Investigations into Replacing Calcined Clay with Sewage Sludge Ash in Limestone Calcined Clay Cement (LC3)
Abstract
1. Introduction
2. Materials and Methods
2.1. SSA Preparation
2.2. Raw Materials
2.3. Mixture Design
2.4. Sample Preparation and Characterizations
2.4.1. Compressive Strength
2.4.2. The Setting Time
2.4.3. XRD, TG/DTG and SEM
2.4.4. Toxicity Characteristic Leaching Procedure (TCLP)
3. Results
3.1. The Setting Times
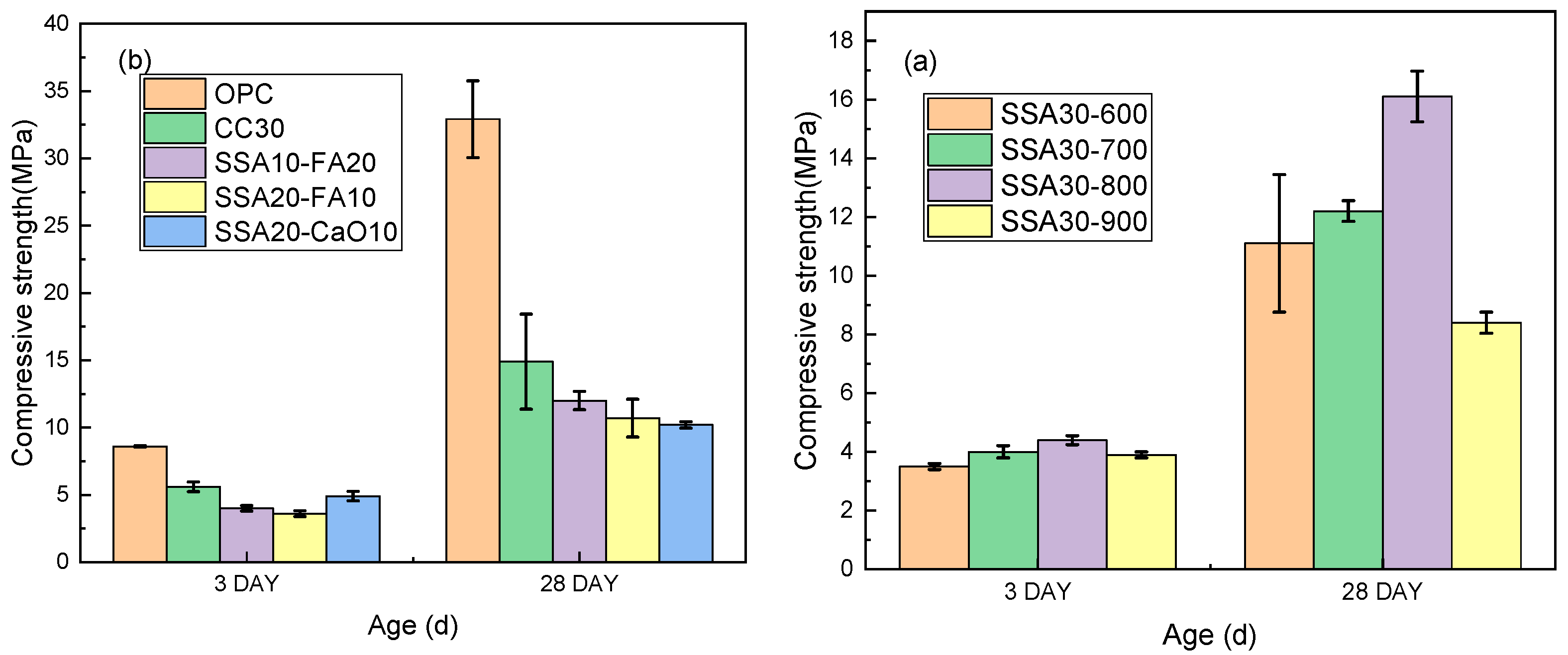
3.2. Compressive Strengths
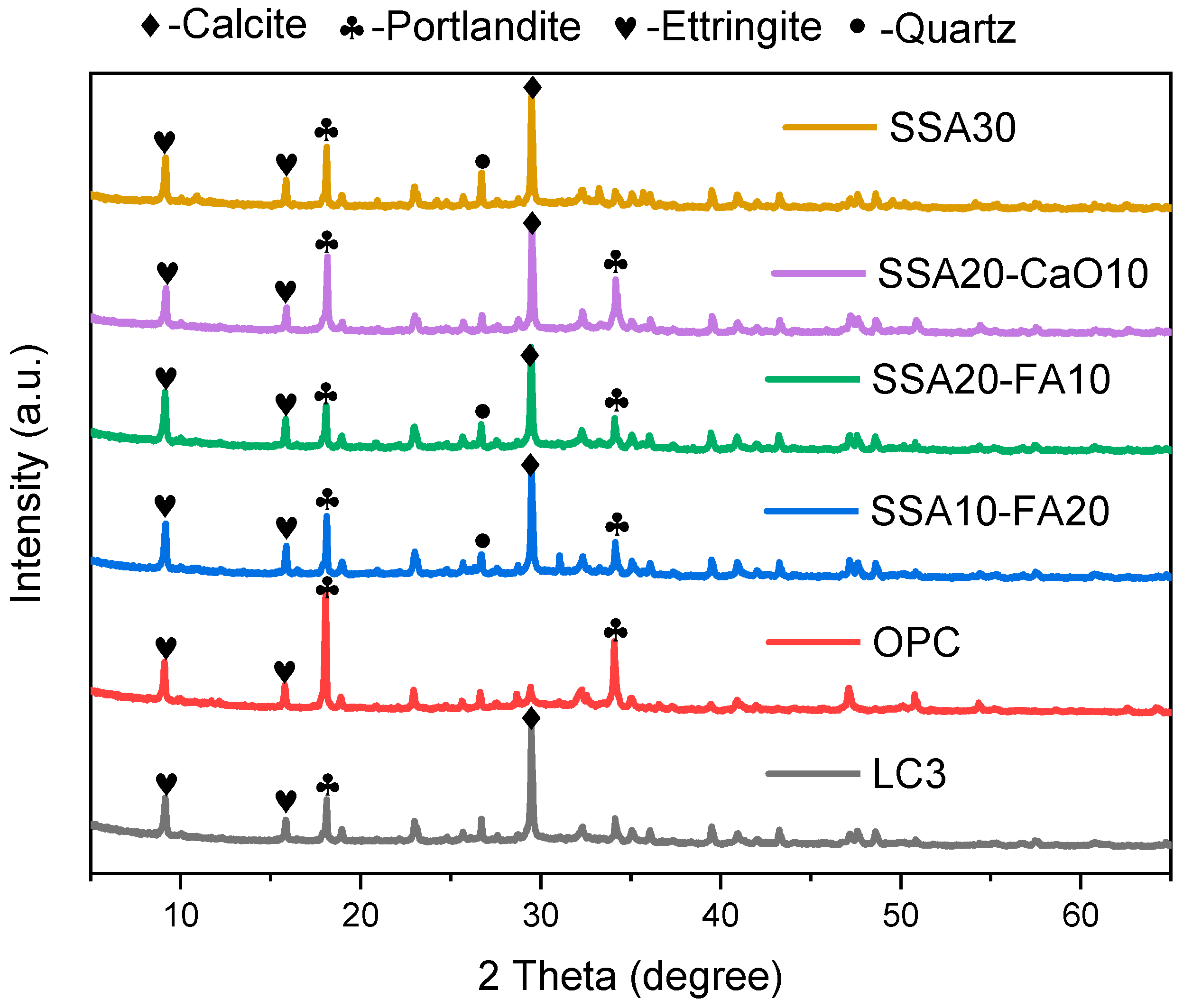
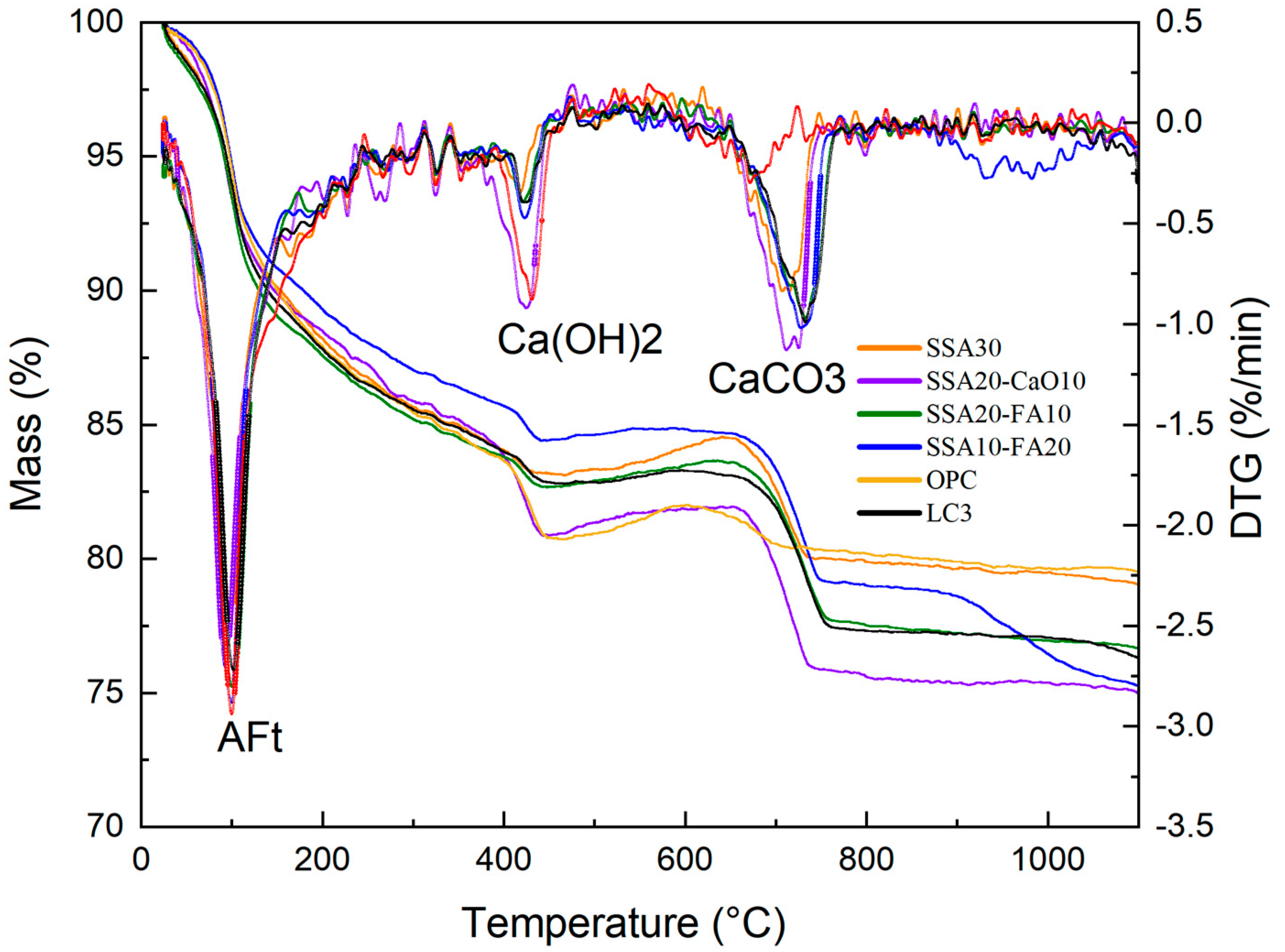
3.3. The Hydration Products
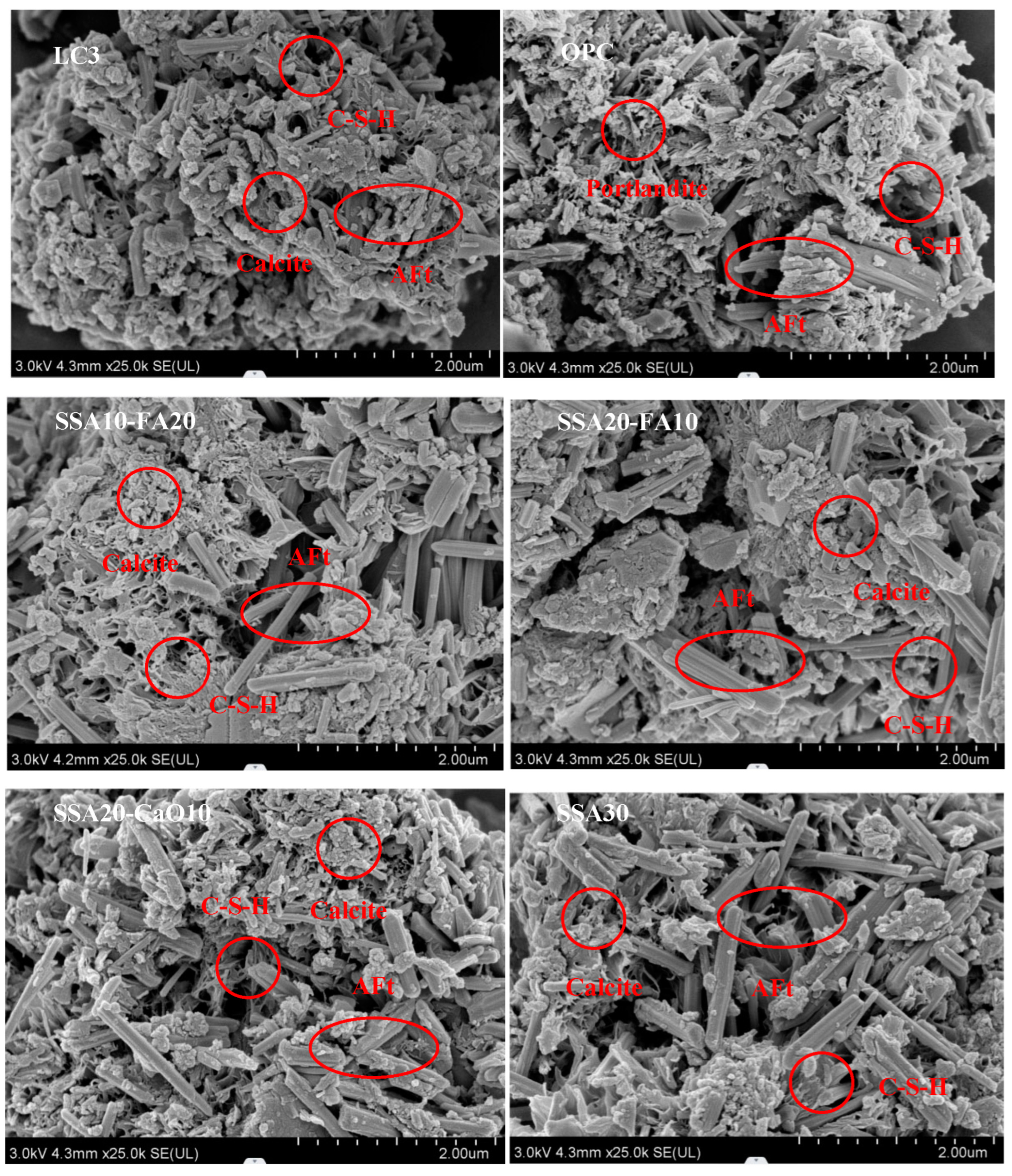
3.4. The Microstructure Observations
3.5. Environmental Impacts
4. Discussions
5. Conclusions
- (1)
- The introduction of SSA into modified LC3 pastes led to a slight prolongation of the final setting time. However, with 30% SSA incorporation, the final setting time of LC3 pastes approximated that of ordinary cement slurry, while exhibiting an earlier initial setting time. Replacing calcined clay with calcined SSA led to a reduction in the compressive strength of LC3 samples.
- (2)
- As the calcination temperature rose, the amorphous phase content within the calcined SSA increased, peaking at an optimal calcination temperature of 800 °C. The primary chemical compositions of SSA, namely Fe2O3, SiO2, Al2O3, and CaO, which mirror the chemical compositions of the clay utilized in LC3, albeit with a higher Fe2O3 and P2O5 content in SSA.
- (3)
- The incorporation of SSA into LC3 did not produce notable alterations in the phase compositions of the modified pastes compared to normal LC3. The primary crystalline minerals observed in the modified LC3 pastes include calcite (CaCO3), portlandite (Ca(OH)2), ettringite (AFt), and quartz (SiO2). Notably, compared to the pure OPC group, the Ca(OH)2 content was lower, while the calcite content was higher in LC3 pastes with SSA.
- (4)
- In samples containing SSA, an increased content of CaCO3 was observed, and the Ca(OH)2 exhibited a radial morphology. The addition of SSA altered the morphologies of Ca(OH)2 and CaCO3, and modified the microstructure of the LC3 paste. Furthermore, LC3 pastes with SSA appeared to possess a more porous microstructure than the normal LC3 group.
- (5)
- Heavy metal ions in SSA can be mostly immobilized in the LC3 pastes containing calcined SSA, thereby alleviating any possible environmental risks associated with utilizing SSA as a calcined clay substitute in LC3.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, G.; Zhang, G.; Wang, H. Current state of sludge production, management, treatment and disposal in China. Water Res. 2015, 78, 60–73. [Google Scholar] [CrossRef]
- Chakraborty, S.; Jo, B.W.; Jo, J.H.; Baloch, Z. Effectiveness of sewage sludge ash combined with waste pozzolanic minerals in developing sustainable construction material: An alternative approach for waste management. J. Clean. Prod. 2017, 153, 253–263. [Google Scholar] [CrossRef]
- Chang, Z.; Long, G.; Zhou, J.L.; Ma, C. Valorization of sewage sludge in the fabrication of construction and building materials: A review. Resour. Conserv. Recycl. 2020, 154, 104606. [Google Scholar] [CrossRef]
- Grobelak, A.; Czerwińska, K.; Murtaś, A. General Considerations on Sludge Disposal. In Industrial and Municipal Sludge; Butterworth-Heinemann: Oxford, UK, 2019; pp. 135–153. [Google Scholar]
- Wang, J. Steady-state chloride diffusion coefficient and chloride migration coefficient of cracks in concrete. J. Mater. Civ. Eng. 2017, 29, 0417117. [Google Scholar] [CrossRef]
- Yin, J.; Tan, X.; Liao, G.; Zhai, J.; Liu, G. Characteristics and Disposal Status of Municipal Sewage Sludge in China. China Water Waste Water 2003, 19, 21–24. [Google Scholar]
- Lynn, C.J.; Dhir, R.K.; Ghataora, G.S.; West, R.P. Sewage sludge ash characteristics and potential for use in concrete. Constr. Build. Mater. 2015, 98, 767–779. [Google Scholar] [CrossRef]
- Juenger, M.C.G.; Siddique, R. Recent advances in understanding the role of supplementary cementitious materials in concrete. Cem. Concr. Res. 2015, 78, 71–80. [Google Scholar] [CrossRef]
- Monzό, J.; Paya, J.; Borrachero, M.V.; Cόrcoles, A. Use of sewage sludge ash (SSA)–cement admixtures in mortars. Cem. Concr. Res. 1996, 26, 1389–1398. [Google Scholar] [CrossRef]
- Tay, J.H. Sludge Ash as Filler for Portland Cement Concrete. J. Environ. Eng. 1987, 113, 345–351. [Google Scholar] [CrossRef]
- Tay, J.-H.; Yip, W.-K. Sludge Ash as Lightweight Concrete Material. J. Environ. Eng. 1989, 115, 56–64. [Google Scholar] [CrossRef]
- Tay, J.-H.; Yip, W.-K.; Show, K.Y. Clay-Blended Sludge as Lightweight Aggregate Concrete Material. J. Environ. Eng. 1991, 117, 834–844. [Google Scholar] [CrossRef]
- Alleman, J.E.; Berman, N.A. Constructive sludge management: Biobrick. J. Environ. Eng. 1984, 110, 301–311. [Google Scholar] [CrossRef]
- Bhatty, J.I.; Reid, K.J. Compressive strength of municipal sludge ash mortars. Aci Mater. J. 1989, 86, 394–400. [Google Scholar] [CrossRef]
- Idris, A.; Saed, K. Characteristics of slag produced from incinerated hospital waste. J. Hazard. Mater. 2002, 93, 201–208. [Google Scholar] [CrossRef]
- Tantawy, M.A.; El-Roudi, A.M.; Abdalla, E.M.; Abdelzaher, M.A. Evaluation of the Pozzolanic Activity of Sewage Sludge Ash. Int. Sch. Res. Not. 2012, 2012, 487037. [Google Scholar] [CrossRef]
- Antoni, M.; Rossen, J.; Martirena, F.; Scrivener, K. Cement substitution by a combination of metakaolin and limestone. Cem. Concr. Res. 2012, 42, 1579–1589. [Google Scholar] [CrossRef]
- Sharma, M.; Bishnoi, S.; Martirena, F.; Scrivener, K. Limestone calcined clay cement and concrete: A state-of-the-art review. Cem. Concr. Res. 2021, 149, 106564. [Google Scholar] [CrossRef]
- Lin, K.L.; Chiang, K.Y.; Lin, C.Y. Hydration characteristics of waste sludge ash that is reused in eco-cement clinkers. Cem. Concr. Res. 2005, 35, 1074–1081. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Yu, J.; Xu, L.; Li, M.; Cheng, J.; Li, Z. Effects of sodium sulfate and potassium sulfate on the properties of calcium sulfoaluminate (CSA) cement based grouting materials. Constr. Build. Mater. 2022, 353, 129045. [Google Scholar] [CrossRef]
- Scrivener, K.L. Options for the future of cement. Indian Concr. J. 2014, 88, 11–21. [Google Scholar]
- Scrivener, K.; Martirena, F.; Bishnoi, S.; Maity, S. Calcined clay limestone cements (LC3). Cem. Concr. Res. 2018, 114, 49–56. [Google Scholar] [CrossRef]
- Hou, P. The role of limestone and calcined clay on the rheological properties of LC3. Cem. Concr. Compos. 2020, 107, 103516. [Google Scholar] [CrossRef]
- Avet, F.; Scrivener, K. Investigation of the calcined kaolinite content on the hydration of Limestone Calcined Clay Cement (LC3). Cem. Concr. Res. 2018, 107, 124–135. [Google Scholar] [CrossRef]
- Zunino, F.; Scrivener, K. The reaction between metakaolin and limestone and its effect in porosity refinement and mechanical properties. Cem. Concr. Res. 2021, 140, 106307. [Google Scholar] [CrossRef]
- Dhandapani, Y.; Sakthivel, T.; Santhanam, M.; Gettu, R.; Pillai, R.G. Mechanical properties and durability performance of concretes with Limestone Calcined Clay Cement (LC3). Cem. Concr. Res. 2018, 107, 136–151. [Google Scholar] [CrossRef]
- Naamane, S.; Rais, Z.; Taleb, M. The effectiveness of the incineration of sewage sludge on the evolution of physicochemical and mechanical properties of Portland cement. Constr. Build. Mater. 2016, 112, 783–789. [Google Scholar] [CrossRef]
- GB175-2007; Common Portland Cement. Standardization Administration of the People’s Republic of China: Beijing, China, 2007.
- BS EN197-1:2011; Cement Part 1: Composition, Specifications and Conformity Criteria for Common Cements. European Committee for Standardization: Brussels, Belgium, 2011.
- Xu, L.; Wang, J.; Li, K.; Hao, T.; Li, Z.; Li, L.; Ran, B.; Du, H. New insights on dehydration at elevated temperature and rehydration of GGBS blended cement. Cem. Concr. Compos. 2023, 139, 105068. [Google Scholar] [CrossRef]
- EN 196-1:2016; Methods of Testing Cement-Part 1: Determination of Strength. European Committee for Standardization: Brussels, Belgium, 2016.
- ASTM. Standard test method for compressive strength of hydraulic cement mortars. C109. In Annual Book of ASTM Standards; ASTM International: West Conshohocken, PA, USA, 2016. [Google Scholar]
- GB/T 50081-2019; Standards for Test Methods of Physical and Mechanical Properties of Concrete. China Architecture and Building Press: Beijing, China, 2019.
- ISO 9597:2008, EN; Cement-Test Methods-Determination of Setting Time and Soundness. ISO: Geneva, Switzerland, 2008.
- Wang, J.; Xu, L.; Li, M.; Wang, Y.; He, H.; Xiang, D.; Li, K.; Hao, T. Investigations on factors influencing physical properties of recycled cement and the related carbon emissions and energy consumptions. J. Clean. Prod. 2023, 414, 137715. [Google Scholar] [CrossRef]
- Lisk, D.J. Compressive strength of cement containing ash from municipal refuse or sewage sludge incinerators. Bull. Environ. Contam. Toxicol. 1989, 42, 540. [Google Scholar] [CrossRef]
- Wang, J.; Liu, E. The relationship between steady-state chloride diffusion and migration coefficients in cementitious materials. Mag. Concr. Res. 2020, 72, 1016–1026. [Google Scholar] [CrossRef]
- Pinarli, V.; Kaymal, G. An innovative sludge disposal option-reuse of sludge ash by incorporation in construction materials. Environ. Technol. Lett. 1994, 15, 843–852. [Google Scholar] [CrossRef]
- Xie, J.; Wang, J.; Li, M.; Xu, L.; Xiang, D.; Wang, Y.; He, H.; Zhu, Y.; Zhao, J. Estimation of chloride diffusion coefficient from water permeability test of cementitious materials. Constr. Build. Mater. 2022, 340, 127816. [Google Scholar] [CrossRef]
- Tamboue, B. Etude de L’utilisation Dans Le Béton Des Cendres De Boues De Lastation D’épuration Des Eaux Usées De La Communauté Urbaine de Montréal. Master’s Thesis, Sherbrooke University, Sherbrooke, QC, Canada, 1995. (In French). [Google Scholar]
- Khanbilvardi, R.; Afshari, S. Sludge ash as fine aggregate for concrete mix. J. Environ. Eng. 1995, 121, 633–638. [Google Scholar] [CrossRef]
- Monzo, J.; Paya, J.; Borrachero, M.V.; Bellver, A.; Peris-Mora, E. Study of cement-based mortars containing Spanish ground sewage sludge ash. In Proceedings of the International Conference on the Environmental and Technical Implications of Construction with Alternative Materials, WASCON ’97, Houthem St. Gerlach, The Netherlands, 4–6 June 1997; Goumans, J.J.J.M., Senden, G.J., van der Sloot, H.A., Eds.; Elsevier Science Publishers: Amsterdam, The Netherlands, 1997; p. 349. [Google Scholar]
- Monzo, J.; Paya, J.; Borrachero, M.V. Experimental basic aspects for reusing sewage sludge ash (SSA) in concrete production. In Exploiting Wastes in Concrete: Proceedings of the International Seminar Held at the University of Dundee, Scotland, UK, 7 September 1999; Dhir, R.K., Jappy, T.G., Eds.; In Thomas Telford: London, UK, 1999; pp. 47–56. [Google Scholar]
- Monzo, J.; Paya, J.; Borrachero, M.V.; Peris-Mora, E. Mechanical behavior of mortars containing sewage sludge ash (SSA) and Portland cements with different tricalcium aluminate content. Cem. Concr. Res. 1999, 29, 87–94. [Google Scholar] [CrossRef]
- Paya, J.; Monzo, J.; Borrachero, M.V.; Amahjour, F.; Girbes, I.; Velazquez, S.; Ordonez, L.M. Advantages in the use of fly ashes in cements containing pozzolanic combustion residues: Silica fume, sewage sludge ash, spent fluidized bed catalyst and rice husk ash. J. Chem. Technol. Biotechnol. 2002, 77, 331–335. [Google Scholar] [CrossRef]
- Monzo, J.; Paya, J.; Borrachero, M.V.; Girbes, I. Reuse of sewage sludge ashes (SSA) in cement mixtures: The effect of SSA on the workability of cement mortars. Waste Manag. 2003, 23, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Al Khaja, W. Effect of sludge ash on the mechanical properties of concrete. Model. Meas. Control. B Solid Fluid Mech. Thermics Mech. Syst. 1997, 63, 9–14. [Google Scholar]
- Wang, Y.; Yu, J.; Wang, J.; Xiang, D.; Gu, H.; Cheng, J. Effects of sodium aluminate and quicklime on the properties of CSA grouting materials. J. Build. Eng. 2022, 58, 105060. [Google Scholar] [CrossRef]
- Dyer, T.D.; Halliday, J.E.; Dhir, R.K. Hydration reaction of sewage sludge ash for use as a cement component in concrete production, recycling and reuse of sewage sludge. In Proceedings of the International Symposium, Dundee, UK, 19–20 March 2001; Thomas Telford: London, UK, 2001; pp. 227–238. [Google Scholar]
- Wang, Y.; Tang, H.; Su, J.; He, H.; Zhao, Y.; Wang, J. Effect of sodium sulfate and gypsum on performances of expansive grouting material with aluminum as expansion agent. Constr. Build. Mater. 2023, 394, 132212. [Google Scholar] [CrossRef]
- Pan, S.C.; Tseng, D.H. Sewage sludge ash characteristics and its potential applications. Water Sci. Technol. 2001, 44, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.C.; Tseng, D.H.; Lee, C. Use of sewage sludge ash as fine aggregate and pozzolan in Portland cement mortar. J. Solid Waste Technol. Manag. 2002, 28, 121–130. [Google Scholar]
- Feng, X.; Garboczi, E.J.; Bentz, D.P.; Stutzman, P.E.; Mason, T.O. Estimation of the degree of hydration of blended cement pastes by a scanning electron microscope point-counting procedure. Cem. Concr. Res. 2004, 34, 1787–1793. [Google Scholar] [CrossRef]
- Jiang, D.; Li, X.; Lv, Y.; Zhou, M.; He, C.; Jiang, W.; Liu, Z.; Li, C. Utilization of limestone powder and fly ash in blended cement: Rheology, strength and hydration characteristics. Construct. Build. Mater 2020, 232, 117228. [Google Scholar] [CrossRef]
- GB 5085.3-2007; Chinese Standard: Identification Standards for Hazardous Wastes-Identification for Extraction Toxicity. PRC Environmental Protection Agency: Beijing, China, 2007.
- Guo, B.; Liu, B.; Yang, J.; Zhang, S. The mechanisms of heavy metal immobilization by cementitious material treatments and thermal treatments: A review. J. Environ. Manag. 2017, 193, 410–422. [Google Scholar] [CrossRef]
- Vespa, M.; Dähn, R.; Wieland, E. Competition behaviour of metal uptake in cementitious systems: An XRD and EXAFS investigation of Nd-and Zn-loaded 11 Å tobermorite. Phys. Chem. Earth Parts ABC 2014, 70, 32–38. [Google Scholar] [CrossRef]
- Lasheras-Zubiate, M.; Navarro-Blasco, I.; Fernandez, J.M.; Alvarez, J.I. Encapsulation, solid-phases identification and leaching of toxic metals in cement systems modified by natural biodegradable polymers. J. Hazard. Mater 2012, 233, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Dermatas, D. Ettringite-induced swelling in soils: State-of-the-art. Appl. Mech. Rev. 1995, 48, 659–673. [Google Scholar] [CrossRef]
- Cyr, M.; Coutand, M.; Clastres, P. Technological and environmental behavior of sewage sludge ash (SSA) in cement-based materials. Cem. Concr. Res. 2007, 37, 1278–1289. [Google Scholar] [CrossRef]
- Yuan, R. Gelling Material Science; Wuhan University of Technology Press: Wuhan, China, 2018. [Google Scholar]
- Liu, J.; Shi, C.; Ma, X.; Khayat, K.H.; Zhang, J.; Wang, D. An overview on the effect of internal curing on shrinkage of high performance cement-based materials. Constr. Build. Mater. 2017, 146, 702–712. [Google Scholar] [CrossRef]
- Yan, P.; Zheng, F.; Peng, J.; Qin, X. Relationship between delayed ettringite formation and delayed expansion in massive shrinkage-compensating concrete. Cem. Concr. Compos. 2004, 26, 687–693. [Google Scholar] [CrossRef]
- Yu, Z.; Zhao, Y.; Ba, H.; Liu, M. Synergistic effects of ettringite-based expansive agent and polypropylene fiber on early-age anti-shrinkage and anti-cracking properties of mortars. J. Build. Eng. 2021, 39, 102275. [Google Scholar] [CrossRef]
- Tran, N.P.; Gunasekara, C.; Law, D.W.; Houshyar, S.; Setunge, S.; Cwirzen, A. A critical review on drying shrinkage mitigation strategies in cement-based materials. J. Build. Eng. 2021, 38, 102210. [Google Scholar] [CrossRef]
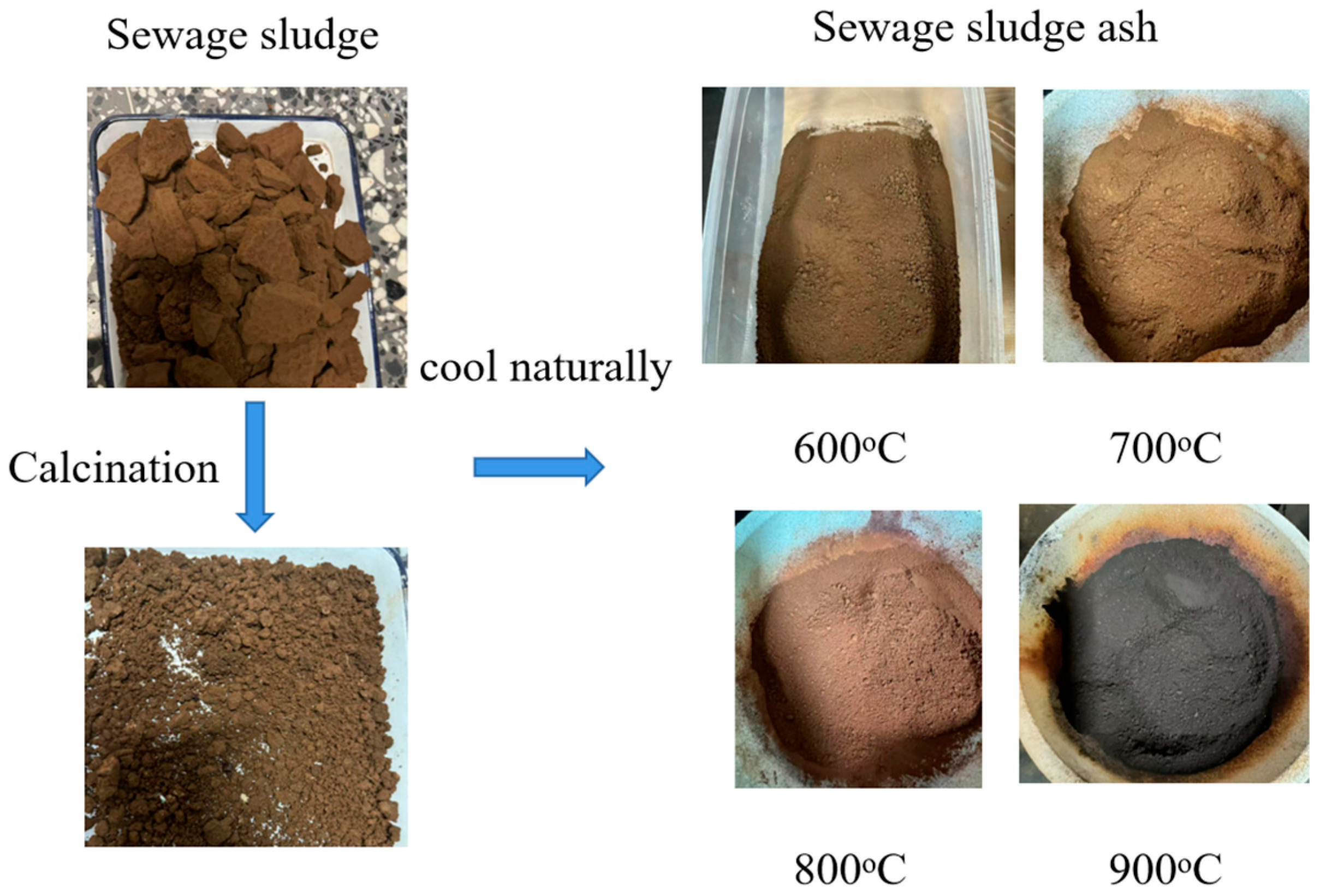
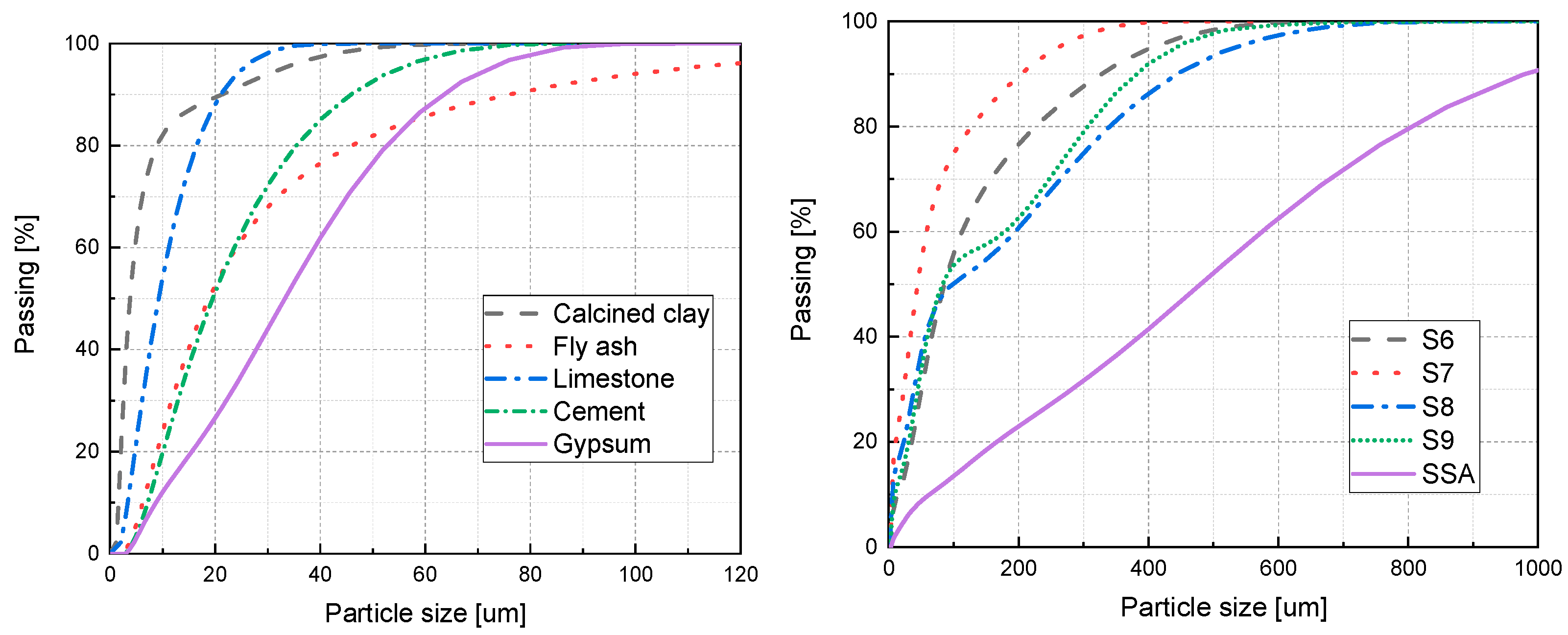

| Material | CC | FA | LS | Cement | Gypsum | S6 | S7 | S8 | S9 | SS |
|---|---|---|---|---|---|---|---|---|---|---|
| Chemical composition (%) | ||||||||||
| SiO2 | 45.54 | 49.89 | 0.10 | 25.48 | 0.37 | 12.53 | 13.96 | 16.06 | 15.82 | 14.67 |
| CaO | 0.10 | 3.53 | 98.03 | 54.45 | 37.09 | 4.57 | 4.12 | 3.61 | 3.69 | 3.23 |
| Al2O3 | 51.18 | 36.01 | 0.05 | 7.89 | 0.10 | 4.16 | 4.39 | 4.98 | 5.23 | 4.05 |
| Fe2O3 | 0.42 | 4.19 | 0.04 | 4.35 | 0.03 | 61.50 | 59.41 | 54.83 | 55.62 | 58.31 |
| MgO | 0.12 | 0.94 | 0.71 | 1.40 | 0.57 | 1.80 | 1.28 | 1.23 | 1.20 | 1.17 |
| Na2O | 0.70 | 0.69 | 0.59 | 0.60 | 0.52 | 1.17 | 1.27 | 1.41 | 1.35 | 1.71 |
| K2O | 0.10 | 1.71 | -- | 0.64 | 0.02 | 0.81 | 0.92 | 1.08 | 1.06 | 0.97 |
| P2O5 | 0.40 | 0.38 | 0.08 | 0.15 | 0.07 | 8.98 | 10.02 | 11.97 | 11.08 | 9.04 |
| SO3 | 0.19 | 1.13 | 0.26 | 4.12 | 61.06 | 3.02 | 2.70 | 2.23 | 2.62 | 3.10 |
| Physical property | ||||||||||
| D50 (μm) | 3.80 | 18.8 | 9.24 | 19.5 | 33.2 | 85.6 | 44.3 | 97.7 | 83.0 | 55.11 |
| Specific surface area (m2/kg) | 1776 | 420.5 | 837.3 | 393.1 | 283.6 | 184.4 | 349.7 | 303.8 | 213.3 | 479 |
| System | w/b | OPC | CC | SSA | FA | CaO | LS | Gypsum | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Proportions | Calcination Temperature | |||||||||
| pre-laboratory | SSA30-600 | 0.5 | 50 | 0 | 30 | 600 | 0 | 0 | 15 | 5 |
| SSA30-700 | 0.5 | 50 | 0 | 30 | 700 | 0 | 0 | 15 | 5 | |
| SSA30-800 | 0.5 | 50 | 0 | 30 | 800 | 0 | 0 | 15 | 5 | |
| SSA30-900 | 0.5 | 50 | 0 | 30 | 900 | 0 | 0 | 15 | 5 | |
| OPC | 0.5 | 95 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | |
| LC3 | 0.5 | 50 | 30 | 0 | 0 | 0 | 0 | 15 | 5 | |
| SSA10-FA20 | 0.5 | 50 | 0 | 10 | 800 | 20 | 0 | 15 | 5 | |
| SSA20-FA10 | 0.5 | 50 | 0 | 20 | 800 | 10 | 0 | 15 | 5 | |
| SSA20-CaO10 | 0.5 | 50 | 0 | 20 | 800 | 0 | 10 | 15 | 5 | |
| System | Initial Setting Time (min) | Final Setting Time (min) |
|---|---|---|
| OPC | 201 | 298 |
| LC3 | 187 | 492 |
| SSA30 | 120 | 283 |
| SSA10-FA20 | 245 | 295 |
| SSA20-FA10 | 200 | 370 |
| SSA20-CaO10 | 28 | 150 |
| Application | Concentration of HM (mg/L) | |||||||
|---|---|---|---|---|---|---|---|---|
| Ag | Cd | Cr | Cu | Pb | Zn | Ba | Ni | |
| OPC | ND | 0.0150 | 0.5576 | 0.4345 | 0.3952 | 0.8894 | 0.7153 | ND |
| SSA30 | ND | 0.0500 | 1.3856 | 1.6498 | 0.9394 | 27.6620 | 1.4744 | 0.2084 |
| SSA10-FA20 | ND | 0.0234 | 0.5529 | 0.7450 | 0.6131 | 7.3584 | 1.9619 | 0.0566 |
| SSA20-FA10 | ND | 0.0383 | 0.9468 | 1.2535 | 0.7756 | 18.4746 | 1.0816 | 0.1657 |
| SSA20-CaO10 | ND | 0.0323 | 0.9819 | 1.0713 | 0.6113 | 16.5055 | 0.8852 | 0.1156 |
| TCLP a Regulatory Limit | 5 | 1 | 5 | 15 | 5 | 25 | 100 | 25 |
| GB 5085.3-2007 b Limit | 5 | 1 | 15 | 100 | 5 | 100 | 100 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, H.; Meng, Z.; Wang, Y.; Gao, X.; Wang, R.; Wang, D.; Sheng, J.; Wang, J. Investigations into Replacing Calcined Clay with Sewage Sludge Ash in Limestone Calcined Clay Cement (LC3). Materials 2025, 18, 782. https://doi.org/10.3390/ma18040782
Gu H, Meng Z, Wang Y, Gao X, Wang R, Wang D, Sheng J, Wang J. Investigations into Replacing Calcined Clay with Sewage Sludge Ash in Limestone Calcined Clay Cement (LC3). Materials. 2025; 18(4):782. https://doi.org/10.3390/ma18040782
Chicago/Turabian StyleGu, Hui, Zhaobo Meng, Yilei Wang, Xiaohui Gao, Ruihua Wang, Dongfang Wang, Jianxiong Sheng, and Junjie Wang. 2025. "Investigations into Replacing Calcined Clay with Sewage Sludge Ash in Limestone Calcined Clay Cement (LC3)" Materials 18, no. 4: 782. https://doi.org/10.3390/ma18040782
APA StyleGu, H., Meng, Z., Wang, Y., Gao, X., Wang, R., Wang, D., Sheng, J., & Wang, J. (2025). Investigations into Replacing Calcined Clay with Sewage Sludge Ash in Limestone Calcined Clay Cement (LC3). Materials, 18(4), 782. https://doi.org/10.3390/ma18040782







