Evolution Mechanism of Multi-Precipitation Regulates Mechanical Properties and High-Temperature Strength in Medium-Alloy Structural Steel
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
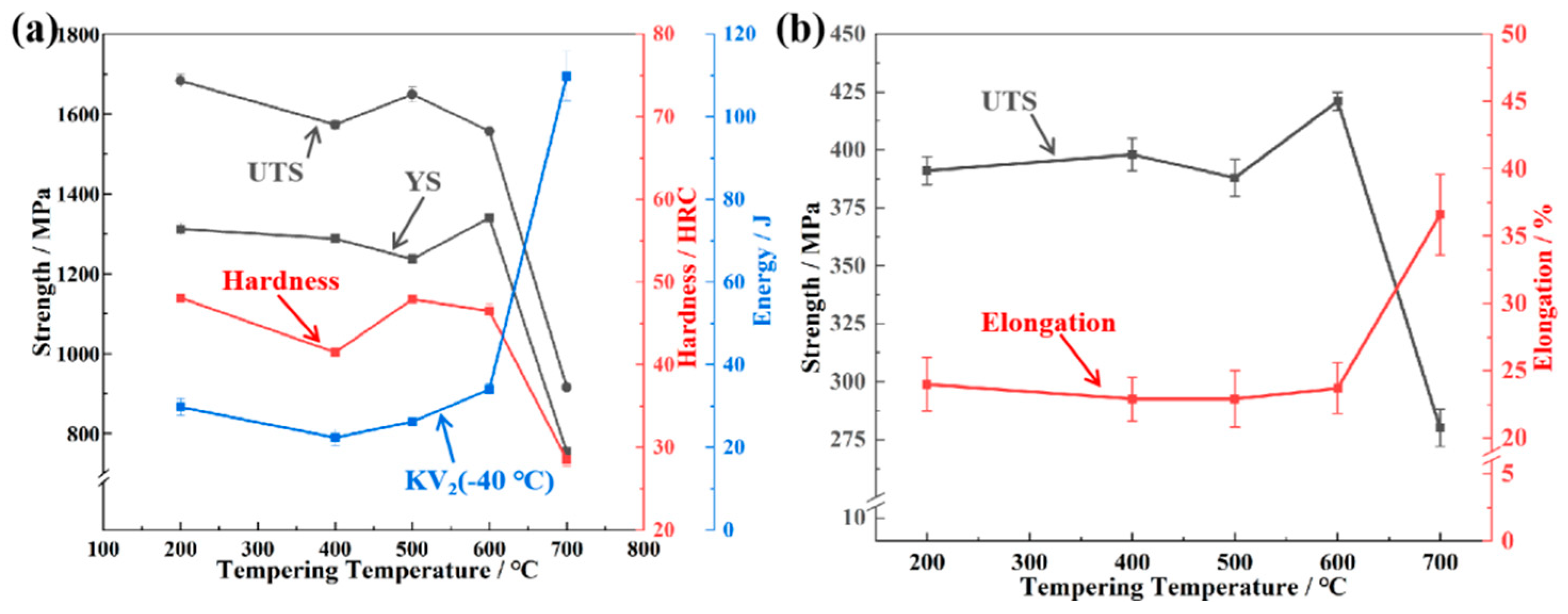
3.1. Mechanical Properties and High-Temperature Strength
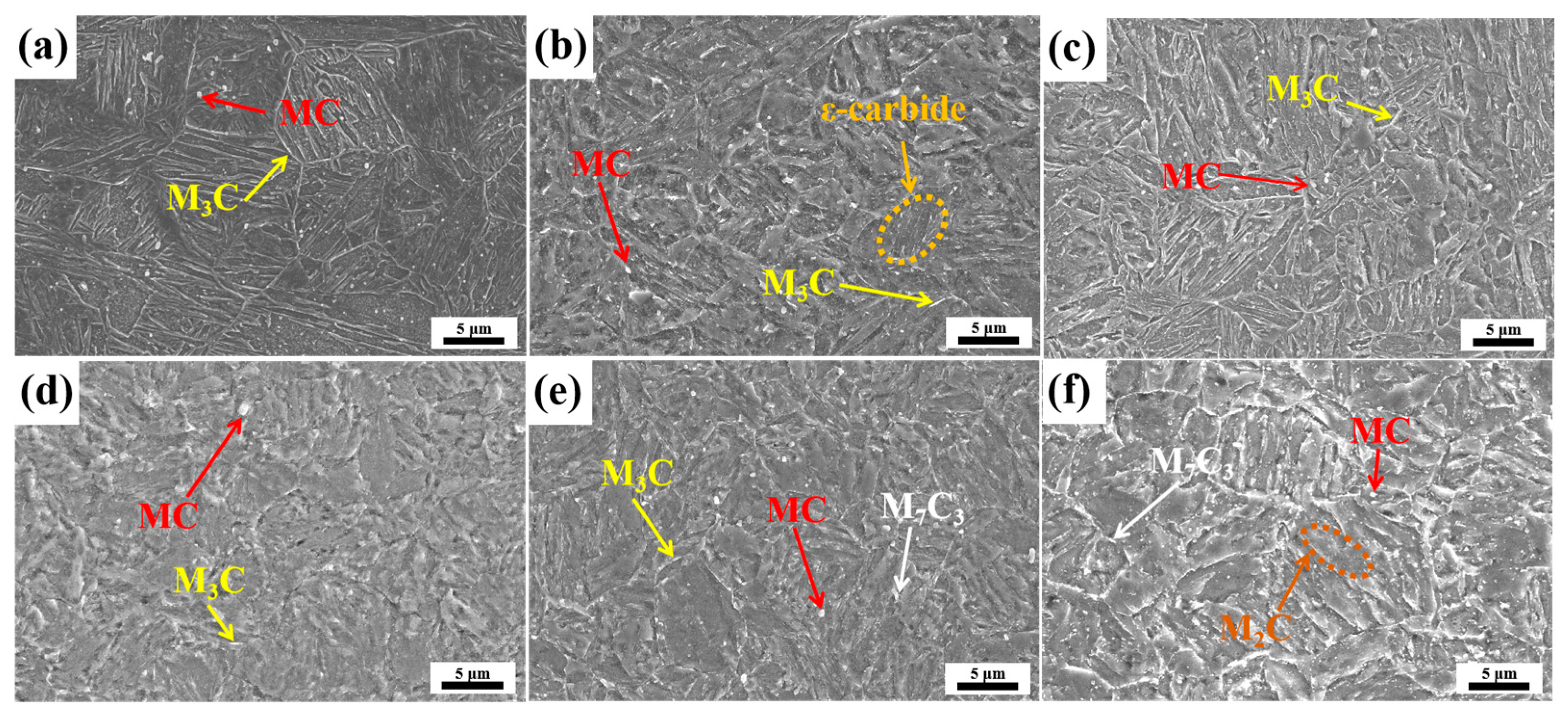
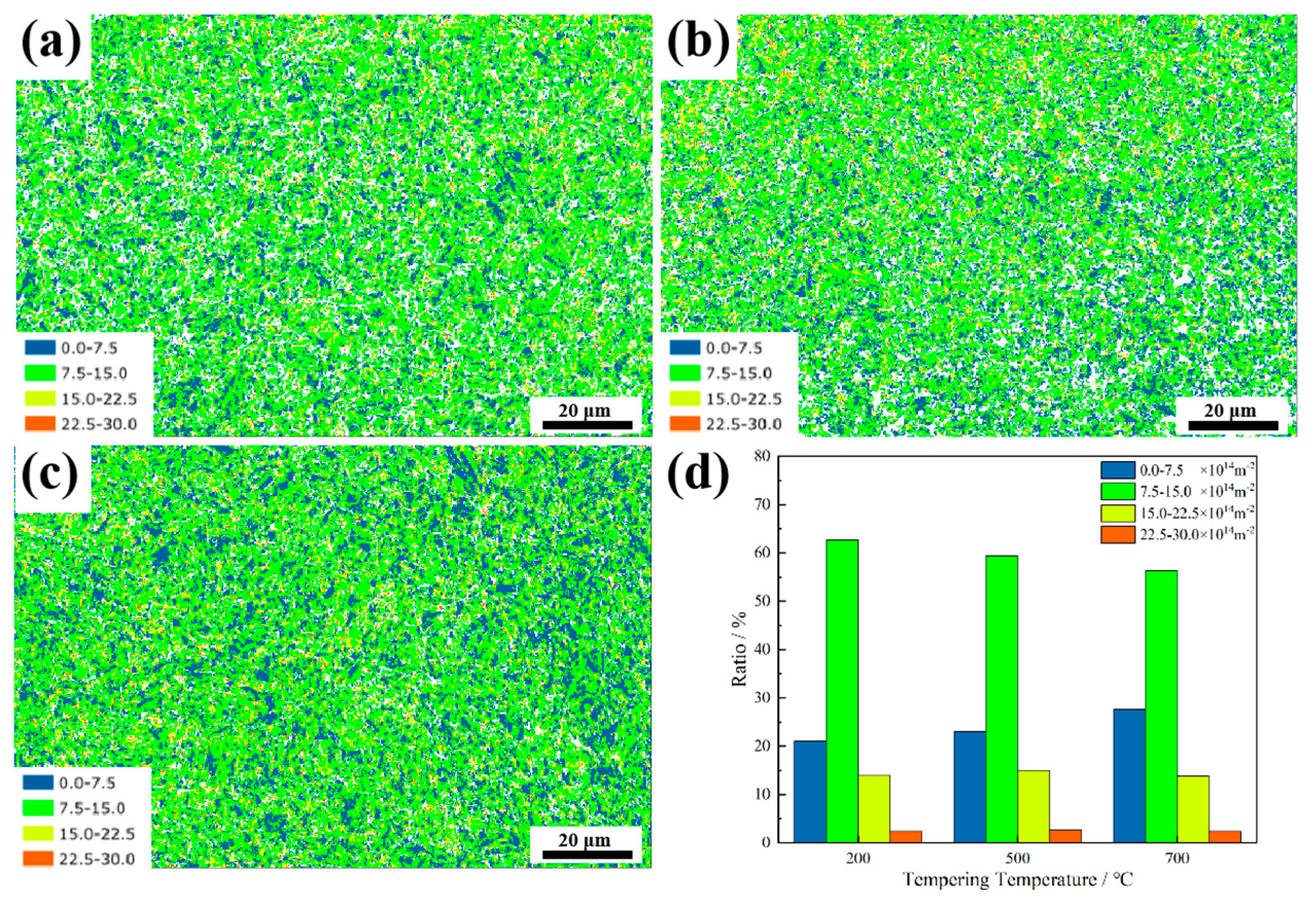
3.2. Microstructure Evolution
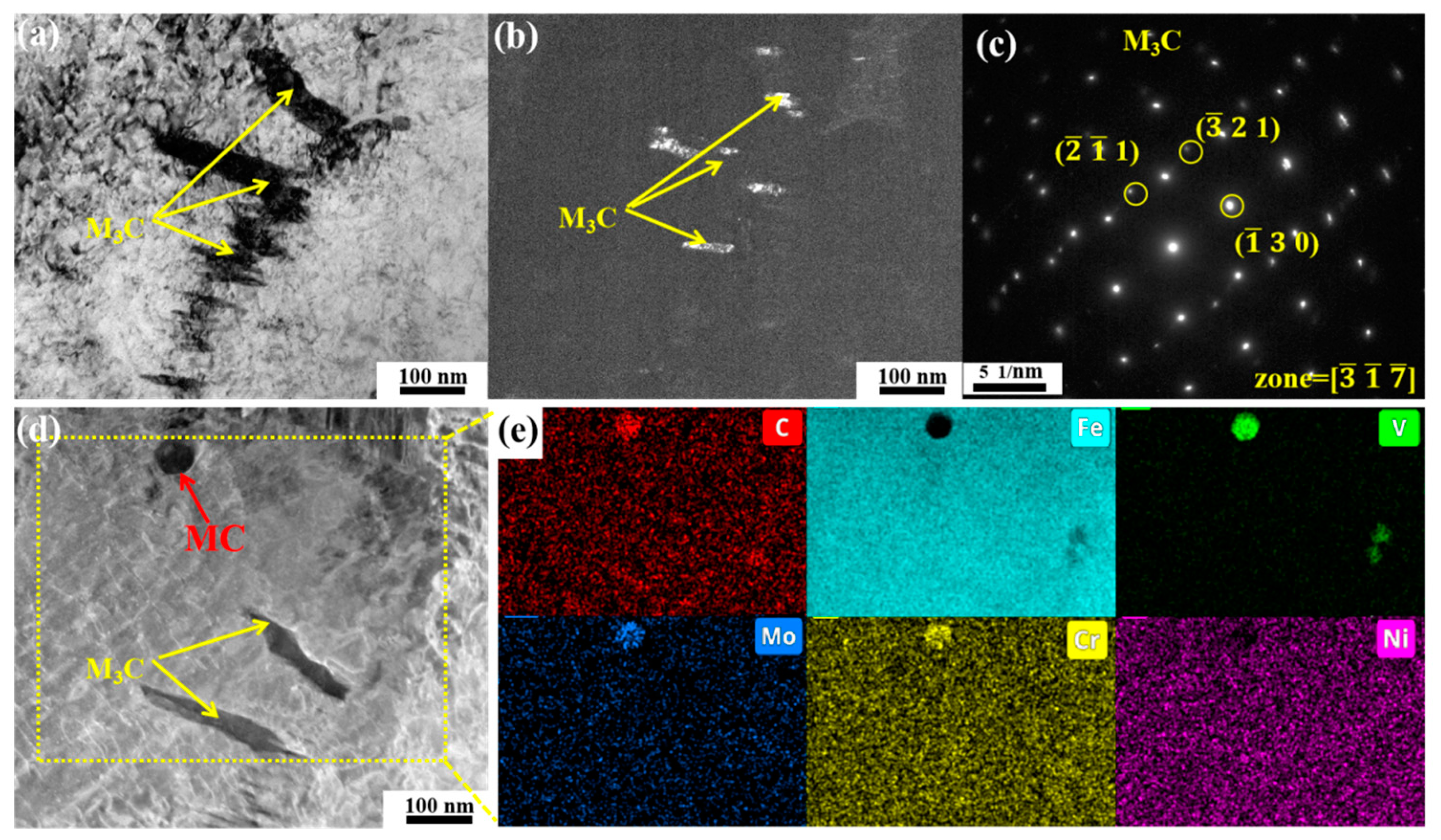
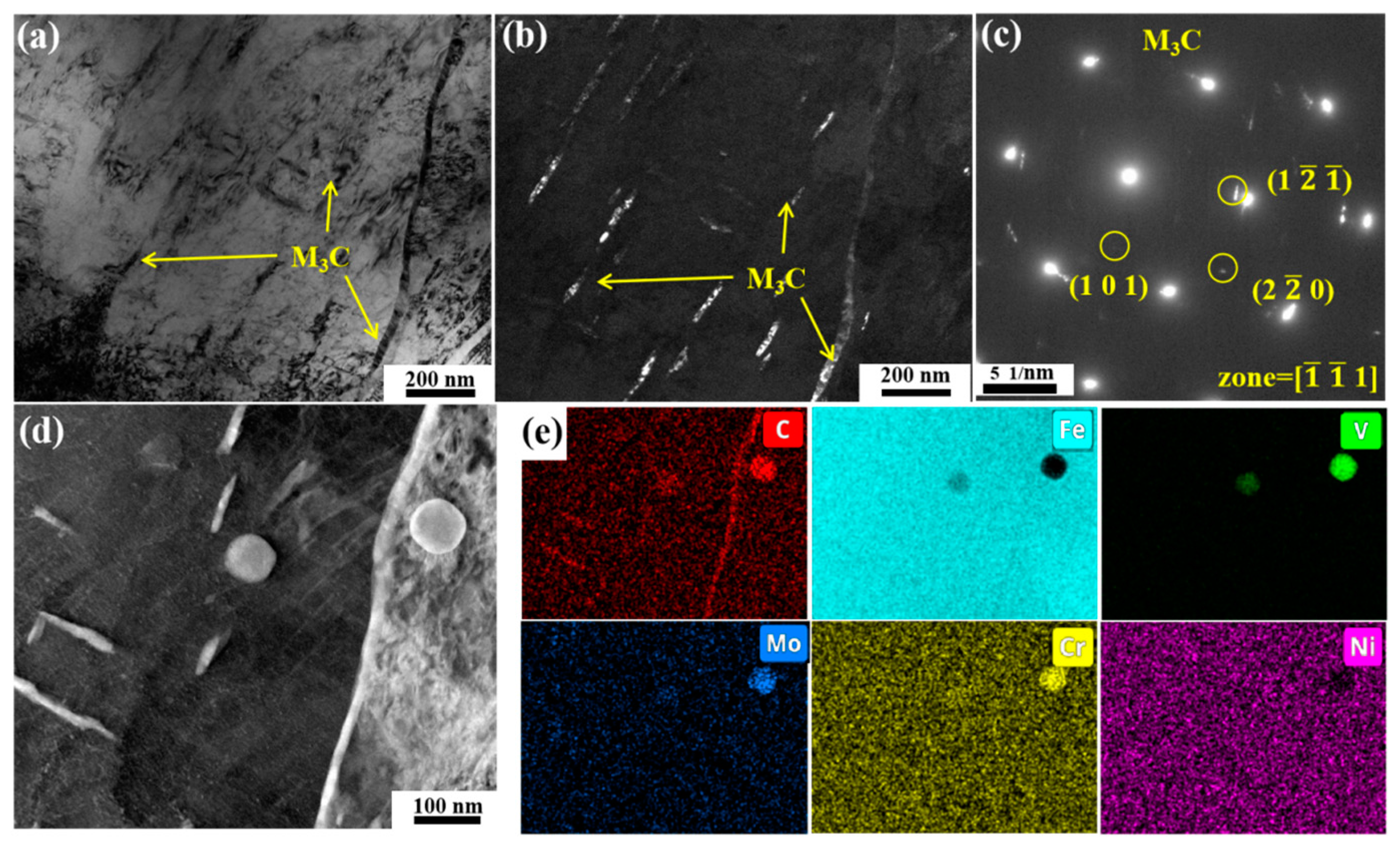
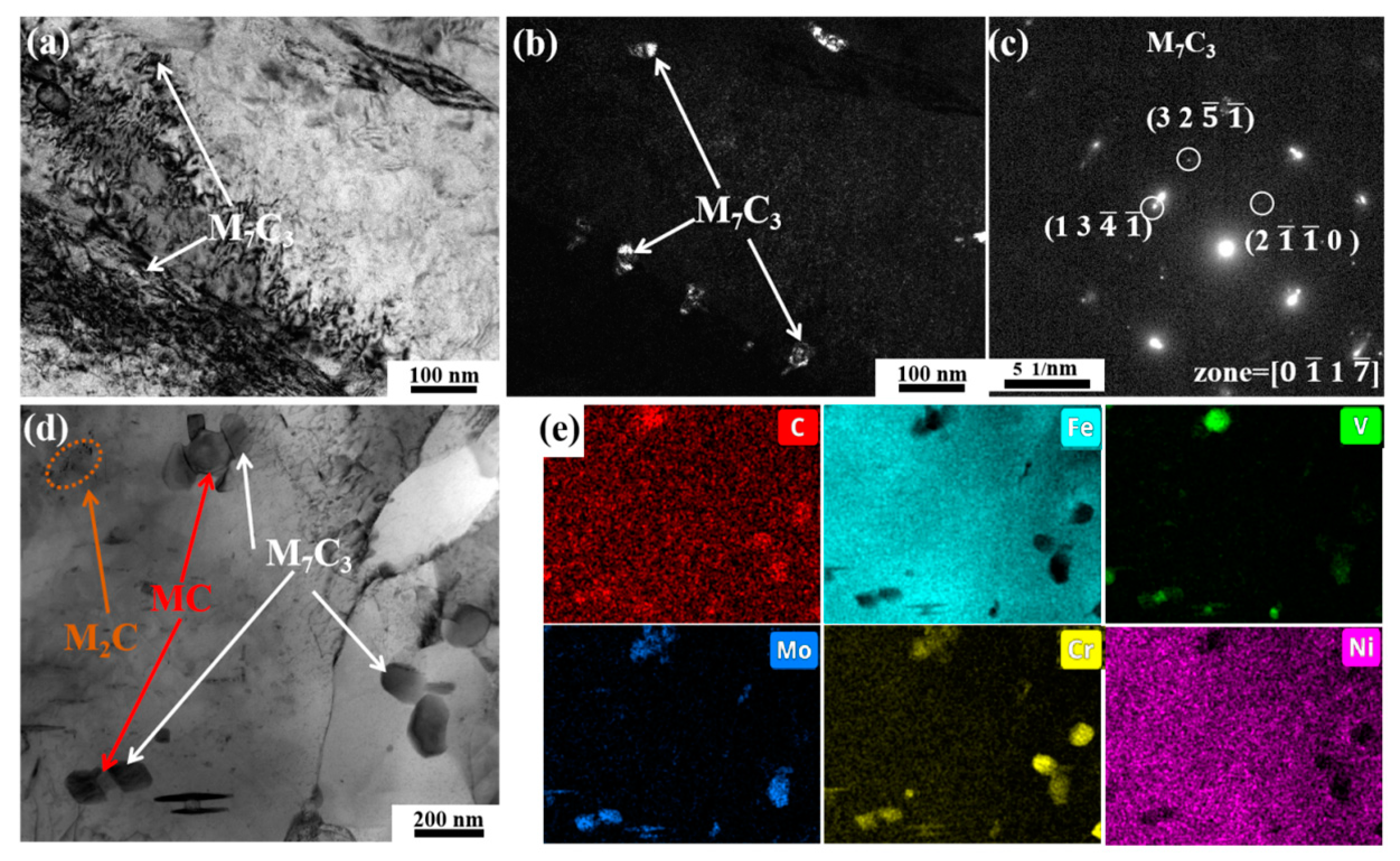
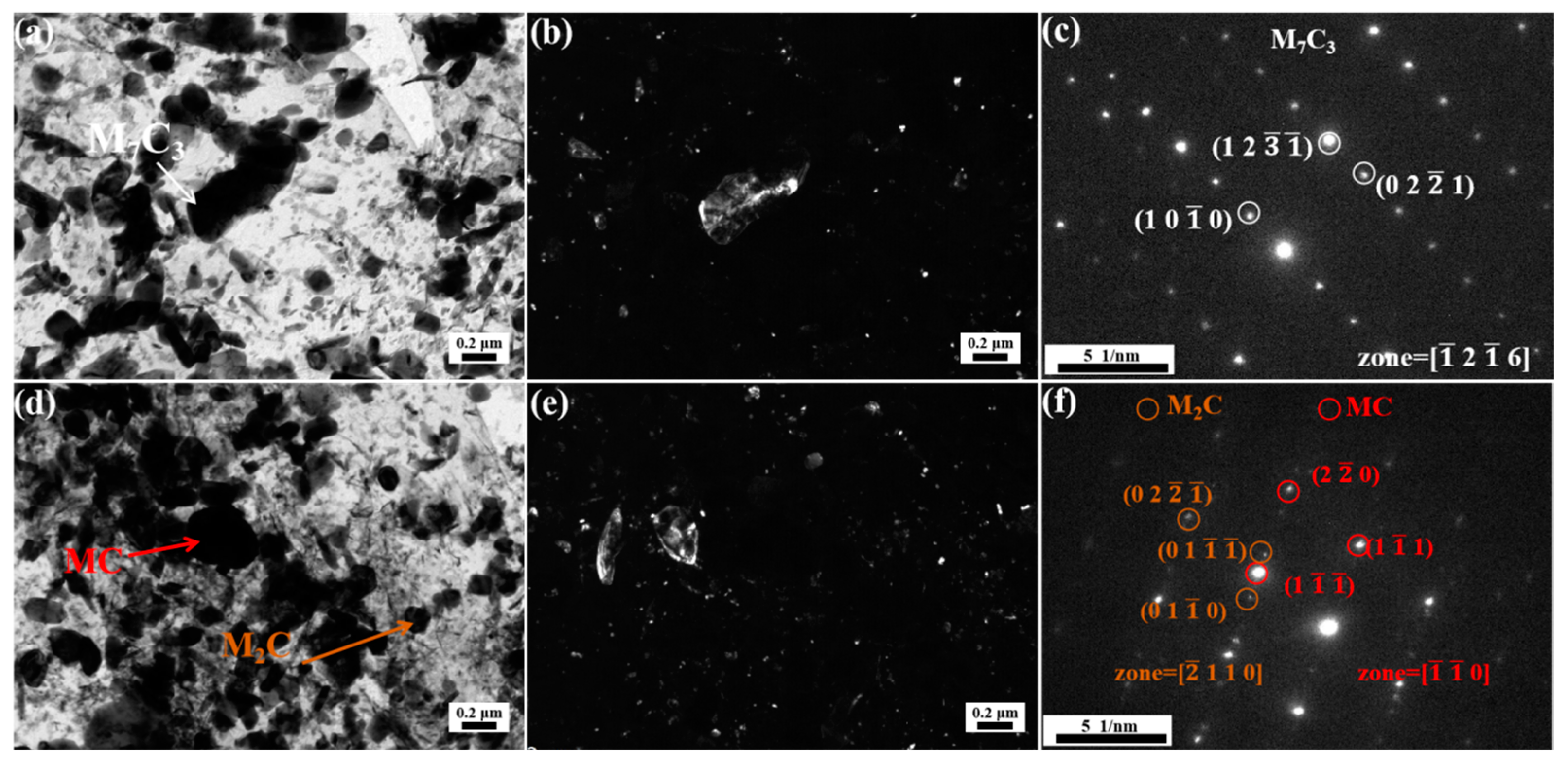
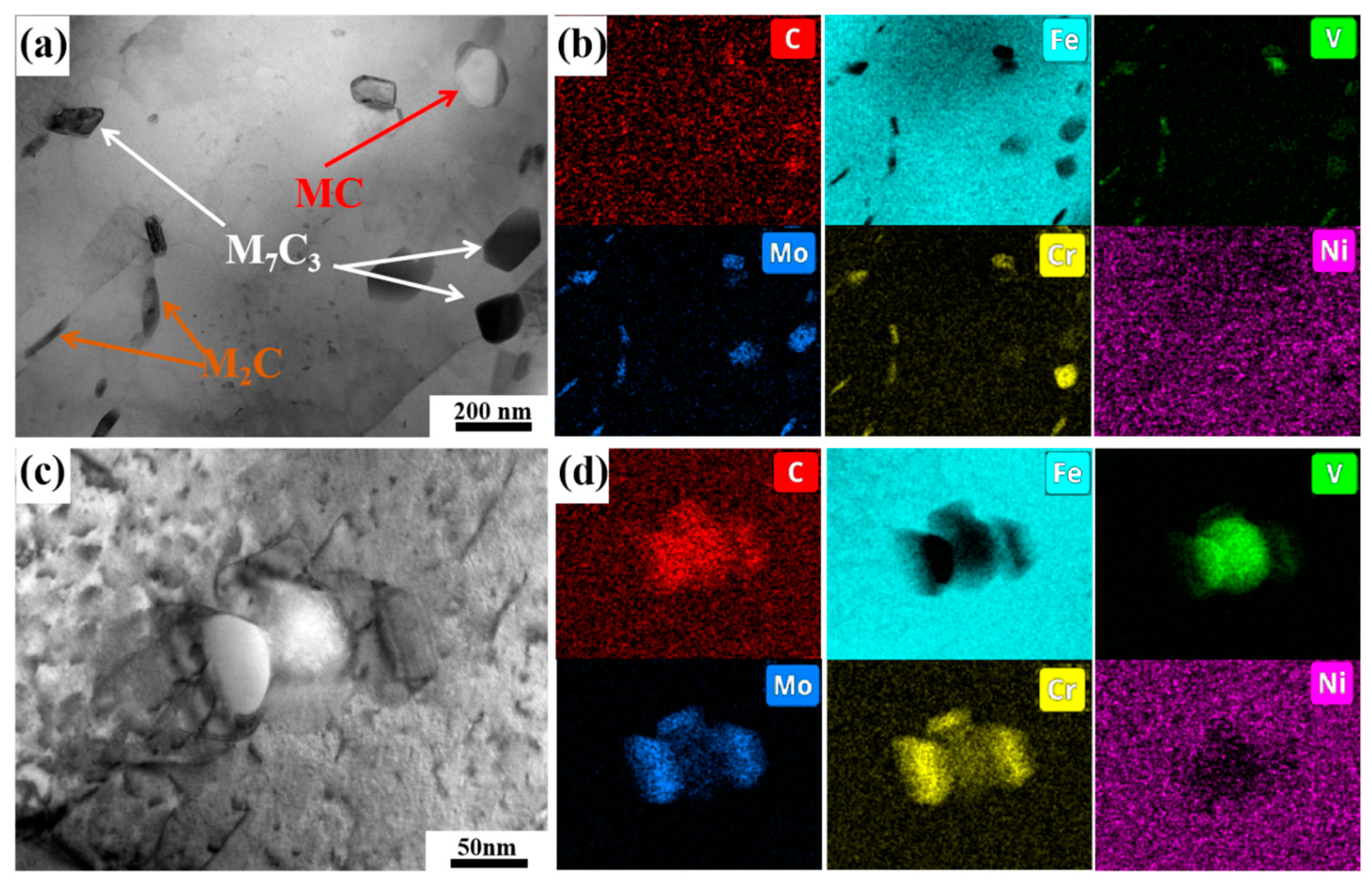
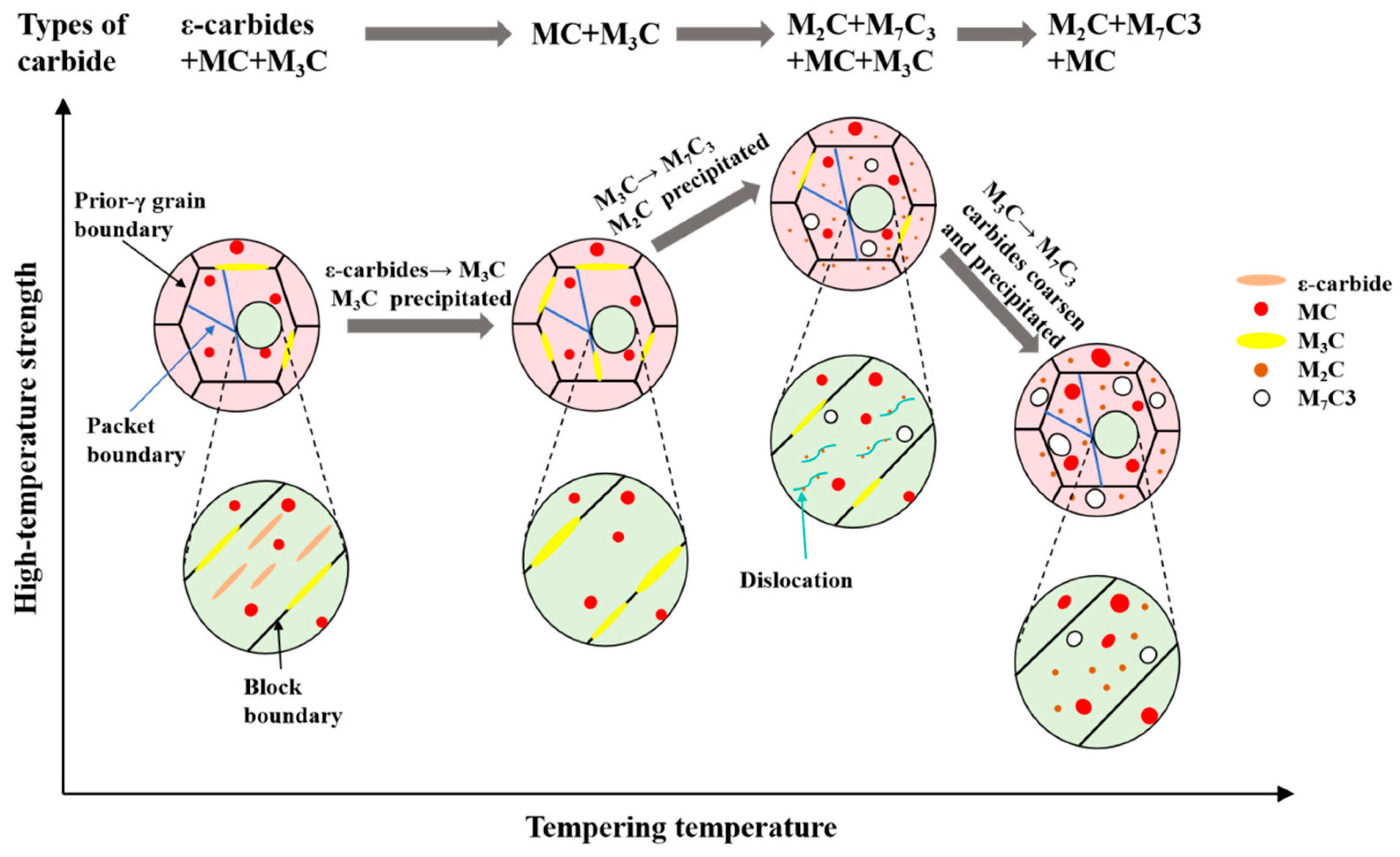
3.3. Carbide Evolution and Strengthening Mechanism
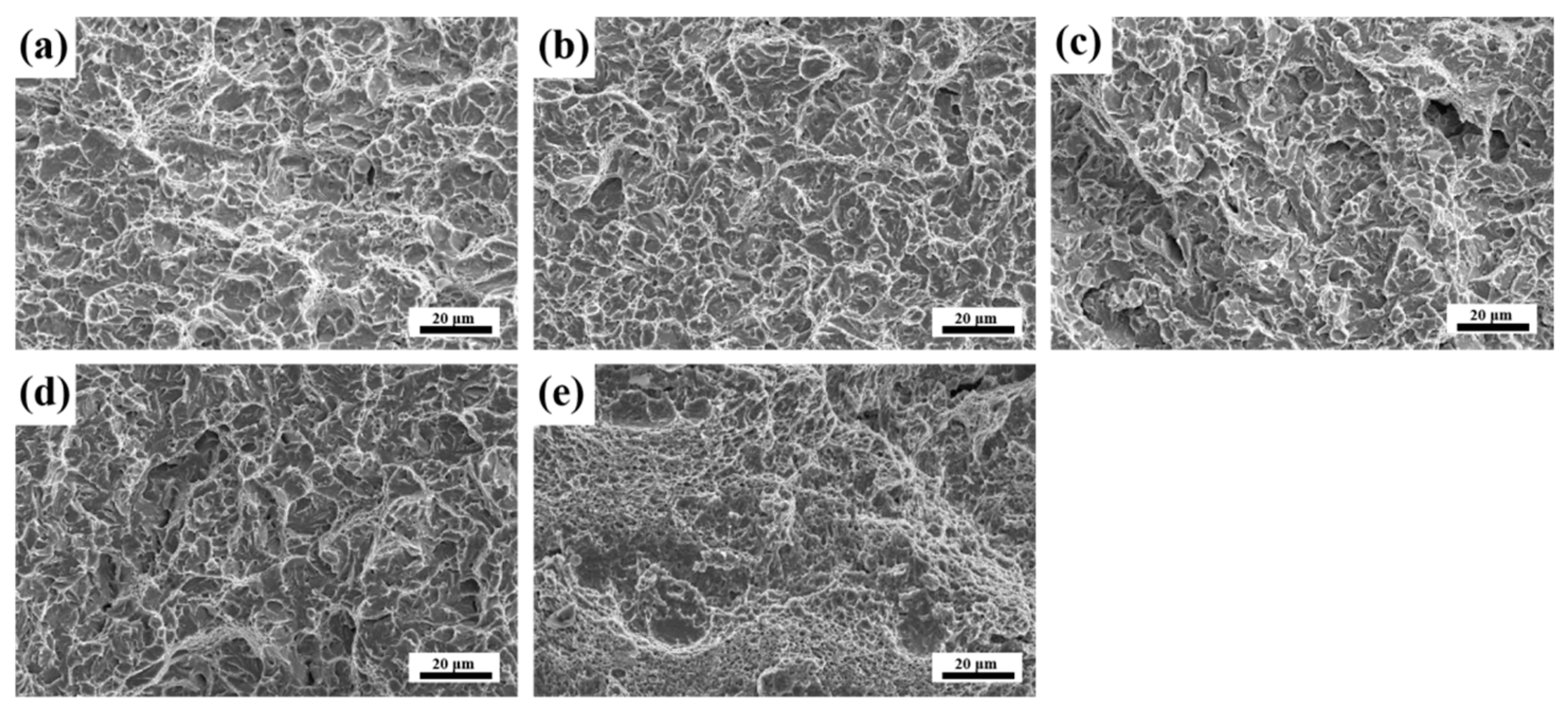
3.4. Fracture Morphology
3.5. High-Temperature Strengthening Mechanism
4. Conclusions
- Oil-cooling after austenitization at 980 °C produces lath martensite. During the tempering process, ε-carbide is the first carbide to precipitate at 200 °C, and transforms into M3C quickly as the temperature rises. Massive precipitation of nanoscale M2C and transformation from M3C to M7C3 occur at 600 °C. At 700 °C, accompanying the decomposition of the substructure, a great amount of carbides precipitate and coarsen. The sequential carbide transitions occurring during tempering develop in this order: ε-carbide + MC + M3C → MC + M3C → MC + M2C + M7C3.
- M3C tends to nucleate and grow at grain boundaries and phase interfaces, and the rod-shaped cementite can be detrimental to toughness, leading to a reduction in toughness from 29.7 ± 1.9 J to 22.4 ± 2.1 J at a tempering temperature of 400 °C. In contrast, fine M2C is less likely to cause stress concentration, which results in improved toughness, increasing KV2 (−40 °C) from 26.2±0.5 J to 34 ± 1.4 J upon tempering at 600 °C.
- At high temperatures such as 700 °C, the contribution of carbides to strength is of great significance in steel. Metastable ε-carbides and relatively coarser M3C carbides are not sufficient to provide high high-temperature strength. Nanoscale M2C with high thermal stability provided both the highest yield strength at room temperature and high-temperature strength, 1340 ± 8 MPa and 421 ± 4 MPa, respectively.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
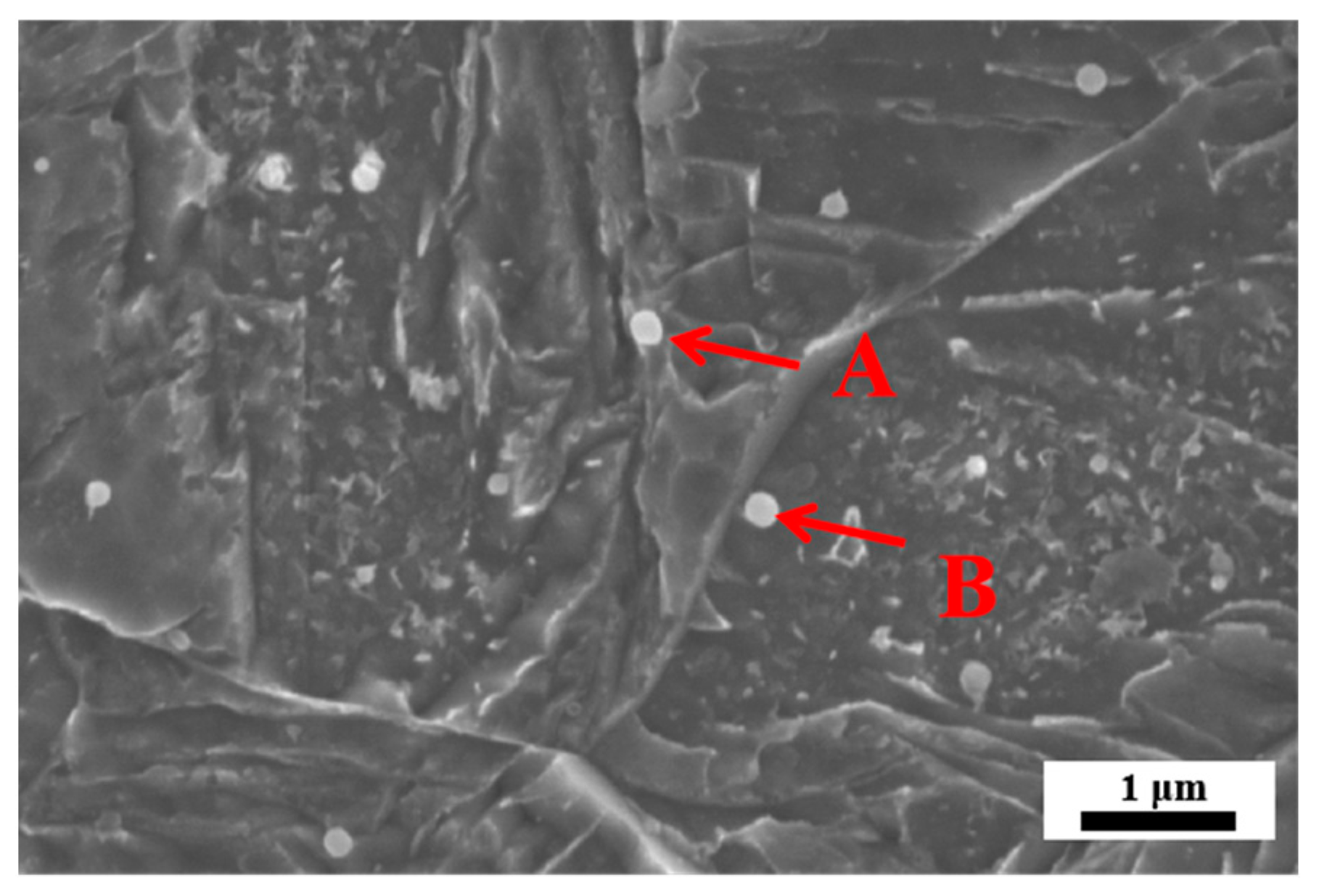

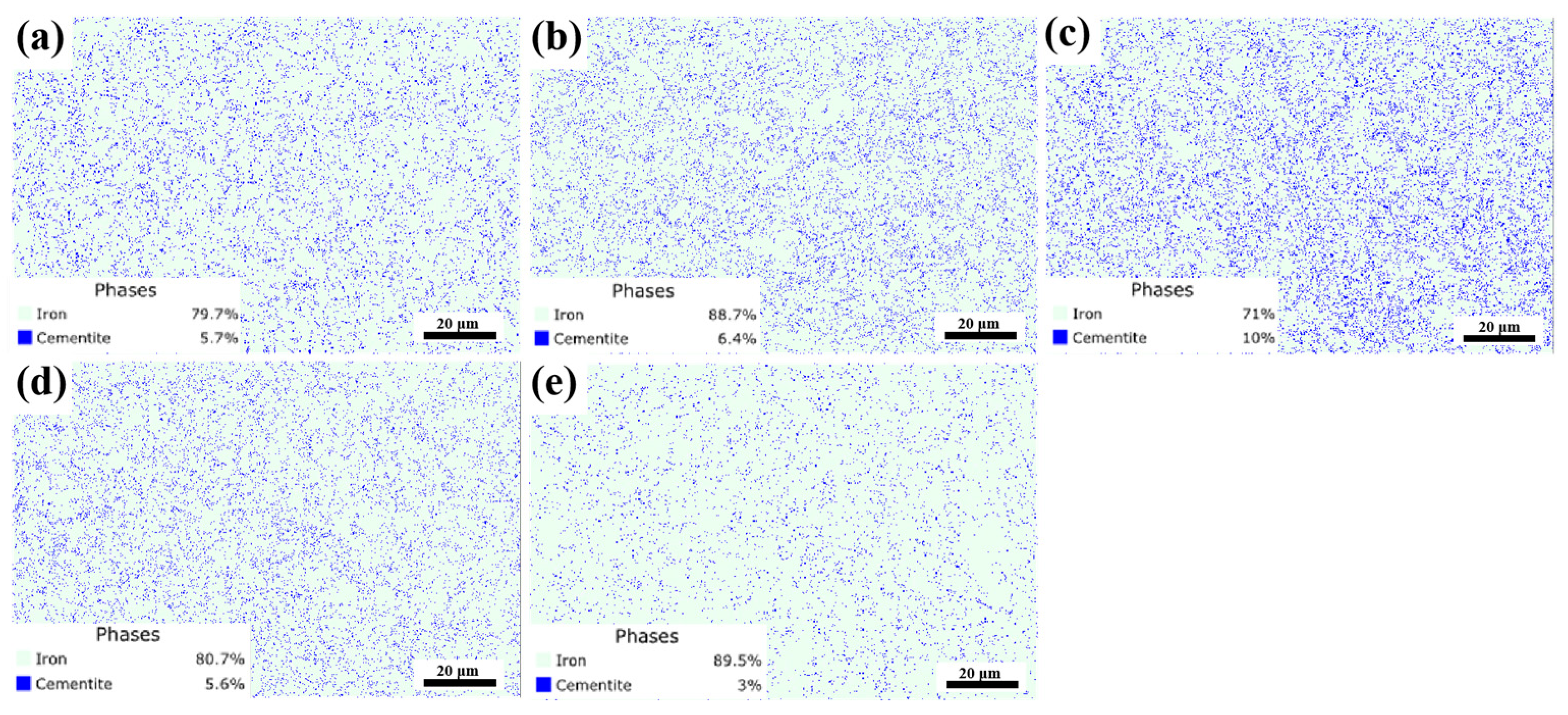
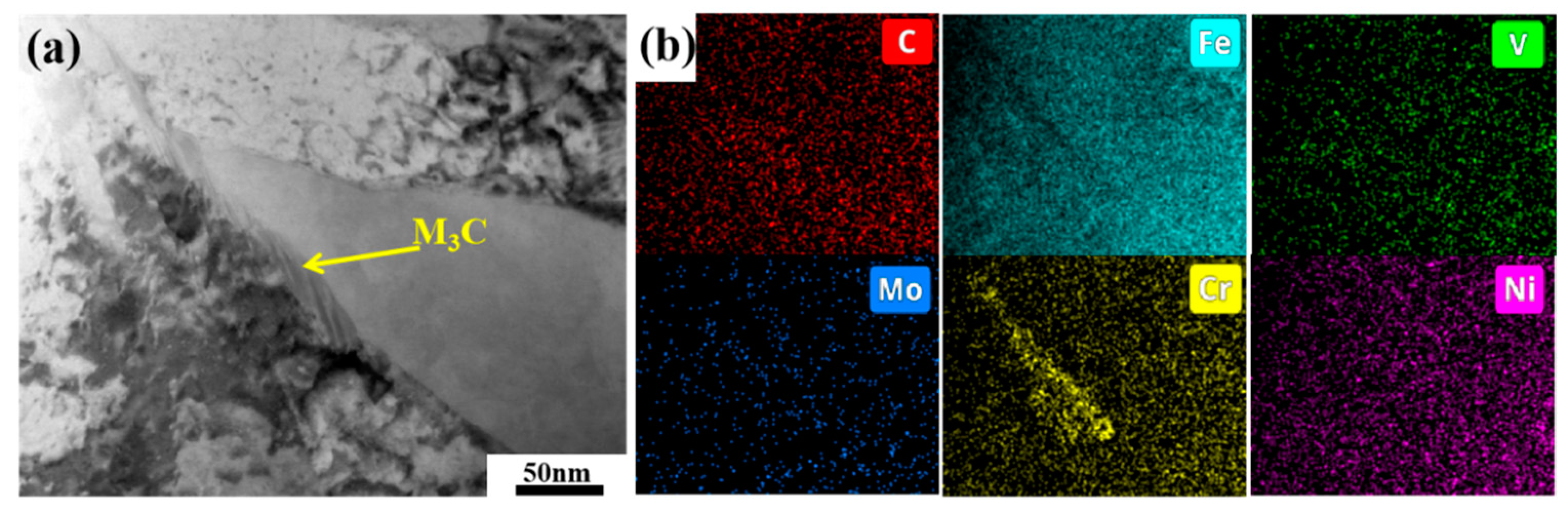
References
- Li, C.; Deng, X.; Li, Y.; Wang, Z. Adding Cr and Mo Simultaneously Enhances the Strength and Impact Toughness of TiC Microparticle-Reinforced Steel Matrix Composites at High Temperatures. J. Alloys Compd. 2023, 931, 167531. [Google Scholar] [CrossRef]
- Ning, A.; Liu, Y.; Gao, R.; Yue, S.; Wang, M.; Guo, H. Effect of Austenitizing Condition on Mechanical Properties, Microstructure and Precipitation Behavior of AISI H13 Steel. J. Iron Steel Res. Int. 2022, 31, 143. [Google Scholar] [CrossRef]
- Ji, Y.; Yang, C.; Xu, T.; Xu, L.; Li, Y.; Hu, C.; Dong, H. Study on the Characteristics and High-Temperature Dissolution Mechanism of Eutectic Carbides in Medium-Alloy Steel. Mater. Res. Technol. 2024, 31, 276–286. [Google Scholar] [CrossRef]
- Maropoulos, S.; Ridley, N.; Karagiannis, S. Structural Variations in Heat Treated Low Alloy Steel Forgings. Mater. Sci. Eng. A 2004, 380, 79–92. [Google Scholar] [CrossRef]
- Du, Y.F.; Lu, H.H.; Shen, X.Q. Coupled Effects of Banded Structure and Carbide Precipitation on Mechanical Performance of Cr–Ni–Mo–V Steel. Mater. Sci. Eng. A 2022, 832, 142478. [Google Scholar] [CrossRef]
- Chen, H.; Li, S.; Ren, Y.; Hou, X.; Yang, H.; Zhang, S. Thermo-Mechanical Fatigue Behavior and Microstructure Evolution of 4Cr5Mo3V Hot Work Die Steel. Int. J. Fatigue 2024, 183, 108263. [Google Scholar] [CrossRef]
- Li, B.; Zheng, Y.; Chen, G.; Itoh, T.; Chen, X. Isothermal and Thermomechanical Fatigue Behavior of 316LN Stainless Steel: Effects of Strain Amplitude. Int. J. Fatigue 2023, 166, 107304. [Google Scholar] [CrossRef]
- Ding, H.; Yuan, Z.; Liu, T.; Chen, L.; Zhou, Y.; Cao, Y.; Cao, F.; Luo, R.; Cheng, X. Microstructure and High-Temperature Tensile Behavior of Modified H13 Steel with the Addition of Tungsten, Molybdenum, and Lowering of Chromium. Mater. Sci. Eng. A 2023, 866, 144655. [Google Scholar] [CrossRef]
- Wang, M.; Dong, H.; Wang, Q.; Fan, C. Low Cycle Fatigue Behavior of High Strength Gun Steels. Int. J. Min. Met. Mater. 2004, 11, 268–272. [Google Scholar]
- Zhang, Y.; Zhao, C.; Sato, M.; Miyamoto, G.; Furuhara, T. Resistance to Temper Softening of Low Carbon Martensitic Steels by Microalloying of V, Nb and Ti. ISIJ Int. 2021, 61, 1641–1649. [Google Scholar] [CrossRef]
- Hu, J.; Li, X.; Meng, Q.; Wang, L.; Li, Y.; Xu, W. Tailoring Retained Austenite and Mechanical Property Improvement in al–Si–V Containing Medium Mn Steel via Direct Intercritical Rolling. Mater. Sci. Eng. A 2022, 855, 143904. [Google Scholar] [CrossRef]
- Barrett, R.A.; O’Donoghue, P.E.; Leen, S.B. A Physically-Based High Temperature Yield Strength Model for 9Cr Steels. Mater. Sci. Eng. A 2018, 730, 410–424. [Google Scholar] [CrossRef]
- Ennis, P.J.; Zielinska-Lipiec, A.; Wachter, O.; Czyrska-Filemonowicz, A. Microstructural Stability and Creep Rupture Strength of the Martensitic Steel P92 for Advanced Power Plant. Acta Mater. 1997, 45, 4901–4907. [Google Scholar] [CrossRef]
- Zhang, X.; Li, W.; Ma, J.; Li, Y.; Zhang, X.; Zhang, X. Modeling the Effects of Grain Boundary Sliding and Temperature on the Yield Strength of High Strength Steel. J. Alloys Compd. 2021, 851, 156747. [Google Scholar] [CrossRef]
- Zhu, C.; Cerezo, A.; Smith, G.D.W. Carbide Characterization in Low-Temperature Tempered Steels. Ultramicroscopy 2009, 109, 545–552. [Google Scholar] [CrossRef]
- Saeglitz, M.; Krauss, G. Deformation, Fracture, and Mechanical Properties of Low-Temperature-Tempered Martensite in SAE 43xx Steels. Metall. Mater. Trans. A 1997, 28, 377–387. [Google Scholar] [CrossRef]
- Kim, B.; Celada, C.; San Martín, D.; Sourmail, T.; Rivera-Díaz-del-Castillo, P.E.J. The Effect of Silicon on the Nanoprecipitation of Cementite. Acta Mater. 2013, 61, 6983–6992. [Google Scholar] [CrossRef]
- Xiao, M.; Lü, X.; Li, D.; Li, S.; Zhao, K.; Yang, M. Carbides Precipitation and Their Evolution of Cr15Co10Mo5-Alloyed Heat-Resistant Bearing Steel after Tempering at Different Temperatures. J. Iron Steel Res. Int. 2019, 26, 1096–1105. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, X.; Dong, C. Effect of Boron on G115 Martensitic Heat Resistant Steel during Aging at 650 °C. Mater. Sci. Eng. A 2020, 787, 139529. [Google Scholar] [CrossRef]
- Kaneko, K.; Fujita, K.; Sadakata, A.; Tomokiyo, Y.; Matsumura, S. Nanostructural and Nanoelemental Analysis of Metastable M3C-Type Carbides with Alloy-Rich Layer in Heat Resistant 2Cr-Martensitic Steel. Scr. Mater. 2003, 48, 761–765. [Google Scholar] [CrossRef]
- Pandey, C.; Giri, A.; Mahapatra, M.M. Evolution of Phases in P91 Steel in Various Heat Treatment Conditions and Their Effect on Microstructure Stability and Mechanical Properties. Mater. Sci. Eng. A 2016, 664, 58–74. [Google Scholar] [CrossRef]
- He, X.; Hu, C.; Wang, Z.; Zhao, H.; Wei, X.; Dong, H. Carbide Transformation Behaviors of a Cr–Mo–V Secondary Hardening Steel during over-Ageing. Mater. Res. Express 2020, 7, 036511. [Google Scholar] [CrossRef]
- Race, J.M.; Bhadeshia, H.K.D.H. Precipitation Sequences during Carburisation of Cr–Mo Steel. Mater. Sci. Technol. 1992, 8, 875–882. [Google Scholar] [CrossRef]
- GB/T228.1-2010; Metallic Materials—Tensile Testing—Part 1: Method of Test at Room Temperature. Standards Press of China: Beijing, China, 2010.
- GB/T228.2-2015; Metallic Materials—Tensile Testing—Part 2: Method of Test at Elevated Temperature. Standards Press of China: Beijing, China, 2015.
- ASTM E23; Standard Test Methods for Notched Bar Impact Testing of Metallic Materials. ASTM International: West Conshohocken, PA, USA, 2024.
- Li, J.; Zhang, C.; Liu, Y. Influence of Carbides on the High-Temperature Tempered Martensite Embrittlement of Martensitic Heat-Resistant Steels. Mater. Sci. Eng. A 2016, 670, 256–263. [Google Scholar] [CrossRef]
- Tsai, M.C.; Yang, J.R. Microstructural Degeneration of Simulated Heat-Affected Zone in 2.25Cr–1Mo Steel during High-Temperature Exposure. Mater. Sci. Eng. A 2003, 340, 15–32. [Google Scholar] [CrossRef]
- Tao, P.; Zhang, C.; Yang, Z.; Takeda, H. Evolution and Coarsening of Carbides in 2.25Cr-lMo Steel Weld Metal during High Temperature Tempering. J. Iron Steel Res. Int. 2010, 17, 74–78. [Google Scholar] [CrossRef]
- Vivas, J.; De-Castro, D.; Altstadt, E.; Houska, M.; San-Martín, D.; Capdevila, C. Design and High Temperature Behavior of Novel Heat Resistant Steels Strengthened by High Density of Stable Nanoprecipitates. Mater. Sci. Eng. A 2020, 793, 139799. [Google Scholar] [CrossRef]
- Speich, G.R.; Leslie, W.C. Tempering of Steel. Metall. Trans. 1972, 3, 1043–1054. [Google Scholar] [CrossRef]
- Clarke, A.J.; Miller, M.K.; Field, R.D.; Coughlin, D.R.; Gibbs, P.J.; Clarke, K.D.; Alexander, D.J.; Powers, K.A.; Papin, P.A.; Krauss, G. Atomic and Nanoscale Chemical and Structural Changes in Quenched and Tempered 4340 Steel. Acta Mater. 2014, 77, 17–27. [Google Scholar] [CrossRef]
- Kawahara, Y.; Kaneko, K.; Sawada, H.; Takahashi, J. Transition from Carbon Clusters to ε, θ-Carbides in a Quenched and Aged Low-Carbon Ferritic Steel. Acta Mater. 2023, 252, 118919. [Google Scholar] [CrossRef]
- Liu, F.; Lin, X.; Song, M.; Yang, H.; Song, K.; Guo, P.; Huang, W. Effect of Tempering Temperature on Microstructure and Mechanical Properties of Laser Solid Formed 300M Steel. J. Alloys Compd. 2016, 689, 225–232. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, T.; Hu, C.; Mu, Y.; Zhao, H.; Dong, H. Developing High Strength/High Toughness Grades Steels by Dual-Precipitates Co-Configuration during Aging Process. Mater. Charact. 2024, 208, 113623. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, X.; Hu, C.; Zhao, H.; Mu, Y.; Wang, G.; Dong, H. Industrially Produced 2.4 GPa Ultra-Strong Steel via Nanoscale Dual-Precipitates Co-Configuration. Mater. Charact. 2024, 208, 113646. [Google Scholar] [CrossRef]
- Kan, L.Y.; Ye, Q.B.; Wang, Q.H.; Tian, Y.; Wang, Z.D.; Wang, Y.M.; Hu, J. Refinement of Cu-M2C Precipitates and Improvement of Strength and Toughness by Ti Microalloying in a Cu-Bearing Steel. Mater. Sci. Eng. A 2021, 802, 140678. [Google Scholar] [CrossRef]
- Mohles, V. Simulations of Dislocation Glide in Overaged Precipitation-Hardened Crystals. Philos. Mag. 2001, 81, 971–990. [Google Scholar] [CrossRef]
- Pandey, C.; Saini, N.; Mahapatra, M.M.; Kumar, P. Study of the Fracture Surface Morphology of Impact and Tensile Tested Cast and Forged (C&F) Grade 91 Steel at Room Temperature for Different Heat Treatment Regimes. Eng. Fail. Anal. 2017, 71, 131–147. [Google Scholar]
- Krauss, G. Tempering of Lath Martensite in Low and Medium Carbon Steels: Assessment and Challenges. Steel Res. Int. 2017, 88, 1700038. [Google Scholar] [CrossRef]
- Chen, J.; Li, C.; Ren, J.; Tu, X.; Chen, L. Strength and Toughness of Fe-1.2Mn-0.3Cr-1.4Ni-0.4Mo-C Tempered Steel Plate in Three Cooling Processes. Mater. Sci. Eng. A 2019, 754, 178–189. [Google Scholar] [CrossRef]
- Shi, G.; Zhao, H.; Zhang, S.; Wang, Q.; Zhang, F. Microstructural Characteristics and Impact Fracture Behaviors of Low-Carbon Vanadium-Microalloyed Steel with Different Nitrogen Contents. Mater. Sci. Eng. A 2020, 769, 138501. [Google Scholar] [CrossRef]
- Ren, J.; Li, C.; Han, Y.; Li, E.; Gao, C.; Qiu, C. Effect of Initial Martensite and Tempered Carbide on Mechanical Properties of 3Cr2MnNiMo Mold Steel. Mater. Sci. Eng. A 2021, 812, 141080. [Google Scholar] [CrossRef]
- Jiang, L.; Yinling, W.; Hu, R.; Liu, R.; Ye, X.X.; Li, Z.; Zhou, X. Formation of Nano-Sized M2C Carbides in Si-Free GH3535 Alloy. Sci. Rep. 2018, 8, 8158. [Google Scholar] [CrossRef]
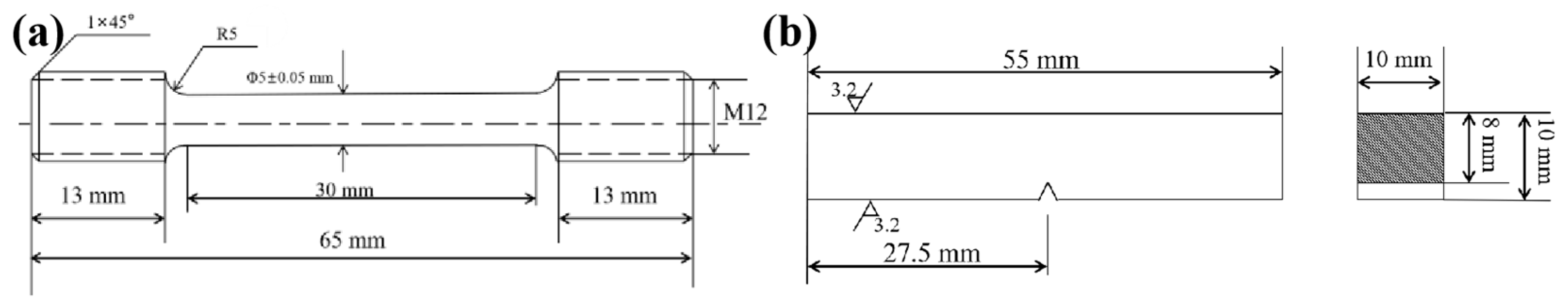





| Tempering Temperature/°C | Mechanical Testing | Microstructural Analysis |
|---|---|---|
| 200 | Room-temperature tensile test + 700 °C high-temperature tensile test + −40 °C Charpy impact test | SEM: microstructure and impact fracture morphology TEM: microstructure EBSD |
| 400 | Room-temperature tensile test + 700 °C high-temperature tensile test + −40 °C Charpy impact test | SEM: microstructure and impact fracture morphology TEM: microstructure and carbon extraction replica EBSD |
| 500 | Room-temperature tensile test + 700 °C high-temperature tensile test + −40 °C Charpy impact test | SEM: microstructure and impact fracture morphology TEM: microstructure and carbon extraction replica EBSD |
| 600 | Room-temperature tensile test + 700 °C high-temperature tensile test + −40 °C Charpy impact test | SEM: microstructure and impact fracture morphology TEM: microstructure and carbon extraction replica EBSD |
| 700 | Room-temperature tensile test + 700 °C high-temperature tensile test + −40 °C Charpy impact test | SEM: microstructure and impact fracture morphology TEM: microstructure and carbon extraction replica EBSD |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheng, J.; Deng, Y.; Cao, X.; Wang, Y.; Hu, C.; Dong, H. Evolution Mechanism of Multi-Precipitation Regulates Mechanical Properties and High-Temperature Strength in Medium-Alloy Structural Steel. Materials 2025, 18, 848. https://doi.org/10.3390/ma18040848
Sheng J, Deng Y, Cao X, Wang Y, Hu C, Dong H. Evolution Mechanism of Multi-Precipitation Regulates Mechanical Properties and High-Temperature Strength in Medium-Alloy Structural Steel. Materials. 2025; 18(4):848. https://doi.org/10.3390/ma18040848
Chicago/Turabian StyleSheng, Junjie, Yahui Deng, Xin Cao, Yangxin Wang, Chundong Hu, and Han Dong. 2025. "Evolution Mechanism of Multi-Precipitation Regulates Mechanical Properties and High-Temperature Strength in Medium-Alloy Structural Steel" Materials 18, no. 4: 848. https://doi.org/10.3390/ma18040848
APA StyleSheng, J., Deng, Y., Cao, X., Wang, Y., Hu, C., & Dong, H. (2025). Evolution Mechanism of Multi-Precipitation Regulates Mechanical Properties and High-Temperature Strength in Medium-Alloy Structural Steel. Materials, 18(4), 848. https://doi.org/10.3390/ma18040848





