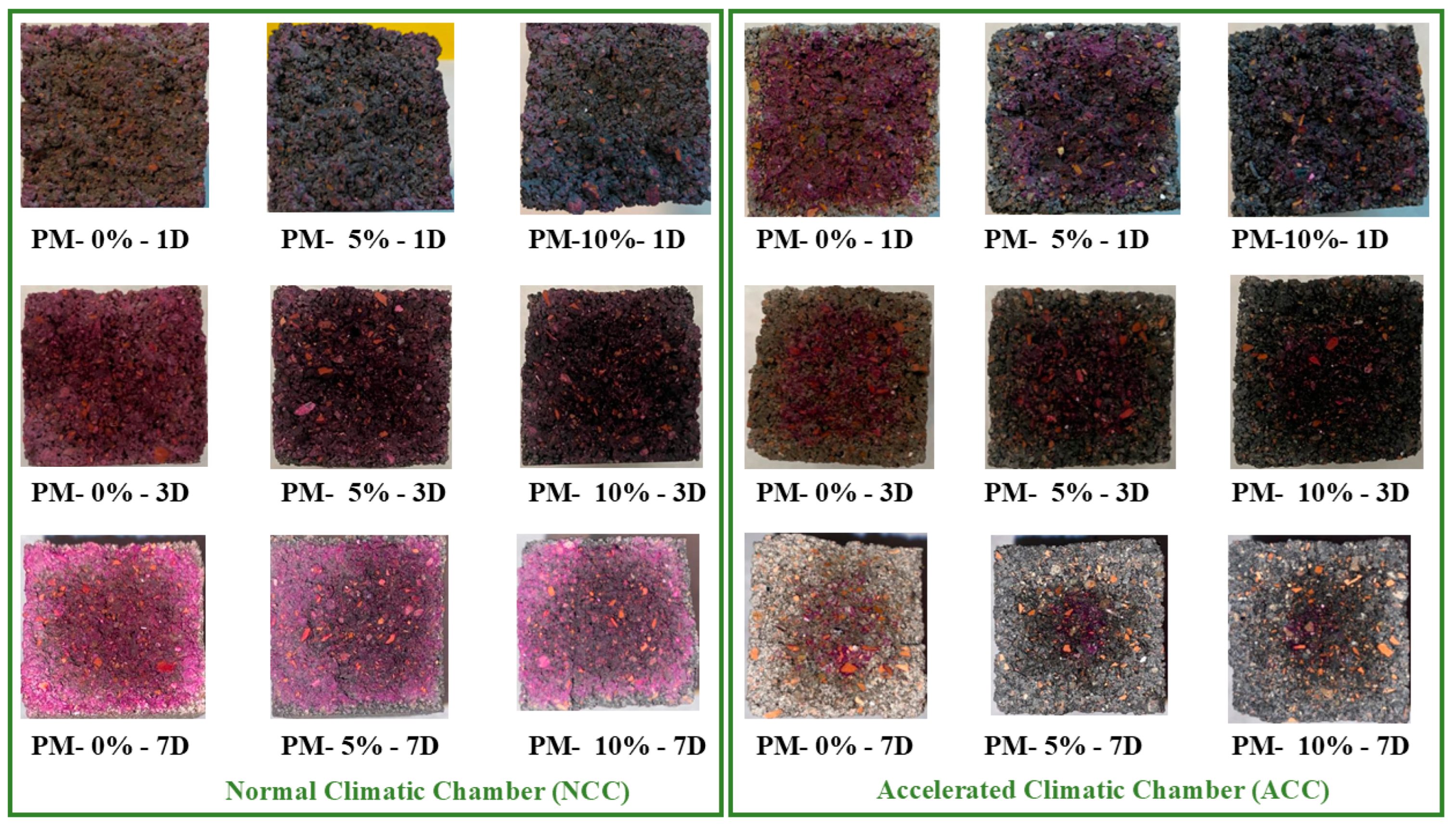
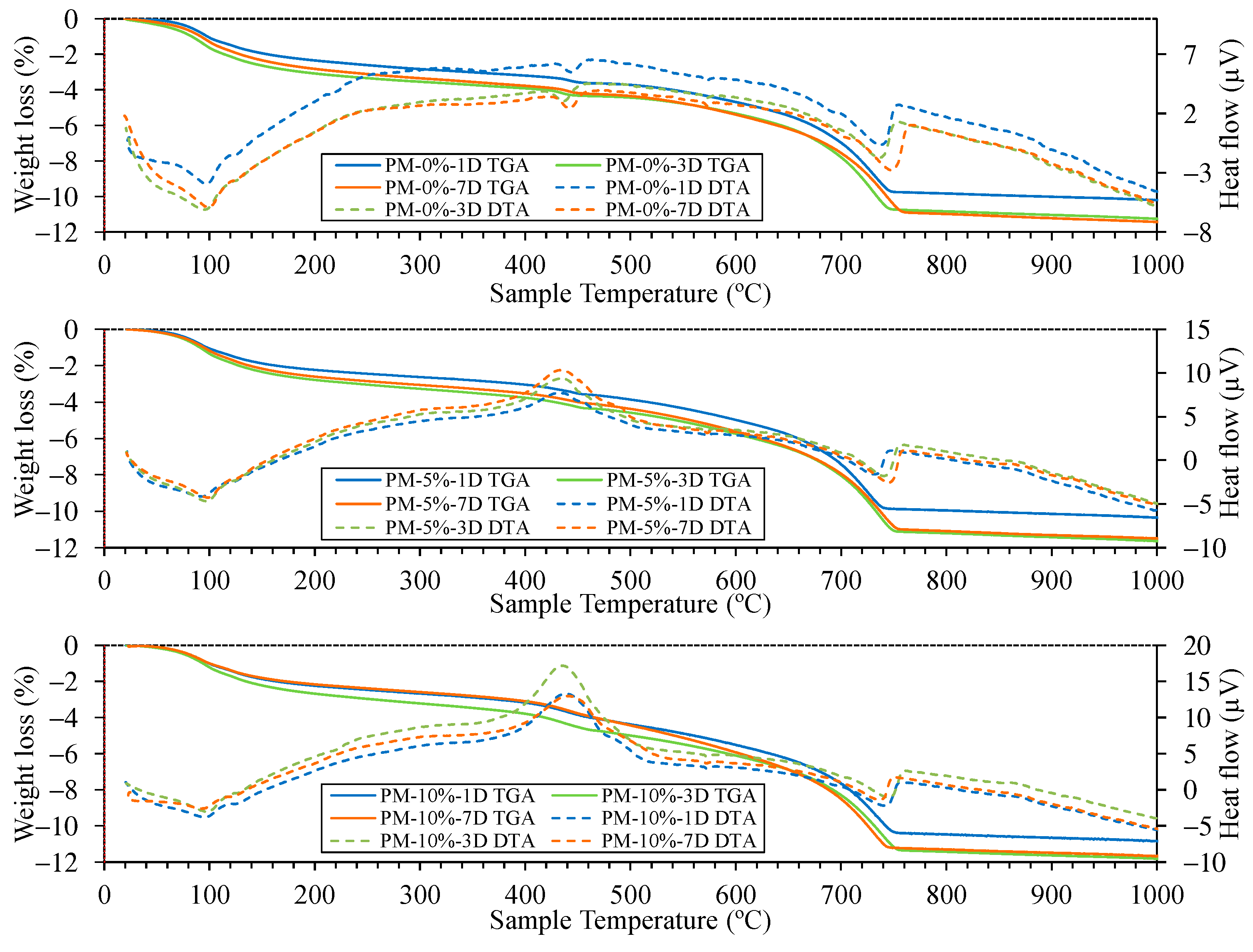
4.4.1. Carbonation Depth
| NCC | ACC | |||||
|---|---|---|---|---|---|---|
| 0%AcB | 5% AcB | 10% AcB | 0% AcB | 5% AcB | 10% AcB | |
| 1 d | 0 | 0 | 0 | 3 | 3.2 | 3.3 |
| 3 d | 0 | 0 | 0 | 4.1 | 4 | 4 |
| 7 d | 1 | 1.4 | 2.1 | 8.7 | 9.1 | 9.3 |
4.4.2. Thermogravimetric Analysis of Mortar
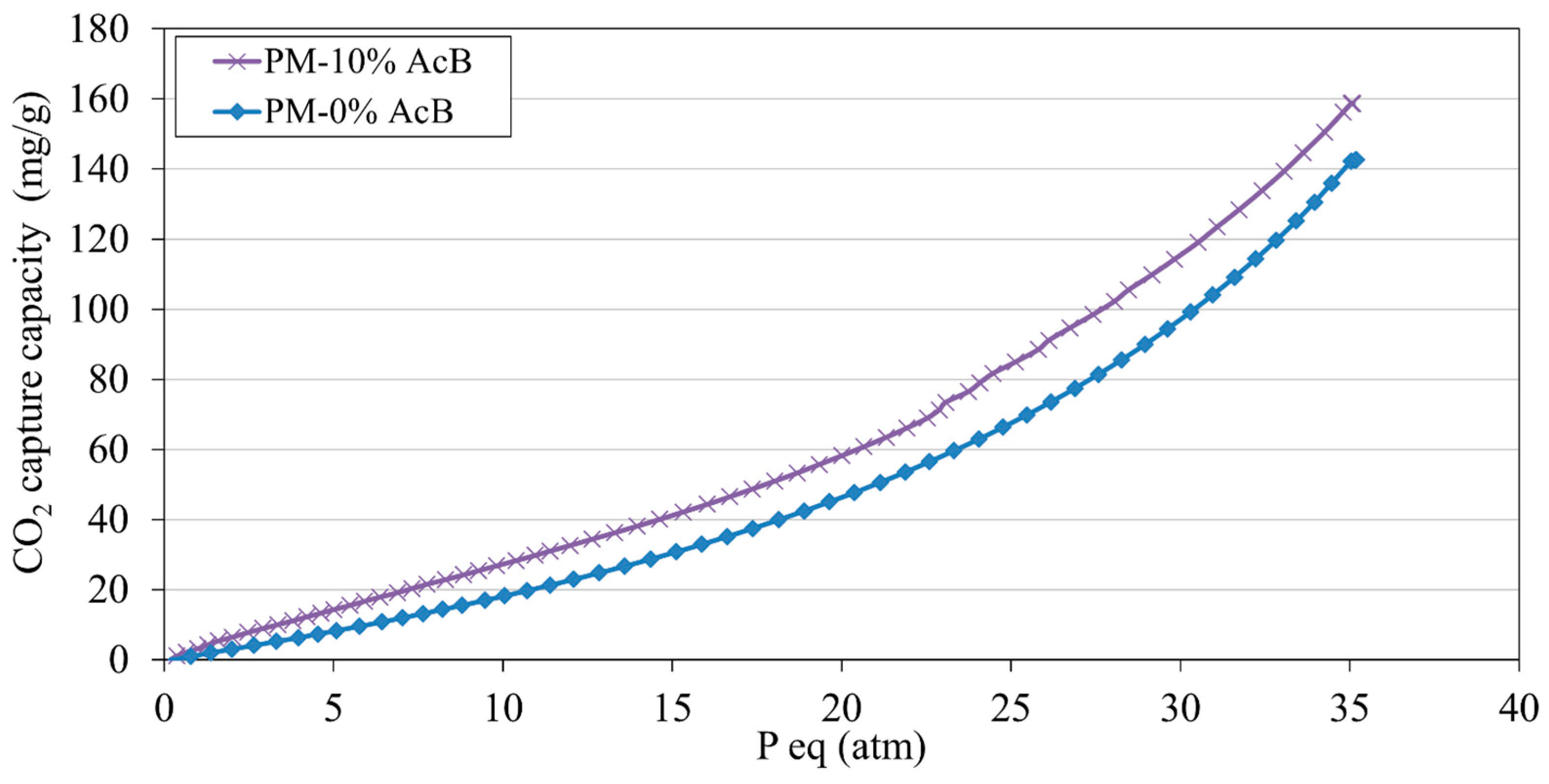
4.4.3. Ultra-Fast Test Method for CO2 Capture Capacity Under High Pressure
5. Conclusions
- The initial setting time (300 min) of the cement samples containing 0.5%, 1%, 3%, 15%, and 20% activated biochar (AcB) did not vary compared to the reference sample (0% AcB), confirming the compatibility of AcB with cement-based materials.
- The incorporation of AcB derived from olive stone waste into porous mortars, along with recycled masonry aggregates (RMA), significantly enhanced the mechanical performance of the materials. Specifically, the addition of 10% biochar increased the compressive strength by 110%, 105%, and 106% after 1, 3, and 7 d of curing, respectively. The flexural strength followed this trend, with higher values observed particularly during the early curing stages.
- Compressive strength due to accelerated carbonation increased with AcB content. The samples containing 10% AcB achieved a compressive strength of 11.32 MPa at 3 days under accelerated carbonation curing, surpassing the 11.29 MPa value at 7 days under normal curing conditions. This highlights the potential of activated biochar in combination with accelerated carbonation, promoting microstructural densification through CaCO3 formation and pore filling.
- The incorporation of activated biochar (AcB) into mortars increased dry bulk density and reduced water-accessible porosity, enhancing mechanical properties due to the pore-filling effect. However, water absorption exhibited an increasing trend with higher AcB content, attributed to its higher fine particle content and larger specific surface area.
- The inclusion of AcB enhanced CO2 sequestration efficiency, increasing carbonate precipitation, which resulted in a 147% increase in CO2 capture in the studied mix containing 10% AcB.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oliveira, T.C.F.; Dezen, B.G.S.; Possan, E. Use of Concrete Fine Fraction Waste as a Replacement of Portland Cement. J. Clean. Prod. 2020, 273, 123126. [Google Scholar] [CrossRef]
- Akhtar, A.; Sarmah, A.K. Construction and Demolition Waste Generation and Properties of Recycled Aggregate Concrete: A Global Perspective. J. Clean. Prod. 2018, 186, 262–281. [Google Scholar] [CrossRef]
- Villoria-Sáez, P.; Porras-Amores, C.; del Río Merino, M. Estimation of Construction and Demolition Waste. In Advances in Construction and Demolition Waste Recycling: Management, Processing and Environmental Assessment; Woodhead Publishing: Sawston, UK, 2020; pp. 13–30. [Google Scholar] [CrossRef]
- Oikonomou, N.D. Recycled Concrete Aggregates. Cem. Concr. Compos. 2005, 27, 315–318. [Google Scholar] [CrossRef]
- Kaptan, K.; Cunha, S.; Aguiar, J.; Mohammed, S.; Barzinjy, A.; Hamad, S.M.; Kaptan, K.; Cunha, S.; Aguiar, J. A Review: Construction and Demolition Waste as a Novel Source for CO2 Reduction in Portland Cement Production for Concrete. Sustainability 2024, 16, 585. [Google Scholar] [CrossRef]
- Villoria Sáez, P.; Osmani, M. A Diagnosis of Construction and Demolition Waste Generation and Recovery Practice in the European Union. J. Clean. Prod. 2019, 241, 118400. [Google Scholar] [CrossRef]
- Wu, L.; Sun, Z.; Cao, Y. Modification of Recycled Aggregate and Conservation and Application of Recycled Aggregate Concrete: A Review. Constr. Build. Mater. 2024, 431, 136567. [Google Scholar] [CrossRef]
- Hoang, N.H.; Ishigaki, T.; Kubota, R.; Yamada, M.; Kawamoto, K. A Review of Construction and Demolition Waste Management in Southeast Asia. J. Mater. Cycles Waste Manag. 2020, 22, 315–325. [Google Scholar] [CrossRef]
- Huang, B.; Wang, X.; Kua, H.; Geng, Y.; Bleischwitz, R.; Ren, J. Construction and Demolition Waste Management in China through the 3R Principle. Resour. Conserv. Recycl. 2018, 129, 36–44. [Google Scholar] [CrossRef]
- Caro, D.; Lodato, C.; Damgaard, A.; Cristóbal, J.; Foster, G.; Flachenecker, F.; Tonini, D. Environmental and Socio-Economic Effects of Construction and Demolition Waste Recycling in the European Union. Sci. Total Environ. 2024, 908, 168295. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Zhang, L. Evolutionary Game Analysis of Construction Waste Recycling Management in China. Resour. Conserv. Recycl. 2020, 161, 104863. [Google Scholar] [CrossRef]
- Moschen-Schimek, J.; Kasper, T.; Huber-Humer, M. Critical Review of the Recovery Rates of Construction and Demolition Waste in the European Union—An Analysis of Influencing Factors in Selected EU Countries. Waste Manag. 2023, 167, 150–164. [Google Scholar] [CrossRef]
- Wijayasundara, M.; Mendis, P.; Crawford, R.H. Integrated Assessment of the Use of Recycled Concrete Aggregate Replacing Natural Aggregate in Structural Concrete. J. Clean. Prod. 2018, 174, 591–604. [Google Scholar] [CrossRef]
- Vargas, P.; Restrepo-Baena, O.; Tobón, J.I. Microstructural Analysis of Interfacial Transition Zone (ITZ) and Its Impact on the Compressive Strength of Lightweight Concretes. Constr. Build. Mater. 2017, 137, 381–389. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, W.; Tang, Z.; Huang, Q. Experimental and Numerical Investigation of Chloride-Induced Reinforcement Corrosion and Mortar Cover Cracking. Cem. Concr. Compos. 2020, 111, 103620. [Google Scholar] [CrossRef]
- Marinković, S.; Radonjanin, V.; Malešev, M.; Ignjatović, I. Comparative Environmental Assessment of Natural and Recycled Aggregate Concrete. Waste Manag. 2010, 30, 2255–2264. [Google Scholar] [CrossRef]
- Brasileiro, K.P.T.V.; Nahime, B.d.O.; Lima, E.C.; Alves, M.M.; Ferreira, W.P.; Santos, I.S.d.; Filho, C.P.B.; Reis, I.C.d. Influence of Recycled Aggregates and Silica Fume on the Performance of Pervious Concrete. J. Build. Eng. 2024, 82, 108347. [Google Scholar] [CrossRef]
- Yoon, S.; Monteiro, P.J.M.; Macphee, D.E.; Glasser, F.P.; Imbabi, M.S.E. Statistical Evaluation of the Mechanical Properties of High-Volume Class F Fly Ash Concretes. Constr. Build. Mater. 2014, 54, 432–442. [Google Scholar] [CrossRef]
- Hu, J.; Zhao, W.; Liu, P.; Huang, Q.; Luo, S. Study on Fracture Characteristics of Recycled Aggregates Asphalt Concrete. Constr. Build. Mater. 2024, 419, 135431. [Google Scholar] [CrossRef]
- Meddah, M.S.; Al-Harthy, A.; Ismail, M.A. Recycled Concrete Aggregates and Their Influences on Performances of Low and Normal Strength Concretes. Buildings 2020, 10, 167. [Google Scholar] [CrossRef]
- Al Sayed, A.A.K.A.; Al-Waked, Q.F.; Shawky, S.M.M.; Al-jabali, H.M.; Fouad Edris, W. Effect of Alkali Activated Limestone-Silica Fume Blended Precursor on Performance Enhancement of Recycled Aggregate Concrete. Case Stud. Constr. Mater. 2023, 19, e02661. [Google Scholar] [CrossRef]
- Tejas, S.; Pasla, D. Assessment of Mechanical and Durability Properties of Composite Cement-Based Recycled Aggregate Concrete. Constr. Build. Mater. 2023, 387, 131620. [Google Scholar] [CrossRef]
- Borges, P.M.; Schiavon, J.Z.; da Silva, S.R.; Rigo, E.; Neves Junior, A.; Possan, E.; Andrade, J.J.d.O. Mortars with Recycled Aggregate of Construction and Demolition Waste: Mechanical Properties and Carbon Uptake. Constr. Build. Mater. 2023, 387, 131600. [Google Scholar] [CrossRef]
- Pu, Y.; Li, L.; Shi, X.; Wang, Q.; Abomohra, A. A Comparative Life Cycle Assessment on Recycled Concrete Aggregates Modified by Accelerated Carbonation Treatment and Traditional Methods. Waste Manag. 2023, 172, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Meng, T.; Lai, Z.; Yang, X.; Dai, D.; Jia, Y.; Yu, H. An Approach to Effectively Improve the Properties of Recycled Concrete Aggregate and Recycled Brick Aggregate by Micro-Nano Particle Reconstruction. Constr. Build. Mater. 2024, 421, 135669. [Google Scholar] [CrossRef]
- Baggio, T.F.; Possan, E.; de Oliveira Andrade, J.J. Physical-Chemical Characterization of Construction and Demolition Waste Powder with Thermomechanical Activation for Use as Supplementary Cementitious Material. Constr. Build. Mater. 2024, 437, 136907. [Google Scholar] [CrossRef]
- Liu, B.; Qin, J.; Shi, J.; Jiang, J.; Wu, X.; He, Z. New Perspectives on Utilization of CO2 Sequestration Technologies in Cement-Based Materials. Constr. Build. Mater. 2021, 272, 121660. [Google Scholar] [CrossRef]
- Khan, Y.; Hassan, T. Promoting Sustainable Development: Evaluating the Influence of Natural Resources, High-Tech Export and Corruption on CO2 Emissions in Developing Economies. Resour. Policy 2024, 88, 104511. [Google Scholar] [CrossRef]
- Chen, F.F.; Wang, Q.S.; Umar, M.; Zheng, L. Towards Sustainable Resource Management: The Role of Governance, Natural Resource Rent and Energy Productivity. Resour. Policy 2023, 85, 104026. [Google Scholar] [CrossRef]
- Hanifa, M.; Agarwal, R.; Sharma, U.; Thapliyal, P.C.; Singh, L.P. A Review on CO2 Capture and Sequestration in the Construction Industry: Emerging Approaches and Commercialised Technologies. J. CO2 Util. 2023, 67, 102292. [Google Scholar] [CrossRef]
- Farahzadi, L.; Kioumarsi, M. Application of Machine Learning Initiatives and Intelligent Perspectives for CO2 Emissions Reduction in Construction. J. Clean. Prod. 2023, 384, 135504. [Google Scholar] [CrossRef]
- Uratani, J.M.; Griffiths, S. A Forward Looking Perspective on the Cement and Concrete Industry: Implications of Growth and Development in the Global South. Energy Res. Soc. Sci. 2023, 97, 102972. [Google Scholar] [CrossRef]
- Supriya; Chaudhury, R.; Sharma, U.; Thapliyal, P.C.; Singh, L.P. Low-CO2 Emission Strategies to Achieve Net Zero Target in Cement Sector. J. Clean. Prod. 2023, 417, 137466. [Google Scholar] [CrossRef]
- Sbahieh, S.; Zaher Serdar, M.; Al-Ghamdi, S.G. Decarbonization Strategies of Building Materials Used in the Construction Industry. Mater. Today Proc. 2023; in press. [Google Scholar] [CrossRef]
- Suescum-Morales, D.; Kalinowska-Wichrowska, K.; Fernández, J.M.; Jiménez, J.R. Accelerated Carbonation of Fresh Cement-Based Products Containing Recycled Masonry Aggregates for CO2 Sequestration. J. CO2 Util. 2021, 46, 101461. [Google Scholar] [CrossRef]
- Liu, Z.; Meng, W. Fundamental Understanding of Carbonation Curing and Durability of Carbonation-Cured Cement-Based Composites: A Review. J. CO2 Util. 2021, 44, 101428. [Google Scholar] [CrossRef]
- Reichenbach, S.; Kromoser, B. State of Practice of Automation in Precast Concrete Production. J. Build. Eng. 2021, 43, 102527. [Google Scholar] [CrossRef]
- Tai, H.W.; Chen, J.H.; Cheng, J.Y.; Hsu, S.C.; Wei, H.H. Learn Curve for Precast Component Productivity in Construction. Int. J. Civil. Eng. 2021, 19, 1179–1194. [Google Scholar] [CrossRef]
- Weng, Y.; Li, M.; Ruan, S.; Wong, T.N.; Tan, M.J.; Ow Yeong, K.L.; Qian, S. Comparative Economic, Environmental and Productivity Assessment of a Concrete Bathroom Unit Fabricated through 3D Printing and a Precast Approach. J. Clean. Prod. 2020, 261, 121245. [Google Scholar] [CrossRef]
- Feng, X.; Zhuo, C.; Yin, S. The Application of C–S–H Accelerators in the Precast Concrete Industry: Early-Age Properties and CO2 Footprint Analysis. J. Clean. Prod. 2024, 435, 140558. [Google Scholar] [CrossRef]
- Vandamme, E.J. Agro-Industrial Residue Utilization for Industrial Biotechnology Products. In Biotechnology for Agro-Industrial Residues Utilisation: Utilisation of Agro-Residues; Springer: Berlin/Heidelberg, Germany, 2009; pp. 3–11. [Google Scholar] [CrossRef]
- Calovi, M.; Rossi, S. Olive Pit Powder as Multifunctional Pigment for Waterborne Paint: Influence of the Bio-Based Filler on the Aesthetics, Durability and Mechanical Features of the Polymer Matrix. Ind. Crops Prod. 2023, 194, 116326. [Google Scholar] [CrossRef]
- Calovi, M.; Rossi, S. Functional Olive Pit Powders: The Role of the Bio-Based Filler in Reducing the Water Uptake Phenomena of the Waterborne Paint. Coatings 2023, 13, 442. [Google Scholar] [CrossRef]
- Tan, X.F.; Liu, S.-B.; Liu, Y.-G.; Gu, Y.-L.; Zeng, G.-M.; Hu, X.-J.; Wang, X.; Liu, S.-H.; Jiang, L.-H. Biochar as Potential Sustainable Precursors for Activated Carbon Production: Multiple Applications in Environmental Protection and Energy Storage. Bioresour. Technol. 2017, 227, 359–372. [Google Scholar] [CrossRef] [PubMed]
- Treviño-Cordero, H.; Juárez-Aguilar, L.G.; Mendoza-Castillo, D.I.; Hernández-Montoya, V.; Bonilla-Petriciolet, A.; Montes-Morán, M.A. Synthesis and Adsorption Properties of Activated Carbons from Biomass of Prunus Domestica and Jacaranda Mimosifolia for the Removal of Heavy Metals and Dyes from Water. Ind. Crops Prod. 2013, 42, 315–323. [Google Scholar] [CrossRef]
- Kołodyńska, D.; Krukowska, J.; Thomas, P. Comparison of Sorption and Desorption Studies of Heavy Metal Ions from Biochar and Commercial Active Carbon. Chem. Eng. J. 2017, 307, 353–363. [Google Scholar] [CrossRef]
- Katish, M.; Allen, S.; Squires, A.; Ferrandiz-Mas, V. Experimental Study of Phase Change Material (PCM) Biochar Composite for Net-Zero Built Environment Applications. Clean. Mater. 2024, 14, 100274. [Google Scholar] [CrossRef]
- Vincevica-Gaile, Z.; Zhylina, M.; Shishkin, A.; Ansone-Bertina, L.; Klavins, L.; Arbidans, L.; Dobkevica, L.; Zekker, I.; Klavins, M. Selected Residual Biomass Valorization into Pellets as a Circular Economy-Supported End-of-Waste. Clean. Mater. 2025, 15, 100295. [Google Scholar] [CrossRef]
- Soda, R.; Wanmolee, W.; Panyapinyopol, B.; Boonyoung, P.; Kraithong, W.; Viriya-empikul, N.; Laosiripojana, N.; Nakason, K. Corn Stover-Derived Biochar Supporting Dual Functional Catalyst for Direct Sorbitol Production from Cellulosic Materials. Clean. Mater. 2024, 13, 100254. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Preparation, Modification and Environmental Application of Biochar: A Review. J. Clean. Prod. 2019, 227, 1002–1022. [Google Scholar] [CrossRef]
- Campion, L.; Bekchanova, M.; Malina, R.; Kuppens, T. The Costs and Benefits of Biochar Production and Use: A Systematic Review. J. Clean. Prod. 2023, 408, 137138. [Google Scholar] [CrossRef]
- Ghanbarpour Mamaghani, Z.; Hawboldt, K.A.; MacQuarrie, S. Adsorption of CO2 Using Biochar—Review of the Impact of Gas Mixtures and Water on Adsorption. J. Environ. Chem. Eng. 2023, 11, 109643. [Google Scholar] [CrossRef]
- Francis, J.C.; Nighojkar, A.; Kandasubramanian, B. Relevance of Wood Biochar on CO2 Adsorption: A Review. Hybrid Adv. 2023, 3, 100056. [Google Scholar] [CrossRef]
- Wen, C.; Liu, T.; Wang, D.; Wang, Y.; Chen, H.; Luo, G.; Zhou, Z.; Li, C.; Xu, M. Biochar as the Effective Adsorbent to Combustion Gaseous Pollutants: Preparation, Activation, Functionalization and the Adsorption Mechanisms. Prog. Energy Combust. Sci. 2023, 99, 101098. [Google Scholar] [CrossRef]
- Luo, Z.; Yao, B.; Yang, X.; Wang, L.; Xu, Z.; Yan, X.; Tian, L.; Zhou, H.; Zhou, Y. Novel Insights into the Adsorption of Organic Contaminants by Biochar: A Review. Chemosphere 2022, 287, 132113. [Google Scholar] [CrossRef]
- Dai, Y.; Zhang, N.; Xing, C.; Cui, Q.; Sun, Q. The Adsorption, Regeneration and Engineering Applications of Biochar for Removal Organic Pollutants: A Review. Chemosphere 2019, 223, 12–27. [Google Scholar] [CrossRef]
- Song, Q.; Kong, F.; Liu, B.F.; Song, X.; Ren, H.Y. Biochar-Based Composites for Removing Chlorinated Organic Pollutants: Applications, Mechanisms, and Perspectives. Environ. Sci. Ecotechnology 2024, 21, 100420. [Google Scholar] [CrossRef]
- Puig-Gamero, M.; Esteban-Arranz, A.; Sanchez-Silva, L.; Sanchez, P. Obtaining Activated Biochar from Olive Stone Using a Bench Scale High-Pressure Thermobalance. J. Environ. Chem. Eng. 2021, 9, 105374. [Google Scholar] [CrossRef]
- Molina-Sabio, M.; RodRíguez-Reinoso, F.; Caturla, F.; Sellés, M.J. Porosity in Granular Carbons Activated with Phosphoric Acid. Carbon 1995, 33, 1105–1113. [Google Scholar] [CrossRef]
- Moreno, N.; Caballero, A.; Hernán, L.; Morales, J. Lithium–Sulfur Batteries with Activated Carbons Derived from Olive Stones. Carbon 2014, 70, 241–248. [Google Scholar] [CrossRef]
- Zabaniotou, A.; Stavropoulos, G.; Skoulou, V. Activated Carbon from Olive Kernels in a Two-Stage Process: Industrial Improvement. Bioresour. Technol. 2008, 99, 320–326. [Google Scholar] [CrossRef]
- Sultana, M.; Rownok, M.H.; Sabrin, M.; Rahaman, M.H.; Alam, S.M.N. A Review on Experimental Chemically Modified Activated Carbon to Enhance Dye and Heavy Metals Adsorption. Clean. Eng. Technol. 2022, 6, 100382. [Google Scholar] [CrossRef]
- Heidarinejad, Z.; Dehghani, M.H.; Heidari, M.; Javedan, G.; Ali, I.; Sillanpää, M. Methods for Preparation and Activation of Activated Carbon: A Review. Environ. Chem. Lett. 2020, 18, 393–415. [Google Scholar] [CrossRef]
- Ramalingam, G.; Priya, A.K.; Gnanasekaran, L.; Rajendran, S.; Hoang, T.K.A. Biomass and Waste Derived Silica, Activated Carbon and Ammonia-Based Materials for Energy-Related Applications—A Review. Fuel 2024, 355, 129490. [Google Scholar] [CrossRef]
- Shabi, A.H.; Prima Hardianto, Y.; Shaheen Shah, S.; Omar Al-Qwairi, F.; Mohamed, M.M.; Nasiruzzaman Shaikh, M.; Saeed Alzahrani, A.; Aziz, M.A. Advancements in Olive-Derived Carbon: Preparation Methods and Sustainable Applications. Chem. Asian J. 2024, 19, e202400045. [Google Scholar] [CrossRef] [PubMed]
- Yakout, S.M.; Sharaf El-Deen, G. Characterization of Activated Carbon Prepared by Phosphoric Acid Activation of Olive Stones. Arab. J. Chem. 2016, 9, S1155–S1162. [Google Scholar] [CrossRef]
- Saleem, J.; Shahid, U.B.; Hijab, M.; Mackey, H.; McKay, G. Production and Applications of Activated Carbons as Adsorbents from Olive Stones. Biomass Convers. Biorefinery 2019, 9, 775–802. [Google Scholar] [CrossRef]
- Estevez, R.; Aguado-Deblas, L.; Montes, V.; Caballero, A.; Bautista, F.M. Sulfonated Carbons from Olive Stones as Catalysts in the Microwave-Assisted Etherification of Glycerol with Tert-Butyl Alcohol. Mol. Catal. 2020, 488, 110921. [Google Scholar] [CrossRef]
- Melo, J.M.; Lütke, S.F.; Igansi, A.V.; Franco, D.S.P.; Vicenti, J.R.M.; Dotto, G.L.; Pinto, L.A.A.; Cadaval, T.R.S.; Felipe, C.A.S. Mass Transfer and Equilibrium Modelings of Phenol Adsorption on Activated Carbon from Olive Stone. Colloids Surf. A Physicochem. Eng. Asp. 2024, 680, 132628. [Google Scholar] [CrossRef]
- Corral-Bobadilla, M.; Lostado-Lorza, R.; Somovilla-Gómez, F.; Escribano-García, R. Effective Use of Activated Carbon from Olive Stone Waste in the Biosorption Removal of Fe(III) Ions from Aqueous Solutions. J. Clean. Prod. 2021, 294, 126332. [Google Scholar] [CrossRef]
- Serafin, J.; Dziejarski, B.; Sreńscek-Nazzal, J. An Innovative and Environmentally Friendly Bioorganic Synthesis of Activated Carbon Based on Olive Stones and Its Potential Application for CO2 Capture. Sustain. Mater. Technol. 2023, 38, e00717. [Google Scholar] [CrossRef]
- Eder, S.; Müller, K.; Azzari, P.; Arcifa, A.; Peydayesh, M.; Nyström, L. Mass Transfer Mechanism and Equilibrium Modelling of Hydroxytyrosol Adsorption on Olive Pit–Derived Activated Carbon. Chem. Eng. J. 2021, 404, 126519. [Google Scholar] [CrossRef]
- Martínez, M.L.; Torres, M.M.; Guzmán, C.A.; Maestri, D.M. Preparation and Characteristics of Activated Carbon from Olive Stones and Walnut Shells. Ind. Crops Prod. 2006, 23, 23–28. [Google Scholar] [CrossRef]
- Wang, Y.; Ge, Y.; Wang, X.; Chen, X.; Li, Q. The Effect of Powder Activated Carbon on Mechanical Properties and Pore Structures of Cement-Based Mortars. Constr. Build. Mater. 2022, 316, 125798. [Google Scholar] [CrossRef]
- Wu, F.; Liu, C.; Zhang, L.; Lu, Y.; Ma, Y. Comparative Study of Carbonized Peach Shell and Carbonized Apricot Shell to Improve the Performance of Lightweight Concrete. Constr. Build. Mater. 2018, 188, 758–771. [Google Scholar] [CrossRef]
- Chin, C.O.; Yang, X.; Kong, S.Y.; Paul, S.C.; Susilawati; Wong, L.S. Mechanical and Thermal Properties of Lightweight Concrete Incorporated with Activated Carbon as Coarse Aggregate. J. Build. Eng. 2020, 31, 101347. [Google Scholar] [CrossRef]
- Justo-Reinoso, I.; Srubar, W.V.; Caicedo-Ramirez, A.; Hernandez, M.T. Fine Aggregate Substitution by Granular Activated Carbon Can Improve Physical and Mechanical Properties of Cement Mortars. Constr. Build. Mater. 2018, 164, 750–759. [Google Scholar] [CrossRef]
- Mahoutian, M.; Lubell, A.S.; Bindiganavile, V.S. Effect of Powdered Activated Carbon on the Air Void Characteristics of Concrete Containing Fly Ash. Constr. Build. Mater. 2015, 80, 84–91. [Google Scholar] [CrossRef]
- Justo-Reinoso, I.; Hernandez, M.T.; Lucero, C.; Srubar, W.V. Dispersion and Effects of Metal Impregnated Granular Activated Carbon Particles on the Hydration of Antimicrobial Mortars. Cem. Concr. Compos. 2020, 110, 103588. [Google Scholar] [CrossRef]
- Haris Javed, M.; Ali Sikandar, M.; Ahmad, W.; Tariq Bashir, M.; Alrowais, R.; Bilal Wadud, M. Effect of Various Biochars on Physical, Mechanical, and Microstructural Characteristics of Cement Pastes and Mortars. J. Build. Eng. 2022, 57, 104850. [Google Scholar] [CrossRef]
- Abdel daiem, M.M.; Rashad, A.M.; Said, N.; Abdel-Gawwad, H.A. An Initial Study about the Effect of Activated Carbon Nano-Sheets from Residual Biomass of Olive Trees Pellets on the Properties of Alkali-Activated Slag Pastes. J. Build. Eng. 2021, 44, 102661. [Google Scholar] [CrossRef]
- Gupta, S.; Kua, H.W.; Low, C.Y. Use of Biochar as Carbon Sequestering Additive in Cement Mortar. Cem. Concr. Compos. 2018, 87, 110–129. [Google Scholar] [CrossRef]
- Sisman, M.; Teomete, E.; Yanik, J.; Malayoglu, U. The Effect of Nano-Biochar Produced from Various Raw Materials on Flow and Mechanical Properties of Mortar. Constr. Build. Mater. 2024, 416, 135040. [Google Scholar] [CrossRef]
- Xu, M.; Zhang, Y.; Yang, S.; Mo, L.; Liu, P. Effects of Internal CO2 Curing Provided by Biochar on the Carbonation and Properties of Steel Slag-Based Artificial Lightweight Aggregates (SALAs). Cem. Concr. Compos. 2023, 142, 105197. [Google Scholar] [CrossRef]
- Chen, T.; Zhao, L.; Gao, X.; Li, L.; Qin, L. Modification of Carbonation-Cured Cement Mortar Using Biochar and Its Environmental Evaluation. Cem. Concr. Compos. 2022, 134, 104764. [Google Scholar] [CrossRef]
- Yang, X.; Wang, X.Y. Strength and Durability Improvements of Biochar-Blended Mortar or Paste Using Accelerated Carbonation Curing. J. CO2 Util. 2021, 54, 101766. [Google Scholar] [CrossRef]
- Senadheera, S.S.; Gupta, S.; Kua, H.W.; Hou, D.; Kim, S.; Tsang, D.C.W.; Ok, Y.S. Application of Biochar in Concrete—A Review. Cem. Concr. Compos. 2023, 143, 105204. [Google Scholar] [CrossRef]
- Gupta, S.; Kua, H.W.; Koh, H.J. Application of Biochar from Food and Wood Waste as Green Admixture for Cement Mortar. Sci. Total Environ. 2018, 619–620, 419–435. [Google Scholar] [CrossRef]
- Gupta, S.; Kua, H.W. Effect of Water Entrainment by Pre-Soaked Biochar Particles on Strength and Permeability of Cement Mortar. Constr. Build. Mater. 2018, 159, 107–125. [Google Scholar] [CrossRef]
- Maljaee, H.; Madadi, R.; Paiva, H.; Tarelho, L.; Ferreira, V.M. Incorporation of Biochar in Cementitious Materials: A Roadmap of Biochar Selection. Constr. Build. Mater. 2021, 283, 122757. [Google Scholar] [CrossRef]
- Legan, M.; Gotvajn, A.Ž.; Zupan, K. Potential of Biochar Use in Building Materials. J. Environ. Manag. 2022, 309, 114704. [Google Scholar] [CrossRef] [PubMed]
- Akinyemi, B.A.; Adesina, A. Recent Advancements in the Use of Biochar for Cementitious Applications: A Review. J. Build. Eng. 2020, 32, 101705. [Google Scholar] [CrossRef]
- Murali, G.; Wong, L.S. A Comprehensive Review of Biochar-Modified Concrete: Mechanical Performance and Microstructural Insights. Constr. Build. Mater. 2024, 425, 135986. [Google Scholar] [CrossRef]
- Maljaee, H.; Paiva, H.; Madadi, R.; Tarelho, L.A.C.; Morais, M.; Ferreira, V.M. Effect of Cement Partial Substitution by Waste-Based Biochar in Mortars Properties. Constr. Build. Mater. 2021, 301, 124074. [Google Scholar] [CrossRef]
- Bai, Y.; Arulrajah, A.; Horpibulsuk, S.; Chu, J. Gasified Olive Stone Biochar as a Green Construction Fill Material. Constr. Build. Mater. 2023, 403, 133003. [Google Scholar] [CrossRef]
- Gupta, S.; Muthukrishnan, S.; Kua, H.W. Comparing Influence of Inert Biochar and Silica Rich Biochar on Cement Mortar—Hydration Kinetics and Durability under Chloride and Sulfate Environment. Constr. Build. Mater. 2021, 268, 121142. [Google Scholar] [CrossRef]
- Suescum-Morales, D.; Jiménez, J.R.; Fernández-Rodríguez, J.M. Use of Carbonated Water as Kneading in Mortars Made with Recycled Aggregates. Materials 2022, 15, 4876. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, J.R.; Ayuso, J.; López, M.; Fernández, J.M.; De Brito, J. Use of Fine Recycled Aggregates from Ceramic Waste in Masonry Mortar Manufacturing. Constr. Build. Mater. 2013, 40, 679–690. [Google Scholar] [CrossRef]
- Ledesma, E.F.; Jiménez, J.R.; Ayuso, J.; Fernández, J.M.; De Brito, J. Maximum Feasible Use of Recycled Sand from Construction and Demolition Waste for Eco-Mortar Production—Part-I: Ceramic Masonry Waste. J. Clean. Prod. 2015, 87, 692–706. [Google Scholar] [CrossRef]
- Caballero, A.; Hernán, L.; Morales, J. Limitations of Disordered Carbons Obtained from Biomass as Anodes for Real Lithium-Ion Batteries. ChemSusChem 2011, 4, 658–663. [Google Scholar] [CrossRef]
- Caballero, A.; Hernán, L.; Morales, J.; Olivares-Marín, M.; Gómez-Serrano, V. Suppressing Irreversible Capacity in Low Cost Disordered Carbons for Li-Ion Batteries. Electrochem. Solid-State Lett. 2009, 12, A167. [Google Scholar] [CrossRef]
- Arrebola, J.C.; Rodríguez-Fernández, N.; Caballero, Á. Decontamination of Wastewater Using Activated Biochar from Agricultural Waste: A Practical Experiment for Environmental Sciences Students. J. Chem. Educ. 2020, 97, 4137–4144. [Google Scholar] [CrossRef]
- UNE-EN-197-1:2011; Part 1: Composition, Specifications and Conformity Criteria for Common Cements. European Committee for Standardization: Brussels, Belgium, 2011.
- UNE-EN 1097-6:2014; Tests for Mechanical and Physical Properties of Aggregates—Part 6: Determination of Particle Density and Water Absorption. British Standard Institution: London, UK, 2014.
- UNE-EN 933-8:2012+A1:2015/1M:2016; Tests for Geometrical Properties of Aggregates—Part 8: Assessment of Fines—Sand Equivalent Test. IPQ: Caparica, Portugal, 2016.
- ASTM-C-144; Standard Specification for Aggregate for Masonry Mortar. ASTM International: West Conshohocken, PA, USA, 2004.
- Suescum-Morales, D.; Fernández-Rodríguez, J.M.; Jiménez, J.R. Use of Carbonated Water to Improve the Mechanical Properties and Reduce the Carbon Footprint of Cement-Based Materials with Recycled Aggregates. J. CO2 Util. 2022, 57, 101886. [Google Scholar] [CrossRef]
- UNE-EN 196-1: 2018; Methods of Testing Cement. Part 1: Determination of Strength. Turkish Standards Institute: Ankara, Turkey, 2018.
- UNE-EN 1015-3:2000; Métodos de Ensayo Para Morteros de Albañilería. Parte 3: Determinación de La Consistencia Del Mortero Fresco (Por La Mesa de Sacudidas). AENOR: Madrid, Spain, 2020.
- Merino-Lechuga, A.M.; González-Caro, Á.; Fernández-Ledesma, E.; Jiménez, J.R.; Fernández-Rodríguez, J.M.; Suescum-Morales, D. Accelerated Carbonation of Vibro-Compacted Porous Concrete for Eco-Friendly Precast Elements. Materials 2023, 16, 2995. [Google Scholar] [CrossRef]
- Suescum-Morales, D.; Fernández-Ledesma, E.; González-Caro, Á.; Merino-Lechuga, A.M.; María Fernández-Rodríguez, J.; Ramón Jiménez, J. Carbon Emission Evaluation of CO2 Curing in Vibro-Compacted Precast Concrete Made with Recycled Aggregates. Materials 2023, 16, 2436. [Google Scholar] [CrossRef] [PubMed]
- Irshidat, M.R.; Al-Nuaimi, N. Industrial Waste Utilization of Carbon Dust in Sustainable Cementitious Composites Production. Materials 2020, 13, 3295. [Google Scholar] [CrossRef]
- González-Caro, Á.; Merino-Lechuga, A.M.; Fernández-Ledesma, E.; Fernández-Rodríguez, J.M.; Jiménez, J.R.; Suescum-Morales, D. The Effect of Acanthocardia Tuberculata Shell Powder as Filler on the Performance of Self-Compacting Mortar. Materials 2023, 16, 1734. [Google Scholar] [CrossRef]
- Saiz Martínez, P.; González Cortina, M.; Fernández Martínez, F.; Rodríguez Sánchez, A. Comparative Study of Three Types of Fine Recycled Aggregates from Construction and Demolition Waste (CDW), and Their Use in Masonry Mortar Fabrication. J. Clean. Prod. 2016, 118, 162–169. [Google Scholar] [CrossRef]
- Reig, L.; Tashima, M.M.; Borrachero, M.V.; Monzó, J.; Cheeseman, C.R.; Payá, J. Properties and Microstructure of Alkali-Activated Red Clay Brick Waste. Constr. Build. Mater. 2013, 43, 98–106. [Google Scholar] [CrossRef]
- Kiełbasa, K.; Bayar, Ş.; Varol, E.A.; Sreńscek-Nazzal, J.; Bosacka, M.; Michalkiewicz, B. Thermochemical Conversion of Lignocellulosic Biomass—Olive Pomace—Into Activated Biocarbon for CO2 Adsorption. Ind. Crops Prod. 2022, 187, 115416. [Google Scholar] [CrossRef]
- Serna-Jiménez, J.A.; Luna-Lama, F.; Caballero, Á.; Martín, M.d.l.Á.; Chica, A.F.; Siles, J.Á. Valorisation of Banana Peel Waste as a Precursor Material for Different Renewable Energy Systems. Biomass Bioenergy 2021, 155, 106279. [Google Scholar] [CrossRef]
- Gonzalez-Corominas, A.; Etxeberria, M. Properties of High Performance Concrete Made with Recycled Fine Ceramic and Coarse Mixed Aggregates. Constr. Build. Mater. 2014, 68, 618–626. [Google Scholar] [CrossRef]
- Camarini, G.; Pinheiro, S.M.M.; Tannous, K. Thermal Analysis of Recycled Gypsum from Construction and Demolition Waste. Appl. Mech. Mater. 2013, 260–261, 977–980. [Google Scholar] [CrossRef]
- Esquinas, A.R.; Ramos, C.; Jiménez, J.R.; Fernández, J.M.; de Brito, J. Mechanical Behaviour of Self-Compacting Concrete Made with Recovery Filler from Hot-Mix Asphalt Plants. Constr. Build. Mater. 2017, 131, 114–128. [Google Scholar] [CrossRef]
- Esquinas, A.R.; Ledesma, E.F.; Otero, R.; Jiménez, J.R.; Fernández, J.M. Mechanical Behaviour of Self-Compacting Concrete Made with Non-Conforming Fly Ash from Coal-Fired Power Plants. Constr. Build. Mater. 2018, 182, 385–398. [Google Scholar] [CrossRef]
- Murthy, I.N.; Rao, J.B. Investigations on Physical and Chemical Properties of High Silica Sand, Fe-Cr Slag and Blast Furnace Slag for Foundry Applications. Procedia Environ. Sci. 2016, 35, 583–596. [Google Scholar] [CrossRef]
- Ashraf, W.; Olek, J. Elucidating the Accelerated Carbonation Products of Calcium Silicates Using Multi-Technique Approach. J. CO2 Util. 2018, 23, 61–74. [Google Scholar] [CrossRef]
- Sáez del Bosque, I.F.; Van den Heede, P.; De Belie, N.; Sánchez de Rojas, M.I.; Medina, C. Carbonation of Concrete with Construction and Demolition Waste Based Recycled Aggregates and Cement with Recycled Content. Constr. Build. Mater. 2020, 234, 117336. [Google Scholar] [CrossRef]
- Ait Kaci Azzou, K.; Terbouche, A.; Ait Ramdane-Terbouche, C.; Belkhalfa, H.; Bachari, K.; Hauchard, D.; Mezaoui, D. Electrochemical Performance of New Hybrid Activated Carbon Materials from Binary and Ternary Date-Olive Pits for Supercapacitor Electrodes. J. Energy Storage 2022, 47, 103559. [Google Scholar] [CrossRef]
- Suescum-Morales, D.; Cantador-Fernández, D.; Jiménez, J.R.; Fernández, J.M. Mitigation of CO2 Emissions by Hydrotalcites of Mg3Al-CO3 at 0 °C and High Pressure. Appl. Clay Sci. 2021, 202, 105950. [Google Scholar] [CrossRef]
- Cantador-Fernandez, D.; Suescum-Morales, D.; Esquivel, D.; Jiménez, J.R.; Fernández-Rodriguez, J.M. CO2 Adsorption by Ethane Periodic Mesoporous Organosilica at Low Temperatures and High Pressure. J. Environ. Chem. Eng. 2023, 11, 110582. [Google Scholar] [CrossRef]
- Suescum-Morales, D.; Cantador-Fernández, D.; Ramón Jiménez, J.; María Fernández, J. Potential CO2 Capture in One-Coat Limestone Mortar Modified with Mg3Al–CO3 Calcined Hydrotalcites Using Ultrafast Testing Technique. Chem. Eng. J. 2021, 415, 129077. [Google Scholar] [CrossRef]
- Suescum-Morales, D.; Fernández, D.C.; Fernández, J.M.; Jiménez, J.R. The Combined Effect of CO2 and Calcined Hydrotalcite on One-Coat Limestone Mortar Properties. Constr. Build. Mater. 2021, 280, 122532. [Google Scholar] [CrossRef]
- Frías, M.; Vigil de la Villa, R.; García, R.; Martínez, S.; Villar, E.; Vegas, I. Effect of a High Content in Activated Carbon Waste on Low Clinker Cement Microstructure and Properties. Constr. Build. Mater. 2018, 184, 11–19. [Google Scholar] [CrossRef]
- Bost, P.; Regnier, M.; Horgnies, M. Comparison of the Accelerating Effect of Various Additions on the Early Hydration of Portland Cement. Constr. Build. Mater. 2016, 113, 290–296. [Google Scholar] [CrossRef]
- JCPDS. Joint Committee on Power Diffraction Standard-International Centre for Diffraction; JCPDS: Newtown Square, PA, USA, 2003. [Google Scholar]
- Zhang, K.; Yio, M.; Wong, H.; Buenfeld, N. Development of More Accurate Methods for Determining Carbonation Depth in Cement-Based Materials. Cem. Concr. Res. 2024, 175, 107358. [Google Scholar] [CrossRef]
- Giulietti, N.; Chiariotti, P.; Cosoli, G.; Mobili, A.; Pandarese, G.; Tittarelli, F.; Revel, G.M. Automated Measurement System for Detecting Carbonation Depth: Image-Processing Based Technique Applied to Concrete Sprayed with Phenolphthalein. Measurement 2021, 175, 109142. [Google Scholar] [CrossRef]
- Gupta, S.; Kua, H.W.; Pang, S.D. Biochar-Mortar Composite: Manufacturing, Evaluation of Physical Properties and Economic Viability. Constr. Build. Mater. 2018, 167, 874–889. [Google Scholar] [CrossRef]
- Rostami, V.; Shao, Y.; Boyd, A.J. Carbonation Curing versus Steam Curing for Precast Concrete Production. J. Mater. Civil. Eng. 2012, 24, 1221–1229. [Google Scholar] [CrossRef]
- Sadeek, S.A.; Mohammed, E.A.; Shaban, M.; Abou Kana, M.T.H.; Negm, N.A. Synthesis, Characterization and Catalytic Performances of Activated Carbon-Doped Transition Metals during Biofuel Production from Waste Cooking Oils. J. Mol. Liq. 2020, 306, 112749. [Google Scholar] [CrossRef]
- PCTPro: Installation and Operations Manual; Merck: Darmstadt, Germany, 2012.
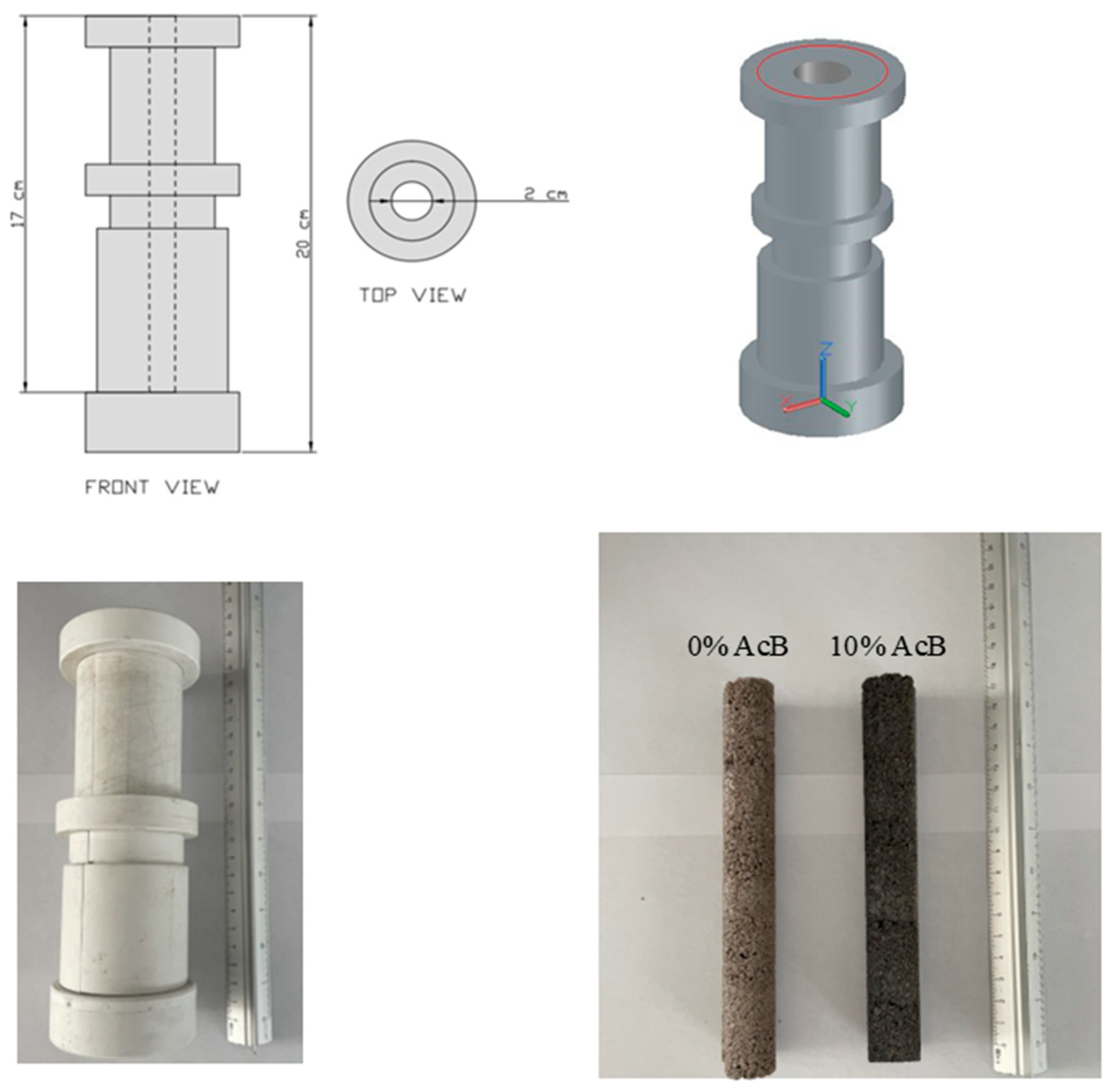


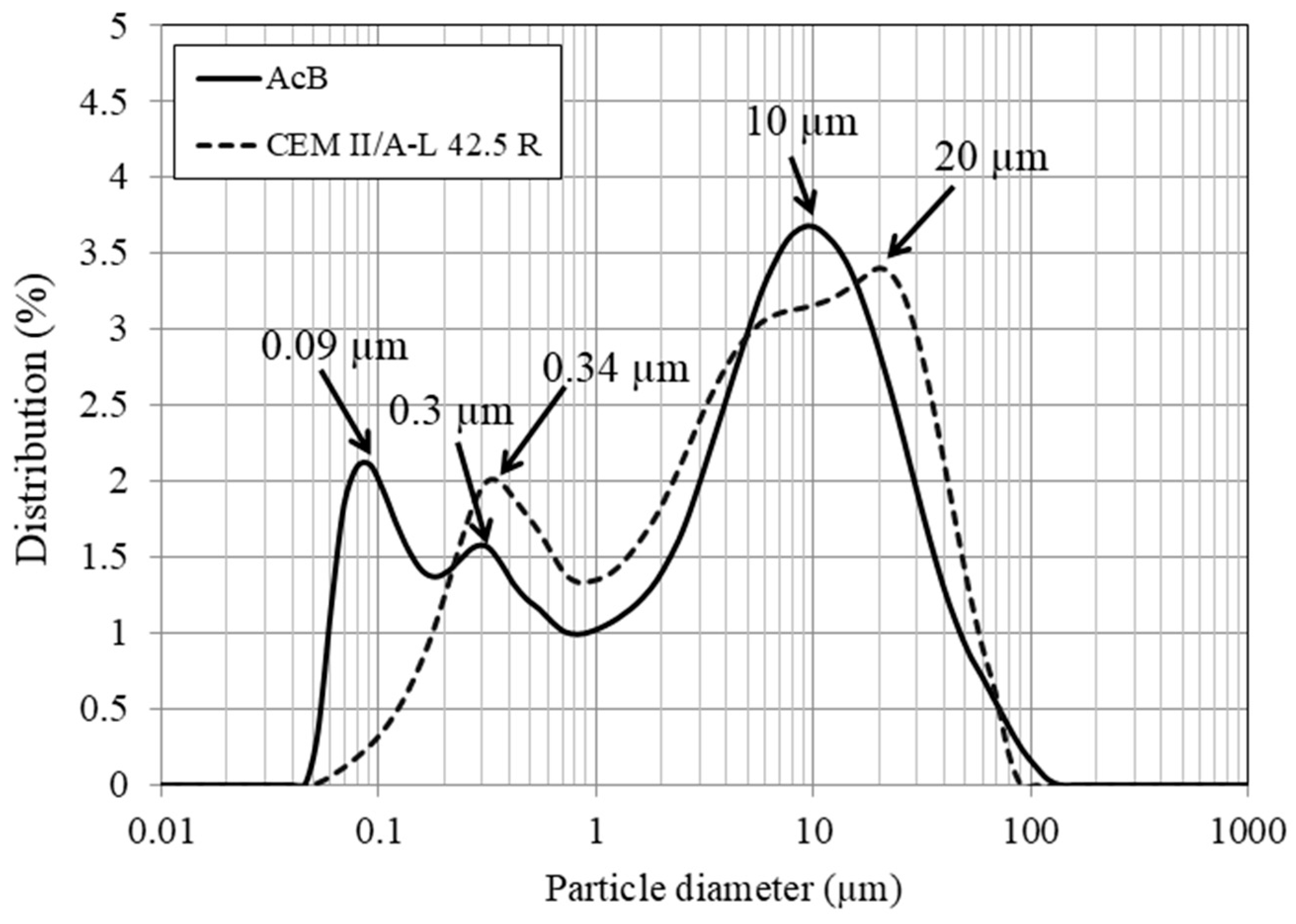
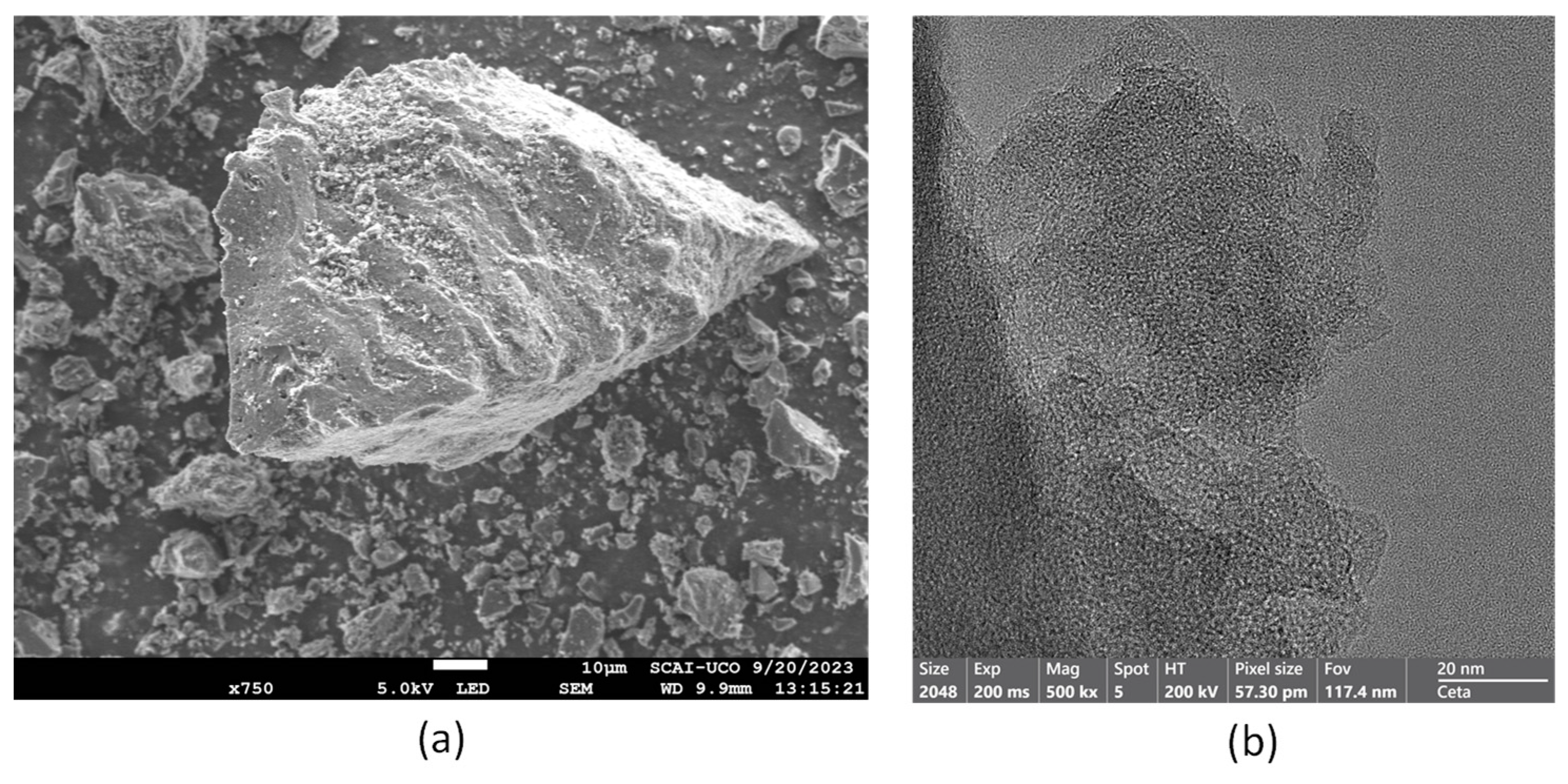
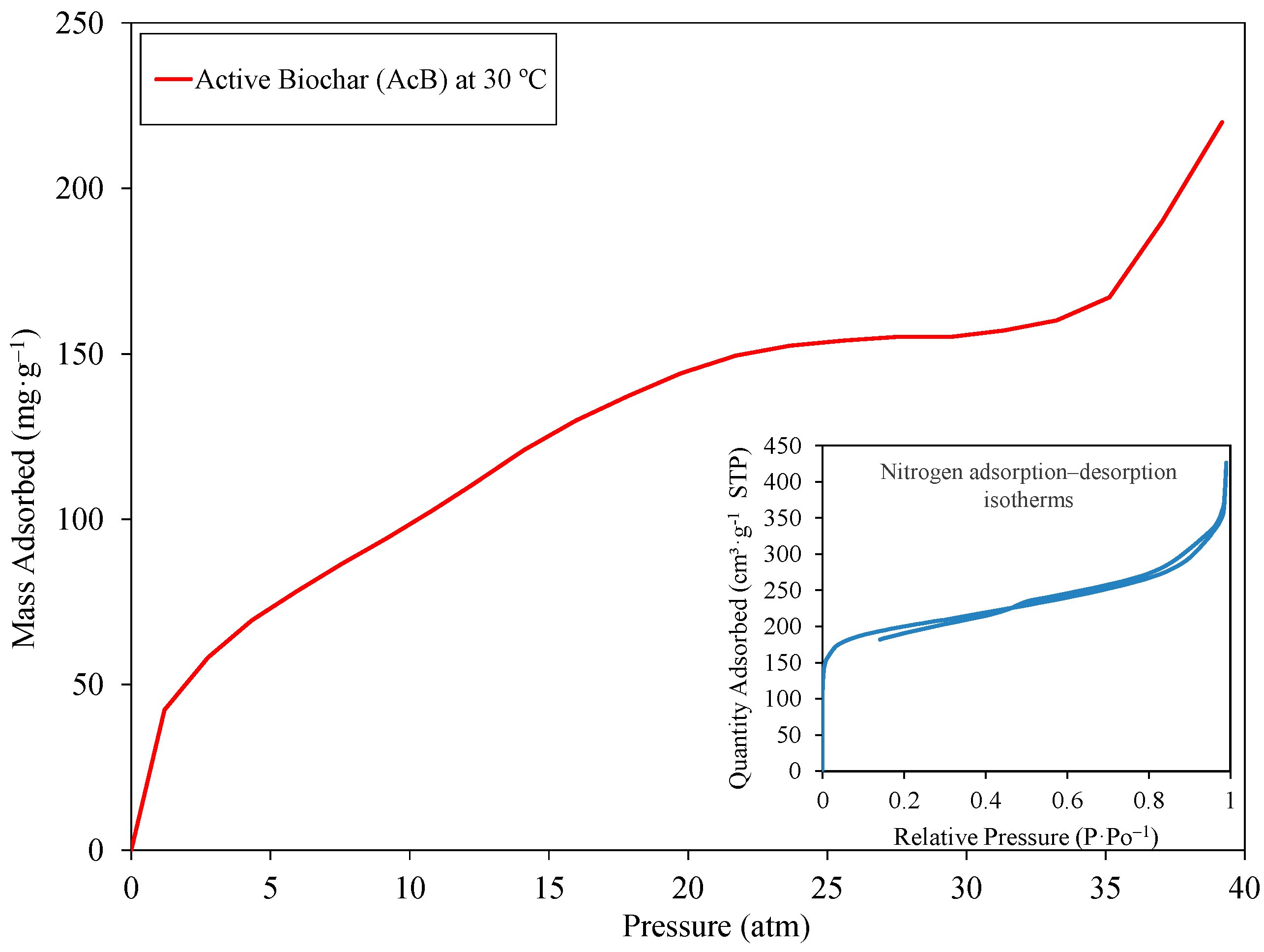



| Material | Dry Bulk Density g/cm3 | Water Absorption % | Sand Equivalent % |
|---|---|---|---|
| RMA | 2.14 | 9.00 | 0.2 |
| PM-0 (kg/m3) | PM-5 (kg/m3) | PM-10 (kg/m3) | |
|---|---|---|---|
| Fraction > 4 | - | - | - |
| Fraction 2/4 | 241.1 | 241.1 | 241.1 |
| Fraction 1/2 | 534.6 | 534.6 | 534.6 |
| Fraction 0.5/1 | 658.1 | 658.1 | 658.1 |
| Fraction 0.25/0.5 | - | - | - |
| Fraction 0.125/0.25 | 174.7 | 174.7 | 174.7 |
| Fraction < 0.125 | - | - | - |
| CEM II/A-L 42.5R | 481.7 | 481.7 | 481.7 |
| Activated Carbon | 0 | 14.4 | 28.8 |
| Absorption water * | 144.8 | 144.8 | 144.8 |
| Effective water | 192.7 | 192.7 | 192.7 |
| w/c ** | 0.3 | 0.3 | 0.3 |
| Oxides | RMA | Cement | AcB |
|---|---|---|---|
| F2O | 0.74 | - | - |
| Na2O | 0.71 | 0.36 | 0.04 |
| MgO | 1.65 | 1.42 | 0.24 |
| Al2O3 | 10.79 | 3.19 | 0.05 |
| SiO2 | 34.44 | 13.48 | 0.18 |
| P2O5 | 0.12 | 0.07 | 0.16 |
| SO3 | 2.52 | 3.17 | 0.17 |
| Cl- | 0.05 | 0.05 | 0.20 |
| K2O | 2.18 | 0.90 | 0.71 |
| CaO | 12.18 | 51.49 | 1.20 |
| TiO2 | 0.54 | 0.12 | - |
| Cr2O3 | 0.02 | - | - |
| MnO2 | 0.06 | 0.08 | - |
| Fe2O3 | 3.55 | 2.10 | 0.06 |
| NiO | - | 0.02 | - |
| ZnO | 0.02 | - | - |
| SrO | 0.03 | 0.05 | - |
| BaO | 0.03 | - | - |
| Balance CO2 | 30.61 | 23.50 | 96.98 |
| Total | 100.00 | 100.00 | 100.00 |
| Age | Mix | CaCO3 | CO2 Uptake | CO2 Uptake |
|---|---|---|---|---|
| (%) | (%) | (g/t) | ||
| PM-0%–1D-NCC | 6.66 | 2.44 | 24,662.00 | |
| 1 Day | PM-0%-1D-ACC | 9.13 | ||
| PM-5%-1D-NCC | 6.79 | 3.38 | 33,783.31 | |
| PM-5%-1D-ACC | 10.16 | |||
| PM-10%-1D-NCC | 6.85 | 4.64 | 46,432.11 | |
| PM-10%-1D-ACC | 11.49 | |||
| PM-0%-3D-NCC | 7.04 | 2.56 | 25,564.77 | |
| 3 Days | PM-0%-3D-ACC | 9.59 | ||
| PM-5%-3D-NCC | 7.38 | 3.86 | 38,575.77 | |
| PM-5%-3D-ACC | 11.24 | |||
| PM-10%-3D-NCC | 7.19 | 4.74 | 47,445.87 | |
| PM-10%-3D-ACC | 11.93 | |||
| 7 Days | PM-0%-7D-NCC | 7.31 | 2.88 | 28,797.82 |
| PM-0%-7D-ACC | 10.19 | |||
| PM-5%-7D-NCC | 7.47 | 5.37 | 53,743.46 | |
| PM-5%-7D-ACC | 12.85 | |||
| PM-10%-7D-NCC | 7.73 | 7.10 | 71,026.66 | |
| PM-10%-7D-ACC | 14.83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merino-Lechuga, A.M.; González-Caro, Á.; Caballero, Á.; Jiménez, J.R.; Fernández-Rodrígez, J.M.; Suescum-Morales, D. Porous Mortars Incorporating Active Biochar from Olive Stone Waste and Recycled Masonry Aggregate: Effects of Accelerated Carbonation Curing. Materials 2025, 18, 904. https://doi.org/10.3390/ma18040904
Merino-Lechuga AM, González-Caro Á, Caballero Á, Jiménez JR, Fernández-Rodrígez JM, Suescum-Morales D. Porous Mortars Incorporating Active Biochar from Olive Stone Waste and Recycled Masonry Aggregate: Effects of Accelerated Carbonation Curing. Materials. 2025; 18(4):904. https://doi.org/10.3390/ma18040904
Chicago/Turabian StyleMerino-Lechuga, Antonio Manuel, Ágata González-Caro, Álvaro Caballero, José Ramón Jiménez, José María Fernández-Rodrígez, and David Suescum-Morales. 2025. "Porous Mortars Incorporating Active Biochar from Olive Stone Waste and Recycled Masonry Aggregate: Effects of Accelerated Carbonation Curing" Materials 18, no. 4: 904. https://doi.org/10.3390/ma18040904
APA StyleMerino-Lechuga, A. M., González-Caro, Á., Caballero, Á., Jiménez, J. R., Fernández-Rodrígez, J. M., & Suescum-Morales, D. (2025). Porous Mortars Incorporating Active Biochar from Olive Stone Waste and Recycled Masonry Aggregate: Effects of Accelerated Carbonation Curing. Materials, 18(4), 904. https://doi.org/10.3390/ma18040904









