Use of EDTA and CaCl2 Extraction Methods to Predict the Bioavailability of Heavy Metals in Soils Polluted with Microplastics
Abstract
1. Introduction
2. Method and Materials
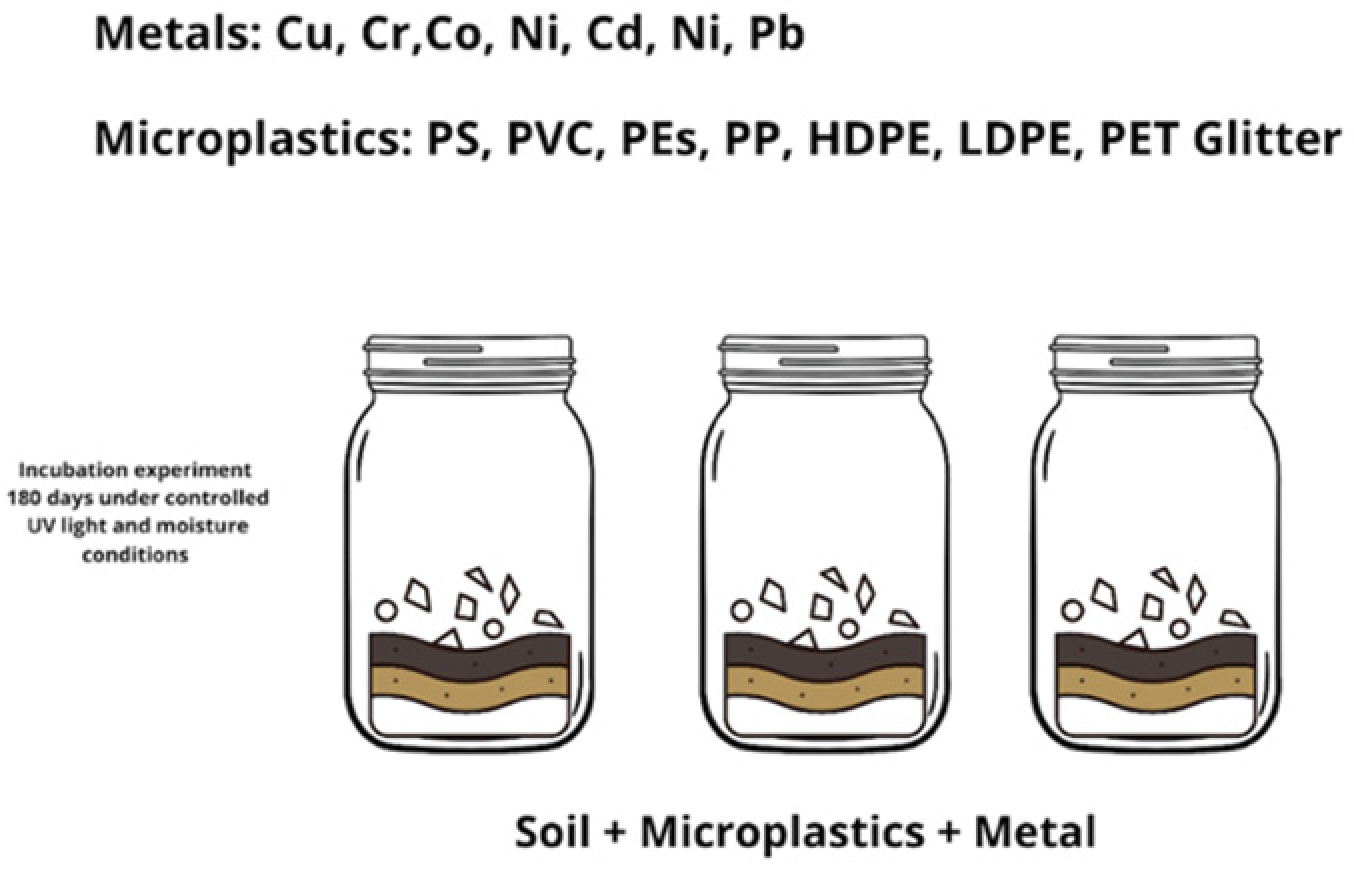
2.1. Incubation Experiment
2.2. Batch Experiment
2.3. Soil Analysis
2.4. Data Analysis
3. Results
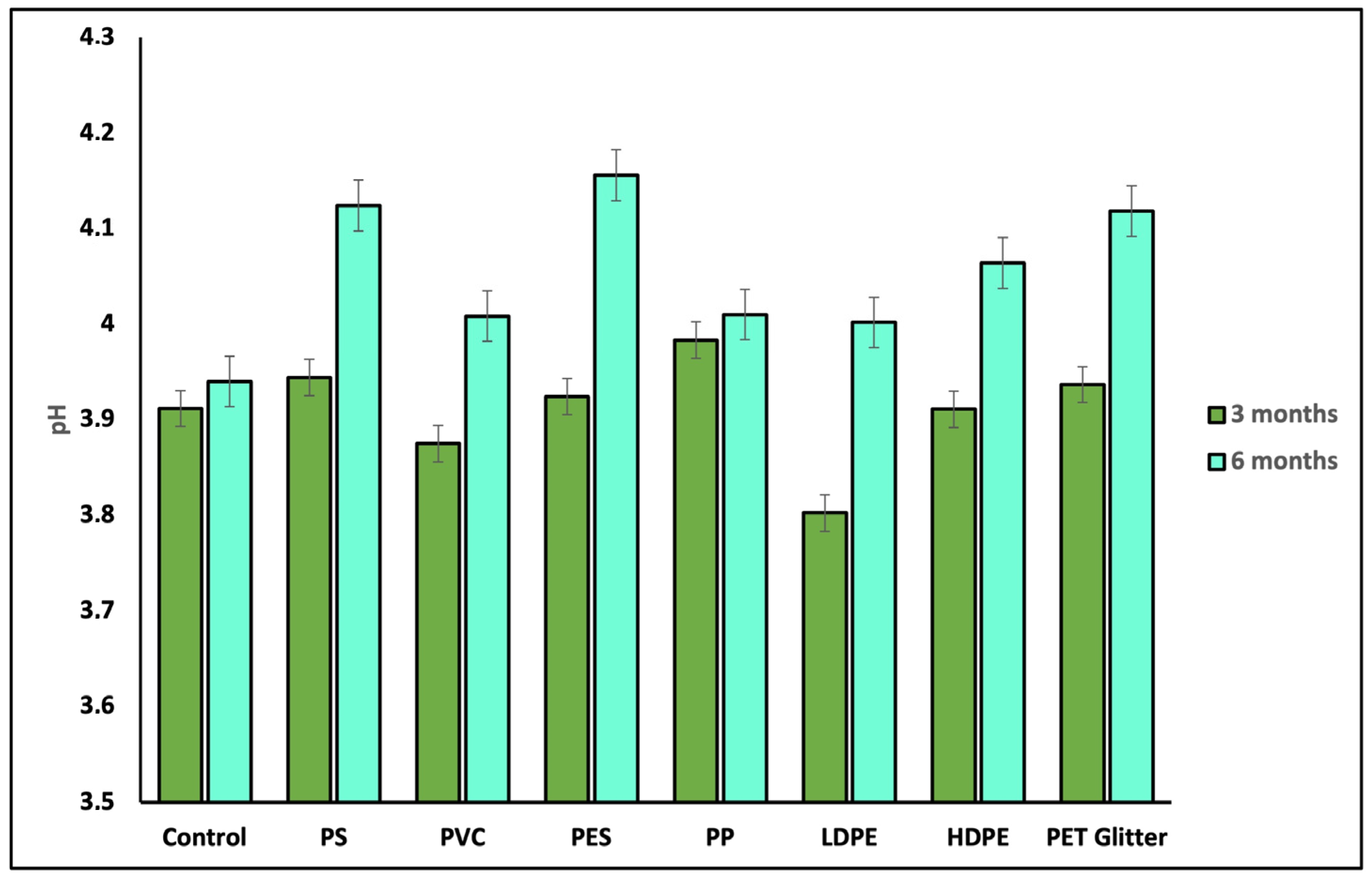
3.1. Soil pH
3.2. Extraction of Available Forms of Heavy Metals
3.3. Sorption of Heavy Metals onto Microplastic and Soil
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MPs | Microplastics |
| HMs | Heavy metals |
| Pb | Lead |
| Cu | Copper |
| Co | Cobalt |
| Ni | Nickel |
| Cr | Chromium |
| Cd | Cadmium |
| PP | Polypropylene |
| PS | Polystyrene |
| PVC | Polyvinyl chloride |
| HDPE | High-density polyethylene |
| LDPE | Low-density polyethylene |
| PES | Polyester |
| PET-Glitter | Polyethylene terephthalate glitter |
| EDTA | Ethylenediaminetetraacetic acid |
| NH4NO3 | Ammonium nitrate |
| NH4OAc | Ammonium acetate |
| CaCl2 | Calcium chloride |
| POM | Polyoxymethylene |
| PTEs | Potentially toxic elements |
| DTPA | Diethylenetriaminepentaacetic acid |
| MP-AES | Microwave Plasma Atomic Emission Spectroscopy |
| RSD | Relative standard deviation |
| AI | Artificial intelligence |
| TEA | Triethylamine |
| pH | Potential of hydrogen |
| SOM | Soil organic matter |
| DOM | Dissolved organic matter |
| POPs | Persistent organic pollutants |
| AOM | Algal organic matter |
References
- Khalid, N.; Aqeel, M.; Noman, A.; Khan, S.M.; Akhter, N. Interactions and Effects of Microplastics with Heavy Metals in Aquatic and Terrestrial Environments. Environ. Pollut. 2021, 290, 118104. [Google Scholar] [CrossRef]
- Dong, Y.; Gao, M.; Song, Z.; Qiu, W. As (III) Adsorption onto Different-Sized Polystyrene Microplastic Particles and Its Mechanism. Chemosphere 2020, 239, 124792. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Tu, C.; He, D.; Zhang, A.; Sun, J.; Li, J.; Xu, J.; Pan, X. Interactions between Microplastics and Contaminants: A Review Focusing on the Effect of Aging Process. Sci. Total Environ. 2023, 899, 165615. [Google Scholar] [CrossRef]
- Chen, Q.; Zhao, H.; Liu, Y.; Jin, L.; Peng, R. Factors Affecting the Adsorption of Heavy Metals by Microplastics and Their Toxic Effects on Fish. Toxics 2023, 11, 490. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Zhao, M.; Ma, X.; Song, Y.; Zuo, S.; Li, H.; Deng, W. A Critical Review on the Interactions of Microplastics with Heavy Metals: Mechanism and Their Combined Effect on Organisms and Humans. Sci. Total Environ. 2021, 788, 147620. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Gui, X.; Xu, X.; Zhao, L.; Qiu, H.; Cao, X. Microplastics in the Soil-Groundwater Environment: Aging, Migration, and Co-Transport of Contaminants-A Critical Review. J. Hazard. Mater. 2021, 419, 126455. [Google Scholar] [CrossRef]
- Lu, Q.; Zhou, Y.; Sui, Q.; Zhou, Y. Mechanism and Characterization of Microplastic Aging Process: A Review. Front. Environ. Sci. Eng. 2023, 17, 100. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, S.; Liang, X.; Hou, Z.; Lin, X.; Chao, L. The Effect of Different Aging Methods on the Heavy Metal Adsorption Capacity of Microplastics. Land Degrad. Dev. 2024. [Google Scholar] [CrossRef]
- Liu, H.; Yang, J.; Jiang, Y.; Sun, R.; Zhou, P.; Li, J. Behavior of Microplastics and Nanoplastics in Farmland Soil Environment and Mechanisms of Interaction with Plants. Pol. J. Environ. Stud. 2024, 33, 2499–2513. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Huang, M.; Wang, Q.; Sun, Y.; Zhao, Y.; Huang, Y. LDPE Microplastics Significantly Alter the Temporal Turnover of Soil Microbial Communities. Sci. Total Environ. 2020, 726, 138682. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, B.; Medyńska-Juraszek, A. Microplastic and Nanoplastic in Crops: Possible Adverse Effects to Crop Production and Contaminant Transfer in the Food Chain. Plants 2024, 13, 2526. [Google Scholar] [CrossRef]
- Ivanic, F.M.; Guggenberger, G.; Woche, S.K.; Bachmann, J.; Hoppe, M.; Carstens, J.F. Soil Organic Matter Facilitates the Transport of Microplastic by Reducing Surface Hydrophobicity. Colloids Surf. A Physicochem. Eng. Asp. 2023, 676, 132255. [Google Scholar] [CrossRef]
- Aydinalp, C.; Katkat, A.V. The Comparison of Extraction Methods for Evaluating Some Heavy Metals in Polluted Soils. Plant Soil Environ. 2004, 50, 212–217. [Google Scholar] [CrossRef]
- Sun, B.; Zhao, F.J.; Lombi, E.; McGrath, S.P. Leaching of Heavy Metals from Contaminated Soils Using EDTA. Environ. Pollut. 2001, 113, 111–120. [Google Scholar] [CrossRef]
- Zhang, M.-K.; Liu, Z.-Y.; Wang, H. Use of Single Extraction Methods to Predict Bioavailability of Heavy Metals in Polluted Soils to Rice. Commun. Soil Sci. Plant Anal. 2010, 41, 820–831. [Google Scholar] [CrossRef]
- Huang, F.; Hu, J.; Chen, L.; Wang, Z.; Sun, S.; Zhang, W.; Jiang, H.; Luo, Y.; Wang, L.; Zeng, Y.; et al. Microplastics May Increase the Environmental Risks of Cd via Promoting Cd Uptake by Plants: A Meta-Analysis. J. Hazard. Mater. 2023, 448, 130887. [Google Scholar] [CrossRef] [PubMed]
- An, Q.; Zhou, T.; Wen, C.; Yan, C. The Effects of Microplastics on Heavy Metals Bioavailability in Soils: A Meta-Analysis. J. Hazard. Mater. 2023, 460, 132369. [Google Scholar] [CrossRef]
- Binda, G.; Kalčíková, G.; Allan, I.J.; Hurley, R.; Rødland, E.; Spanu, D.; Nizzetto, L. Microplastic Aging Processes: Environmental Relevance and Analytical Implications. TrAC Trends Anal. Chem. 2024, 172, 117566. [Google Scholar] [CrossRef]
- Turner, A.; Holmes, L.A. Adsorption of Trace Metals by Microplastic Pellets in Fresh Water. Environ. Chem. 2015, 12, 600–606. [Google Scholar] [CrossRef]
- Llorca, M.; Ábalos, M.; Vega-Herrera, A.; Adrados, M.A.; Abad, E.; Farré, M. Adsorption and Desorption Behaviour of Polychlorinated Biphenyls onto Microplastics’ Surfaces in Water/Sediment Systems. Toxics 2020, 8, 59. [Google Scholar] [CrossRef]
- Hüffer, T.; Metzelder, F.; Sigmund, G.; Slawek, S.; Schmidt, T.C.; Hofmann, T. Polyethylene Microplastics Influence the Transport of Organic Contaminants in Soil. Sci. Total Environ. 2019, 657, 242–247. [Google Scholar] [CrossRef]
- Pueyo, M.; López-Sánchez, J.F.; Rauret, G. Assessment of CaCl2, NaNO3 and NH4NO3 extraction procedures for the study of Cd, Cu, Pb and Zn extractability in contaminated soils. Anal. Chim. Acta 2004, 504, 217–226. [Google Scholar] [CrossRef]
- Medyńska-Juraszek, A.; Jadhav, B. Influence of Different Microplastic Forms on pH and Mobility of Cu2+ and Pb2+ in Soil. Molecules 2022, 27, 1744. [Google Scholar] [CrossRef]
- Campillo-Cora, C.; Conde-Cid, M.; Arias-Estévez, M.; Fernández-Calviño, D.; Alonso-Vega, F. Specific Adsorption of Heavy Metals in Soils: Individual and Competitive Experiments. Agronomy 2020, 10, 1113. [Google Scholar] [CrossRef]
- Houba, V.J.G.; Temminghoff, E.J.M.; Gaikhorst, G.A.; van der Lee, J.J. Soil Analysis Procedures Using 0.01 M Calcium Chloride as Extraction Reagent. Commun. Soil Sci. Plant Anal. 2000, 31, 1299–1396. [Google Scholar] [CrossRef]
- Kołodyńska, D. The Effect of the Novel Complexing Agent in Removal of Heavy Metal Ions from Waters and Waste Waters. Chem. Eng. J. 2010, 165, 835–845. [Google Scholar] [CrossRef]
- Lebrun, M.; Száková, J.; Drábek, O.; Tejnecký, V.; Hough, R.L.; Beesley, L.; Wang, H.; Trakal, L. EDTA as a Legacy Soil Chelatant: A Comparative Study to a More Environmentally Sensitive Alternative for Metal Removal by Pistia stratiotes L. Environ. Sci. Pollut. Res. 2023, 30, 74314–74326. [Google Scholar] [CrossRef] [PubMed]
- Kaurin, A.; Gluhar, S.; Tilikj, N.; Lestan, D. Soil Washing with Biodegradable Chelating Agents and EDTA: Effect on Soil Properties and Plant Growth. Chemosphere 2020, 260, 127673. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, J.P.; Duarte, A.C.; Santos-Echeandía, J. Interaction of microplastics with metal(oid)s in aquatic environments: What is done so far? J. Hazard. Mater. Adv. 2022, 6, 100072. [Google Scholar] [CrossRef]
- Tang, S.; Lin, L.; Wang, X.; Yu, A.; Sun, X. Interfacial interactions between collected nylon microplastics and three divalent metal ions Cu (II), Ni (II), Zn (II)) in aqueous solutions. J. Hazard. Mater. 2021, 403, 123548. [Google Scholar] [CrossRef]
- Purwiyanto, A.I.S.; Suteja, Y.; Trisno; Ningrum, P.S.; Putri, W.A.E.; Rozirwan; Agustriani, F.; Fauziyah; Cordova, M.R.; Koropitan, A.F. Concentration and adsorption of Pb and Cu in microplastics: Case study in aquatic environment. Mar. Pollut. Bull. 2020, 158, 111380. [Google Scholar] [CrossRef] [PubMed]
- Medyńska-Juraszek, A.; Rivier, P.-A.; Rasse, D.; Joner, E.J. Biochar affects heavy metal uptake in plants through interactions in the rhizosphere. Appl. Sci. 2020, 10, 5105. [Google Scholar] [CrossRef]
- Liu, W.-J.; Guo, Y.-F.; Deng, W.-B. Effect of Photoaging on Adsorption of Cu(II) by Polystyrene Microplastics with Different Particle Sizes. Huanjing Kexue 2024, 45, 3708–3715. [Google Scholar] [CrossRef]
- Xie, A.; Chen, S.; Liang, X.; Li, L.; Song, Y.; Lv, M.; Liang, F.; Zhou, W. Effect of Microplastics on the Adsorption and Desorption Characteristics of Heavy Metal Ni(II) under Different Aging Modes. Res. Sq. 2024, preprint. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Li, D.; Wang, J.; Ding, Y.; Wang, Y.; Feng, L.; Hu, Y. Aging Properties of Polyethylene and Polylactic Acid Microplastics and Their Adsorption Behavior of Cd(II) and Cr(VI) in Aquatic Environments. Chemosphere 2024, 363, 142833. [Google Scholar] [CrossRef]
- Chen, L.; Xie, N.; Yuan, S.; Shao, H. Adsorption Mechanism of Hexavalent Chromium on Electron Beam-Irradiated Aged Microplastics: Novel Aging Processes and Environmental Factors. Chemosphere 2024, 363, 142741. [Google Scholar] [CrossRef]
- Chen, Z.-Y.; Sun, W.; Wang, S.; Yang, J.; Huang, W.; Huang, D.; Jiang, K.; Zhang, X.; Sun, X. Interactions between Microplastics and Organic Contaminants: The Microbial Mechanisms for Priming Effects of Organic Compounds on Microplastic Biodegradation. Water Res. 2024, 267, 122523. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Sang, W.; Abbas, M.; Xu, C.; Jiang, Z.; Ma, Y.; Shi, J.; Feng, M.; Ni, L.; Li, S. The Interaction Mechanisms of Algal Organic Matter (AOM) and Various Types and Aging Degrees of Microplastics. J. Hazard. Mater. 2024, 477, 135273. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Feng, R.; Hu, K.; Hu, X.; Qu, Q.; Mu, L.; Wen, J.; Ma, C. Polystyrene Microplastics Facilitate Formation of Refractory Dissolved Organic Matter and Reduce CO₂ Emissions. Environ. Int. 2024, 190, 108809. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Fang, L.; Gardea-Torresdey, J.L.; Zhou, X.; Yan, B. Microplastic-Derived Dissolved Organic Matter: Generation, Characterization, and Environmental Behaviors. Sci. Total Environ. 2024, 54, 865–882. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Ding, L.; Qiu, X.; Liang, X.; Zhang, Y.; Shan, X.; Chen, Q.; Guo, X. Interactions between Iron Minerals and Dissolved Organic Matter Derived from Microplastics Inhibited the Ferrihydrite Transformation as Revealed at the Molecular Scale. Environ. Sci. Technol. 2024, 58, 13478–13489. [Google Scholar] [CrossRef]
- Teuten, E.L.; Saquing, J.M.; Knappe, D.R.U.; Barlaz, M.A.; Jonsson, S.; Björn, A.; Takada, H. Transport and Release of Chemicals from Plastics to the Environment and to Wildlife. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2027–2045. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Lin, L.; Wang, X.; Feng, A.; Yu, A. Pb (II) uptake onto nylon microplastics: Interaction mechanism and adsorption performance. J. Hazard. Mater. 2020, 386, 121960. [Google Scholar] [CrossRef] [PubMed]
- Kalčíková, G.; Skalar, T.; Marolt, G.; Jemec Kokalj, A. An environmental concentration of aged microplastics with adsorbed silver significantly affects aquatic organisms. Water Res. 2020, 175, 115644. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Hu, Y.; Yuan, Y.; Hu, B.; Wang, B. Comparative analysis of kinetics and mechanisms for Pb(II) sorption onto three kinds of microplastics. Ecotoxicol. Environ. Saf. 2021, 208, 111451. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Hu, G.; Fan, X.; Jia, H. Sorption properties of cadmium on microplastics: The common practice experiment and a two-dimensional correlation spectroscopic study. Ecotoxicol. Environ. Saf. 2020, 190, 110118. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Wang, S.; Yu, X.; Vogt, R.D.; Feng, J.; Zhai, L.; Ma, W.; Zhu, L.; Lu, X. Kinetics and size effects on adsorption of Cu(II), Cr(III), and Pb(II) onto polyethylene, polypropylene, and polyethylene terephthalate microplastic particles. Front. Mar. Sci. 2021, 8, 785146. [Google Scholar] [CrossRef]
- Godoy, V.; Blázquez, G.; Calero, M.; Quesada, L.; Martín-Lara, M.A. The potential of microplastics as carriers of metals. Environ. Pollut. 2019, 255, 113363. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Tan, Z.; Qi, Y.; Ouyang, C. Sorption of tri-n-butyl phosphate and tris(2-chloroethyl) phosphate on polyethylene and polyvinyl chloride microplastics in seawater. Mar. Pollut. Bull. 2019, 149, 110490. [Google Scholar] [CrossRef]
- Loncarski, M.; Tubic, A.; Kragulj-Isakovski, M.; Jovic, B.; Apostolovic, T.; Nikic, J.; Agbaba, J. Modelling of the adsorption of chlorinated phenols on polyethylene and polyethylene terephthalate microplastic. J. Serb. Chem. Soc. 2020, 85, 697–709. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Su, F.; Wang, Y.; Peng, L.; Liu, D. Adsorption behaviour of microplastics on the heavy metal Cr(VI) before and after ageing. Chemosphere 2022, 302, 134865. [Google Scholar] [CrossRef]
- Wang, F.; Wang, X.; Song, N. Polyethylene microplastics increase cadmium uptake in lettuce (Lactuca sativa L.) by altering the soil microenvironment. Sci. Total Environ. 2021, 784, 147133. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Han, B.; Sun, Y.; Wang, F. Microplastics influence the adsorption and desorption characteristics of Cd in agricultural soil. J. Hazard. Mater. 2020, 388, 121775. [Google Scholar] [CrossRef] [PubMed]
- Holmes, L.A.; Turner, A.; Thompson, R.C. Interactions between trace metals and plastic production pellets under estuarine conditions. Mar. Chem. 2014, 167, 25–32. [Google Scholar] [CrossRef]
- Rochman, C.M.; Hentschel, B.T.; Teh, S.J. Long-term sorption of metals is similar among plastic types: Implications for plastic debris in aquatic environments. PLoS ONE 2014, 9, e85433. [Google Scholar] [CrossRef] [PubMed]
- Frost, H.; Bond, T.; Sizmur, T.; Felipe-Sotelo, M. Sorption of metal ions onto PET-derived microplastic fibres. Environ. Sci. Process. Impacts 2024, 26, 2309–2319. [Google Scholar] [CrossRef] [PubMed]
- Seyfi, S.; Katibeh, H.; Heshami, M. Investigation of the process of adsorption of heavy metals in coastal sands containing micro-plastics, with special attention to the effect of aging process and bacterial spread in micro-plastics. Arch. Environ. Prot. 2023, 47, 50–59. [Google Scholar] [CrossRef]
- Tenea, A.-G.; Dinu, C.; Rus, P.A.; Ionescu, I.A.; Gheorghe, S.; Iancu, V.I.; Vasile, G.G.; Pascu, L.F.; Chiriac, F.L. Exploring adsorption dynamics of heavy metals onto varied commercial microplastic substrates: Isothermal models and kinetics analysis. Heliyon 2024, 10, e35364. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Wang, Q.; Hu, X. Extraction of heavy metals from water-stable soil aggregates using EDTA. Procedia Environ. Sci. 2013, 18, 679–685. [Google Scholar] [CrossRef][Green Version]
- Moral, R.; Gilkes, R.J.; Moreno-Caselles, J. A comparison of extractants for heavy metals in contaminated soils from Spain. Commun. Soil Sci. Plant Anal. 2002, 33, 2781–2791. [Google Scholar] [CrossRef]
- Lehmann, A.; Fitschen, K.; Rillig, M.C. Abiotic and Biotic Factors Influencing the Effect of Microplastic on Soil Aggregation. Soil Syst. 2021, 5, 12. [Google Scholar] [CrossRef]
- Ahmad, M.; Lee, S.; Dou, X.; Mohan, D.; Sung J., E.; Yang, J.; Ok, Y.S. Effects of pyrolysis temperature on soybean stover- and peanut shell-derived biochar properties and TCE adsorption in water. Bioresour. Technol. 2012, 118, 536–544. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jadhav, B.; Medyńska-Juraszek, A. Use of EDTA and CaCl2 Extraction Methods to Predict the Bioavailability of Heavy Metals in Soils Polluted with Microplastics. Materials 2025, 18, 760. https://doi.org/10.3390/ma18040760
Jadhav B, Medyńska-Juraszek A. Use of EDTA and CaCl2 Extraction Methods to Predict the Bioavailability of Heavy Metals in Soils Polluted with Microplastics. Materials. 2025; 18(4):760. https://doi.org/10.3390/ma18040760
Chicago/Turabian StyleJadhav, Bhakti, and Agnieszka Medyńska-Juraszek. 2025. "Use of EDTA and CaCl2 Extraction Methods to Predict the Bioavailability of Heavy Metals in Soils Polluted with Microplastics" Materials 18, no. 4: 760. https://doi.org/10.3390/ma18040760
APA StyleJadhav, B., & Medyńska-Juraszek, A. (2025). Use of EDTA and CaCl2 Extraction Methods to Predict the Bioavailability of Heavy Metals in Soils Polluted with Microplastics. Materials, 18(4), 760. https://doi.org/10.3390/ma18040760






