Understanding the Effect of Waiting for the Dissolution of Sodium Hydroxide in Geopolymer Concrete Mixes
Highlights
- Optimised mixing regimes and temperature-controlled alkaline activators significantly enhance the mechanical properties and durability of geopolymer concrete.
- Alkaline activator temperature control and systematic ingredient addition order are critical parameters governing compressive strength development in geopolymer concrete.
- Segregation and early hydration issues in specific mixes necessitate careful ingredient monitoring during preparation.
- Mix G-(0.5W-S) demonstrates superior strength performance compared to other formulations.
- Carefully controlled mixing regimes are essential for the successful practical implementation of geopolymer concrete.
Abstract
1. Introduction
2. Materials
3. Methodology
3.1. Mix Design
3.1.1. Approach 1: Optimizing the Preparation of the Alkaline Activator
3.1.2. Approach 2: Optimizing the Mixing Order of the Geopolymer Concrete (GPC) Ingredients
3.2. Alkaline Activator Preparation
3.3. Geopolymer Concrete Specimen Preparation and Testing Methods
4. Results and Discussion
4.1. Alkaline Activator Temperature (Approach 1)
4.2. Workability
4.3. Unconfined Compressive Strength
5. Conclusions
- ▪
- The use of an alkaline activator is crucial for the geopolymerization process; its temperature at the time of mixing needs to be controlled to ensure proper reaction kinetics, essential for achieving optimal compressive strength in geopolymer concrete.
- ▪
- The temperature of the SH solution increased significantly after adding SF due to the start of the exothermic chemical rection to form the new bonds of the sodium silicate alternative and water.
- ▪
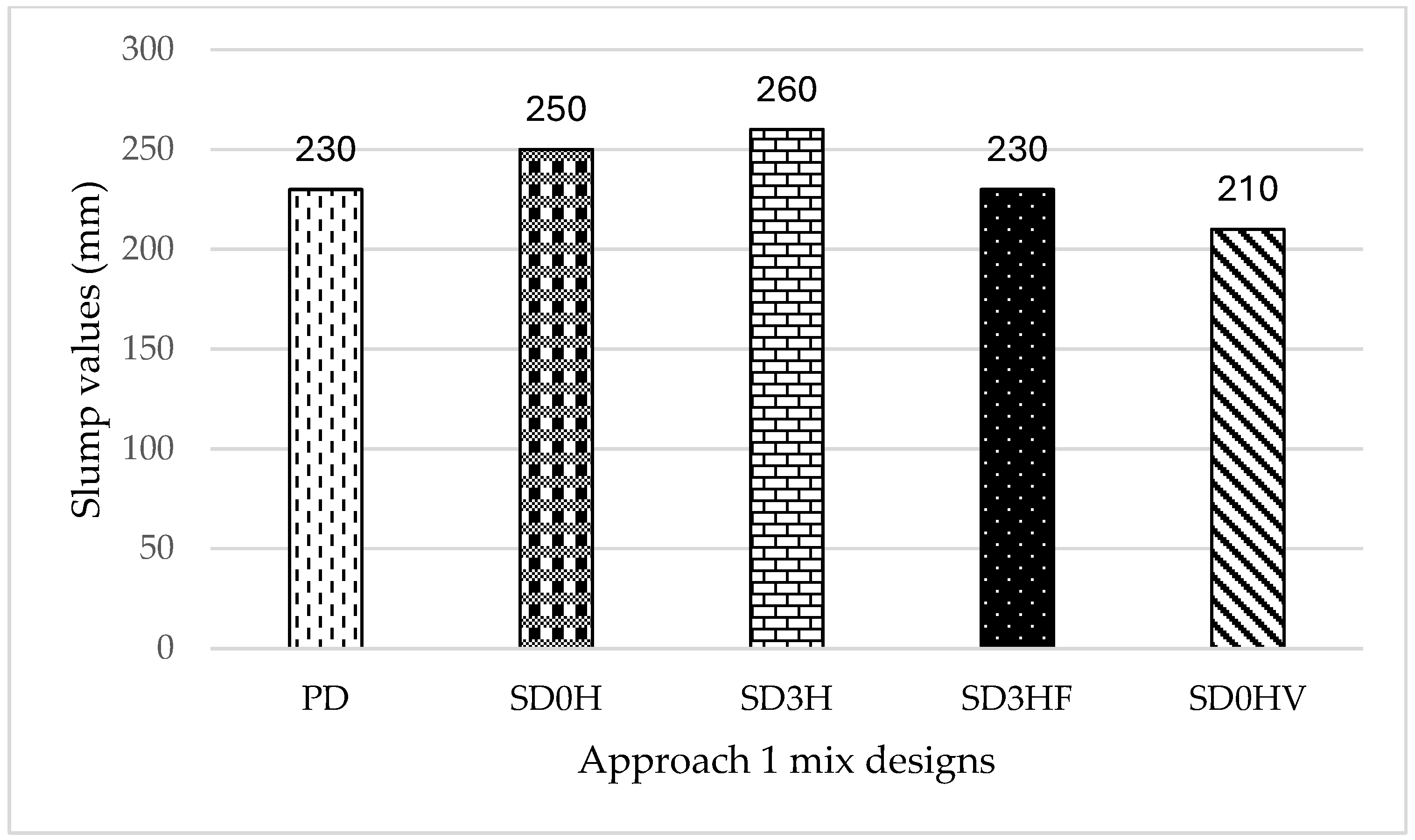
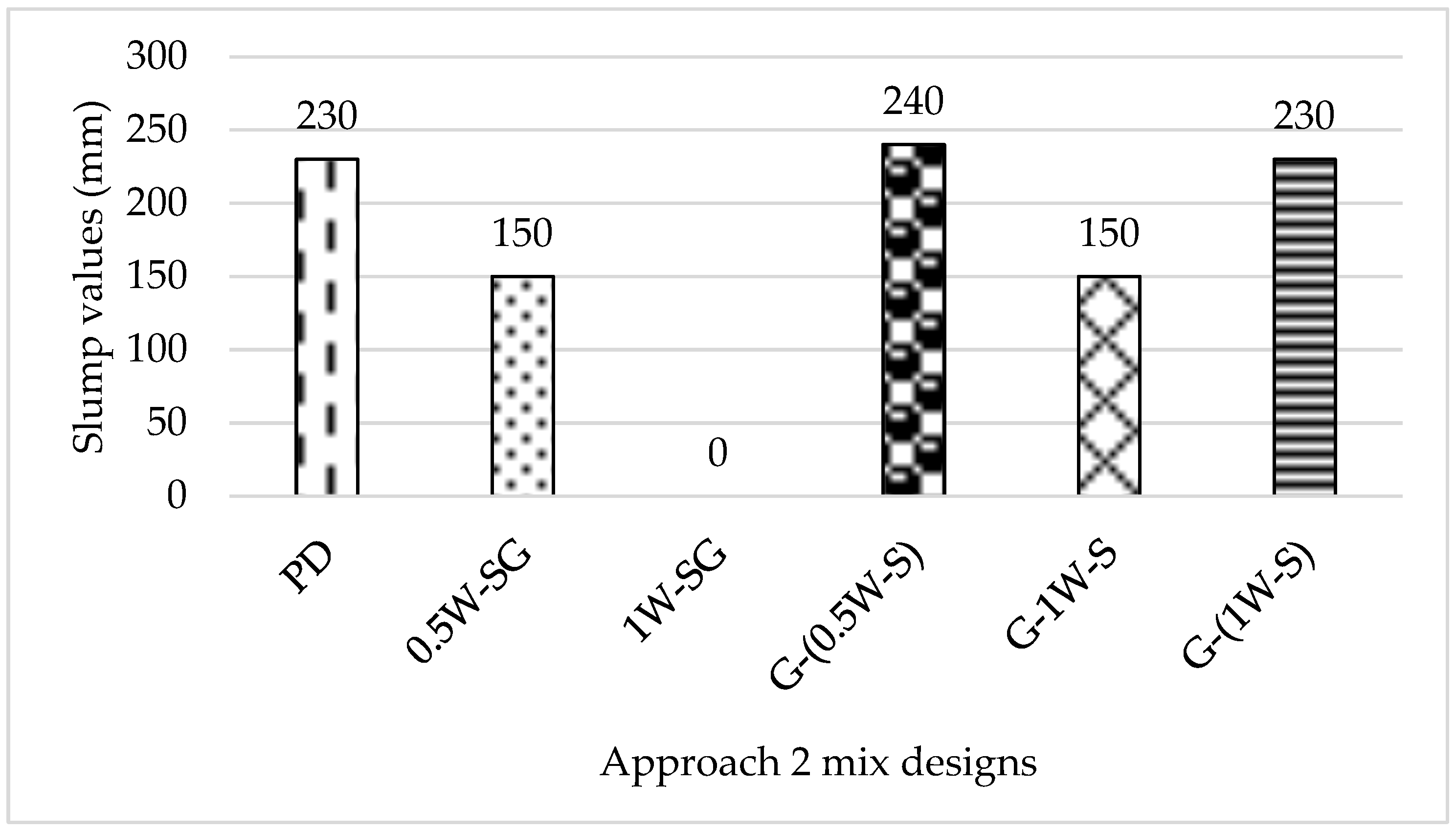
- All mixes exhibited satisfactory workability properties ranging between 150 mm and 260 mm, except those that did not contain the NaOH solution (SD0HV and 1W-SG).
- ▪
- High segregation was shown by mix (SD0HV), where no alkaline activator preparation was required beforehand; instead, the geopolymer ingredients were added to the mix in various orders.
- ▪
- Mixing the total water content with the NaOH pellets, GGBS, and SF caused mix (1W-SG) to fail at the first stage (during chemical preparation) due to the early start of the hydration process, with the presence of GGBS aided by the heat generated from the SH dissolution in water.
- ▪
- The highest slump value was achieved in mix (SD3H), which contained an alkaline activator with a temperature equivalent to room temperature after cooling for 2 h.
- ▪
- The alkaline activator used to prepare GPC with acceptable fresh and hardened properties does not necessarily need 24 h to fully reach; it can be used when it has cooled and reached room temperature.
- ▪
- An inverse relation was observed between the alkaline activator temperature at mixing time and the compressive strength value—as the alkaline activator temperature decreased, the compressive strength value increased.
- ▪
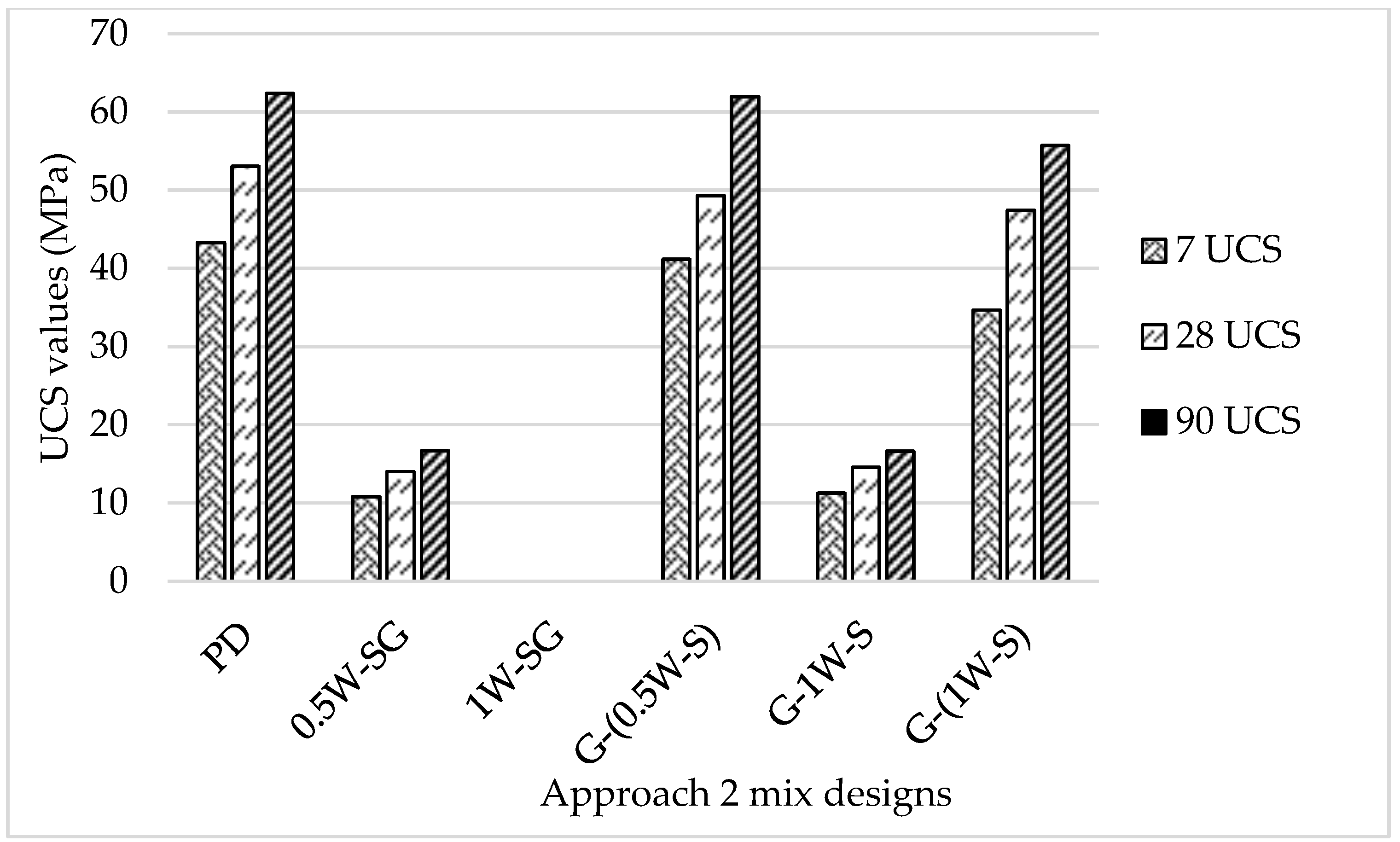
- The mixes consisting of alkaline activators achieved the highest compressive strength values despite the varying concentrations of NaOH and SF in the mix.
- ▪
- The compressive strength values of all mixes continued to increase with time.
- ▪
- Up to 64% of the compressive strength was achieved at 7 days due to the relatively high content of MgO in the GGBS, which contributed to the formation of the hydrotalcite (Mg6Al2(OH)16CO3), which plays a significant role in the early strength development of slag-based geopolymer concrete (Wang et al., 2020) [32].
- ▪
- Mix G-(0.5W-S) (which was formulated by adding the aggregates + GGBS, then half of the water mixed with NaOH pellets + SF, and then the other half of water) was considered the optimized mix in terms of its mechanical performance and properties.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scrivener, K.L.; John, V.M.; Gartner, E.M.; UN Environment. Eco-efficient cements: Potential economically viable solutions for a low-CO2 cement-based materials industry. Cem. Concr. Res. 2018, 114, 2–26. [Google Scholar] [CrossRef]
- Rodríguez-Benavides, D.; Andrés-Rosales, R.; Álvarez-García, J.; Bekun, F.V. Convergence of clubs between per capita carbon dioxide emissions from fossil fuels and cement production. Energy Policy 2024, 186, 114007. [Google Scholar] [CrossRef]
- Supriya; Chaudhury, R.; Sharma, U.; Thapliyal, P.C.; Singh, L.P. Low-CO2 emission strategies to achieve net zero target in cement sector. J. Clean. Prod. 2023, 417, 137466. [Google Scholar] [CrossRef]
- Huseien, G.F.; Khamehchi, M.; Kubba, Z.; ∙Benjeddou, O.; ∙Mahmoodi, M.J. Freeze-thaw cycle and abrasion resistance of alkali-activated FA and POFA-based mortars: Role of high volume GBFS incorporation. Heliyon 2023, 9, e17672. [Google Scholar] [CrossRef] [PubMed]
- Huseien, G.F.; Sam, A.R.M.; Algaifi, H.A.; Alyousef, R. Development of a sustainable concrete incorporated with effective microorganism and fly Ash: Characteristics and modeling studies. Constr. Build. Mater. 2021, 285, 122899. [Google Scholar] [CrossRef]
- Garces, J.I.T.; Dollente, I.J.; Beltran, A.B.; Tan, R.R.; Promentilla, M.A.B. Life cycle assessment of self-healing geopolymer concrete. Clean. Eng. Technol. 2021, 4, 100147. [Google Scholar] [CrossRef]
- Huang, D.; Yuan, Q.; Chen, P.; Tian, X.; Peng, H. Effect of activator properties on drying shrinkage of alkali-activated fly ash and slag. J. Build. Eng. 2022, 62, 105341. [Google Scholar] [CrossRef]
- Zhang, B.; Xiong, T.; Peng, H. Flexural behavior of FRP bars reinforced seawater coral aggregate concrete beams incorporating alkali-activated materials. Mater. Struct./Mater. Constr. 2024, 57, 26. [Google Scholar] [CrossRef]
- Bodur, B.; Bayraktar, O.Y.; Benli, A.; Kaplan, G.; Tobbala, D.E.; Tayeh, B. Effect of using wastewater from the ready-mixed concrete plant on the performance of one-part alkali-activated GBFS/FA composites: Fresh, mechanical and durability properties. J. Build. Eng. 2023, 76, 107167. [Google Scholar] [CrossRef]
- Tian, L.; He, D.; Zhao, J.; Wang, H. Durability of geopolymers and geopolymer concretes: A review. Reviews on Advanced Materials Science. 2021, 60, 1–14. [Google Scholar] [CrossRef]
- Adediran, A.; Yliniemi, J.; Carvelli, V.; Adesanya, E.; Illikainen, M. Durability of alkali-activated Fe-rich fayalite slag-based mortars subjected to different environmental conditions. Cem. Concr. Res. 2022, 162, 106984. [Google Scholar] [CrossRef]
- Luhar, S.; Nicolaides, D.; Luhar, I. Fire Resistance Behaviour of Geopolymer Concrete: An Overview. Buildings 2021, 11, 82. [Google Scholar] [CrossRef]
- Shi, J.; Wu, M.; Ming, J. Long-term corrosion resistance of reinforcing steel in alkali-activated slag mortar after exposure to marine environments. Corros. Sci. 2021, 179, 109175. [Google Scholar] [CrossRef]
- Fu, Y.; Cai, L.; Yonggen, W. Freeze–thaw cycle test and damage mechanics models of alkali-activated slag concrete. Constr. Build. Mater. 2011, 25, 3144–3148. [Google Scholar] [CrossRef]
- Zhang, Z.; Provis, J.L.; Ma, X.; Reid, A.; Wang, H. Efflorescence and subflorescence induced microstructural and mechanical evolution in fly ash-based geopolymers. Cem. Concr. Compos. 2018, 92, 165–177. [Google Scholar] [CrossRef]
- Asghar, R.; Khan, M.A.; Alyousef, R.; Javed, M.F.; Ali, M. Promoting the green Construction: Scientometric review on the mechanical and structural performance of geopolymer concrete. Constr. Build. Mater. 2023, 368, 130502. [Google Scholar] [CrossRef]
- Ranjbar, N.; Kuenzel, C.; Spangenberg, J.; Mehrali, M. Hardening evolution of geopolymers from setting to equilibrium: A review. Cem. Concr. Compos. 2020, 114, 103729. [Google Scholar] [CrossRef]
- Mahmood, A.H.; Foster, S.J.; Castel, A. Effects of mixing duration on engineering properties of geopolymer concrete. Constr. Build. Mater. 2021, 303, 124449. [Google Scholar] [CrossRef]
- Gao, X.; Yu, Q.L.; Brouwers, H.J.H. Reaction kinetics, gel character and strength of ambient temperature cured alkali activated slag–fly ash blends. Constr. Build. Mater. 2015, 80, 105–115. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymer Chemistry and Applications, 5th ed.; Geopolymer Institute: Saint-Quentin, France, 2008; 680p, Available online: https://www.researchgate.net/publication/265076752 (accessed on 12 May 2023).
- BS EN 15167-1:2006; Ground Granulated Blast Furnace Slag for Use in Concrete, Mortar and Grout. Part 1: Definitions, Specifications and Conformity Criteria. BSI Standards Ltd.: London, UK, 2006.
- Billong, N.; Kinuthia, J.; Oti, J.; Melo, U.C. Performance of sodium silicate free geopolymers from metakaolin (MK) and Rice Husk Ash (RHA): Effect on tensile strength and microstructure. Constr. Build. Mater. 2018, 189, 307–313. [Google Scholar] [CrossRef]
- BS EN 12620:2002+A1:2008; Aggregates for Concrete. BSI Standards Limited: London, UK, 2008.
- Adeleke, B.O.; Kinuthia, J.M.; Oti, J.; Ebailila, M. Physico-Mechanical Evaluation of Geopolymer Concrete Activated by Sodium Hydroxide and Silica Fume-Synthesised Sodium Silicate Solution. Materials 2023, 16, 2400. [Google Scholar] [CrossRef]
- Billong, N.; Oti, J.; Kinuthia, J. Using silica fume based activator in sustainable geopolymer binder for building application. Constr. Build. Mater. 2021, 275, 122177. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, C.; Zhan, Z.; Li, N. An overview on the reuse of waste glasses in alkali-activated materials. Resour. Conserv. Recycl. 2019, 144, 297–309. [Google Scholar] [CrossRef]
- BS EN 12350-2:2019; Testing Fresh Concrete. Part 1: Slump Test. BSI Standards Ltd.: London, UK, 2019.
- BS EN 206:2013+A2:2021; Concrete Specification, Performance, Production and Conformity. BSI Standards Ltd.: London, UK, 2021.
- BS EN 12390-3:2019; Testing Hardened Concrete. Part 3: Compressive Strength of Test Specimens. BSI Standards Limited: London, UK, 2019.
- Alnahhal, M.F.; Hamdan, A.; Hajimohammadi, A.; Castel, A.; Kim, T. Hydrothermal synthesis of sodium silicate from rice husk ash: Effect of synthesis on silicate structure and transport properties of alkali-activated concrete. Cem. Concr. Res. 2024, 178, 107461. [Google Scholar] [CrossRef]
- Korde, C.; Cruickshank, M.; West, R.P. Activation of slag: A comparative study of cement, lime, calcium sulfate, GGBS fineness and temperature. Mag. Concr. Res. 2020, 73, 15–31. [Google Scholar] [CrossRef]
- Wang, J.; Xie, J.; Wang, C.; Zhao, J.; Liu, F.; Fang, C. Study on the optimum initial curing condition for fly ash and GGBS based geopolymer recycled aggregate concrete. Constr. Build. Mater. 2020, 247, 118540. [Google Scholar] [CrossRef]
- Özbayrak, A.; Kucukgoncu, H.; Aslanbay, H.H.; Aslanbay, Y.G.; Atas, O. Comprehensive experimental analysis of the effects of elevated temperatures in geopolymer concretes with variable alkali activator ratios. J. Build. Eng. 2023, 68, 106108. [Google Scholar] [CrossRef]
- Mohamed, O.A.; Najm, O.; Zuaiter, H.A.; Saleem, S.K.; Ivak, S.; Al-Aribe, K. Effect of activator concentration on setting time, workability and compressive strength of sustainable concrete with alkali-activated slag binder. Mater. Today Proc. 2024; in press. [Google Scholar] [CrossRef]


| Oxide | Composition (wt %) | |
|---|---|---|
| GGBS | SF | |
| SiO2 | 35.54 | 97.1 |
| Al2O3 | 11.46 | 0.2 |
| Fe2O3 | 0.42 | 0.1 |
| Na2O | 0.37 | - |
| CaO | 37.99 | 0.2 |
| MgO | 8.78 | 0.1 |
| K2O | 0.43 | 0.2 |
| TiO2 | 0.7 | - |
| V2O5 | 0.04 | - |
| P2O5 | 0.02 | 0.03 |
| SO3 | 1.54 | 0.1 |
| Mn2O3 | 0.43 | - |
| BaO | 0.09 | - |
| L.O.I | 2.00 | 0.5 |
| Property | GGBS | SF |
|---|---|---|
| Specific gravity | 2.9 | 3.15 |
| Bulk density (kg/m3) | 1200 | 300 |
| Insoluble residue | 0.3 | - |
| Glass content (%) | 90 | - |
| Blaine fineness (m2/kg) | 450 | - |
| Alkalinity value (pH) | 10.4 | 7 |
| Color | Off-white | Grey |
| Physical form | Fine powder | powder |
| Physical Properties | Coarse Aggregate | Fine Aggregate (Sand) | |
|---|---|---|---|
| 20 mm | 10 mm | ||
| Uniformity coefficient (CU) | 1.3 | 3.3 | 0.11 |
| Curvature coefficient (CC) | 7.5 | 1.5 | 1.75 |
| Flakiness index (%) | 23 | 30–35 | - |
| Elongation index (%) | 12 | 17–22 | - |
| Shape index (%) | 7 | 12 | - |
| Impact value | 15 | 23 | - |
| Fineness modulus (mm) | - | 4 | 1.54 |
| Uncompacted bulk density (kg/m3) | 2570 | 1350 | 1500 |
| Pre-dried particle density (kg/m3) | - | 2690 | 2600 |
| Water absorption (%) | 1.1 | 2 | 21 |
| Mix | Description |
|---|---|
| PD | The alkaline activator was prepared 24 h prior to the mixing day. |
| SD0H | Reducing the activator preparation period from the previous day (24 h) to same day mixing, immediately before mixing without cooling. |
| SD3H | Reducing the activator preparation period from the previous day (24 h) to same day mixing, up to 3 h prior to mixing. |
| SD3HF | Reducing the activator preparation period from the previous day (24 h) to same day mixing, aided by cooling in the freezer for up to 3 h. |
| SD0HV | Reducing the activator preparation period from the previous day (24 h) to adding all activator ingredients in various orders during mixing. |
| Mix Design | Alkaline Activator | Precursor (kg) | Sand (kg) | Aggregate (kg) | Water (W3) (L) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| SSA | SH Sol. (kg) | |||||||||
| SH Sol. (mL) | SF (kg) | |||||||||
| Water (W1) (L) | SH Pellets (kg) | Water (W2) (L) | SH Pellets (kg) | 10 mm | 20 mm | |||||
| PD, SD0H, SD3H, SD3HF | 0.455 | 0.182 | 0.281 | 0.455 | 0.182 | 2.67 | 8 | 4 | 8 | 1.29 |
| SD0HV | 0 | 0 | 0.281 | 0 | 0.364 | 2.67 | 8 | 4 | 8 | 2.2 |
| Mix | Description |
|---|---|
| PD | The control mix, the same control mix prepared in Approach 1, consisted of an alkaline activator prepared 24 h prior to the mixing day. |
| 0.5W-SG | Investigating the effect of changing the addition order of the GPC ingredients, starting by adding the aggregates, then the NaOH solution (half water amount + NaOH pellets), and then the mixture of SF and GGBS with the other half of water. |
| 1W-SG | Investigating the effect of changing the addition order of the GPC ingredients, starting by adding the aggregates and then a solution of the total amount of water + NaOH pellets + GGBS + SF. |
| G-(0.5W-S) | Investigating the effect of changing the addition order of the GPC ingredients, starting by adding the aggregates + GGBS, then half of the water mixed with NaOH pellets + SF, and then the other half of water. |
| G-1W-S | Investigating the effect of changing the addition order of the GPC ingredients, starting by adding the aggregates + GGBS to the NaOH solution (total amount of water + NaOH pellets) and then adding SF at the end. |
| G-(1W-S) | Investigating the effect of changing the addition order of the GPC ingredients, starting by adding the aggregates + GGBS, then the slurry (total amount of water + NaOH pellets + SF). |
| Mix Design | Alkaline Activator | Precursor (kg) | Sand (kg) | Aggregate (kg) | Water (W3) (L) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| SSA | SH Sol. (kg) | |||||||||
| SH Sol. (mL) | SF (kg) | |||||||||
| Water (W1) (L) | SH Pellets (kg) | Water (W2) (L) | SH Pellets (kg) | 10 mm | 20 mm | |||||
| PD | 0.455 | 0.182 | 0.281 | 0.455 | 0.182 | 2.67 | 8 | 4 | 8 | 1.29 |
| 0.5W-SG, G-(0.5W-S) | 1.1 | 0.364 | 0.281 | 0 | 0 | 2.67 | 8 | 4 | 8 | 1.1 |
| 1W-SG, G-1W-S, G-(1W-S) | 0 | 0.364 | 0.281 | 0 | 0 | 2.67 | 8 | 4 | 8 | 2.2 |
| Time | Temp. °C (PD) | Temp. °C (SD0H) | Temp. °C (SD3H) | Temp. °C (SD3HF) | Temp. °C (SD0HV) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| NaOH | SSA | NaOH | SSA | NaOH | SSA | NaOH | SSA | NaOH | SSA | |
| Prep. time (0 min.) | 92 | 103 | 91 | 103.4 | 93 | 104.7 | 93 | 105 | No alkaline activator | |
| 30 min. after prep. | N/A | N/A | N/A | N/A | 62 | 77 | 54 | 61 | No alkaline activator | |
| 60 min. after prep. | N/A | N/A | N/A | N/A | 44.6 | 47.6 | 21.4 | 25.7 | No alkaline activator | |
| 90 min. after prep. | N/A | N/A | N/A | N/A | 38 | 39.9 | 14.2 | 12.5 | No alkaline activator | |
| Mixing time | room temp. (25 ± 2) (24 h) | room temp. (25 ± 2) (24 h) | 73.2 (15 min) | 88.6 (15 min) | 31 (2 h) | 32 (2 h) | 15.5 (1 h and 45 min) | 13 (1 h and 45 min) | No alkaline activator | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altameemi, S.; Adeleke, B.O.; Kinuthia, J.M.; Oti, J. Understanding the Effect of Waiting for the Dissolution of Sodium Hydroxide in Geopolymer Concrete Mixes. Materials 2025, 18, 849. https://doi.org/10.3390/ma18040849
Altameemi S, Adeleke BO, Kinuthia JM, Oti J. Understanding the Effect of Waiting for the Dissolution of Sodium Hydroxide in Geopolymer Concrete Mixes. Materials. 2025; 18(4):849. https://doi.org/10.3390/ma18040849
Chicago/Turabian StyleAltameemi, Samara, Blessing O. Adeleke, John M. Kinuthia, and Jonathan Oti. 2025. "Understanding the Effect of Waiting for the Dissolution of Sodium Hydroxide in Geopolymer Concrete Mixes" Materials 18, no. 4: 849. https://doi.org/10.3390/ma18040849
APA StyleAltameemi, S., Adeleke, B. O., Kinuthia, J. M., & Oti, J. (2025). Understanding the Effect of Waiting for the Dissolution of Sodium Hydroxide in Geopolymer Concrete Mixes. Materials, 18(4), 849. https://doi.org/10.3390/ma18040849








