Evaluation of the Properties of Bioactive Mesoporous Glasses Doped with Cerium and Loaded with Polyphenols
Highlights
- Polyphenol-loaded MBGsCe.
- Evaluation of the loading and antioxidant activity of loaded MBGsCe.
- Successfully loaded MBGsCe with improved antioxidant properties.
- Polyphenol loaded MBGsCe retain their bioactivity.
- Polyphenol loaded MBGsCe candidates for drug delivery systems on biological systems.
Abstract
1. Introduction
2. Materials and Methods
2.1. MBGsCe Preparation and Biomolecules’ Loading
2.2. Surface Activation
2.3. Elemental Analysis (EA)
2.4. Folin–Ciocalteu (FC) Method
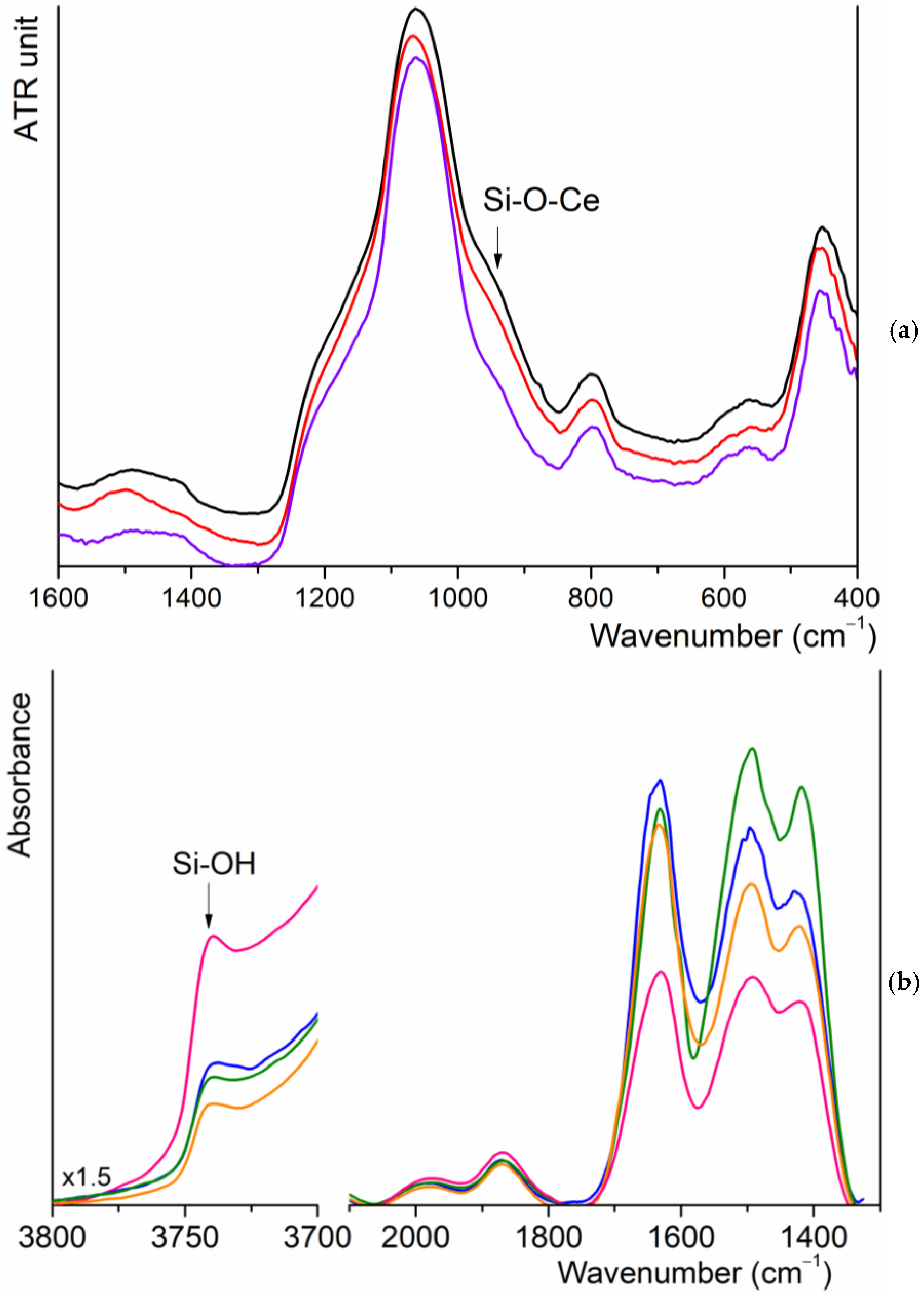
2.5. FT-IR Spectroscopy
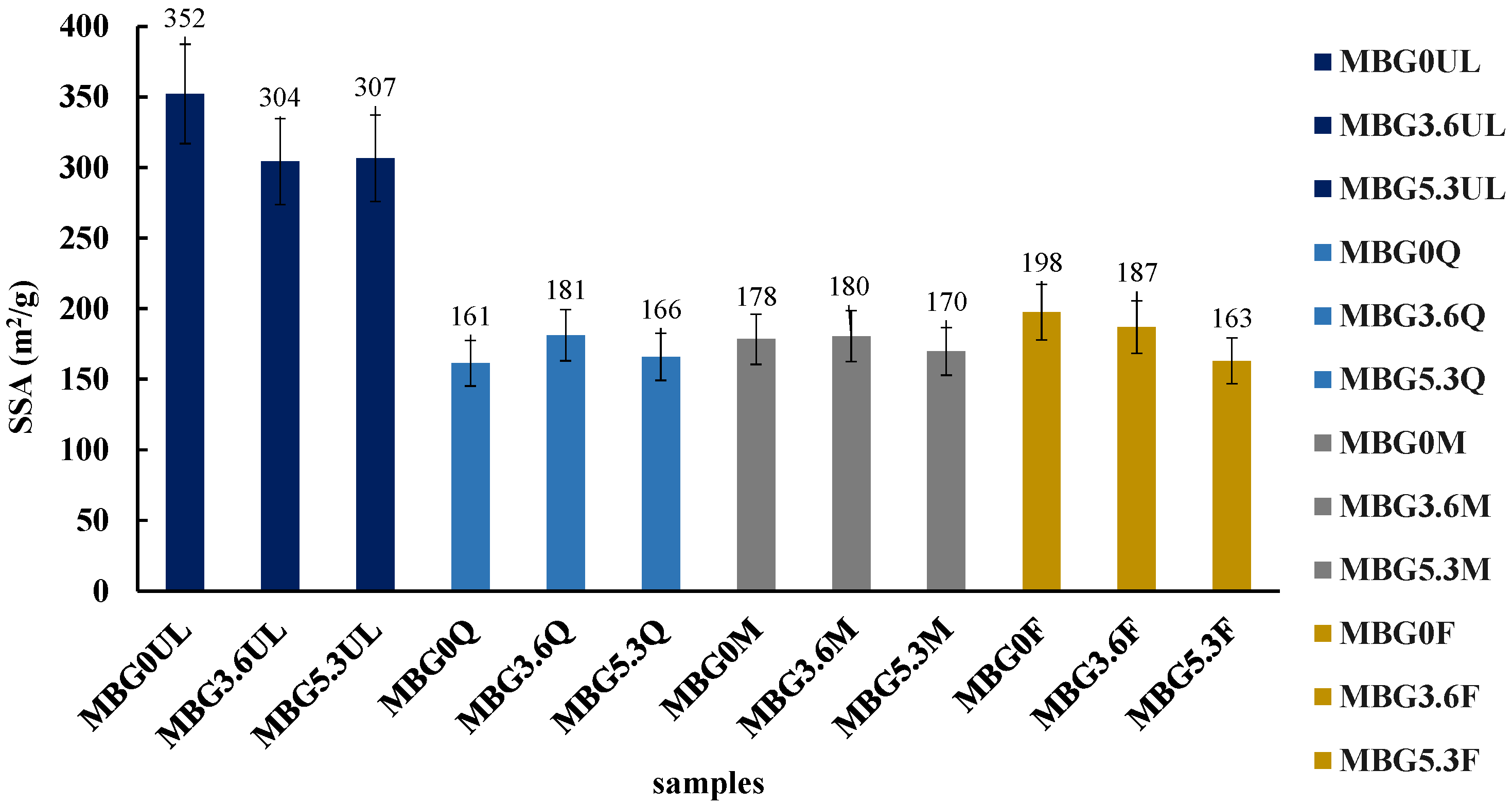
2.6. Specific Surface Area (SSA) Determination
2.7. Antioxidant Activity Assays
SOD-like Activity
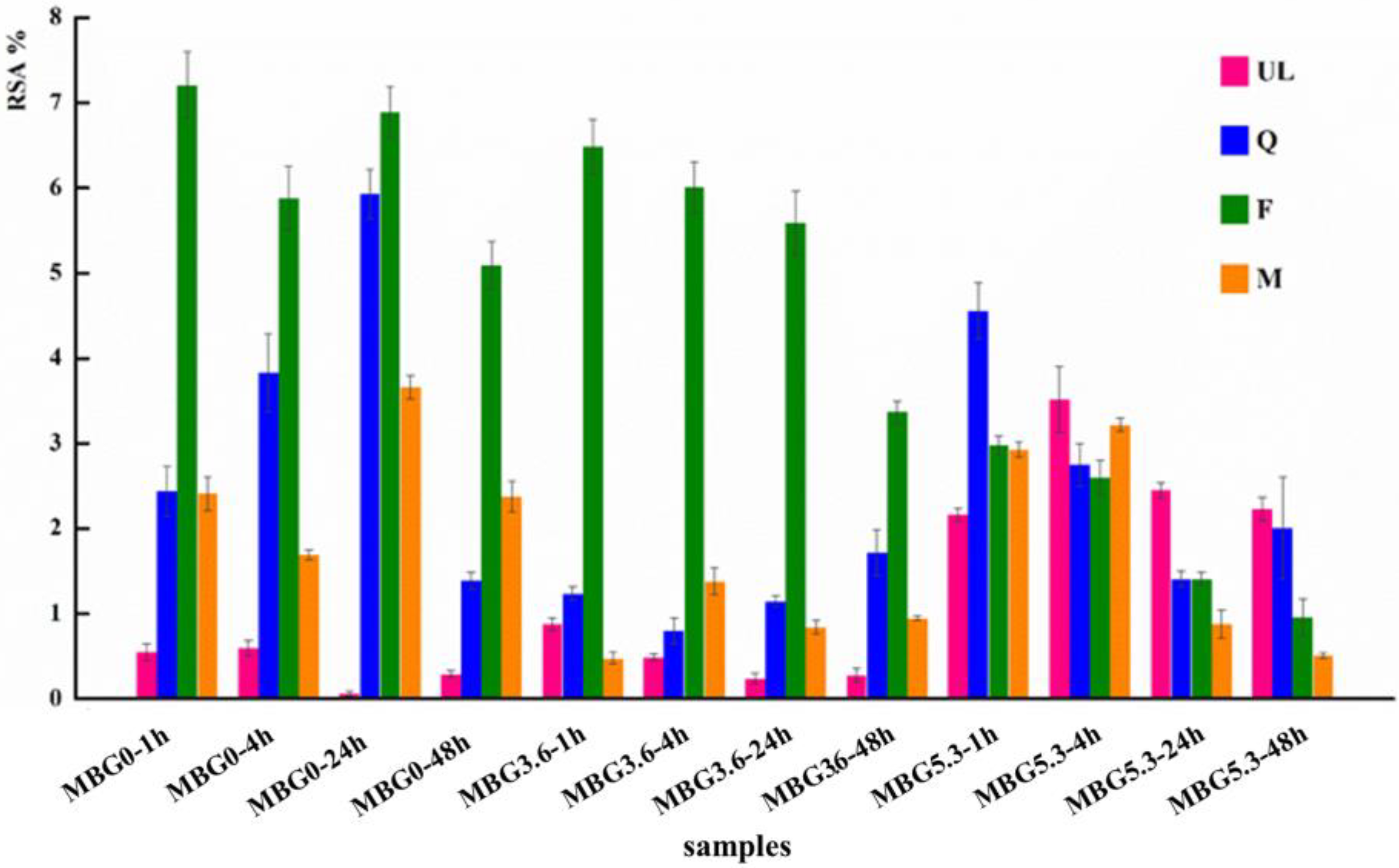
2.8. RSA
2.9. In Vitro Bioactivity Assessment
3. Results and Discussion
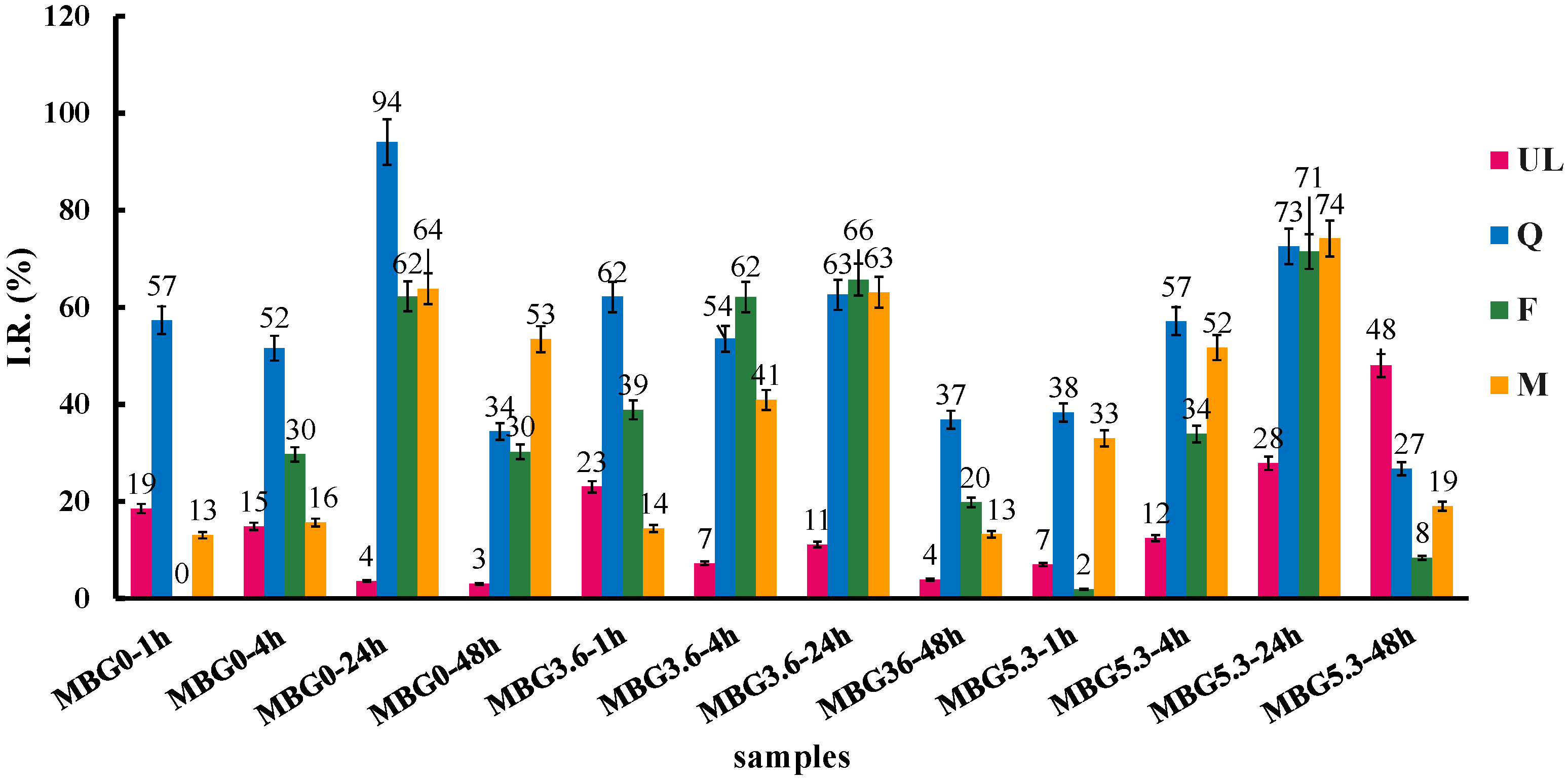
3.1. Loading Evaluation
EA and BET
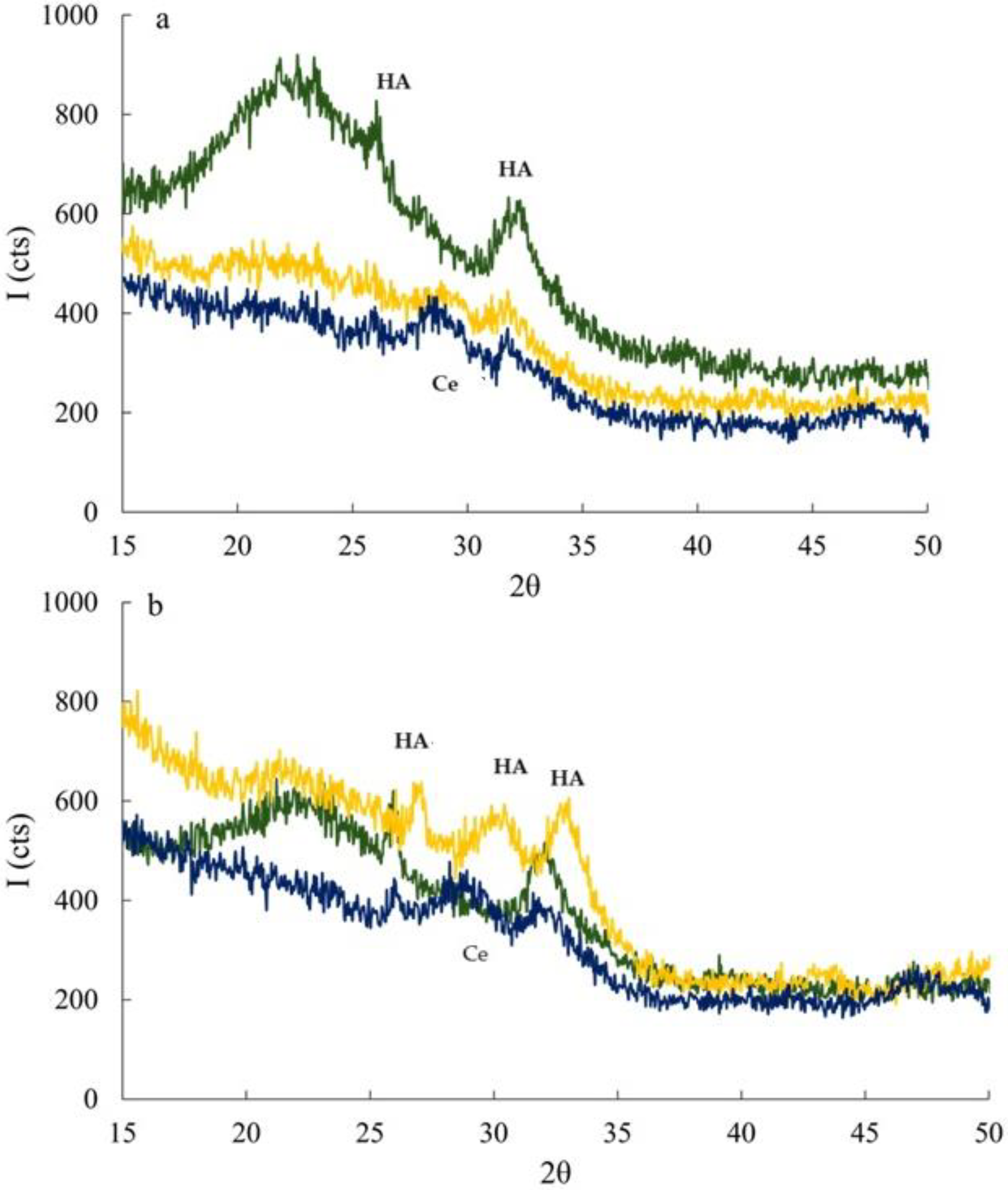
3.2. FT-IR
3.3. Antioxidant Properties
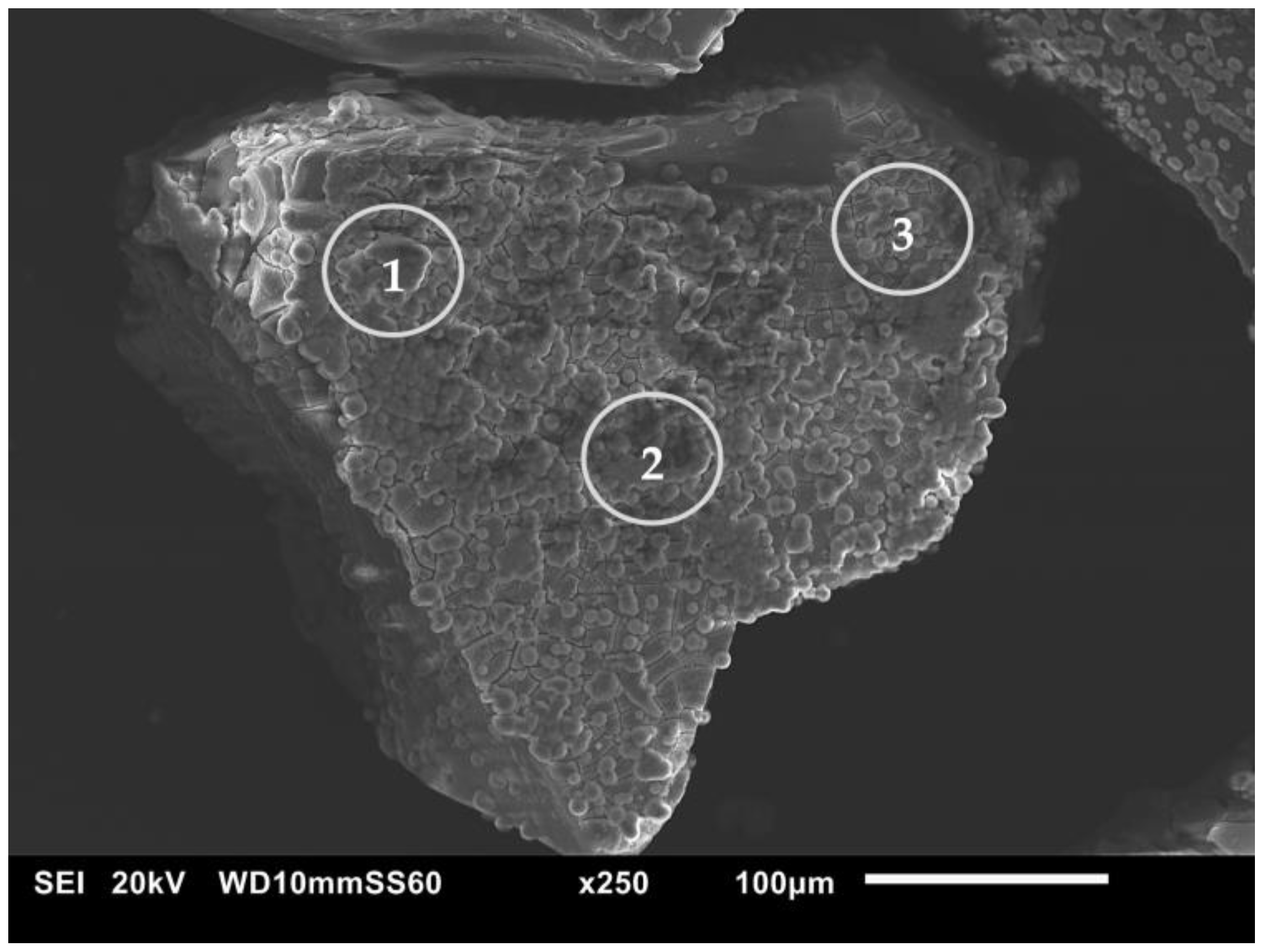
3.4. In Vitro Bioactivity Assessment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hench, L.L. Bioceramics: From Concept to Clinic. J. Am. Ceram. Soc. 1991, 74, 1487–1510. [Google Scholar] [CrossRef]
- Hoppe, A.; Güldal, N.S.; Boccaccini, A.R. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials 2011, 32, 2757–2774. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeldt, F.; Wilson, M.; Lee, G.; Kure, C.; Ou, R.; Braun, L.; de Haan, J. Oxidative Stress in Surgery in an Ageing Population: Pathophysiology and Therapy. Exp. Gerontol. 2013, 48, 45–54. [Google Scholar] [CrossRef]
- Kelly, F.J. Oxidative Stress: Its Role in Air Pollution and Adverse Health Effects. Occup. Environ. Med. 2003, 60, 612–616. [Google Scholar] [CrossRef] [PubMed]
- Ferraris, S.; Corazzari, I.; Turci, F.; Cochis, A.; Rimondini, L.; Vernè, E. Antioxidant Activity of Silica-Based Bioactive Glasses. ACS Biomater. Sci. Eng. 2021, 7, 2309–2316. [Google Scholar] [CrossRef] [PubMed]
- Baino, F.; Hamzehlou, S.; Kargozar, S. Bioactive Glasses: Where Are We and Where Are We Going? J. Funct. Biomater. 2018, 9, 25. [Google Scholar] [CrossRef]
- Hoppe, A.; Mouriño, V.; Boccaccini, A.R. Therapeutic Inorganic Ions in Bioactive Glasses to Enhance Bone Formation and Beyond. Biomater. Sci. 2013, 1, 254–256. [Google Scholar] [CrossRef]
- Westhauser, F.; Rehder, F.; Decker, S.; Kunisch, E.; Moghaddam, A.; Zheng, K.; Boccaccini, A. Ionic Dissolution Products of Cerium-Doped Bioactive Glass Nanoparticles Promote Cellular Osteogenic Differentiation and Extracellular Matrix Formation of Human Bone Marrow Derived Mesenchymal Stromal Cells. Biomed. Mater. 2021, 16, 035028. [Google Scholar] [CrossRef]
- Zambon, A.; Malavasi, G.; Pallini, A.; Fraulini, F.; Lusvardi, G. Cerium Containing Bioactive Glasses: A Review. ACS Biomater. Sci. Eng. 2021, 7, 4388–4401. [Google Scholar] [CrossRef]
- Nicolini, V.; Gambuzzi, E.; Malavasi, G.; Menabue, L.; Menziani, M.C.; Lusvardi, G.; Pedone, A.; Benedetti, F.; Luches, P.; D’Addato, S.; et al. Evidence of Catalase Mimetic Activity in Ce3+/Ce4+ Doped Bioactive Glasses. J. Phys. Chem. B 2015, 119, 4009–4019. [Google Scholar] [CrossRef]
- Lusvardi, G.; Fraulini, F.; D’Addato, S.; Zambon, A. Loading with Biomolecules Modulates the Antioxidant Activity of Cerium-Doped Bioactive Glasses. ACS Biomater. Sci. Eng. 2022, 8, 2890–2898. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Torre, E.; Bari, A.; Taccardi, N.; Cassinelli, C.; Morra, M.; Fiorilli, S.; Vitale-Brovarone, C.; Iviglia, G.; Boccaccini, A.R. Antioxidant Mesoporous Ce-Doped Bioactive Glass Nanoparticles with Anti-Inflammatory and pro-Osteogenic Activities. Mater. Today Bio 2020, 5, 100041. [Google Scholar] [CrossRef] [PubMed]
- Nicolini, V.; Malavasi, G.; Lusvardi, G.; Zambon, A.; Benedetti, F.; Cerrato, G.; Valeri, S.; Luches, P. Mesoporous Bioactive Glasses Doped with Cerium: Investigation over Enzymatic-like Mimetic Activities and Bioactivity. Ceram. Int. 2019, 45, 20910–20920. [Google Scholar] [CrossRef]
- Migneco, C.; Fiume, E.; Verné, E.; Baino, F. A Guided Walk through the World of Mesoporous Bioactive Glasses (MBGs): Fundamentals, Processing, and Applications. Nanomaterials 2020, 10, 2571. [Google Scholar] [CrossRef] [PubMed]
- Baino, F.; Fiorilli, S.; Mortera, R.; Onida, B.; Saino, E.; Visai, L.; Verné, E.; Vitale-Brovarone, C. Mesoporous Bioactive Glass as a Multifunctional System for Bone Regeneration and Controlled Drug Release. J. Appl. Biomater. Funct. Mater. 2012, 10, 12–21. [Google Scholar] [CrossRef]
- Vallet-Regí, M. Ordered Mesoporous Materials in the Context of Drug Delivery Systems and Bone Tissue Engineering. Chem. Eur. J. 2006, 12, 5934–5943. [Google Scholar] [CrossRef]
- Xia, W.; Chang, J. Well-Ordered Mesoporous Bioactive Glasses (MBG): A Promising Bioactive Drug Delivery System. J. Control. Release 2006, 110, 522–530. [Google Scholar] [CrossRef]
- Hum, J.; Boccaccini, A.R. Bioactive Glasses as Carriers for Bioactive Molecules and Therapeutic Drugs: A Review. J. Mater. Sci. Mater. Med. 2012, 23, 2317–2333. [Google Scholar] [CrossRef]
- Leonelli, C.; Lusvardi, G.; Malavasi, G.; Menabue, L.; Tonelli, M. Synthesis and Characterization of Cerium-Doped Glasses and in Vitro Evaluation of Bioactivity. J. Non Cryst. Solids 2003, 316, 198–216. [Google Scholar] [CrossRef]
- Fraulini, F.; Raimondi, S.; Candeliere, F.; Ranieri, R.; Zambon, A.; Lusvardi, G. Ce-MBGs Loaded with Gentamicin: Characterization and in Vitro Evaluation. J. Funct. Biomater. 2023, 14, 129. [Google Scholar] [CrossRef]
- Raimondi, S.; Zambon, A.; Ranieri, R.; Fraulini, F.; Amaretti, A.; Rossi, M.; Lusvardi, G. Investigation on the Antimicrobial Properties of Cerium-Doped Bioactive Glasses. J. Biomed. Mater. Res. A 2022, 110, 504–508. [Google Scholar] [CrossRef] [PubMed]
- Torre, E.; Iviglia, G.; Cassinelli, C.; Morra, M. Potentials of Polyphenols in Bone-Implant Devices. In Polyphenols; Wong, J., Ed.; IntechOpen: Rijeka, Croatia, 2018; Chapter 4; ISBN 978-1-78923-425-1. [Google Scholar]
- Zambon, A.; Fraulini, F.; Raimondi, S.; Lusvardi, G. Dual Loaded Ce-MBGs with Bioactivity, Antioxidant and Antibacterial Properties. Ceram. Int. 2023, 49, 30875–30880. [Google Scholar] [CrossRef]
- Cazzola, M.; Vernè, E.; Cochis, A.; Sorrentino, R.; Azzimonti, B.; Prenesti, E.; Rimondini, L.; Ferraris, S. Bioactive Glasses Functionalized with Polyphenols: In Vitro Interactions with Healthy and Cancerous Osteoblast Cells. J. Mater. Sci. 2017, 52, 9211–9223. [Google Scholar] [CrossRef]
- Riccucci, G.; Cazzola, M.; Ferraris, S.; Gobbo, V.A.; Miola, M.; Bosso, A.; Örlygsson, G.; Ng, C.H.; Verné, E.; Spriano, S. Surface Functionalization of Bioactive Glasses and Hydroxyapatite with Polyphenols from Organic Red Grape Pomace. J. Am. Ceram. Soc. 2022, 105, 1697–1710. [Google Scholar] [CrossRef]
- Butun, B.; Topcu, G.; Ozturk, T. Recent Advances on 3-Hydroxyflavone Derivatives: Structures and Properties. Mini-Rev. Med. Chem. 2017, 17, 1. [Google Scholar] [CrossRef]
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouységu, L. Plant Polyphenols: Chemical Properties, Biological Activities, and Synthesis. Angew. Chem. Int. Ed. 2011, 50, 586–621. [Google Scholar] [CrossRef]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.; Wang, S.; Liu, H.; Yin, Y. Quercetin, Inflammation and Immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef]
- Ali, S.; Wang, X.; Yan, H.-C. Morin Hydrate: A Comprehensive Review on Novel Natural Dietary Bioactive Compound with Versatile Biological and Pharmacological Potential. Biomed. Pharmacother. 2021, 13, 111511. [Google Scholar] [CrossRef]
- Varini, E.; Sánchez-Salcedo, S.; Malavasi, G.; Lusvardi, G.; Vallet-Regí, M.; Salinas, A.J. Cerium (III) and (IV) Containing Mesoporous Glasses/Alginate Beads for Bone Regeneration: Bioactivity, Biocompatibility and Reactive Oxygen Species Activity. Mater. Sci. Eng. C 2019, 105, 109971. [Google Scholar] [CrossRef]
- Lusvardi, G.; Fraulini, F.; Cavazzoli, C.; Zambon, A. Evaluation of the behaviour of hydrogels containing mesoporous glasses doped with cerium and loaded with polyphenols. Ceram. Int. 2024, 50, 33937–33945. [Google Scholar] [CrossRef]
- Verné, E.; Vitale-Brovarone, C.; Bui, E.; Bianchi, C.L.; Boccaccini, A.R. Surface Functionalization of Bioactive Glasses. J. Biomed. Mater. Res. A 2009, 90A, 981–992. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ferraris, S.; Prenesti, E.; Verné, E. Surface Functionalization of Bioactive Glasses with Natural Molecules of Biological Significance, Part I: Gallic Acid as Model Molecule. Appl. Surf. Sci. 2013, 287, 329–340. [Google Scholar] [CrossRef]
- Zhang, X.; Ferraris, S.; Prenesti, E.; Verné, E. Surface Functionalization of Bioactive Glasses with Natural Molecules of Biological Significance, Part II: Grafting of Polyphenols Extracted from Grape Skin. Appl. Surf. Sci. 2013, 287, 341–348. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Karakoti, A.; Singh, S.; Dowding, J.M.; Seal, S.; Self, W.T. Redox-Active Radical Scavenging Nanomaterials. Chem. Soc. Rev. 2010, 39, 4422–4432. [Google Scholar] [CrossRef]
- Gulcin, İ.; Alwasel, S.H. DPPH Radical Scavenging Assay. Processes 2023, 11, 2248. [Google Scholar] [CrossRef]
- Xie, J.; Schaich, K.M. Re-Evaluation of the 2,2-Diphenyl-1-Picrylhydrazyl Free Radical (DPPH) Assay for Antioxidant Activity. J. Agric. Food Chem. 2014, 62, 4251–4260. [Google Scholar] [CrossRef]
- Foti, M.C. Use and Abuse of the DPPH• Radical. J. Agric. Food Chem. 2015, 63, 8765–8776. [Google Scholar] [CrossRef]
- Maçon, A.L.B.; Kim, T.; Valliant, E.; Goetschius, K.; Brow, R.; Day, D.; Hoppe, A.; Boccaccini, A.; Kim, I.; Ohtsuki, C.; et al. A Unified in Vitro Evaluation for Apatite-Forming Ability of Bioactive Glasses and Their Variants. J. Mater. Sci. Mater. Med. 2015, 26, 5403. [Google Scholar] [CrossRef]
- Kokubo, T.; Kushitani, H.; Sakka, S.; Kitsugi, T.; Yamamuro, T. Solutions Able to Reproduce in Vivo Surface-Structure Changes in Bioactive Glass-Ceramic A-W3. J. Biomed. Mater. Res. 1990, 24, 721–734. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How Useful Is SBF in Predicting in Vivo Bone Bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.M.; Al-Rashidy, Z.M. Synergistic Effect of Cerium and Structure Directing Agent on Drug Release Behavior and Kinetics. J. Solgel Sci. Technol. 2023, 105, 430–442. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Chan, P.T.; Ho, K.Y.; Fung, K.P.; Wang, J. Antioxidant Activity of Natural Flavonoids Is Governed by Number and Location of Their Aromatic Hydroxyl Groups. Chem. Phys. Lipids 1996, 79, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Caroli, C.; Baron, G.; Cappellucci, G.; Brighenti, V.; Della Vedova, L.; Fraulini, F.; Oliaro-Bosso, S.; Alessandrini, A.; Zambon, A.; Lusvardi, G.; et al. Extraction, Purification and in Vitro Assessment of the Antioxidant and Anti-Inflammatory Activity of Policosanols from Non-Psychoactive Cannabis sativa L. Heliyon 2024, 10, e30291. [Google Scholar] [CrossRef]
- Dudonné, S.; Vitrac, X.; Coutière, P.; Woillez, M.; Mérillon, J.-M. Comparative Study of Antioxidant Properties and Total Phenolic Content of 30 Plant Extracts of Industrial Interest Using DPPH, ABTS, FRAP, SOD, and ORAC Assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef]
- Gates-Rector, S.; Blanton, T. The Powder Diffraction File: A Quality Materials Characterization Database. Powder Diffr. 2019, 34, 352–360. [Google Scholar] [CrossRef]
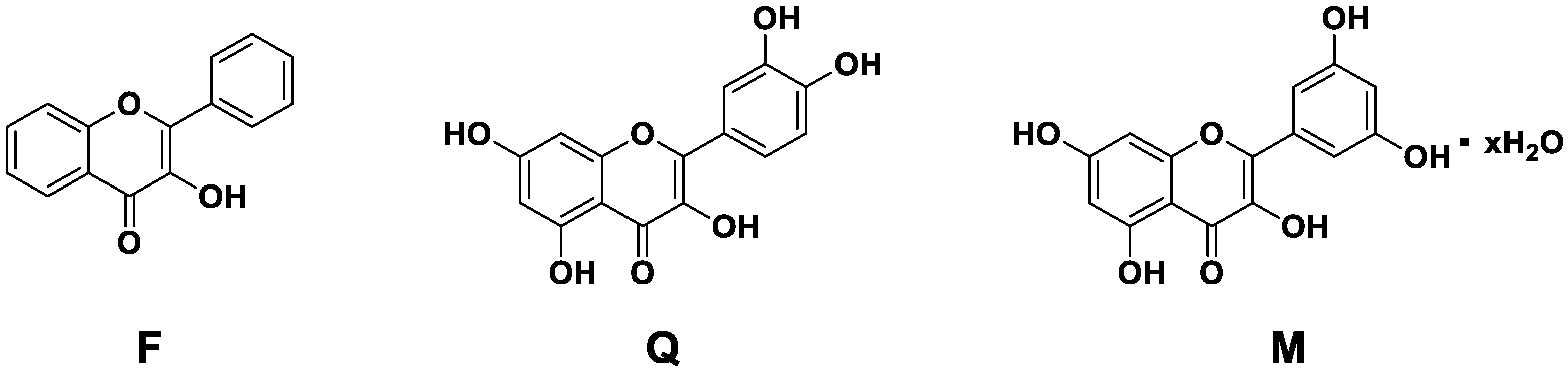
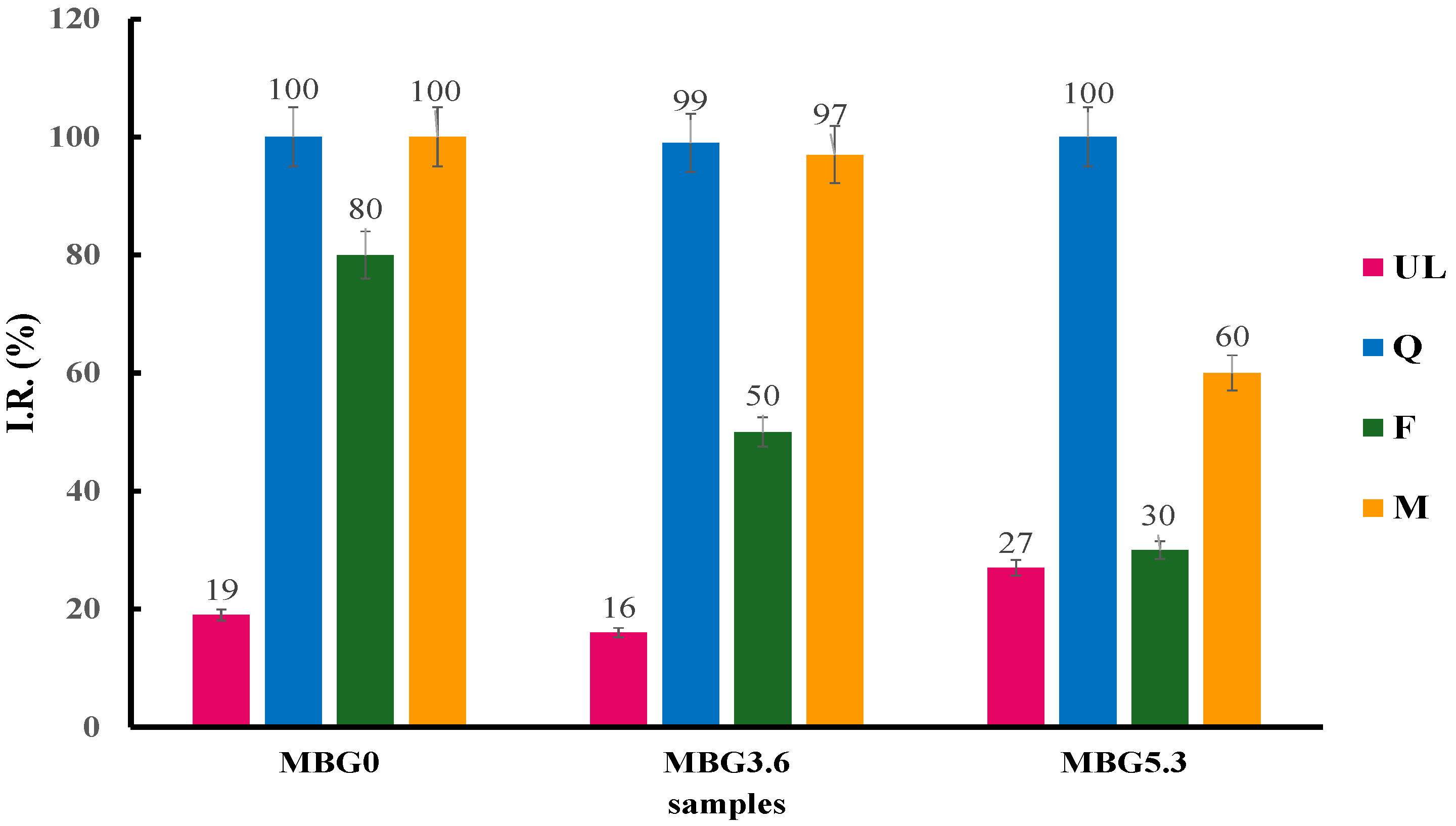
| MBGsCe | SiO2 | CaO | P2O5 | CeO2 |
|---|---|---|---|---|
| MBG0 | 80 | 15 | 5 | - |
| MBG3.6 | 77.1 | 14.5 | 4.8 | 3.6 |
| MBG5.3 | 75.8 | 14.2 | 4.7 | 5.3 |
| MBGsCepoly | LC (%) | LE (%) |
|---|---|---|
| MBG0Q | 2.0 | 39.6 |
| MBG3.6Q | 1.2 | 23.2 |
| MBG5.3Q | 1.0 | 19.5 |
| MBG0M | 1.1 | 21.7 |
| MBG3.6M | 0.5 | 10.3 |
| MBG5.3M | 0.6 | 11.7 |
| MBG0F | 1.7 | 34.9 |
| MBG3.6F | 1.0 | 20.1 |
| MBG5.3F | 0.6 | 11.9 |
| MBGsCe | Wavenumber (cm−1) | |||||
|---|---|---|---|---|---|---|
| 605 | 565 | 605 | 565 | 605 | 565 | |
| MBG0 | + | + | +++ | +++ | +++ | +++ |
| MBG3.6 | + | + | ++ | ++ | +++ | +++ |
| MBG5.3 | + | + | ++ | ++ | +++ | +++ |
| MBG0Q | + | + | + | + | + | + |
| MBG3.6Q | − | − | − | − | + | + |
| MBG5.3Q | − | − | − | − | + | + |
| MBG0F | +++ | +++ | +++ | +++ | +++ | +++ |
| MBG3.6F | − | − | + | + | +++ | +++ |
| MBG5.3F | − | − | ++ | ++ | +++ | +++ |
| MBG0M | +++ | +++ | +++ | +++ | +++ | +++ |
| MBG3.6M | − | − | − | − | + | + |
| MBG5.3M | − | − | − | − | + | + |
| MBG3.6F | SiO2 | P2O5 | CaO | CeO2 |
|---|---|---|---|---|
| before | 77.1 | 4.8 | 14.5 | 3.6 |
| after (circle 1) | 2.6 | 23.6 | 73.8 | - |
| after (circle 2) | 3.9 | 20.7 | 75.4 | - |
| after (circle 3) | 3.3 | 23.3 | 73.4 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giordana, A.; Cavazzoli, C.; Fraulini, F.; Zardi, P.; Zambon, A.; Cerrato, G.; Lusvardi, G. Evaluation of the Properties of Bioactive Mesoporous Glasses Doped with Cerium and Loaded with Polyphenols. Materials 2025, 18, 709. https://doi.org/10.3390/ma18030709
Giordana A, Cavazzoli C, Fraulini F, Zardi P, Zambon A, Cerrato G, Lusvardi G. Evaluation of the Properties of Bioactive Mesoporous Glasses Doped with Cerium and Loaded with Polyphenols. Materials. 2025; 18(3):709. https://doi.org/10.3390/ma18030709
Chicago/Turabian StyleGiordana, Alessia, Chiara Cavazzoli, Francesca Fraulini, Paolo Zardi, Alfonso Zambon, Giuseppina Cerrato, and Gigliola Lusvardi. 2025. "Evaluation of the Properties of Bioactive Mesoporous Glasses Doped with Cerium and Loaded with Polyphenols" Materials 18, no. 3: 709. https://doi.org/10.3390/ma18030709
APA StyleGiordana, A., Cavazzoli, C., Fraulini, F., Zardi, P., Zambon, A., Cerrato, G., & Lusvardi, G. (2025). Evaluation of the Properties of Bioactive Mesoporous Glasses Doped with Cerium and Loaded with Polyphenols. Materials, 18(3), 709. https://doi.org/10.3390/ma18030709











