Innovative Approaches to Tin Recovery from Low-Grade Secondary Resources: A Focus on (Bio)hydrometallurgical and Solvometallurgical Methods
Abstract
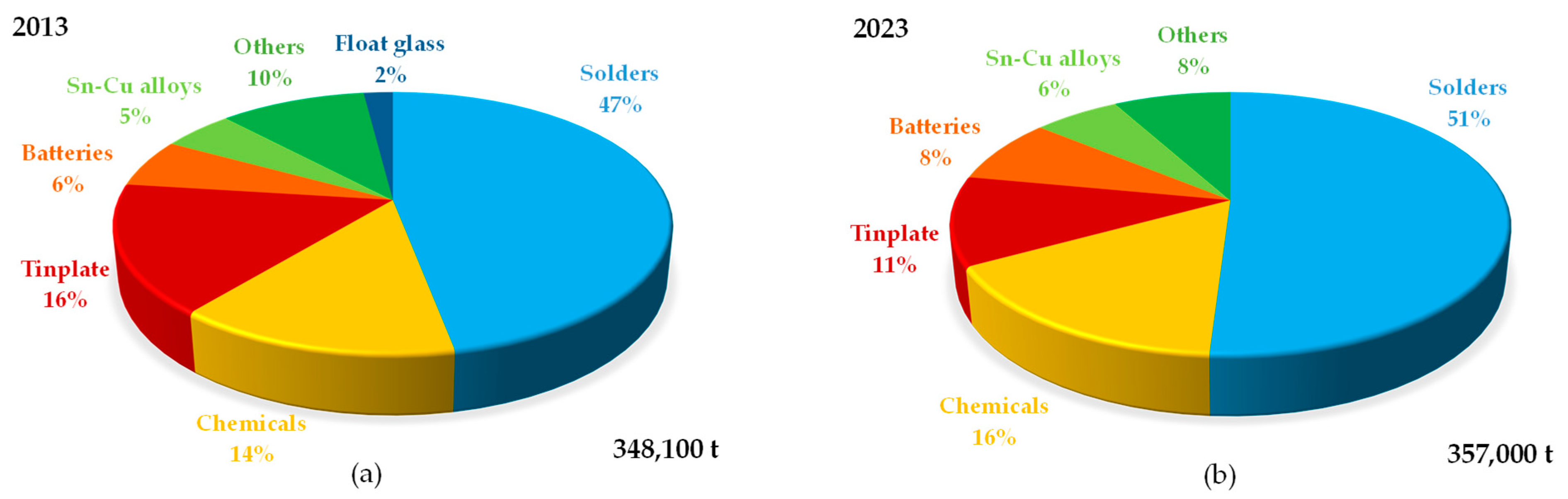
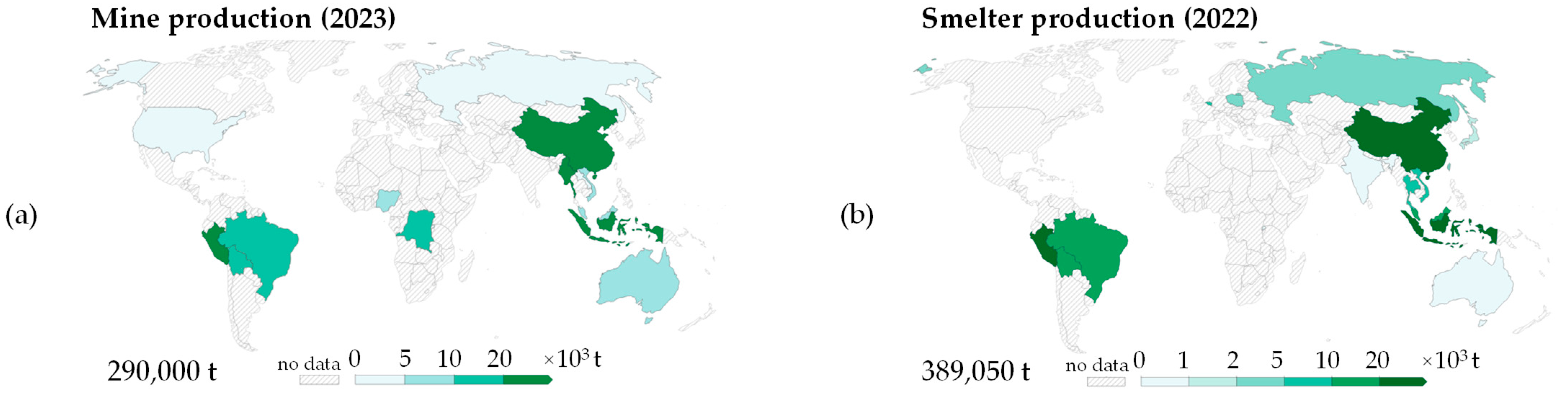
1. Introduction
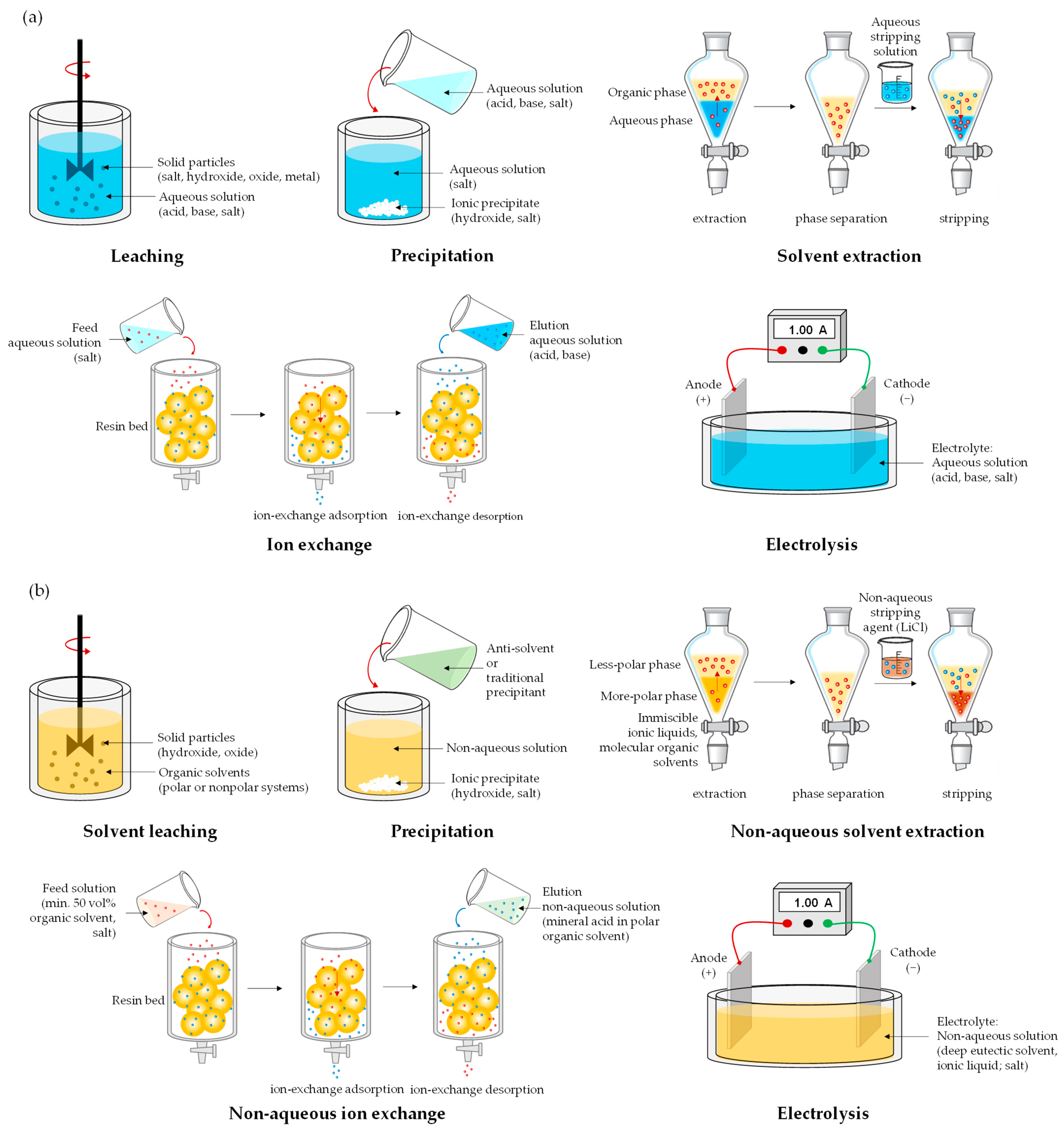
2. Materials and Methods
3. Hydrometallurgy Versus Solvometallurgy
4. Tin Recovery from Waste Printed Circuit Boards
4.1. Tin in Printed Circuit Boards
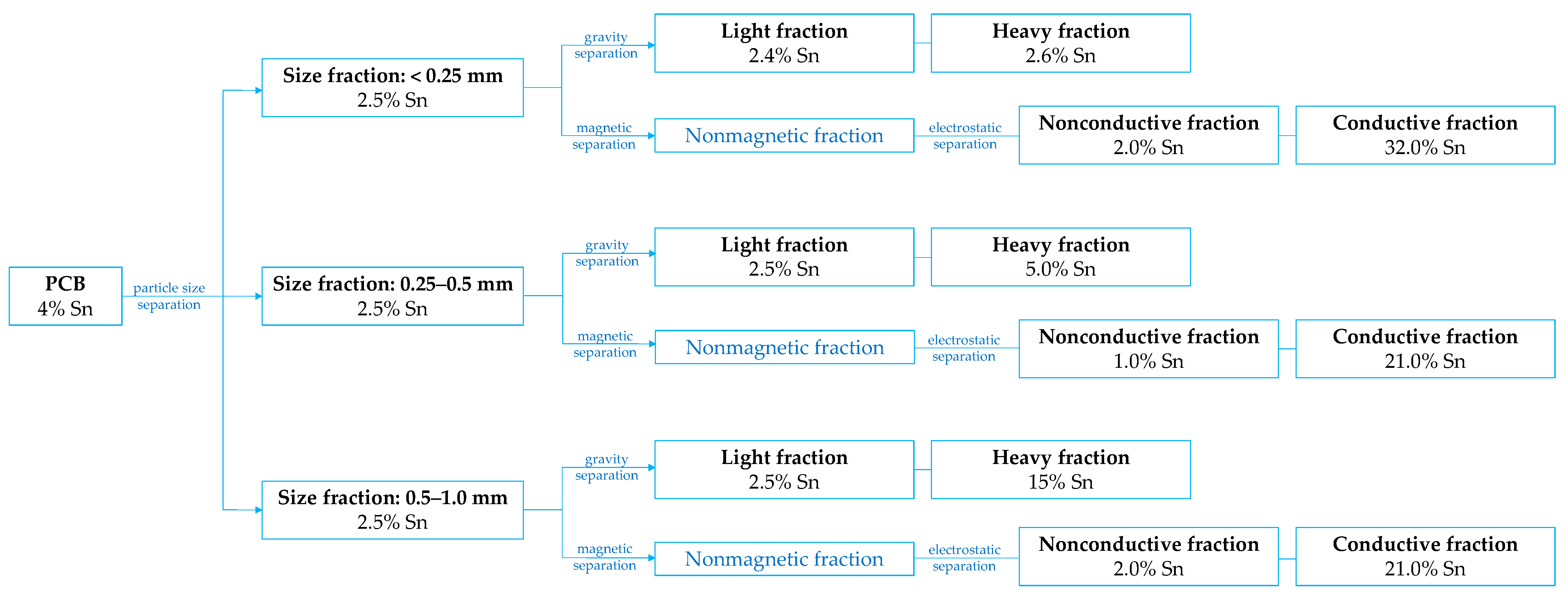
4.2. Physical Pre-Treatment
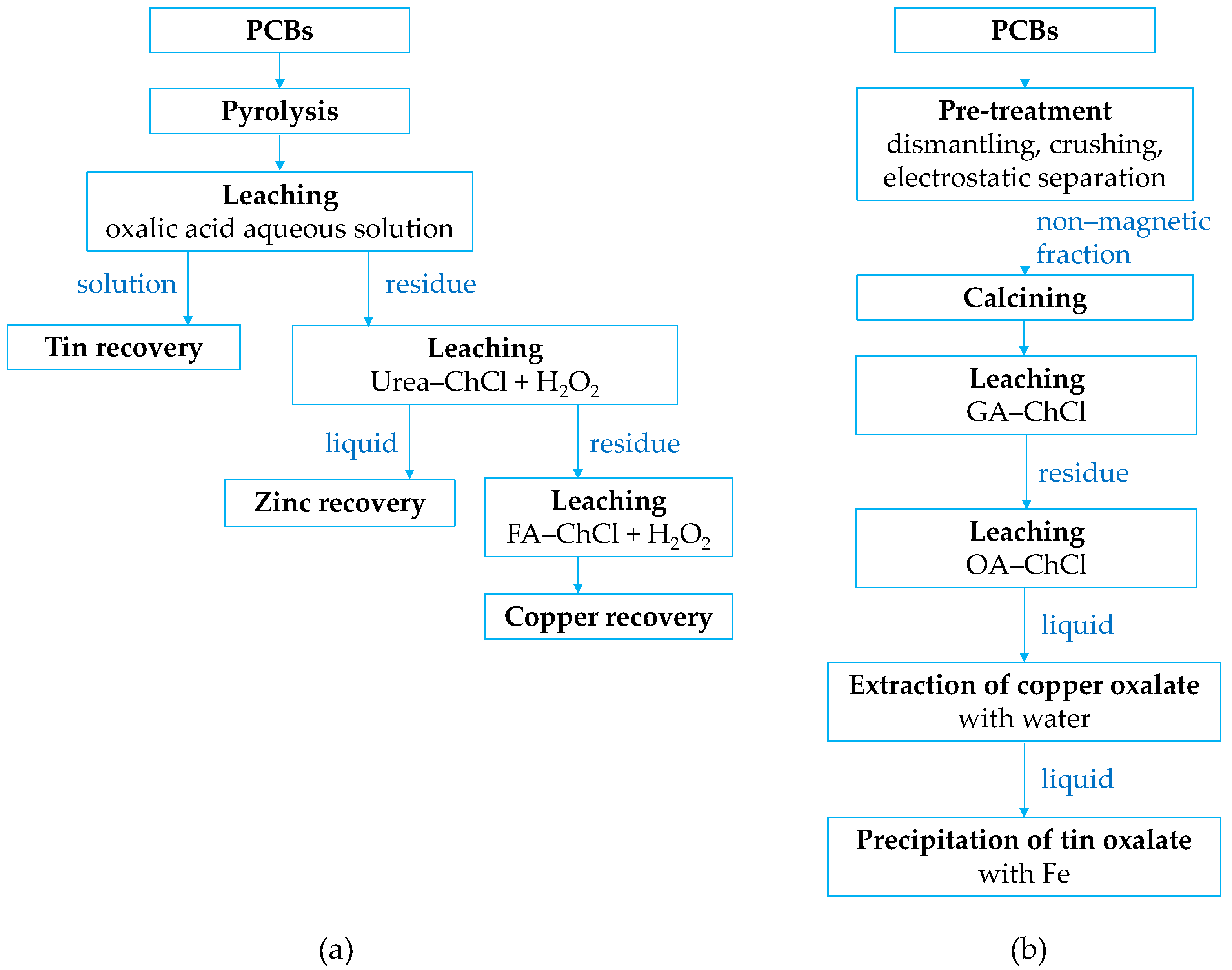
4.3. Hydrometallurgical Treatment
4.4. Biohydrometallurgical Treatment
4.5. Solvometallurgical Treatment
5. Tin Recovery from LCD
5.1. Tin in Liquid Crystal Displays
5.2. Physical Pre-Treatment
5.3. Hydrometallurgical Treatment
5.4. Biohydrometallurgical Treatment
5.5. Solvometallurgical Treatment
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Muhly, J.D. Sources of tin and the beginnings of bronze metallurgy. Am. J. Archeol. 1985, 89, 275–291. [Google Scholar] [CrossRef]
- Enghag, P. Encyclopedia of the Elements; Wiley-VCH: Weinheim, Germany, 2004. [Google Scholar]
- Dey, S.; Agrawal, M.K. Tinplate as a sustainable packaging material: Recent innovation and developments to remain environment friendly and cost effective. Int. J. Res. IT Manag. Eng. 2016, 6, 9–22. [Google Scholar]
- Deshwal, G.K.; Panjagari, N.R. Review on metal packaging: Materials, forms, food applications, safety and recyclability. J. Food Sci. Technol. 2020, 57, 2377–2392. [Google Scholar] [CrossRef] [PubMed]
- Dele-Afolabi, T.T.; Ansari, M.N.M.; Azmah Hanim, M.A.; Oyekanmi, A.A.; Ojo-Kupoluyi, O.J.; Atiqah, A. Recent advances in Sn-based lead-free solder interconnections for microelectronics packaging: Materials and technologies. J. Mater. Res. Technol. 2023, 25, 4231–4263. [Google Scholar] [CrossRef]
- Teja, M.B.K.; Sharma, A.; Das, S.; Das, K. A review on nanodispersed lead-free solders in electronics: Synthesis, microstructure and intermetallic growth characteristics. J. Mater. Sci. 2022, 57, 8597–8633. [Google Scholar] [CrossRef]
- Wang, M.; Chen, T.; Liao, T.; Zhang, X.; Zhu, B.; Tang, H.; Dai, C. Tin dioxide-based nanomaterials as anodes for lithium-ion batteries. RSC Adv. 2020, 11, 1200–1221. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Pan, Z.; Su, X.; An, L. Tin-based materials as versatile anodes for alkali (earth)-ion batteries. J. Power Sources 2018, 395, 41–59. [Google Scholar] [CrossRef]
- Liu, L.; Xie, F.; Lyu, J.; Zhao, T.; Li, T.; Choi, B.G. Tin-based anode materials with well-designed architectures for next-generation lithium-ion batteries. J. Power Sources 2016, 321, 11–35. [Google Scholar] [CrossRef]
- Godeke, A.; den Ouden, A.; Nijhuis, A.; ten Kate, H.H.J. state of the art powder-in-tube niobium-tin superconductors. Cryogenics 2008, 48, 308–316. [Google Scholar] [CrossRef]
- Xu, X. A review and prospects for Nb3Sn superconductor development. Supercond. Sci. Technol. 2017, 30, 093001. [Google Scholar] [CrossRef]
- Shivasharma, T.K.; Sahu, R.; Rath, M.C.; Keny, S.J.; Sankapal, B.R. Exploring tin oxide based materials: A critical review on synthesis, characterizations and supercapacitative energy storage. Chem. Eng. J. 2023, 477, 147191. [Google Scholar] [CrossRef]
- Mahajan, P.; Datt, R.; Tsoi, W.C.; Gupta, V.; Tomar, A.; Arya, S. Recent progres, fabrication challenges and stability issues of lead-free tin-based perovskite thin films in the field pf photovoltaics. Coord, Chem. Rev. 2021, 429, 213633. [Google Scholar] [CrossRef]
- Dalapati, G.K.; Sharma, H.; Guchhait, A.; Chakrabarty, N.; Bamola, P.; Liu, Q.; Saianand, G.; Krishna, A.M.S.; Mukhopadhyay, S.; Dey, A.; et al. Tin oxide for optoelectronic, photovoltaic and energy storage devices: A review. J. Mater. Chem. A 2021, 9, 16621–16684. [Google Scholar] [CrossRef]
- Critical Minerals Policy Tracker. International Energy Agency. Available online: https://www.iea.org/data-and-statistics/data-tools/critical-minerals-policy-tracker (accessed on 22 December 2024).
- Regulation (EU) 2024/1252 of the European Parliament and of the Council of 11 April 2024 Establishing a Framework for Ensuring a Secure and Sustainable Supply of Critical Raw Materials and Amending Regulations (EU) No 168/2013, (EU) 2018/858, (EU) 2018/1724 and (EU) 2019/1020 (Text with EEA Relevance). Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=OJ:L_202401252 (accessed on 22 December 2024).
- Critical, Strategic and Advanced Materials. Available online: https://rmis.jrc.ec.europa.eu/eu-critical-raw-materials (accessed on 22 December 2024).
- Bradley, J.E.; Auping, W.L.; Kleijn, R.; Kwakkel, J.H. Reassessing tin circularity and criticality. J. Ind. Ecol. 2024, 28, 232–246. [Google Scholar] [CrossRef]
- Olafsdottir, A.H.; Sverdrup, H.U. Modelling global mining, secondary extraction, supply, stocks-in-society, recycling, market price and resources, using the WORLD6 Model; tin. BioPhys. Econ. Res. Qual. 2018, 3, 11. [Google Scholar] [CrossRef]
- Tin Use in Recovery Cycle. International Tin Association. Available online: https://www.internationaltin.org/ita-study-tin-use-in-recovery-cycle/ (accessed on 22 December 2024).
- Rudnick, R.L.; Gao, S. Composition of the continental crust. In Treatise on Geochemistry. The Crust; Rudnick, R.L., Ed.; Elsevier: Oxford, UK, 2005; Volume 3, pp. 1–64. [Google Scholar]
- Angadi, S.I.; Sreenivas, T.; Jeon, H.S.; Baek, S.H.; Mishra, B.K. A review of cassiterite beneficiation fundamentals and plant practices. Min. Eng. 2015, 70, 178–200. [Google Scholar] [CrossRef]
- Fosu, A.Y.; Bartier, D.; Diot, F.; Kanari, N. Insight into the extractive metallurgy of tin from cassiterite. Materials 2024, 17, 3312. [Google Scholar] [CrossRef]
- Lehmann, B. Formation of tin ore deposits: A reassessment. Lithos 2021, 402–403, 105756. [Google Scholar] [CrossRef]
- Schwartz, M.O.; Rajah, S.S.; Askury, A.K.; Putthapiban, P.; Djaswadi, S. The Southeast Asian Tin Belt. Earth Sci. Rev. 1995, 38, 95–293. [Google Scholar] [CrossRef]
- BGS—World Mineral Statistics (2023)—With Major Processing by Our World in Data. “Tin production” (Dataset) British Geological Survey, “World Mineral Statistics” (Original Data). Available online: https://ourworldindata.org/metals-minerals (accessed on 12 December 2024).
- Global tin Demand: Upside in Energy Transition? Thunder Said Energy. Available online: https://thundersaidenergy.com/downloads/global-tin-demand-upside-in-energy-transition/ (accessed on 22 December 2024).
- 2020 Report on Global Tin Resources and Reserves. International Tin Association. Available online: https://www.internationaltin.org/reports/2020-report-on-global-tin-resources-reserves/ (accessed on 22 December 2024).
- Li, H.; Qin, W.; Li, J.; Tian, Z.; Jiao, F.; Yang, C. Tracing the global tin flow network: Highly concentrated production and consumption. Res. Cons. Rec. 2021, 169, 105495. [Google Scholar] [CrossRef]
- Rahayu, D.P.; Rahayu, S.; Faisal; Yanto, A. Countering illegal tin mining with a legal formulation of law based on local wisdom in Bangka Belitung, Indonesia. Cog. Soc. Sci. 2024, 10, 2311053. [Google Scholar] [CrossRef]
- Binnemans, K.; Jones, P.T. The twelve principles of circular hydrometallurgy. J. Sustain. Metall. 2023, 9, 1–25. [Google Scholar] [CrossRef]
- Quijada-Maldonado, E.; Olea, F.; Sepulveda, R.; Castillo, J.; Cabezas, R.; Merlet, G.; Romero, J. Possibilities and challenges for ionic liquids in hydrometallurgy. Sep. Pur. Technol. 2020, 251, 117289. [Google Scholar] [CrossRef]
- Binnemans, K.; Jones, P.T. Solvometallurgy: An emerging branch of extractive metallurgy. J. Sustain. Metall. 2017, 3, 570–600. [Google Scholar] [CrossRef]
- Kaksonen, A.H.; Deng, X.; Bohu, T.; Zea, L.; Khaleque, H.N.; Gumulya, Y.; Boxall, N.J.; Morris, C.; Cheng, K.Y. Prospective directions for biohydrometallurgy. Hydrometallurgy 2020, 195, 105376. [Google Scholar] [CrossRef]
- Nkuna, R.; Ijoma, G.N.; Matambo, T.S.; Chimwani, N. Accessing metals from low-grade ores and the environmental impact considerations: A review of the perspectives of conventional versus bioleaching strategies. Minerals 2022, 12, 506. [Google Scholar] [CrossRef]
- Kaksonen, A.H.; Boxall, N.J.; Gumulya, Y.; Khaleque, H.N.; Morris, C.; Bohu, T.; Cheng, K.Y.; Usher, K.M.; Lakaniemi, A.M. Recent progress in biohydrometallurgy and microbial characterization. Hydrometallurgy 2018, 180, 7–25. [Google Scholar] [CrossRef]
- Habashi, F. A short history of hydrometallurgy. Hydrometallurgy 2005, 79, 15–22. [Google Scholar] [CrossRef]
- Abbott, A.P.; Frisch, G.; Hartley, J.; Ryder, K.S. Processing of metals and metal oxides using ionic liquids. Green Chem. 2011, 13, 471–481. [Google Scholar] [CrossRef]
- Park, J.; Jung, Y.; Kusumah, P.; Lee, J.; Kwon, K.; Lee, C.K. Application of ionic liquids in hydrometallurgy. Int. J. Mol. Sci. 2014, 15, 15320–15343. [Google Scholar] [CrossRef]
- Richter, J.; Ruck, M. Ionometallurgy: An academic exercise or promising approach? RSC Sustain. 2024, 2, 1202–1214. [Google Scholar] [CrossRef]
- Binnemans, K.; Jones, P.T. Ionic liquids and deep-eutectic solvents in extractive metallurgy: Mismatch between academic research and industrial applicability. J. Sustain. Metall. 2023, 9, 423–438. [Google Scholar] [CrossRef]
- Lin, P.; Werner, J.; Groppo, J.; Yang, X. Material characterization and physical processing of a general type of waste printed circuit boards. Sustainability 2022, 14, 13479. [Google Scholar] [CrossRef]
- Oke, E.A.; Potgieter, H. Discarded e-waste/printed circuit boards: E review of their recent methods of disassembly, sorting and environmental impacts. J. Mater. Cycl. Waste Mgmt. 2024, 26, 1277–1293. [Google Scholar] [CrossRef]
- Anić-Vučinić, A.; Bedeković, G.; Sarc, R.; Premur, V. Determining metal content in waste printed circuit boards and their electronic components. J. Sustain. Devel. En Wat. Environ. Sys. 2020, 8, 590–602. [Google Scholar] [CrossRef]
- Szałatkiewicz, J. Metals content in printed circuit board waste. Pol. J. Environ. Stud. 2014, 23, 2365–2369. [Google Scholar]
- Yuan, C.Y.; Zhang, H.C.; McKenna, G.; Korzeniowski, C.; Li, J. Experimental studies on cryogenic recycling of printed circuit board. Int. J. Adv. Manuf. Technol. 2007, 34, 357–666. [Google Scholar] [CrossRef]
- Ogunniyi, I.O.; Vermaak, M.K.G.; Groot, D.R. Chemical composition and liberation characterization of printed circuit board comminution fines for beneficiation investigations. Waste Manag. 2009, 29, 2140–2146. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Eksteen, J.; Oraby, E. Hydrometallurgical recovery of metals from waste printed circuit boards (WPCBs): Current status and perspectives—A review. Res. Conserv. Rec. 2018, 139, 122–139. [Google Scholar] [CrossRef]
- Touze, S.; Hubau, A.; Ghestem, J.P.; Moreau, P.; Lafaurie, N.; Noireaux, J. Estimation of the uncertainty of metal content in a batch of waste printed circuit boards from computer motherboards. Waste Mgmt. 2024, 189, 325–333. [Google Scholar] [CrossRef]
- Cui, H.; Anderson, C. Hydrometallurgical treatment of waste printed circuit boards: Bromine leaching. Metals 2020, 10, 462. [Google Scholar] [CrossRef]
- Van Yken, J.; Cheng, K.Y.; Boxall, N.J.; Sheedy, C.; Nikoloski, A.N.; Moheimani, N.R.; Kaksonen, A.H. A comparison of methods for the characterization of waste-printed circuit boards. Metals 2021, 11, 1935. [Google Scholar] [CrossRef]
- Markets Insider. Available online: https://markets.businessinsider.com/commodities/tin-price (accessed on 8 January 2025).
- Yang, C.; Tan, Q.; Liu, L.; Dong, Q.; Li, J. Recycling tin from electronic waste: A problem that needs more attention. ACS Sustain. Chem. Eng. 2017, 5, 9586–9598. [Google Scholar] [CrossRef]
- Arshadi, M.; Yaghmaei, S.; Mousavi, S.M. Content evaluation of different waste PCBs to enhance basic metals recycling. Res. Cons. Rec. 2018, 139, 298–306. [Google Scholar] [CrossRef]
- Meeh, P. Immersion tin: A proven final finish for printed circuits boards providing reliable solderability and marginal formation of tin-whiskers. Circuit World 2004, 31, 28–40. [Google Scholar] [CrossRef]
- Cheng, S.; Huang, C.M.; Pecht, M. A review of lead-free solders for electronic applications. Microel. Rel. 2017, 75, 77–95. [Google Scholar] [CrossRef]
- Marques, A.C.; Cabrera, J.M.; de Fraga Malfatti, C. Printed circuits boards: A review on the perspective of sustainability. J. Environ. Mgmt. 2013, 131, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Restriction of Hazardous Substances in Electrical and Electronic Equipment (RoHS). Available online: https://environment.ec.europa.eu/topics/waste-and-recycling/rohs-directive_en (accessed on 8 January 2025).
- Desye, B.; Tesfaye, A.H.; Berihun, G.; Ademas, A.; Sewunet, B. A systematic review of the health effects of lead exposure from electronic waste in children. Front. Public Health 2023, 11, 1113561. [Google Scholar] [CrossRef] [PubMed]
- Beula, D.; Sureshkumar, M. A review on the toxic E-waste killing health and environment—Today’s global scenario. Mater. Today Proc. 2021, 47, 2168–2174. [Google Scholar] [CrossRef]
- Ankit; Saha, L.; Kumar, V.; Tivari, J.; Sweta; Rawat, S.; Singh, J.; Bauddh, K. Electronic waste and their leachates impact on human health and environment: Global ecological threat and management. Environ. Technol. Innov. 2021, 24, 102049. [Google Scholar] [CrossRef]
- Adie, G.U.; Sun, L.; Zeng, X.; Zheng, L.; Osibanjo, O.; Li, J. Examining the evolution of metals utilized in printed circuit boards. Environ. Technol. 2017, 38, 1696–1701. [Google Scholar] [CrossRef]
- Fatema, K.; Hassan, M.N.; Hasan, S.; Roy, H. E-waste recycling in an optimized way for copper recovery by leaching and a case study on E-waste generation and management. Heliyon 2025, 11, e41453. [Google Scholar] [CrossRef]
- Maluleke, M.D.; Kotsiopoulous, A.; Govender-Optiz, E.; Harrison, S.T.L. Microbial immobilization and adaptation to Cu2+ enhances microbial Fe2+ oxidation for bioleaching of printed circuit boards in the presence of mixed metal ions. Res. Microbiol. 2024, 175, 104148. [Google Scholar] [CrossRef] [PubMed]
- Holgersson, S.; Steenari, B.M.; Björkman, M.; Cullbrand, K. Analysis of the metal content of small-size waste electric and electronic equipment (WEEE) printed circuit boards—Part 1: Internet routers, mobile phones and smartphones. Res. Cons. Rec. 2018, 133, 300–308. [Google Scholar] [CrossRef]
- Bizzo, W.A.; Figueiredo, R.A.; de Andrade, V.F. Characterization of printed circuit boards for metal and energy recovery after milling and mechanical separation. Materials 2014, 7, 4555–4566. [Google Scholar] [CrossRef]
- Tunali, M.; Tunali, M.M.; Yenigun, O. Characterization of different types of electronic waste: Heavy metal, precious metal and rare earth element content by comparing different digestion methods. J. Mater. Cycl. Waste Mgmt. 2021, 23, 149–157. [Google Scholar] [CrossRef]
- Das, S.; Ting, Y.-P. Evaluation of wet digestion methods for quantification of metal content in electronic scrap material. Resources 2017, 6, 64. [Google Scholar] [CrossRef]
- Jha, R.; Agrawal, M.; Jena, A.; Mishra, G.; Verma, H.R.; Meshram, A.; Singh, K.K. A review on existing pre-treatment techniques of waste printed circuit boards. Can. Metall. Quart. 2024, 64, 336–352. [Google Scholar] [CrossRef]
- Das, S.K.; Ellamparuthy, G.; Kundu, T.; Angadi, S.I.; Rath, S.S. A comprehensive review of the mechanical separation of waste printed circuit boards. Proc. Safety Environ. Prot. 2024, 187, 221–239. [Google Scholar]
- Raman, P.R.; Shanmugam, R.R.; Saminathan, S. Review on the role of density-based separation in PCBs recycling. Chem. Eng. J. 2024, 496, 154339. [Google Scholar] [CrossRef]
- Bhoi, N.K. Advancements in E-waste recycling technologies: A comprehensive overview of strategies and mechatronics integration for future development. Sustain. Mater. Technol. 2024, 42, e01182. [Google Scholar] [CrossRef]
- Puttlitz, K.J.; Salter, K.A. Handbook of Lead-Free Solder Technology For Microelectronic Assemblies; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Properties of Solder Alloys. Available online: https://invacu.com/solder-alloys (accessed on 8 January 2025).
- Kaya, M. Waste printed circuit board (WPCB) recovery technology disassembly and desoldering approach. In Encyclopedia of Renevable and Sustainable Materials; Hashmi, S., Choudhury, I.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 4, pp. 658–676. [Google Scholar]
- Zhao, W.; Xu, J.; Fei, W.; Liu, Z.; He, W.; Li, G. The reuse of electronic components from waste printed circuit boards: A critical review. Environ. Sci. Adv. 2023, 2, 196–214. [Google Scholar] [CrossRef]
- Hossain, R.; Nekuoei, R.K.; Mansuri, I.; Sahajwalla, V. Sustainable recovery of Cu and Sn from problematic global waste: Exploring value from waste printed circuit boards. ACS Sustain. Chem. Eng. 2019, 7, 1006–1017. [Google Scholar] [CrossRef]
- Meng, L.; Gao, J.; Zhong, Y.; Wang, Z.; Chen, K.; Guo, Z. supergravity separation for recovering Pb and Sn from electronic waste. Sep. Pur. Technol. 2018, 191, 375–383. [Google Scholar] [CrossRef]
- Veit, H.M.; Pereira, C.C.; Bernardes, A.M. Using mechanical processing in recycling printed wiring boards. JOM 2002, 54, 45–47. [Google Scholar] [CrossRef]
- Veit, H.M.; Diehl, T.R.; Salami, A.P.; Rodrigues, J.S.; Bernardes, A.M.; Tenório, J.A.S. Utilization of magnetic and electrostatic separation in the recycling of printed circuit board scrap. Waste Mgmt. 2005, 25, 67–74. [Google Scholar] [CrossRef]
- Chao, G.; Hui, W.; Wei, L.; Jiangang, F.; Xin, Y. Liberation characteristics and physical separation of printed circuit board (PCB). Waste Mgmt. 2011, 31, 2161–2166. [Google Scholar]
- Yao, Y.; He, J.; Yang, B.; Zhao, Y.; Zhu, L. Study on particle characteristics and metal distribution of waste printed circuit boards based on a shear crusher. Powder Technol. 2023, 415, 118103. [Google Scholar] [CrossRef]
- Barnwal, A.; Dhawan, N. Evaluation of fluidization process for recovery of metals from discarded printed circuit boards. J. Sustain. Metall. 2019, 5, 519–527. [Google Scholar] [CrossRef]
- Franke, D.M.; Kar, U.; Suponik, T.; Siudyga, T. Evaluation of the use of flotation for the separation of ground printed circuit boards. Min. Res. Mgmt. 2022, 38, 171–188. [Google Scholar]
- Barnwal, A.; Mir, S.; Dhawan, N. Processing of discarded printed circuit board fines via flotation. J. Sustain. Metall. 2020, 6, 631–642. [Google Scholar] [CrossRef]
- Das, S.K.; Ellamparuthy, G.; Kundu, T.; Ghosh, M.K.; Angadi, S.I. Critical analysis of metallic and non-metallic fractions in the flotation of waste printed circuit boards. Powder Technol. 2021, 389, 450–459. [Google Scholar] [CrossRef]
- Smith, P.J. Chemistry of Tin, 2nd ed.; Springer Science+Business Media: Dordrecht, The Netherlands, 1998. [Google Scholar]
- Bielański, A. Podstawy Chemii Nieorganicznej, 2nd ed.; Wydawnictwo Naukowe PWN: Warszawa, Polska, 1994. [Google Scholar]
- Ranitović, M.; Kamberović, Ž.; Korać, M.; Jovanović, N.; Mihajlović, A. Hydrometallurgical recovery of tin and lead from waste printed circuit boards (WPCBs): Limitations and opportunities. Metallurgija 2016, 55, 153–156. [Google Scholar]
- Jha, M.K.; Choubey, P.K.; Jha, A.K.; Kumari, A.; Lee, J.; Kumar, V.; Jeong, J. Leaching studies for tin recovery from waste e-scrap. Waste Mgmt. 2012, 32, 1919–1925. [Google Scholar] [CrossRef]
- Moosakazemi, F.; Ghassa, S.; Mohammadi, M.R.T. Environmentally friendly hydrometallurgical recovery of tin and lead from waste printed circuit boards: Thermodynamic and kinetics studies. J. Clean. Prod. 2019, 228, 185–196. [Google Scholar] [CrossRef]
- Moosakazemi, F.; Ghassa, S.; Soltani, F.; Mohammadi, M.R.T. Regeneration of Sn-Pb solder from waste printed circuit boards: A hydrometallurgical approach to treating waste with waste. J. Hazard. Mater. 2020, 385, 121589. [Google Scholar] [CrossRef]
- Castro, L.A.; Martins, A.H. Recovery of tin and copper by recycling of printed circuit boards from obsolete computers. Braz. J. Chem. Eng. 2009, 26, 649–657. [Google Scholar] [CrossRef]
- Hao, J.; Wang, X.; Wang, Y.; Guo, F.; Wu, F. Optimization, kinetic studies of tin leaching from waste printed circuit boards and selective tin recovery from its pregnant solution. Metals 2022, 12, 954. [Google Scholar] [CrossRef]
- Mecucci, A.; Scott, K. Leaching and electrochemical recovery of copper, lead and tin from scrap printed circuit boards. J. Chem. Technol. Biotechnol. 2002, 77, 449–457. [Google Scholar] [CrossRef]
- Guo, X.; Qin, H.; Tian, Q.; Li, D. Recovery of metals from waste printed circuit boards by selective leaching combined with cyclone electrowinning process. J. Hazard. Mater. 2020, 384, 121355. [Google Scholar] [CrossRef]
- Abdo, D.M.; Adbelbasir, S.M.; El-Sheltawy, S.T.; Ibrahim, I.A. Recovery of tin as tin oxide nanoparticles from waste printed circuit boards for photocatalytic dye degradation. Korean J. Chem. Eng. 2021, 38, 1934–1945. [Google Scholar] [CrossRef]
- Lisińska, M.; Wojtal, T.; Saternus, M.; Willner, J.; Rzelewska-Piekut, M.; Nowacki, K. Two-stage leaching of PCBs using sulfuric acid and nitric acid with the addition of hydrogen peroxide and ozone. Materials 2024, 17, 219. [Google Scholar] [CrossRef]
- Vlasopoulos, D.; Oustadakis, P.; Remoundaki, E.; Agatzini-Leonardou, S. Hydrometallurgical recovery of tin from waste-printed circuit boards. Mater. Proc. 2023, 15, 90. [Google Scholar]
- Zhao, J.; Liu, Z.; He, C.; Yang, Y.; Li, J.; Fujita, T.; Wang, G.; Shen, F. Improved leaching of Cu, Sn, Pb, Zn, and Al from waste printed circuit boards by electro-generated Cl2 in HCl solution. Waste Mgmt. 2022, 153, 386–396. [Google Scholar] [CrossRef]
- Jung, M.; Yoo, K.; Alorro, R.D. Dismantling of electric and electronic components from waste printed circuit boards by hydrochloric acid leaching with stannic ions. Mat. Trans. 2017, 58, 1076–1080. [Google Scholar] [CrossRef]
- Zhang, X.; Guan, J.; Guo, Y.; Yan, X.; Yuan, H.; Xu, J.; Guo, J.; Zhou, Y.; Su, R.; Guo, Z. Selective desoldering separation of tin-lead alloy for dismantling of electronic components from printed circuits boards. ACS Sustain. Chem. Eng. 2015, 3, 1696–1700. [Google Scholar] [CrossRef]
- Zhang, X.; Guan, J.; Guo, Y.; Cao, Y.; Guo, J.; Yuan, H.; Su, R.; Liang, B.; Gao, G.; Zhou, Y.; et al. Effective dismantling of waste printed circuit board assembly with methanesulfonic acid containing hydrogen peroxide. Environ. Prog. Sustain. En. 2017, 36, 873–878. [Google Scholar] [CrossRef]
- Mishra, S.; Hunter, T.N.; Pant, K.K.; Harbottle, D. Green deep eutectic solvents (DESs) for sustainable metal recovery from thermally treated PCBs: A greener alternative to conventional methods. ChemSusChem 2024, 17, e202301418. [Google Scholar] [CrossRef]
- Yang, T.; Zhu, P.; Liu, W.; Chen, L.; Zhang, D. Recovery of tin from metal powders of waste printed circuit boards. Waste Mgmt. 2017, 68, 449–457. [Google Scholar] [CrossRef]
- Tang, C.; Deng, X.; Chen, Y.; Li, Y.; Deng, C.; Zhu, Q.; Liu, J.; Yang, S. Electrochemical dissolution and recovery of tin from printed circuit board in methane-sulfonic acid solution. Hydrometallurgy 2021, 205, 105726. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, C.; Zheng, F.; Ma, E.; Wang, R.; Bai, J.; Yuan, W.; Wang, J. Alkaline electrochemical leaching of Sn and Pb from the surface of waste printed circuit board and the stripping of gold by methanesulfonic acid. Environ. Prog. Sustain. En. 2020, 39, e133324. [Google Scholar] [CrossRef]
- Liu, B.; Shi, C.; Huang, Y.; Han, G.; Sun, G.; Zhang, L. Intensifying separation of Pb and Sn from waste Pb-Sn alloy by ultrasound-assisted acid leaching: Selective dissolution and sonochemistry mechanism. Ultrason. Sonochem. 2024, 102, 106758. [Google Scholar] [CrossRef] [PubMed]
- Gande, V.V.; Vats, S.; Bhatt, N.; Pushpavanam, S. Sequential recovery of metals from waste printed circuit boards using a zero-charge hydrometallurgical process. Clean. Eng. Technol. 2021, 4, 100143. [Google Scholar] [CrossRef]
- Thagarajan, V.; Srinivasan, S.; Sukesh, R.L.; Ramesh, L.; Ranjani, P.; Pushpavanam, S. Scale up of a process for extraction of tin, lead, and copper from waste printed circuit boards by simultaneous electrolysis. ACS Sustain. Resour. Mgmt. 2024, 1, 732–742. [Google Scholar]
- ILyas, S.; Srivastava, R.R.; Kim, H.; Iluas, N. Biotechnological recycling of hazardous waste PCBs using Sulfobactillus thermosulfidooxidans through pretreatment of toxicant metals: Process optimization and kinetics studies. Chemosphere 2022, 286, 131978. [Google Scholar] [CrossRef]
- Havlik, T.; Orac, D.; Petranikova, M.; Miskufova, A.; Kukurugya, F.; Takacova, Z. Leaching of copper and tin from used printed circuits boards after thermal treatment. J. Hazard. Mater. 2010, 183, 866–873. [Google Scholar] [CrossRef]
- Kim, S.; Lee, J.; Yoo, K. Leaching of tin from waste Pb-free solder in hydrochloric acid solution with stannic chloride. Hydrometallurgy 2016, 165, 143–147. [Google Scholar] [CrossRef]
- Jadhav, U.; Hocheng, H. Hydrometallurgical recovery of metals from large printed circuit board pieces. Sci. Rep. 2015, 5, 14574. [Google Scholar] [CrossRef]
- Konaté, P.O.; Vitry, V.; Yonli, A.H. Leaching base materials in PCBs and copper cementation by iron powder. J. Hazard. Mater. Adv. 2024, 15, 100449. [Google Scholar]
- Ping, Z.; Liu, X.; Tao, Q.; Ma, Y.; Wang, Y.; Li, Z.; Wang, J.; Cao, Z.; Hao, Y.; Qian, G. Mechanism of dissolving tin solders from waste printed circuit board assemblies by cyclic fluoroboric acid composite system. Environ. Eng. Sci. 2019, 36, 903–911. [Google Scholar] [CrossRef]
- Fogarasi, S.; Imre-Lucaci, A.; Imre-Lucaci, F. Dismantling of waste printed circuit boards with the simultaneous recovery of copper: Experimental study and process modeling. Materials 2021, 14, 5186. [Google Scholar] [CrossRef]
- Yazici, E.Y.; Deveci, H. Ferric sulphate leaching of metals from waste printed circuit boards. Int. J. Min. Proc. 2014, 133, 39–45. [Google Scholar] [CrossRef]
- Yang, C.; Li, J.; Tan, Q.; Liu, L.; Dong, Q. Green process of metal recycling: Coprocessing waste printed circuit boards and spent tin stripping solution. ACS Sustain. Chem. Eng. 2017, 5, 3524–3534. [Google Scholar] [CrossRef]
- Tan, Q.; Liu, L.; Yu, M.; Li, J. An innovative method of recycling metals in printed circuit board (PCB) using solutions from PCB production. J. Hazard. Mater. 2020, 390, 121892. [Google Scholar] [CrossRef]
- Nan, T.; Yang, J.; Aromaa-Atubb, R.; Zhu, Q.; Lundström, M. Process simulation and life cycle assessment of hydrometallurgical recycling routes of waste printed circuit boards. J. Clean. Prod. 2024, 435, 140458. [Google Scholar] [CrossRef]
- Segura-Bailon, B.; Lapidus, G.T. Selective recovery of copper contained in waste PCBs from cellphones with impurity inhibition in the citrate-phosphate system. Hydrometallurgy 2021, 203, 105699. [Google Scholar] [CrossRef]
- Segura-Bailon, B.; Lapidus, G.T. Selective leaching of base/precious metals from E-waste of cellphone printed circuit boards (EWPCB): Advantages and challenges in case study. Hydrometallurgy 2023, 217, 106040. [Google Scholar] [CrossRef]
- Fogarasi, S.; Imre-Lucaci, F.; Fogarasi, M.; Imre-Lucaci, A. Technical and environmental assessment of selective recovery of tin and lead from waste solder alloy using direct anodic oxidation. J. Clean. Prod. 2019, 213, 872–883. [Google Scholar] [CrossRef]
- Rudnik, E.; Bayaraa, E. Electrochemical dissolution of smelted low-grade electronic scraps in acid sulfate-chloride solutions. Hydrometallurgy 2016, 159, 110–119. [Google Scholar] [CrossRef]
- Rudnik, E.; Dashbold, N. Study on copper recovery from smelted low-grade e-scrap using hydrometallurgical methods. Min. Metall. Proc. 2017, 34, 20–29. [Google Scholar] [CrossRef]
- Rudnik, E.; Dobosz, I.; Włoch, G. Ammoniacal dissolution of polymetallic alloy produced from waste electroscrap. Russ. J. Nonferr. Met. 2018, 59, 476–485. [Google Scholar] [CrossRef]
- Rudnik, E. Application of ammoniacal solutions for leaching and electrochemical dissolution of metals from alloys produced from low-grade e-scrap. Arch. Metall. Mater. 2017, 63, 1679–1686. [Google Scholar] [CrossRef]
- Rudnik, E.; Pierzynka, M.; Handzlik, P. Ammoniacal leaching and recovery of copper from alloyed low-grade e-waste. J. Mater. Cycl. Waste. Mgmt. 2016, 18, 318–328. [Google Scholar] [CrossRef]
- Rudnik, E.; Kołczyk, K.; Kutyła, D. Comparative studies on hydrometallurgical treatment of smelted low-grade electronic scraps for selective copper recovery. Trans. Nonferr. Met. Soc. China 2015, 25, 2763–2771. [Google Scholar] [CrossRef]
- Groot, D.R.; van der Linde, J.A.N. The processing of e-waste. Part 1: The preparation and characterization of a metallic alloy derived from the smelting of printed circuit boards. J. South. Afr. Inst. Min. Metall. 2009, 109, 697–700. [Google Scholar]
- Groot, D.R.; van der Linde, J.A.N. The processing of e-waste. Part 2: The electrochemical leaching behaviour of a metallic alloy derived from the smelting of waste printed circuit boards. J. South. Afr. Inst. Min. Metall. 2009, 109, 701–707. [Google Scholar]
- Lim, Y.; Kwon, O.; Lee, J.; Yoo, K. The ammonia leaching of alloy produced from waste printed circuit boards smelting process. Geosys. Eng. 2013, 16, 216–224. [Google Scholar] [CrossRef]
- Guo, X.; Liu, J.; Qin, H.; Liu, Y.; Tian, Q.; Li, D. Recovery of metal values from waste printed circuit boards using an alkali fusion-leaching-separation process. Hydrometallurgy 2015, 156, 199–205. [Google Scholar] [CrossRef]
- Rao, M.D.; Singh, K.K.; Morrison, C.A.; Love, J.B. Selective recovery of nickel from obsolete mobile phone PCBs. Hydrometallurgy 2022, 210, 105843. [Google Scholar] [CrossRef]
- da Silva, M.S.B.; de Melo, R.A.C.; Lopes-Moriyama, A.L.; Souza, C.P. electrochemical extraction of tin and copper from acid leachate of printed circuit boards using copper electrodes. J. Environ. Mgmt. 2019, 246, 410–417. [Google Scholar] [CrossRef]
- Baniasadi, M.; Vakilchap, F.; Bahaloo-Horeh, N.; Mousavi, S.M.; Farnaud, S. Advances in bioleaching as a sustainable method for metal recovery from e-waste: A review. J. Ind. Eng. Chem. 2019, 76, 75–90. [Google Scholar] [CrossRef]
- Dusengemungu, L.; Kasali, G.; Gwanama, C.; Mubema, B. Overview of fungal bioleaching of metals. Environ. Adv. 2021, 5, 100083. [Google Scholar] [CrossRef]
- Hu, S.; Wang, H.; Li, X.; He, w.; Ma, J.; Xu, Y.; Xu, Y.; Ming, W. Recent advances in bioleaching and biosorption of metals from waste printed circuit boards: A review. J. Environ. Mgmt. 2024, 371, 123008. [Google Scholar] [CrossRef] [PubMed]
- Brandl, H.; Bosshard, R.; Wegmann, M. Computer-munching microbes: Metal leaching from electronic scrap by bacteria and fungi. Hydrometallurgy 2001, 59, 319–326. [Google Scholar] [CrossRef]
- Bryan, C.G.; Watkin, E.L.; McCredden, T.J.; Wong, Z.R.; Harrison, S.T.L.; Kaksonen, A.H. The use of pyrite as a source of lixiviant in the bioleaching of electronic waste. Hydrometallurgy 2015, 152, 33–43. [Google Scholar] [CrossRef]
- Jadhav, U.; Hocheng, H. Leaching of metals from printed circuit board powder by an Aspergillus niger culture supernatant and hydrogen peroxide. RSC Adv. 2016, 6, 43442–43452. [Google Scholar] [CrossRef]
- Xia, M.C.; Wang, Y.P.; Peng, T.J.; Shen, L.; Yu, R.L.; Liu, Y.D.; Chen, M.; Li, J.K.; Wu, X.L.; Zeng, W.M. Recycling of metals from pretreated waste printed circuit boards effectively in stirred tank reactor by a moderately thermophilic culture. J. Biosci. Bioeng. 2017, 123, 714–721. [Google Scholar] [CrossRef]
- Santaolalla, A.; Lens, P.N.L.; Barona, A.; Rojo, N.; Ocio, A.; Gallastequi, G. Metal extraction and recovery from mobile phone PCBs by a combination of bioleaching and precipitation processes. Minerals 2021, 11, 1004. [Google Scholar] [CrossRef]
- Magoda, K.; Nomngongo, P.N.; Mekuto, L. Two-step bio-dissolution of metals from printed circuit boards using acidophilic iron- and sulfur-oxidizing mesophiles. Recycling 2024, 9, 6. [Google Scholar] [CrossRef]
- Alias, C.; Bulgari, D.; Bilo, F.; Borgese, L.; Gianoncelli, A.; Ribaudo, G.; Gobbi, E.; Alessandri, I. Food waste-assisted metal extraction from printed circuit boards: The Aspergillus niger route. Microorganisms 2021, 9, 895. [Google Scholar] [CrossRef] [PubMed]
- Barrueto, Y.; Hernández, P.; Jiménez, Y.P.; Morales, J. Properties and application of ionic liquids in leaching base/precious metals from e-waste. A review. Hydrometallurgy 2022, 212, 105895. [Google Scholar] [CrossRef]
- Guo, M.; Deng, R.; Gao, M.; Xu, C.; Zhang, Q. Sustainable recovery of metals from e-waste using deep eutectic solvents: Advances, challenges, and perspectives. Curr. Op. Green Sustain. Chem. 2024, 47, 100913. [Google Scholar] [CrossRef]
- Gómez, M.; Grimes, S.; Fowler, G. Novel hydrometallurgical process for the recovery of copper from end-of-life mobile phone printed circuit boards using ionic liquids. J. Clean. Prod. 2023, 420, 138379. [Google Scholar] [CrossRef]
- Łukomska, A.; Wiśniewska, A.; Dąbrowski, Z.; Lach, J.; Wróbel, K.; Kolasa, D.; Domańska, U. Recovery of metals from electronic waste-printed circuit boards by ionic liquids, DESs and organophosphorus-based acid extraction. Molecules 2022, 27, 4984. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, S.; Huang, J.; Chen, H. Lead during the leaching process of copper from waste printed circuit boards by five typical ionic liquid acids. J. Clean. Prod. 2015, 95, 142–147. [Google Scholar] [CrossRef]
- Zhu, P.; Chen, Y.; Wang, L.Y.; Zhou, M. A new technology for recycling solder from waste printed circuit boards using ionic liquid. Waste Mgmt. Res. 2012, 30, 1222–1226. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Li, J.; Xie, H.; Liu, L. A novel dismantling process of waste printed circuit boards using water-soluble ionic liquid. Chemosphere 2013, 93, 1288–1294. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Ma, S.; Ho, W.; Wang, Y.; Ho, J.Y.T.; Shih, K. Simple and environmentally friendly metal recovery from waste printed circuit boards by using deep eutectic solvents. J. Clean. Prod. 2023, 421, 138508. [Google Scholar] [CrossRef]
- Display Panel Production Capacity from 2016 to 2025, by Type, Statista. Available online: https://www.statista.com/statistics/1057441/display-panel-production-capacity-type/ (accessed on 22 January 2025).
- Liu, Z.; Xu, Z.; Huang, H.; Li, B. A study of waste liquid crystal display generation in mainland China. Waste Mgmt. Res. 2016, 34, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Kalmykova, Y.; Patricio, J.; Rosado, L.; Berg, P.E.O. Old with the old, out with the new—The effect of transitions in TVs and monitors technology on consumption and WEEE generation in Sweden 1996–2014. Waste Mgmt. 2015, 46, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Akcil, A.; Agcasulu, I.; Swain, B. Valorization of waste LCD and recovery of critical raw material for circular economy: A review. Res. Cons. Rec. 2019, 149, 622–637. [Google Scholar] [CrossRef]
- Chen, J.; Cranton, W.; Fihn, M. Handbook of Visual Display Technology; Springer–Verlag: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Ueberschaar, M.; Schlummer, M.; Jalalpoor, D.; Kaup, N.; Rotter, V.S. Potential and recycling strategies for LCD panels from WEEE. Recycling 2017, 2, 7. [Google Scholar] [CrossRef]
- Illés, I.B.; Kékesi, T. The application of selective leaching and complex anion exchange in a novel aqueous process to produce pure indium from waste liquid crystal display panels. J. Environ. Chem. Eng. 2022, 10, 108420. [Google Scholar] [CrossRef]
- Kobylarz, D.; Jurowski, K. measurement of hazardous elements as emerging contaminants from discarded Liquid Crystal Displays (LCDs) from mobile phones available in Poland using portable XRF as “white” analytical technique. Measurement 2025, 244, 116492. [Google Scholar] [CrossRef]
- Schuster, J.; Ebin, B. Investigation of indium and other valuable metals leaching from unground waste LCD screens by organic and inorganic acid leaching. Sep. Pur. Technol. 2021, 279, 119659. [Google Scholar] [CrossRef]
- Europe Tin Market Report by Product Type (Metal, Alloy, Compounds), Application (Soldering, Tin Plating, Chemicals, and Others), End Use Industry (Automotive, Electronics, Packaging (Food and Beverages), Glass, and Others), and Country 2025–2033. IMARC. Available online: https://www.imarcgroup.com/europe-tin-market-report (accessed on 22 January 2025).
- Zhang, K.; Wu, Y.; Wang, W.; Li, B.; Zhang, Y.; Zuo, T. Recycling indium from waste LCDs: A review. Res. Cons. Rec. 2015, 104, 276–290. [Google Scholar] [CrossRef]
- Wang, X.; Lu, X.; Zhang, S. Study of waste liquid crystal display treatment: Focus on the resource recovery. J. Hazard. Mat. 2013, 244–245, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Gao, S.; Duan, H.; Liu, L. Recovery of valuable materials from waste liquid crystal display panel. Waste Mgmt. 2009, 29, 2033–2039. [Google Scholar] [CrossRef] [PubMed]
- Ruan, J.; Guo, Y.; Qiao, Q. Recovery of indium from scrap TFT-LCDs by solvent extraction. Proc. Environ. Sci. 2012, 16, 545–551. [Google Scholar] [CrossRef]
- Dobiba, G.; Nagai, H.; Wang, L.P.; Okaya, K.; Fujita, T. Leaching of indium from obsolete liquid crystal displays: Comparing grinding with electrical disintegration in context of LCA. Waste Mgmt. 2012, 32, 1937–1944. [Google Scholar] [CrossRef]
- Rocchetti, L.; Amato, A.; Fonti, V.; Ubaldini, S.; De Michelis, I.; Kopacek, B.; Vegilio, F.; Beolchini, F. Cross-current leaching indium from end-of-life LCD panels. Waste Mgmt. 2015, 42, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Li, B.; Wu, Y.; Wang, W.; Li, R.; Zhang, Y.; Zuo, T. Recycling of indium from waste LCD: A promising non-crushing leaching with the aid of ultrasonic wave. Waste. Mgmt. 2017, 64, 236–243. [Google Scholar] [CrossRef]
- Stępień, M.; Palimąka, P.; Bukowska, A. Impact of selected parameters on extraction of indium from LCD screens. Metall. Found. Eng. 2017, 43, 305–311. [Google Scholar] [CrossRef]
- Souada, M.; Louage, C.; Doisy, J.-Y.; Meunier, L.; Benderrag, A.; Ouddane, B.; Bellayer, S.; Nins, N.; Traisnel, M.; Maschke, U. Extraction of indium-tin oxide from end-of life LCD panels using ultrasound assisted leaching. Ultrason. Sonochem. 2018, 40, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Yáñez-López, A.; Alonso, A.; Vengoechea-Pimienta, A.; Ramírez-Muñoz, J. Indium and tin recovery from waste LCD panels using citrate as complexing agent. Waste Mgmt. 2019, 96, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Zhang, L.; Xu, Z. Indium recovery from In-Sn-Cu-Al mixed system of waste liquid crystal display panels via acid leaching and two-step electrodeposition. J. Hazard. Mat. 2020, 381, 120973. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Ning, S.; Fujita, T.; Wei, Y.; Zhang, S.; Lu, S. Leaching of indium and tin from waste LCD by a time-efficient method assisted planetary high energy ball milling. Waste Mgmt. 2021, 120, 193–201. [Google Scholar] [CrossRef]
- Toache-Pérez, A.D.; Lapidus, G.T.; Bolarín-Miró, A.M.; Sánchez De Jesús, F. Selective leaching and recovery of Er, Gd, Sn, and In from liquid crystal display screen waste by sono-leaching assisted by magnetic separation. ACS Omega 2022, 7, 31897–31904. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Ning, S.; Zeng, J.; He, Z.; Hu, F.; Li, Y.; Fujita, T.; Wei, Y. Leaching behavior and process optimization of tin recovery from waste liquid crystal display under mechanical activation. J. Clean. Prod. 2023, 399, 136640. [Google Scholar] [CrossRef]
- Yang, J.; Retegan, T.; Ekberg, C. Indium recovery from discarded LCD panel glass by solvent extraction. Hydrometallurgy 2013, 137, 68–77. [Google Scholar] [CrossRef]
- Kang, H.N.; Kim, K.Y.; Kim, J.Y. Recovery and purification of indium from waste sputtering target by selective solvent extraction of Sn. Green Chem. 2013, 15, 2200–2207. [Google Scholar] [CrossRef]
- Cui, J.; Zhu, N.; Luo, D.; Li, Y.; Wu, P.; Dang, Z.; Hu, X. The role of oxalic acid in the leaching system for recovering indium from waste liquid crystal display panels. ACS Sustain. Chem. Eng. 2019, 7, 3849–3857. [Google Scholar] [CrossRef]
- Kato, T.; Igarashi, S.; Ishiwatari, Y.; Furukawa, M.; Yamaguchi, H. Separation and concentration of indium from a liquid crystal display via homogenous liquid-liquid extraction. Hydrometallurgy 2013, 137, 148–155. [Google Scholar] [CrossRef]
- Qin, J.; Ning, S.; Xu, J.; Guo, F.; Li, Z.; Wei, Y.; Dodiba, G.; Fujita, T. Study on the adsorption behavior of tin from waste liquid crystal display using novel macroporous silica-based adsorbent in one-step separation. Sep. Pur. Technol. 2022, 292, 121006. [Google Scholar] [CrossRef]
- Wang, F.; Xu, S.; Wang, N.; Fujita, T.; Ning, S.; Wei, Y.; Wang, X. Precise target capture and the dynamic separation of Sn(IV) in highly acidic media by combining N and P donor covalent organic framework silica-based composite adsorbents. J. Clean. Prod. 2024, 747, 143596. [Google Scholar] [CrossRef]
- Grimes, S.M.; Yashri, N.G.; Chaudhary, A.J. Recovery of critical metals from dilute leach solutions-separation of indium from tin and lead. Inorg. Chim. Acta 2017, 461, 161–166. [Google Scholar] [CrossRef]
- Jowkar, M.J.; Bahaloo-Horeh, N.; Mousavi, S.M.; Pourhossein, F. Bioleaching of indium from discarded liquid crystal displays. J. Clean. Prod. 2018, 180, 417–429. [Google Scholar] [CrossRef]
- Cui, J.; Zhu, N.; Li, Y.; Luo, D.; Wu, P.; Dang, Z. Rapid and green process for valuable materials recovery from waste liquid crystal displays. Res. Cons. Rec. 2020, 153, 104544. [Google Scholar] [CrossRef]
- Golzar-Ahmani, M.; Mousavi, S.M. Extraction of valuable metals from discarded AMOLED displays in smartphones using Bacillus foraminis as as alkali-tolerant strain. Waste Mgmt. 2021, 131, 226–236. [Google Scholar] [CrossRef]
- Constantin, A.; Pourhossein, F.; Ray, D.; Farnsud, S. Investigating the acidophilic microbial community’s adaptation for enhancement indium bioleaching from high pulp density shredded discarded LCD panels. J. Environ. Mgmt. 2024, 365, 121521. [Google Scholar] [CrossRef] [PubMed]
- Parsa, A.; Bahaloo-Horeh, N.; Mousavi, S.M. A kinetic study of indium, aluminum, arsenic, and strontium extraction from LCDs using biometabolites produced by Aspergillus niger. Min. Eng. 2024, 205, 108441. [Google Scholar] [CrossRef]
- Willner, J.; Fornalczyk, A.; Gajda, B.; Saternus, M. Bioleaching of indium and tin from used LCD panels. Physicochem. Probl. Miner. Process. 2018, 54, 639–645. [Google Scholar]
- Willner, J.; Fornalczyk, A.; Saternus, M.; Sedlakova-Kadukova, J.; Gajda, B. LCD panels bioleaching with pure and mixed culture of Acidithiobactillus. Physicochem. Probl. Miner. Process. 2022, 58, 15–23. [Google Scholar]
- Jin, X.; Liu, G.; Jin, B.; Rao, L.; Cao, K.; Huang, Z.; Chen, F.; Huang, Q. Separation of indium and tin from ITO powders with short-chain dicarboxylic acid-ChCl deep eutectic solvents: Indium tin leaching and splitting mechanism. Proc. Safety Environ. Prot. 2024, 185, 1268–1276. [Google Scholar] [CrossRef]
- Deferm, C.; Van de Voorde, M.; Luyten, J.; Oosterhof, H.; Fransaer, J.; Binnemans, K. Purification of indium by solvent extraction with undiluted ionic liquids. Green Chem. 2016, 18, 4116–4127. [Google Scholar] [CrossRef]
- Dhiman, S.; Gupta, B. Cyphos IL 104 assisted extraction of indium and recycling of indium, tin and zinc from discarded LCD screen. Sep. Pur. Technol. 2020, 237, 116407. [Google Scholar] [CrossRef]
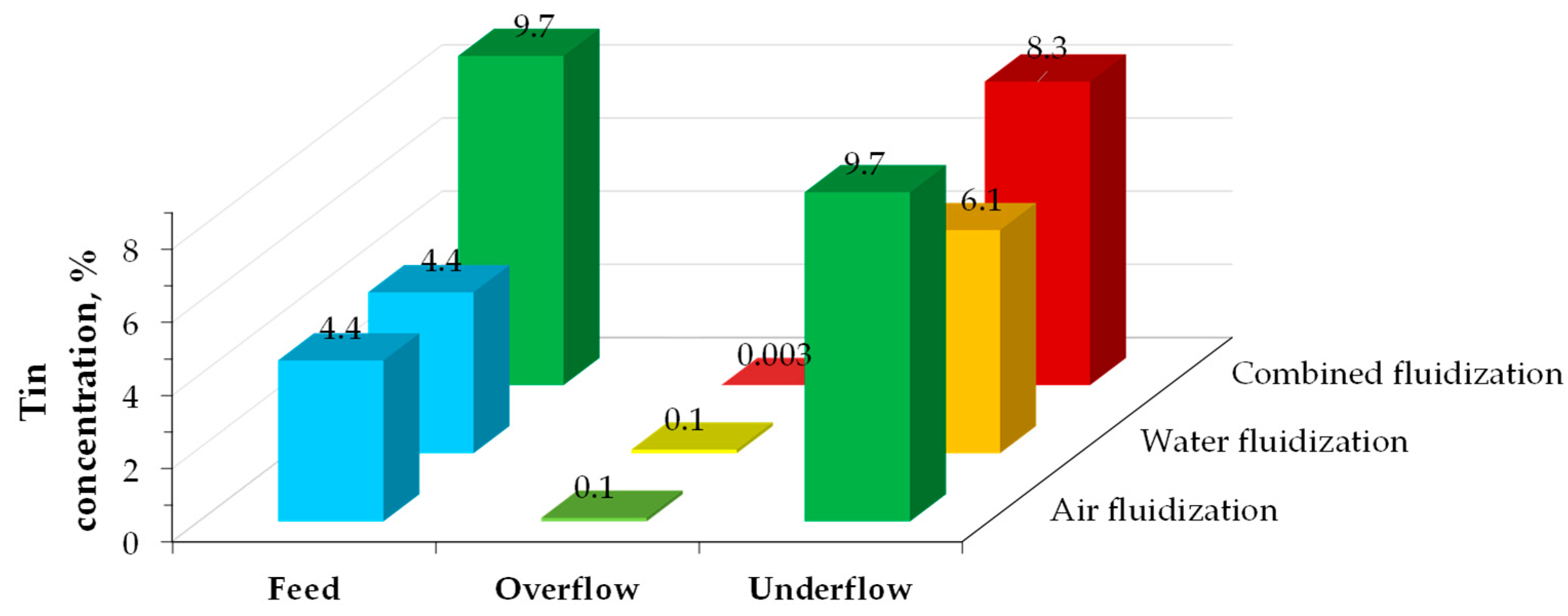
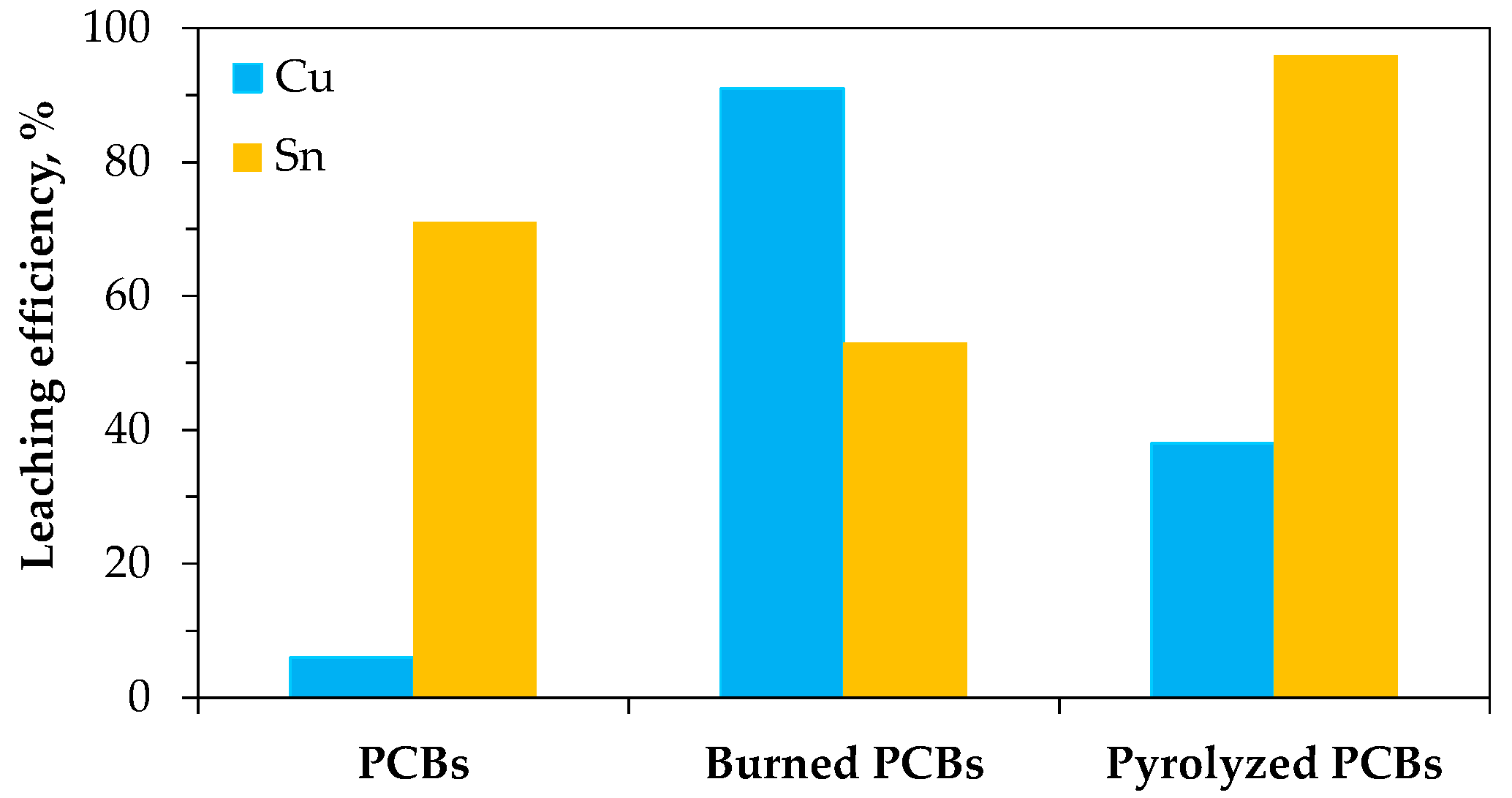
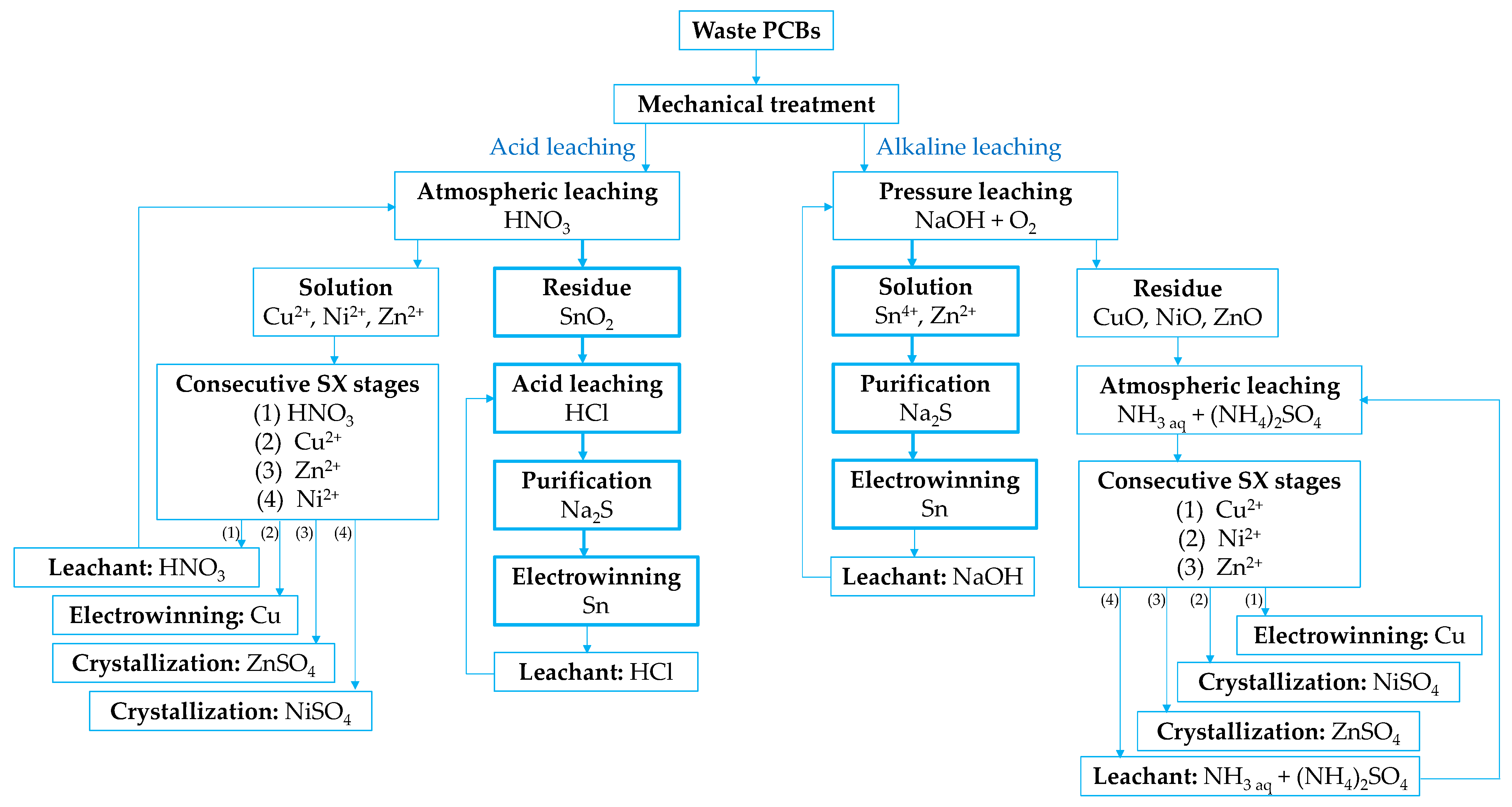
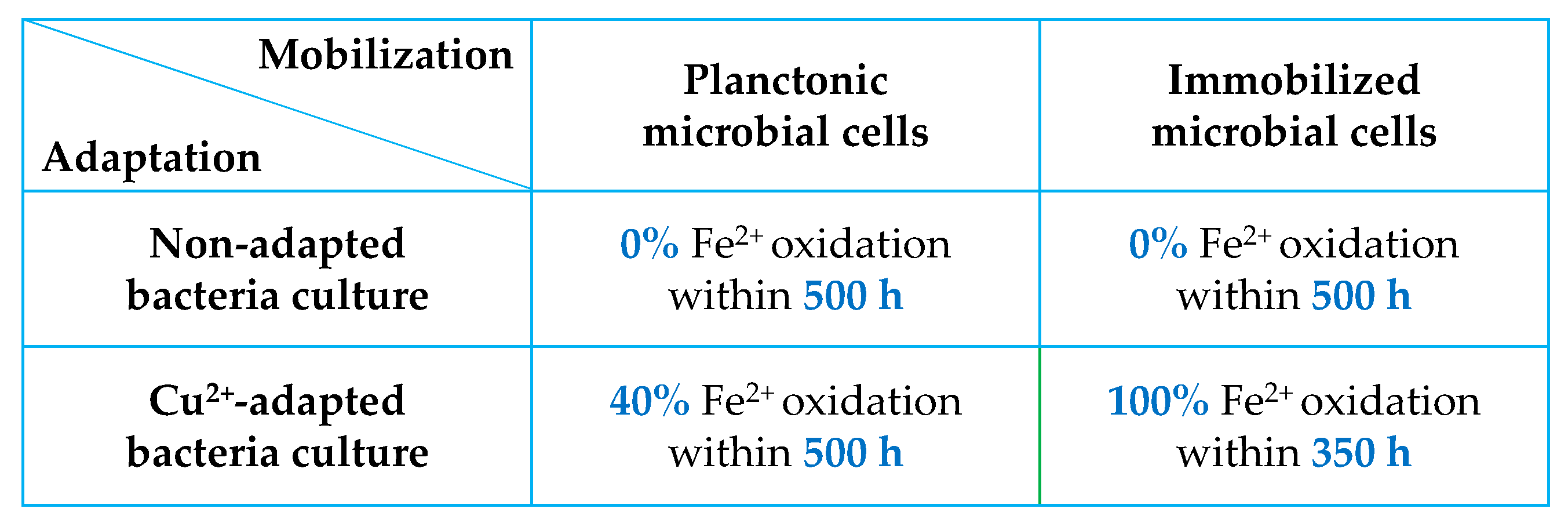


| Routers | Computers | Mobile Phones, Smartphones | TV Board | Copy Machine | Fax Machine | Printer | Central Processing Unit |
|---|---|---|---|---|---|---|---|
| 1.3–9.3 | 0.7–10.3 | 0.02–4.7 | 1.4–6.4 | 2.5 | 3.0 | 1.0 | 1.8 |
| Material * | Melting Point, °C | Density, g/cm3 | Electrical Resistivity, μΩ∙m | Thermal Conductivity, W/m∙K | Brinell Hardness, HB |
|---|---|---|---|---|---|
| Sn metal | 232 | 7.3 | 0.12 | 73 | 4 |
| Sn-37Pb alloy | 183 | 8.4 | 0.25 | 50 | 17 |
| Sn-Ag alloys | ~221 | 7.4 | 0.12 | 55 | 15 |
| Sn-Cu alloys | ~227 | 7.3 | 0.12 | 66 | 9 |
| Sn-Ag-Cu alloys | ~217 | 7.4 | 0.13 | 58–62 | 15 |
| Sn-Bi alloys | 138 | 8.1 | 0.35 | 30 | 24 |
| Tin Content in PCBs, % | Leaching Conditions | Leaching Rate, % | Tin Recovery Conditions | Final Product | Recovery Rate, % | Ref. |
|---|---|---|---|---|---|---|
| Acid Leaching | ||||||
| 3.3 | L/S 5, 2 M HCl, 80 °C, 6 h | 91.8 | - | - | - | [89] |
| ? | L/S 20, 4.5 M HCl, 90 °C, 1 h | 97.8 | Precipitation with NaOH at pH 1.9 | Na2SnO3 | ? | [90] |
| 2.6 | L/S 10, 2 M HCl, 75 °C, 1 h | 88.0 | Cementation with Al | Sn-Pb | 97.0 | [91,92] |
| 3.1 | L/S 10, 3 M HCl, 60 °C, 2 h | 89.1 | Precipitation with NaOH under pH control | ? | 98.2 | [93] |
| L/S 10, 3 M HCl + 1 M HNO3, 60 °C, 2 h | 98.1 | SnO, SnO2 | 85.8 | |||
| L/S 10, 3 M HCl + 1 M H2SO4, 60 °C, 2 h | 90.5 | 96.3 | ||||
| 2.5 | L/S 12.5, 4.9 M HCl, 74 °C, 3 h | 97.6 | Precipitation with NaOH at pH 3; calcination | SnO2 | 99.9 | [94] |
| 0.1 | L/S 3, 1–6 M HNO3, 80 °C, 6 h | <1% | SnO2·H2O dissolution in 1.5 M HCl; electrowinning | Sn | 100 | [95] |
| 4.6 | L/S 20, 1 M H2SO4, 55 °C, 1.5 h | 26.7 | - | - | - | [96] |
| L/S 10, 1 M H2SO4 + CuSO4 (nCu/nSn 1.6), 65 °C, 1.5 h | 95.2 | Hydrolysis precipitation: 10% H2O2, 80 °C, pH 3, 2 h | SnO2 | 92.7 | ||
| 16.2 * | L/S 20, 2 M H2SO4, 80 °C, 3 h | ~20 | - | - | - | [97] |
| 2.5 | L/S 10, 2 M H2SO4, 80 °C, 8 h | 100 | - | - | - | [98] |
| 12.7 * | L/S 3.3, 6 M HCl + 3 M NaCl, 25 °C, 24 h | 94.8 | Precipitation with NaOH at pH 3 | amorphous | 97.4 | [99] |
| 6.2 * | L/S 45, 5 M HCl + Cl2 (anode, at 4 A), 30 °C, 1 h | 99.4 | - | - | - | [100] |
| 2.2 | L/S 13, 1 M HCl + 0.08 M SnCl4, 50 °C, 4 h | 93.5 | - | - | - | [101] |
| 3.8 | L/S 20, 2 M HCl + 1.17 M NaBr + 0.77 M Br2, 23 °C, 10 h | 96.8 | - | - | - | [50] |
| L/S 20, 1.2 M HNO3 + 1.17 M NaBr + 0.77 M Br2, 23 °C, 10 h | 97.1 | - | - | - | ||
| L/S 20, 2.7 M H2SO4 + 1.17 M NaBr + 0.77 M Br2, 23 °C, 10 h | 99.2 | - | - | - | ||
| ? | 3 M HBF4, 0.4 M H2O2, 20 °C, 0.5 h | 98.5 | - | - | - | [102] |
| ? | 3.5 M CH4SO3, 0.5 M H2O2, 20 °C, 0.75 h | 99.2 | - | - | - | [103] |
| 7.9 ** | L/S 20, 1 M H2C2O4, 80 °C, 1 h | 92.3 | - | - | - | [104] |
| Alkaline Leaching | ||||||
| 3.3 | L/S 5, 1 M NaOH, 90 °C, 2 h | 62.4 | - | - | - | [89] |
| 8.6 | L/S 4, 2.5 M NaOH, pO2 2 MPa, 150 °C, 3 h | 98.2 | Precipitation of PbS and ZnS; electrowinning | Sn | 86.2 | [105] |
| Chelating Leaching | ||||||
| 16.2 * | L/S 30, 0.1 M Na2-EDTA, pH 5, 80 °C, 3 h | 100 | Precipitation with NaOH at pH 9, 60 °C | SnO2 nanoparticles | ? | [97] |
| Electrochemical dissolution of PCB Anode | ||||||
| 13.8 | Sn2+-CH4SO3, 40 °C, 2 A/dm2 | 85 | Simultaneous cathodic deposition | Sn | ? | [106] |
| 2.6 | 3 M NaOH, 80 °C, 3 A/dm2, 2 h | 100 | Sn | ? | [107] | |
| Microorganism | Leaching Agents | Bioleaching Conditions | Tin Content in PCBs, % | Leaching Efficiency, % | Ref. |
|---|---|---|---|---|---|
| Bacteria Leaching | |||||
| Acidithiobacillus ferrooxidans | Fe3+, H2SO4 | L/S 1000, pH 2.6, 30 °C, 10 days | 2.3 | 0 * | [140] |
| Acidithiobacillus thiooxidans | H2SO4 | ||||
| Acidithiobacillus ferrooxidans | Fe3+, H2SO4 | L/S 285, pH 1.8, 30 °C, 4 days | 3.3 | 0 ** | [144] |
| Leptospirillum ferriphilum | Fe3+ | L/S 1000, 38–150 μm, pH 1.8, 30 °C, 2 days | 18 | 21 | [145] |
| Acidithiobacillus ferrooxidans | Fe3+, H2SO4 | ||||
| Acidithiobacillus caldus | H2SO4 | ||||
| Leptospirillum ferriphilum | Fe3+ | L/S 1000, pH 1.4, 37 °C, 3 h | 0.1–3 g/L | 0 * | [64] |
| Acidiplasma cupricumulans | Fe3+, H2SO4 | ||||
| Acidithiobacillus caldus | H2SO4 | ||||
| Fungal Leaching | |||||
| Aspergillus niger | H2C2O4, H8C6O7, H12C6O7 | L/S 1000, pH 3.0–3.5, 30 °C, 21 days | 2.3 | 38 | [140] |
| Penicillium simplicissimum | 65 | ||||
| Aspergillus niger | L/S 500, 30 °C, 1 day | 0.98 | 1.5 | [142] | |
| Computer Monitor | TV Monitor | Smartphone | iPhone | Mobile Phones | Tablet | Notebook | Laptop |
|---|---|---|---|---|---|---|---|
| 10–46 | 18 ± 7 | 3 ± 2 | 1276 | 15–4470 | 16 ± 10 | 11 ± 4 | 110 |
| Tin Content in LCDs, ppm | Leaching Conditions | Leaching Rate, % | Tin Recovery Conditions | Final Product | Recovery Rate, % | Ref. |
|---|---|---|---|---|---|---|
| Acid Leaching | ||||||
| 260 ± 30 | L/S 5, 2 M H2SO4, 80 °C, 1 h | 60 | - | - | - | [170] |
| 100 ± 20 | L/S 10, 2 M H2SO4, 70 °C, 8 h | 60 | - | [172] | ||
| ? * | L/S ?, 18 M H2SO4, 60 °C, 0.5 h | 50 | - | - | - | [173] |
| L/S ?, 18 M H2SO4, 60 °C, 0.5 h, us | 70 | |||||
| L/S ?, 9 M H2SO4, 60 °C, 0.5 h, us | 100 | |||||
| 12,800 ** | L/S 8, 4 M H2SO4, 70 °C, 2 h | 80 | - | - | - | [175] |
| L/S 8, 1 M H2SO4, 70 °C, 2 h | 3 | hydrolytic precipitation during leaching | SnO2 | 97 | ||
| 100 | L/S 6, 3 M H2SO4, 85 °C, 1 h | 86 | - | - | - | [176,178] |
| 4000 * | L/S 10, 0.8 M HCl, 25 °C, 1 h, us | 31 | - | - | - | [171] |
| Chelating Leaching | ||||||
| 1392 | L/S 50, 1 M H5C6O7Na3, 0.2 M N2H4, pH 5 (H2SO4), 25 °C, 12 h | 40 | - | - | - | [174] |
| L/S 50, 1 M H5C6O7Na3, 0.2 M N2H4, pH 5 (HNO3), 25 °C, 3 h | 40 | |||||
| L/S 50, 1 M H5C6O7Na3, 1.5 M H2O2, pH 5 (HNO3), 25 °C, 3 h | 40 | |||||
| L/S 50, 0.5 M H8C6O7, 0.2 M N2H4, pH 5 (NaOH), 25 °C, 3 h | 95 | |||||
| 835 | L/S 50, 0.05 M Na4P2O7, 3% H2O2, pH 3, 25 °C, 2 h, us | 1 | magnetic separation from solid residue | Sn | 72 | [177] |
| L/S 50, 0.05 M Na4P2O7, 3% H2O2, pH 6, 25 °C, 1 h, us | 23 | - | - | - | ||
| Microorganism | Leaching Agents | Bioleaching Conditions * | Tin Content in PCBs, ppm | Leaching Efficiency, % | Ref. |
|---|---|---|---|---|---|
| Acidithiobacillus ferrooxidans | Fe3+, H2SO4 | L/S 100, 9 K, 30 °C, 14 days | 250 | 90 | [191] |
| Acidithiobacillus thiooxidans | L/S 100, 9 K, H2SO4, 30 °C, 21 days | 10 | |||
| Acidithiobacillus ferrooxidans | Fe3+, H2SO4 | L/S 100, 9 K, 30 °C, 15 days | 98 | [192] | |
| Acidithiobacillus thiooxidans | H2SO4 | L/S 100, WJ, 30 °C, 35 days | 65 | ||
| Acidithiobacillus ferrooxidans | Fe3+, H2SO4 | L/S 100, 9 K, 30 °C, 15 days | 98 | ||
| Acidithiobacillus thiooxidans |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rudnik, E. Innovative Approaches to Tin Recovery from Low-Grade Secondary Resources: A Focus on (Bio)hydrometallurgical and Solvometallurgical Methods. Materials 2025, 18, 819. https://doi.org/10.3390/ma18040819
Rudnik E. Innovative Approaches to Tin Recovery from Low-Grade Secondary Resources: A Focus on (Bio)hydrometallurgical and Solvometallurgical Methods. Materials. 2025; 18(4):819. https://doi.org/10.3390/ma18040819
Chicago/Turabian StyleRudnik, Ewa. 2025. "Innovative Approaches to Tin Recovery from Low-Grade Secondary Resources: A Focus on (Bio)hydrometallurgical and Solvometallurgical Methods" Materials 18, no. 4: 819. https://doi.org/10.3390/ma18040819
APA StyleRudnik, E. (2025). Innovative Approaches to Tin Recovery from Low-Grade Secondary Resources: A Focus on (Bio)hydrometallurgical and Solvometallurgical Methods. Materials, 18(4), 819. https://doi.org/10.3390/ma18040819







