Interface Effects on the Electronic and Optical Properties of Graphitic Carbon Nitride (g-C3N4)/SnS2: First-Principles Studies
Highlights
- The g-C3N4/SnS2 has an indirect band gap smaller than the g-C3N4 and SnS2 monolayers.
- There is a typical type-Ⅱ band alignment at the g-C3N4/SnS2 hetero-interface.
- Without a high barrier height, there is effective carrier transformation.
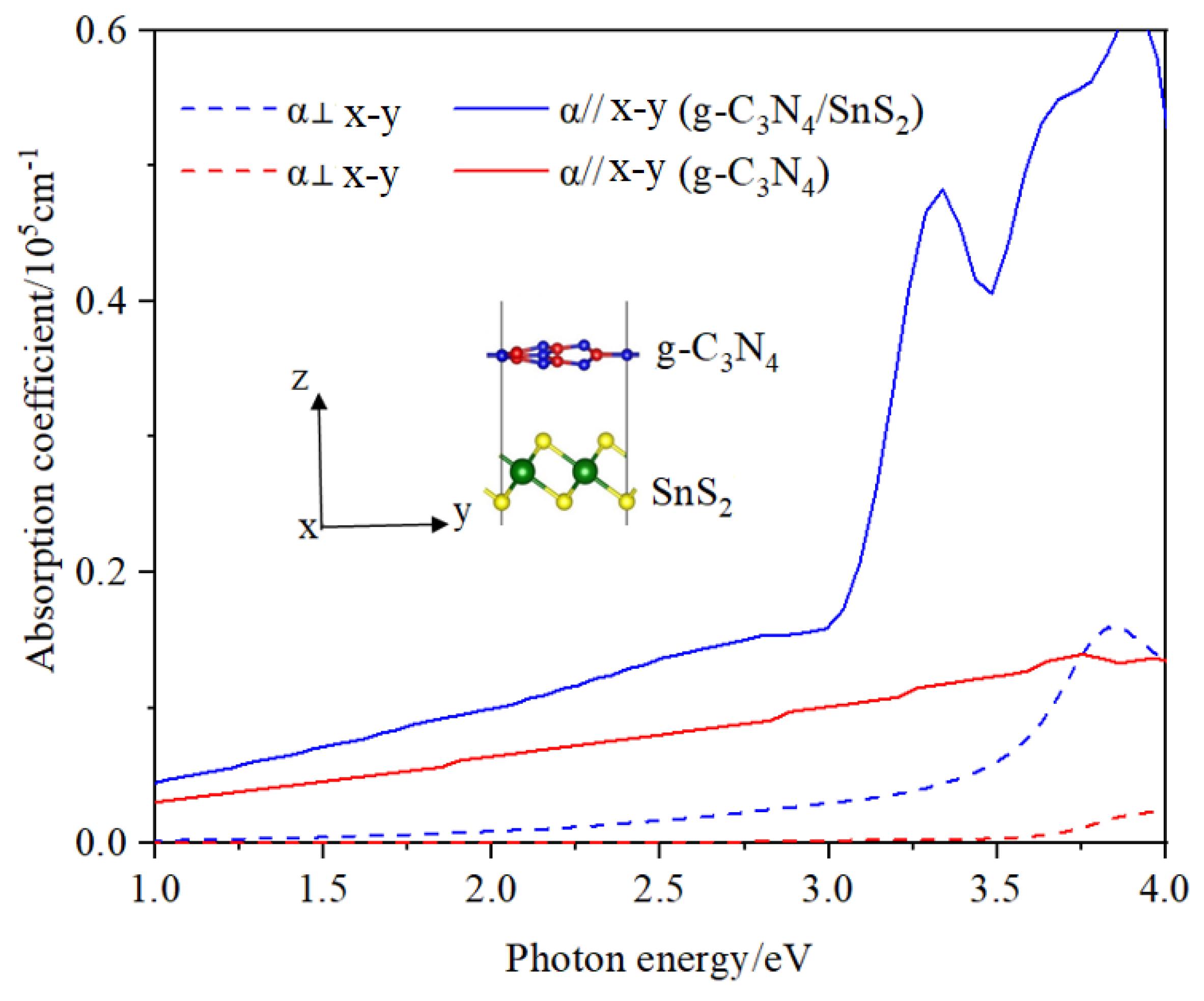
- The absorption spectrum is dependent on the polarization direction.
Abstract
1. Introduction
2. Computational Methods
3. Results and Discussion
3.1. Geometric Structures
3.2. Fat Band and Density of States (DOS)
3.3. Charge Transport
3.4. Optical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bachvarova-Nedelcheva, A.; Iordanova, R.; Kaneva, N. The Solvent Role for the Decomposition of Paracetamol in Distilled and Drinking Water by Pure and Ag-Modified TiO2 Sol–Gel Powders. Materials 2024, 17, 1791. [Google Scholar] [CrossRef]
- Kuranov, D.; Grebenkina, A.; Bogdanova, A.; Platonov, V.; Polomoshnov, S.; Krivetskiy, V.; Rumyantseva, M. Effect of Donor Nb(V) Doping on the Surface Reactivity, Electrical, Optical and Photocatalytic Properties of Nanocrystalline TiO2. Materials 2024, 17, 375. [Google Scholar] [CrossRef]
- Vrithias, N.R.; Katsara, K.; Papoutsakis, L.; Papadakis, V.M.; Viskadourakis, Z.; Remediakis, I.N.; Kenanakis, G. Three-Dimensional-Printed Photocatalytic Sponges Decorated with Mn-Doped ZnO Nanoparticles. Materials 2023, 16, 5672. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antoniett, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef]
- Liu, B.; Nie, X.; Tang, Y.; Yang, S.; Bian, L.; Don, F.G.; He, H.; Zhou, Y.; Liu, K. Objective findings on the K-doped g-C3N4 photocatalysts: The presence and influence of organic byproducts on K-doped g-C3N4 photocatalysis. Langmuir 2021, 37, 4859–4868. [Google Scholar] [CrossRef]
- Zhao, C.; Ding, C.; Han, C.; Yang, X.; Xu, J. Lignin-incorporated supramolecular copolymerization yielding g-C3N4 nanoarchitectures for efficient photocatalytic hydrogen evolution. Sol. RRL 2021, 5, 2000486. [Google Scholar] [CrossRef]
- Li, N.; Huang, M.; Zhou, J.; Liu, M.; Jing, D. MgO and Au nanoparticle Co-modified g-C3N4 photocatalysts for enhanced photoreduction of CO2 with H2O. Chin. J. Catal. 2021, 42, 781–794. [Google Scholar] [CrossRef]
- Li, D.; Huang, J.; Li, R.; Chen, P.; Chen, D.; Cai, M.; Liu, H.; Feng, Y.; Lv, W.; Liu, G. Synthesis of a carbon dots modified g-C3N4/SnO2 Z-scheme photocatalyst with superior photocatalytic activity for PPCPs degradation under visible light irradiation. J. Hazard. Mater. 2021, 401, 123257. [Google Scholar] [CrossRef]
- Liu, X.; Chen, J.; Yang, L.; Yun, S.; Que, M.; Zheng, H.; Zhao, Y.; Yang, T.; Liu, Z. 2D/2D g-C3N4/TiO2 with exposed (001) facets Z-Scheme composites accelerating separation of interfacial charge and visible photocatalytic degradation of rhodamine B. J. Phys. Chem. Solids 2022, 160, 110339. [Google Scholar] [CrossRef]
- Chen, Z.; Guo, F.; Sun, H.; Shi, Y.; Shi, W. Well-designed three-dimensional hierarchical hollow tubular g-C3N4/ZnIn2S4 nanosheets heterostructure for achieving efficient visible-light photocatalytic hydrogen evolution. J. Colloid Interface Sci. 2022, 607, 1391–1401. [Google Scholar] [CrossRef]
- Rugma, T.P.; Aakash, W.; Vijayarajan, V.S.; Sandeep, K.L.; Neppolian, B. synergistic hydrogen evolution activity of NiO/g-C3N4 photocatalysts under direct solar light irradiation. Mater. Lett. 2021, 302, 130292. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, J.; Zhang, M.; Yuan, Q.; Dong, B. Ultrathin hexagonal SnS2 nanosheets coupled with g-C3N4 nanosheets as 2D/2D heterojunction photocatalysts toward high photocatalytic activity. Appl. Catal. B-Environ. 2015, 163, 298–305. [Google Scholar] [CrossRef]
- Liu, E.; Chen, J.; Ma, Y.; Feng, J.; Jia, J.; Fan, J.; Hu, X. Fabrication of 2D SnS2/g-C3N4 heterojunction with enhanced H2 evolution during photocatalytic water splitting. J. Colloid Interface Sci. 2018, 524, 313–324. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 1993, 47, 558–561. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular-dynamics simulation of the liquid-metal-amorphous-semiconductor transition in germanium. Phys. Rev. B 1994, 49, 14251–14269. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mat. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Bučko, T.; Hafner, J.; Lebègue, S.; Ángyán, J.G. Improved description of the structure of molecular and layered crystals: Ab initio DFT calculations with van der waals corrections. J. Phys. Chem. A 2010, 114, 11814–11824. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillonin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Zhu, B.; Tan, H.; Fan, J.; Cheng, B.; Yu, J.; Ho, W. Tuning the strength of built-in electric field in 2D/2D g-C3N4/SnS2 and g-C3N4/ZrS2 S-scheme heterojunctions by nonmetal doping. J. Mater. 2021, 7, 988–997. [Google Scholar] [CrossRef]
- Yang, K.; Deng, Z.Y.; Feng, H.J. Polarization tunable photogenerated carrier transfer of CH3NH3PbI3/polyvinylidene fluoride heterostructure. Appl. Phys. Lett. 2017, 111, 143902. [Google Scholar] [CrossRef]
- Liu, J.; Hua, E. High photocatalytic activity of heptazine-based g-C3N4/SnS2 heterojunction and its origin: Insights from hybrid DFT. J. Phys. Chem. C 2017, 121, 25827–25835. [Google Scholar] [CrossRef]
- Gajdoš, M.; Hummer, K.; Kresse, G.; Furthmüller, J.; Bechstedt, F. Linear optical properties in the projector-augmented wave methodology. Phys. Rev. B 2006, 73, 045112. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qu, L.-H.; Wang, Y.; Xia, S.-W.; Nie, R.; Yin, L.; Zhong, C.-G.; Zhang, S.-L.; Zhang, J.-M.; Xie, Y. Interface Effects on the Electronic and Optical Properties of Graphitic Carbon Nitride (g-C3N4)/SnS2: First-Principles Studies. Materials 2025, 18, 892. https://doi.org/10.3390/ma18040892
Qu L-H, Wang Y, Xia S-W, Nie R, Yin L, Zhong C-G, Zhang S-L, Zhang J-M, Xie Y. Interface Effects on the Electronic and Optical Properties of Graphitic Carbon Nitride (g-C3N4)/SnS2: First-Principles Studies. Materials. 2025; 18(4):892. https://doi.org/10.3390/ma18040892
Chicago/Turabian StyleQu, Li-Hua, Yu Wang, Si-Wen Xia, Ran Nie, Le Yin, Chong-Gui Zhong, Sheng-Li Zhang, Jian-Min Zhang, and You Xie. 2025. "Interface Effects on the Electronic and Optical Properties of Graphitic Carbon Nitride (g-C3N4)/SnS2: First-Principles Studies" Materials 18, no. 4: 892. https://doi.org/10.3390/ma18040892
APA StyleQu, L.-H., Wang, Y., Xia, S.-W., Nie, R., Yin, L., Zhong, C.-G., Zhang, S.-L., Zhang, J.-M., & Xie, Y. (2025). Interface Effects on the Electronic and Optical Properties of Graphitic Carbon Nitride (g-C3N4)/SnS2: First-Principles Studies. Materials, 18(4), 892. https://doi.org/10.3390/ma18040892




