Synthesis of Submicron-Sized TiB2 Powders by Reaction of TiC, B4C, and Ca in Molten CaCl2
Highlights
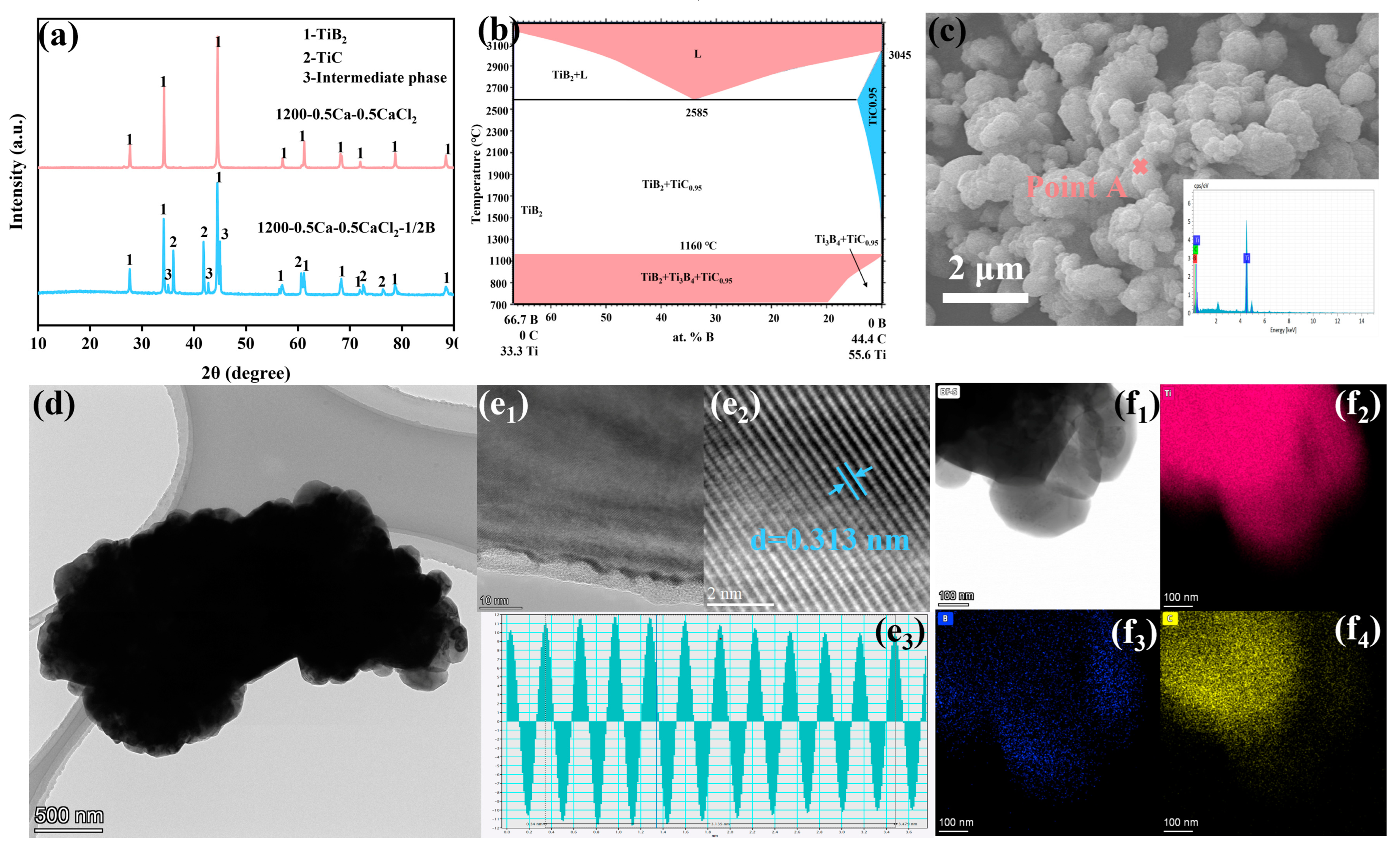
- Submicron TiB2 (300 nm to 1 μm) powders were prepared using TiC, B4C, Ca, and CaCl2.
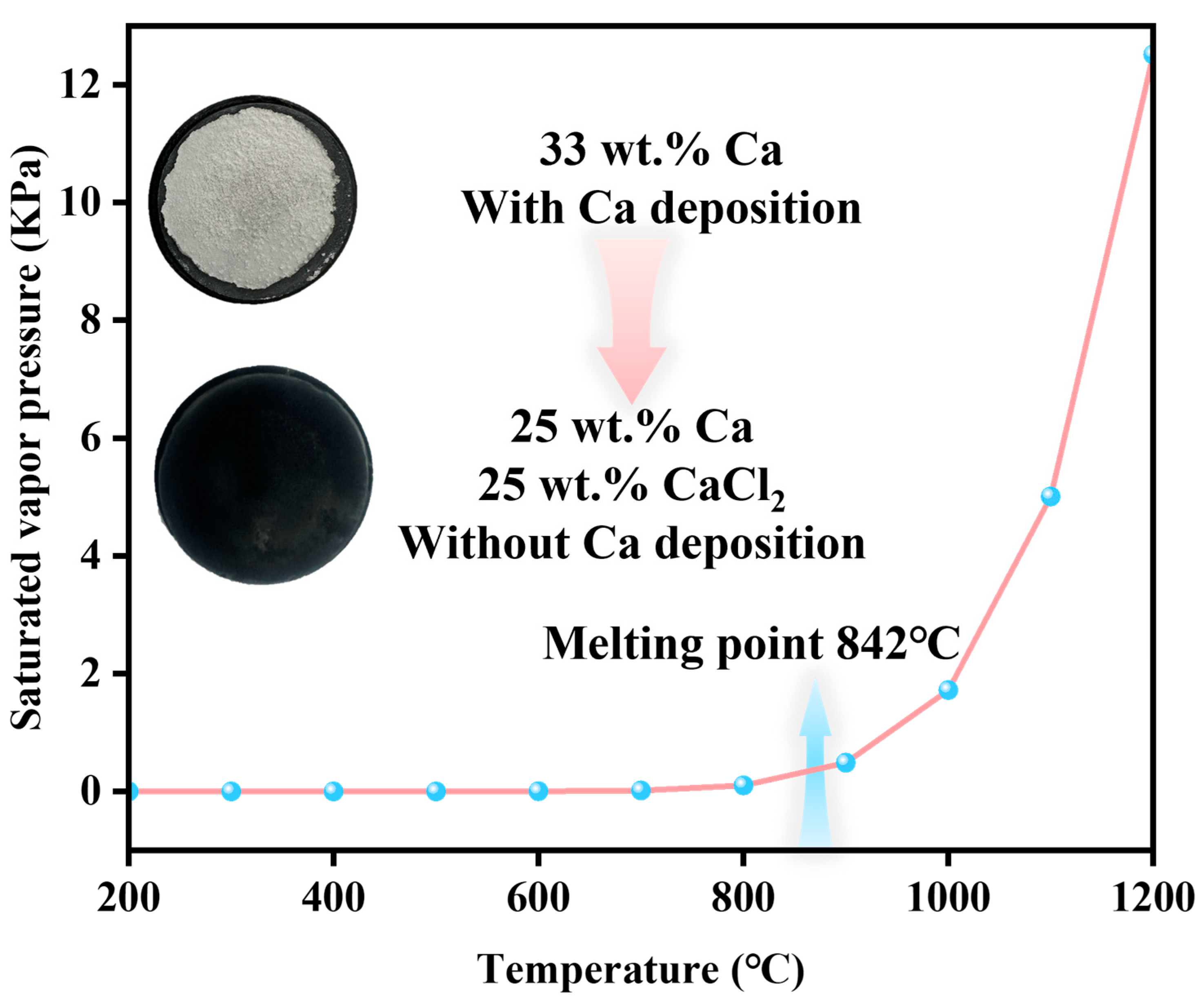
- CaCl2 can promote the reaction and inhibit the volatilization of excess Ca.
- The particle size and morphology of TiB2 were inherited from TiC.
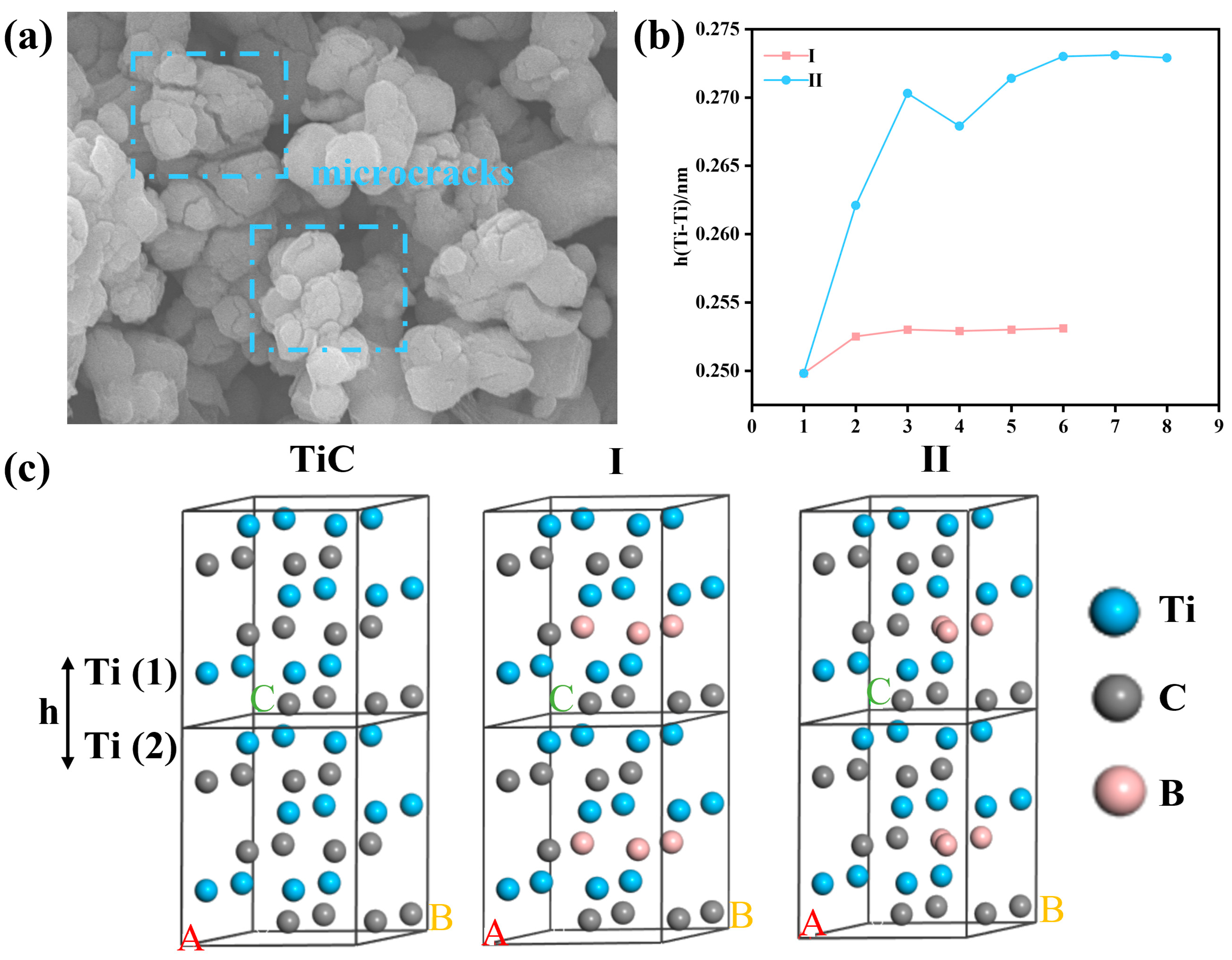
- Boronization was a process that B atoms from B4C replaced C atoms in the TiC lattice.
Abstract
1. Introduction
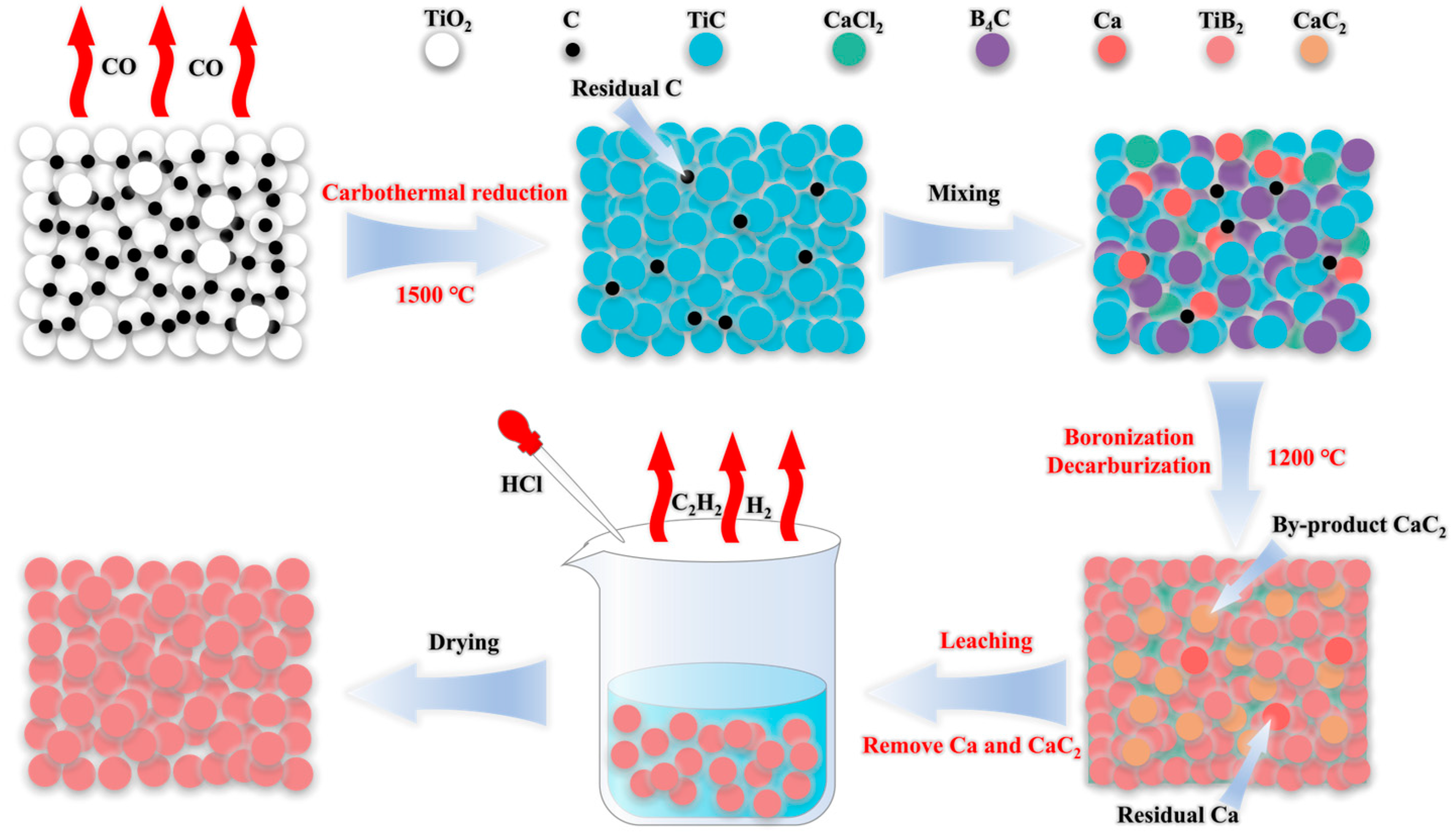
2. Materials and Methods
3. Results and Discussions
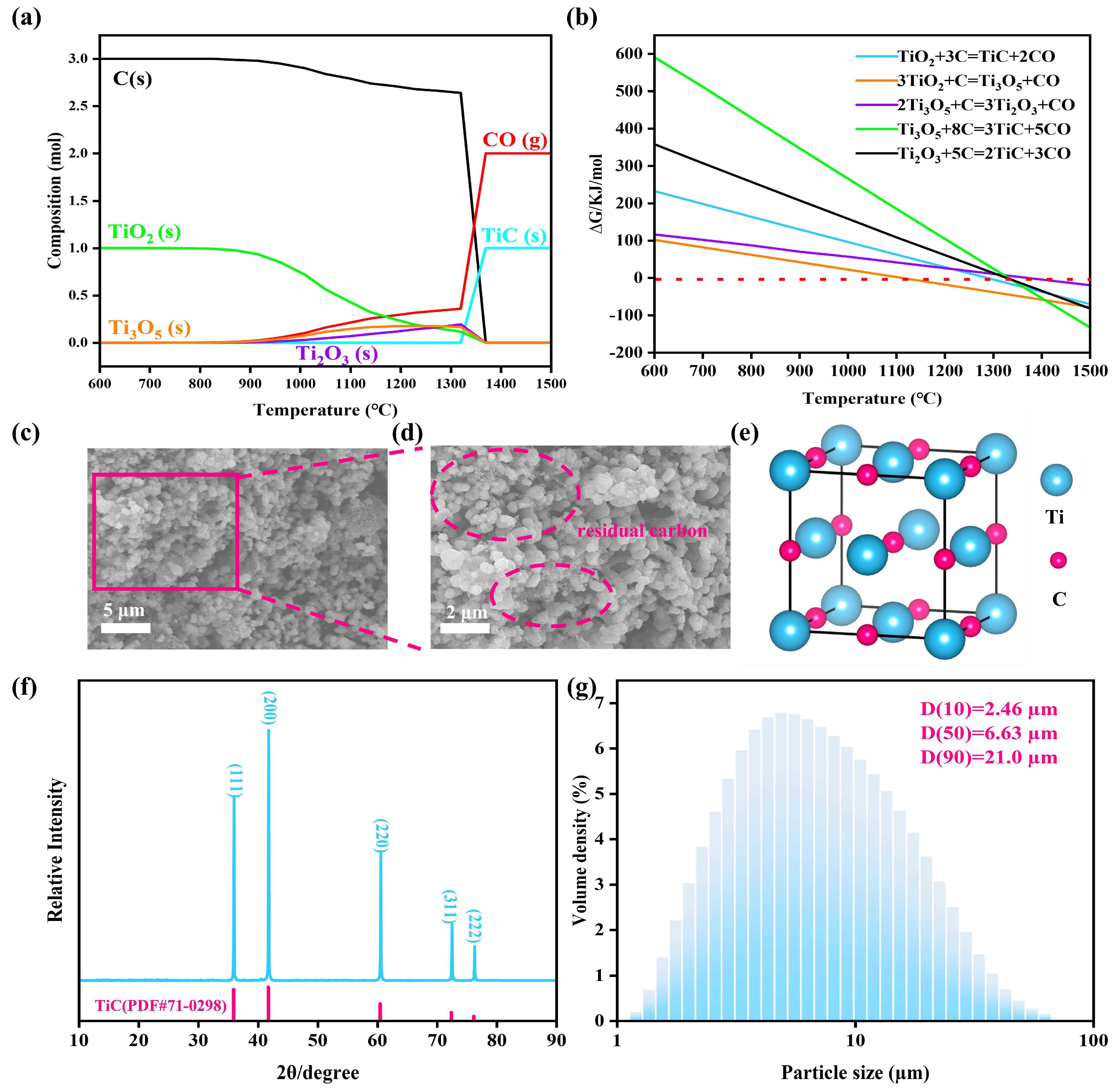
3.1. Carbothermal Reduction
3.2. Effects of Temperature and CaCl2 (Promotion of Thermodynamics as Well as Kinetics)
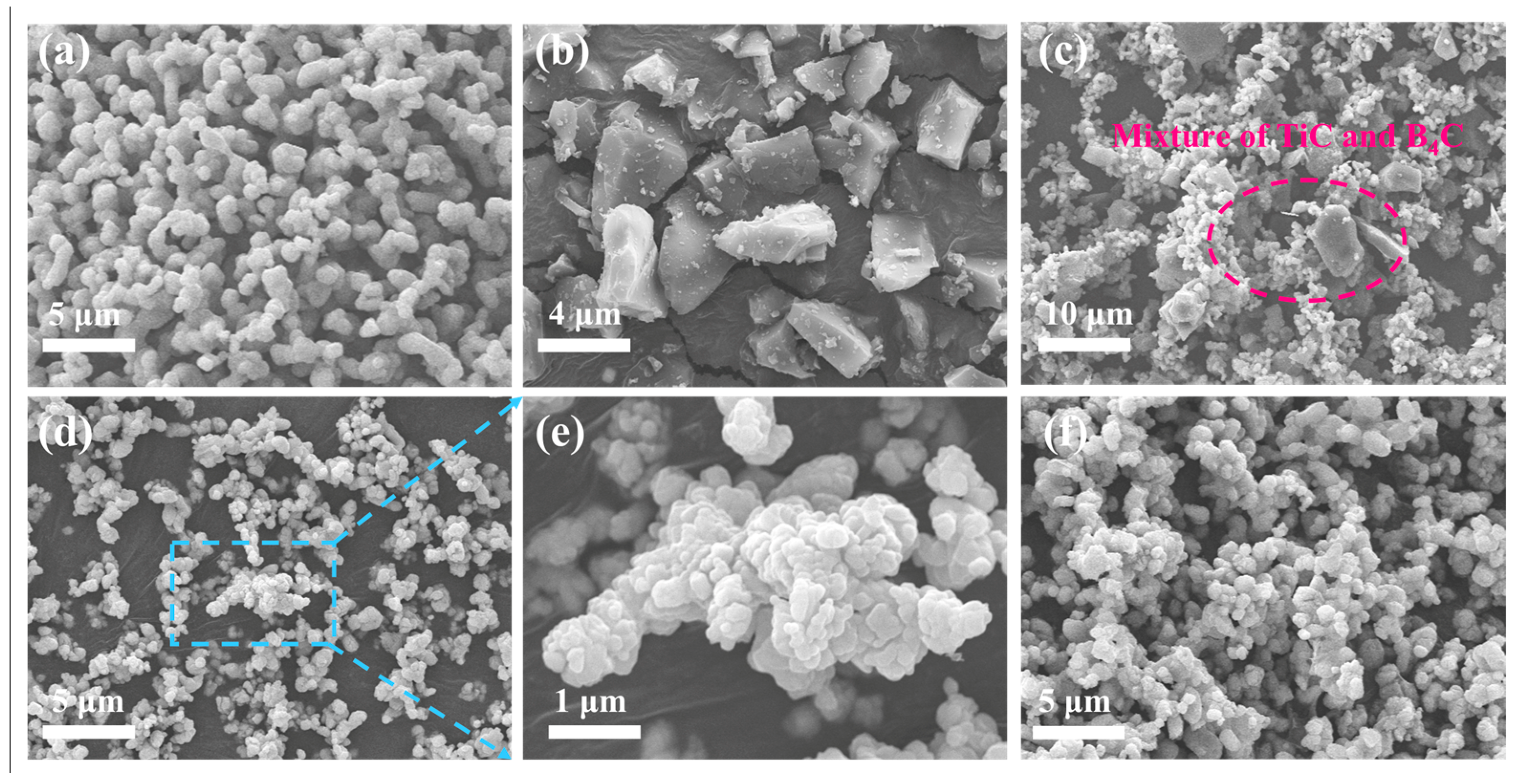
3.3. Morphology and Particle Size of Prepared TiB2 Powder
3.4. Mechanism of Boronization Reaction
4. Conclusions
- (1)
- The introduction of CaCl2 not only promoted the reaction but also reduced the volatilization of excess Ca.
- (2)
- The particle size and morphology of TiB2 were inherited from TiC based on the “template/growth” mechanism, and the particle size of the prepared TiB2 ranged from 300 nm to 1 μm.
- (3)
- Boronization was a process in which B atoms from B4C diffused into the TiC lattice and gradually replaced the C atoms.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, B.; Wei, W.; Gan, Y.; Duan, C.; Cui, H. Preparation mechanical properties and microstructure of TiB2 based ceramic cutting tool material toughened by TiC whisker. Int. J. Refract. Met. Hard Mater. 2020, 93, 105372. [Google Scholar] [CrossRef]
- Yadav, S.; Zhang, Q.; Agrawal, P.; Haridas, R.S.; Morphew, C.; Behera, A.; Mahbooba, Z.; Gong, J.; Mishra, R.S. Effect of ceramic-binder interface on the mechanical properties of TiB2-HEA composites. Mater. Sci. Eng. A 2022, 857, 144059. [Google Scholar] [CrossRef]
- Nayebi, B.; Delbari, S.A.; Asl, M.S.; Ghasali, E.; Parvin, N.; Shokouhimehr, M. A nanostructural approach to the interfacial phenomena in spark plasma sintered TiB2 ceramics with vanadium and graphite additives. Compos. Part B Eng. 2021, 222, 109069. [Google Scholar] [CrossRef]
- Guo, W.M.; Zhang, G.J.; You, Y.; Wu, S.H.; Lin, H.T. TiB2 Powders Synthesis by Borothermal Reduction in TiO2 Under Vacuum. J. Am. Ceram. Soc. 2014, 97, 1359–1362. [Google Scholar] [CrossRef]
- Turan, A.; Bugdayci, M.; Yucel, O. Self-propagating High Temperature Synthesis of TiB2. High Temp. Mater. Process. 2014, 34, 185–193. [Google Scholar] [CrossRef]
- Bača, Ľ.; Stelzer, N. Adapting of sol–gel process for preparation of TiB2 powder from low-cost precursors. J. Eur. Ceram. Soc. 2008, 28, 907–911. [Google Scholar] [CrossRef]
- Liu, D.; Chu, Y.; Jing, S.; Ye, B.; Zhou, X. Low-temperature synthesis of ultrafine TiB2 nanopowders by molten-salt assisted borothermal reduction. J. Am. Ceram. Soc. 2018, 101, 5299–5303. [Google Scholar] [CrossRef]
- Yu, J.; Ma, L.; Zhang, Y.; Gong, H.; Zhou, L. Synthesis of TiB2 powders via carbothermal reduction of TiO2, HBO2 and carbon black. Ceram. Int. 2016, 42, 5512–5516. [Google Scholar] [CrossRef]
- Zhang, S.; Khangkhamano, M.; Zhang, H.; Yeprem, H.A. Novel synthesis of ZrB2 powder via molten-salt-mediated magnesiothermic reduction. J. Am. Ceram. Soc. 2014, 97, 1686–1688. [Google Scholar] [CrossRef]
- Fu, Z.; Koc, R. Synthesis of TiB2 from a carbon-coated precursors method. J. Am. Ceram. Soc. 2017, 100, 2471–2481. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y.-D.; Peng, B.; Wu, K.-H.; Zhang, G.-H. A universal method for the synthesis of refractory metal diborides. Ceram. Int. 2021, 47, 14107–14114. [Google Scholar] [CrossRef]
- Wang, Y.-L.; Deng, X.-C.; Zhang, G.-H. A method to synthesize ultrafineTiB2 and ZrB2 powders. Int. J. Refract. Met. Hard Mater. 2024, 119, 106557. [Google Scholar] [CrossRef]
- Chen, Z.; Suzuki, T.S.; Wang, H. Synthesis of zirconium boride (ZrB2) rod crystals through salt-assisted boro/carbothermal reduction. Ceram. Int. 2023, 49, 28030–28035. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Y.; Yang, L.; Liu, R.; Zeng, C. Synthesis and characterization of nanosized Ti2AlC2 ceramic powder by elemental powders of Ti, Al and C in molten salt. J. Mater. Sci. Technol. 2020, 37, 77–84. [Google Scholar] [CrossRef]
- Wu, K.-H.; Wang, Y.; Yu, J.; Zhang, G.-H.; Jiao, S.-Q.; Chou, K.-C. Low temperature synthesis of titanium diboride nanosheets by molten salt–assisted borothermal reduction of TiO2. J. Nanoparticle Res. 2019, 21, 103. [Google Scholar] [CrossRef]
- Li, X.; Chen, B.; Qiao, J.; Tang, J.; Sahin, F.C.; Yucel, O. Low-temperature synthesis and sinterability of high-purity submicron TiB2 powder via microwave-assisted carbothermal reduction. J. Eur. Ceram. Soc. 2024, 44, 4549–4557. [Google Scholar] [CrossRef]
- Lv, T.; Tian, F.; Hu, T. Preparation of Titanium Carbide by Carburisation of Titanium Dioxide. Processes 2024, 12, 102. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, H.; Jiao, S.; Chou, K.C.; Zhang, G.H. A facile pathway to prepare molybdenum boride powder from molybdenum and boron carbide. J. Am. Ceram. Soc. 2020, 103, 2399–2406. [Google Scholar] [CrossRef]
- Bao, K.; Wen, Y.; Khangkhamano, M.; Zhang, S. Low-temperature preparation of titanium diboride fine powder via magnesiothermic reduction in molten salt. J. Am. Ceram. Soc. 2017, 100, 2266–2272. [Google Scholar] [CrossRef]
- Suzuki, R.O.; Aizawa, M.; Ono, K. Calcium-deoxidation of niobium and titanium in Ca-saturated CaCl2 molten salt. J. Alloys Compd. 1999, 288, 173–182. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, S.; Li, W.; Chen, Z. Low temperature synthesis of LaB6 nanoparticles by a molten salt route. Powder Technol. 2018, 323, 203–207. [Google Scholar] [CrossRef]
- Devesa, S.; Rooney, A.P.; Graça, M.P.; Cooper, D.; Costa, L.C. Williamson-hall analysis in estimation of crystallite size and lattice strain in Bi1.34Fe0.66Nb1.34O6.35 prepared by the sol-gel method. Mater. Sci. Eng. B 2021, 263, 114830. [Google Scholar] [CrossRef]
- Li, Z.-B.; Zhang, H.; Zhang, G.-H.; Chou, K.-C. Superior strength-ductility synergy in a novel tailored Zr-based particle-strengthened medium W content alloys. Compos. Part B Eng. 2022, 236, 109817. [Google Scholar] [CrossRef]
- Vekables, J.D. The nature of precipitates in boron-doped TiC2. Philos. Mag. 1967, 16, 873–890. [Google Scholar] [CrossRef]
- Plyushchay, I.V.; Tsaregrads’ka, T.L.; Kalenyk, O.O.; Plyushchay, O.I. The Theoretical Analysis of Phase-Formation Processes in Amorphous Alloys of Fe—Zr System. Metallofiz. I Noveishie Tekhnologii 2016, 38, 1233–1247. [Google Scholar] [CrossRef]
- Popov, O.; Vishnyakov, V.; Chornobuk, S.; Totsky, I.; Plyushchay, I. Mechanisms of TiB2 and graphite nucleation during TiC–B4C high temperature interaction. Ceram. Int. 2019, 45, 16740–16747. [Google Scholar] [CrossRef]
- Aivazov, M.I.; Domashnev, I.A. Electrophysical properties of titanium diboride and alloys in the system Ti-B-N. Inorg. Mater. (USSR) 1971, 7, 1551–1553. [Google Scholar]
- Witusiewicz, V.T.; Bondar, A.A.; Hecht, U.; Rex, S.; Velikanova, T.Y. The Al–B–Nb–Ti system. J. Alloys Compd. 2008, 448, 185–194. [Google Scholar] [CrossRef]
- Cao, K.; Shi, G.-Y.; Liu, T.-T.; Li, X.; Li, J.-F.; Wang, X.-L.; Su, Y.-H.; Zhang, C.; Jiang, H. Phase transition mechanical and electronic properties of Ti3B4 under high pressure. Vacuum 2023, 217, 112582. [Google Scholar] [CrossRef]
- Rou, S.; Chandran, K.S.R. First principles calculation of single-crystal elastic constants of titanium tetraboride (Ti3B4) and experimental validation. J. Am. Ceram. Soc. 2018, 101, 4308–4320. [Google Scholar] [CrossRef]


| Sample No. | Temperature (°C) | Mass Ratio (wt.%) | ||
|---|---|---|---|---|
| TiC + B4C (Mixture of TiC and B4C) | Ca | CaCl2 | ||
| 1000-0.5Ca-0.5CaCl2 | 1000 | 50 | 25 | 25 |
| 1100-0.5Ca-0.5CaCl2 | 1100 | 50 | 25 | 25 |
| 1200-0.5Ca-0.5CaCl2 | 1200 | 50 | 25 | 25 |
| 1200-0.5Ca-0CaCl2 | 1200 | 66 | 33 | 0 |
| 1200-0.5Ca-1CaCl2 | 1200 | 40 | 20 | 40 |
| 1200-0.5Ca-0.5CaCl2-1/2B | 1200 | 50 (molar ratio of TiC and B4C = 4:1) | 25 | 25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.-L.; Zhang, G.-H. Synthesis of Submicron-Sized TiB2 Powders by Reaction of TiC, B4C, and Ca in Molten CaCl2. Materials 2025, 18, 744. https://doi.org/10.3390/ma18040744
Wang Y-L, Zhang G-H. Synthesis of Submicron-Sized TiB2 Powders by Reaction of TiC, B4C, and Ca in Molten CaCl2. Materials. 2025; 18(4):744. https://doi.org/10.3390/ma18040744
Chicago/Turabian StyleWang, Ya-Long, and Guo-Hua Zhang. 2025. "Synthesis of Submicron-Sized TiB2 Powders by Reaction of TiC, B4C, and Ca in Molten CaCl2" Materials 18, no. 4: 744. https://doi.org/10.3390/ma18040744
APA StyleWang, Y.-L., & Zhang, G.-H. (2025). Synthesis of Submicron-Sized TiB2 Powders by Reaction of TiC, B4C, and Ca in Molten CaCl2. Materials, 18(4), 744. https://doi.org/10.3390/ma18040744







