The Influence of Roughness on the Protective Layer Formation Induced by Marine Microorganisms on 5083 Aluminum Alloy
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment Setup
2.2. Electrochemical Experiment
2.3. SEM/EDX Characterization
2.4. TEM Analysis
2.5. Assessment of Protective Properties Through Pitting Corrosion Tests
2.6. Assessment of Surface Colonization by Microorganisms Using SEM/EDX
3. Results
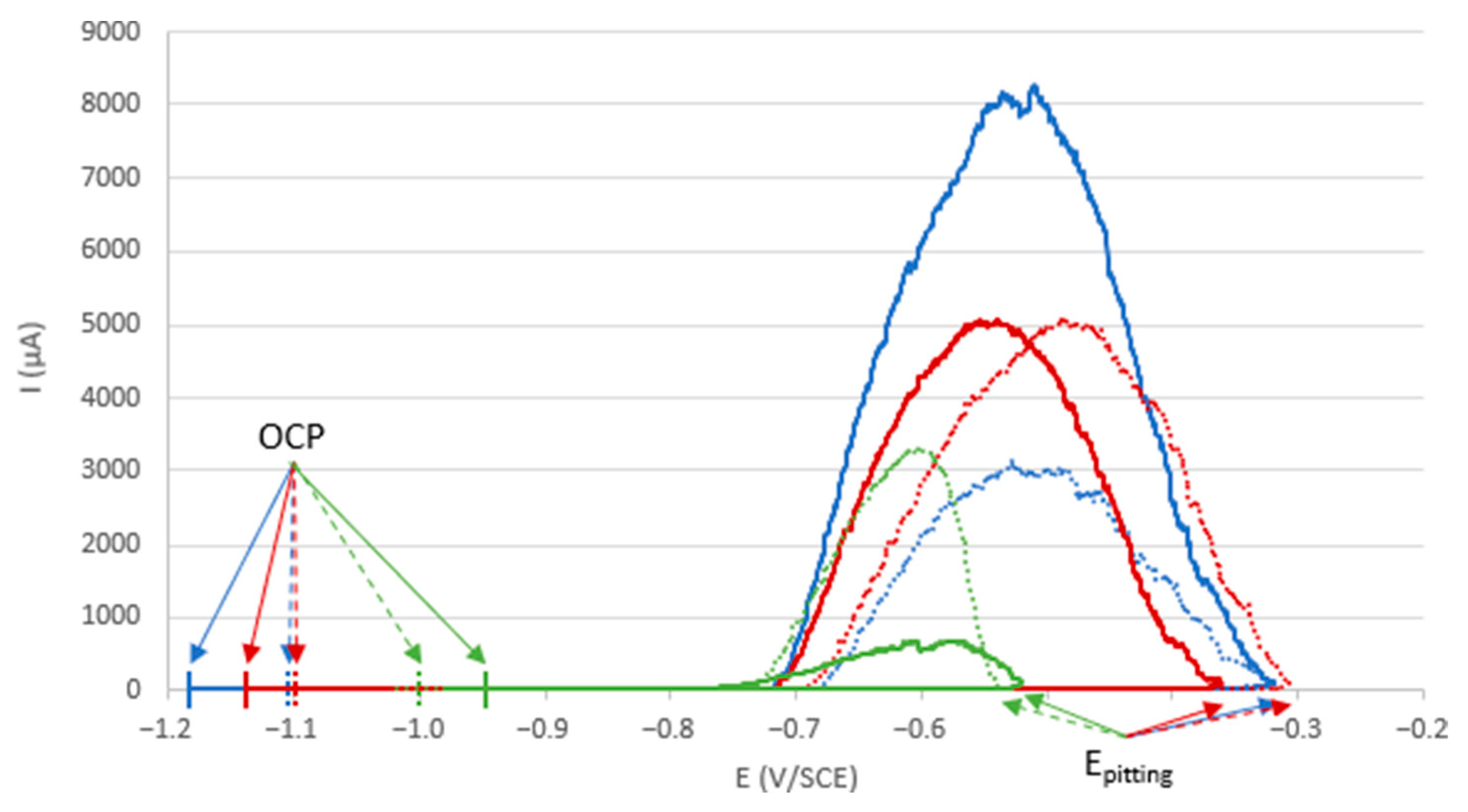
3.1. Electrochemical Study
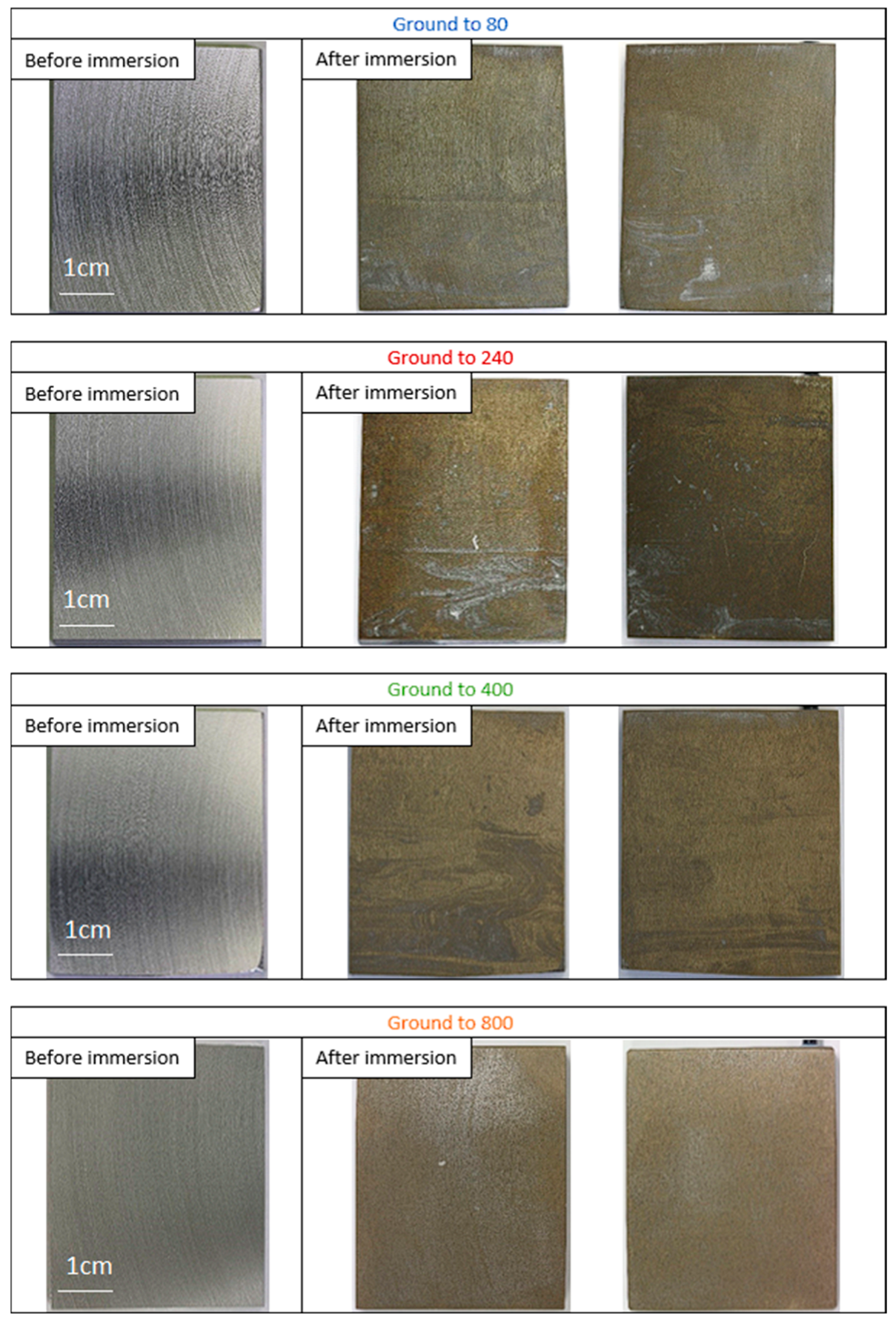
3.2. Photographs of Aluminum Alloy Electrodes
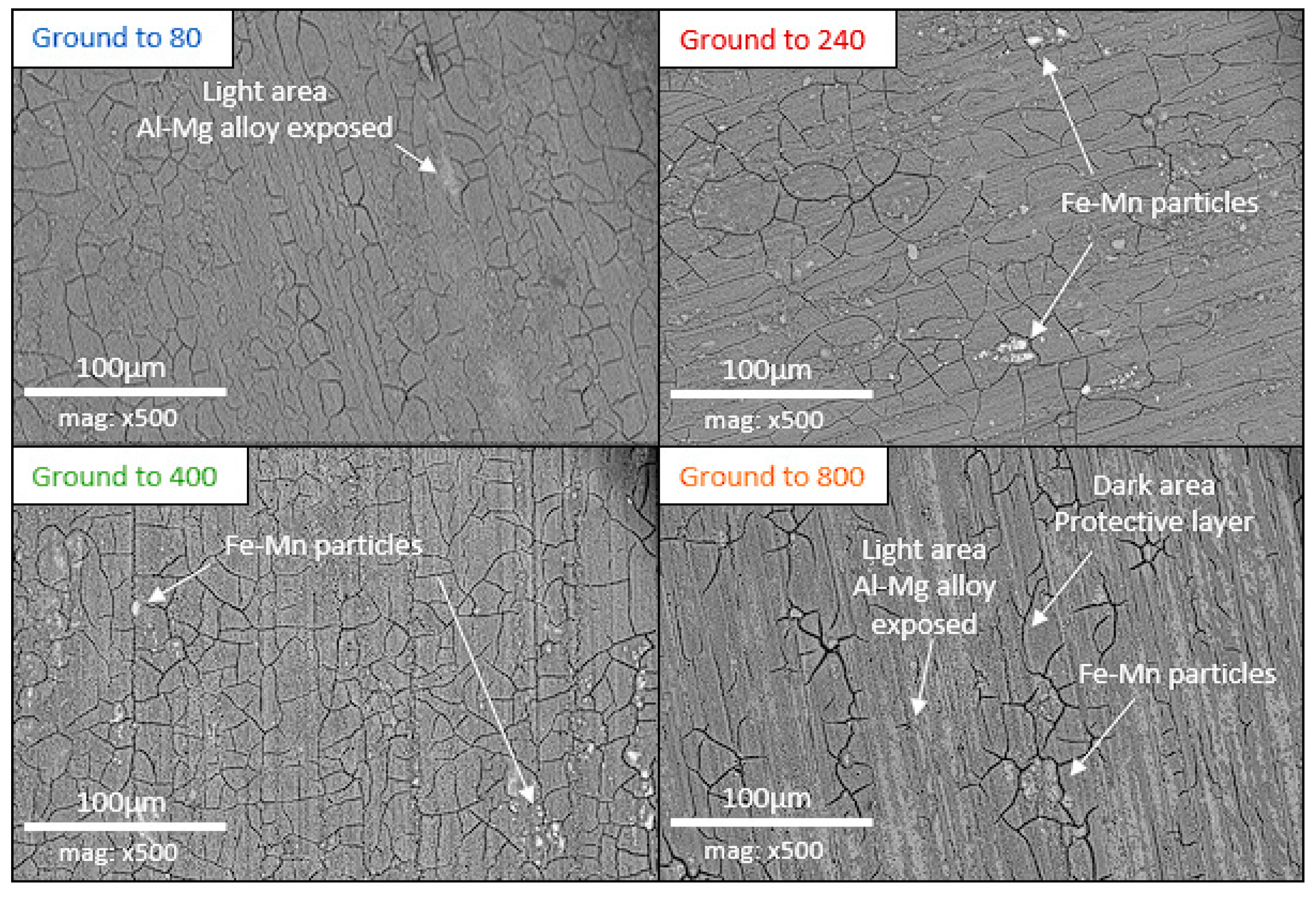
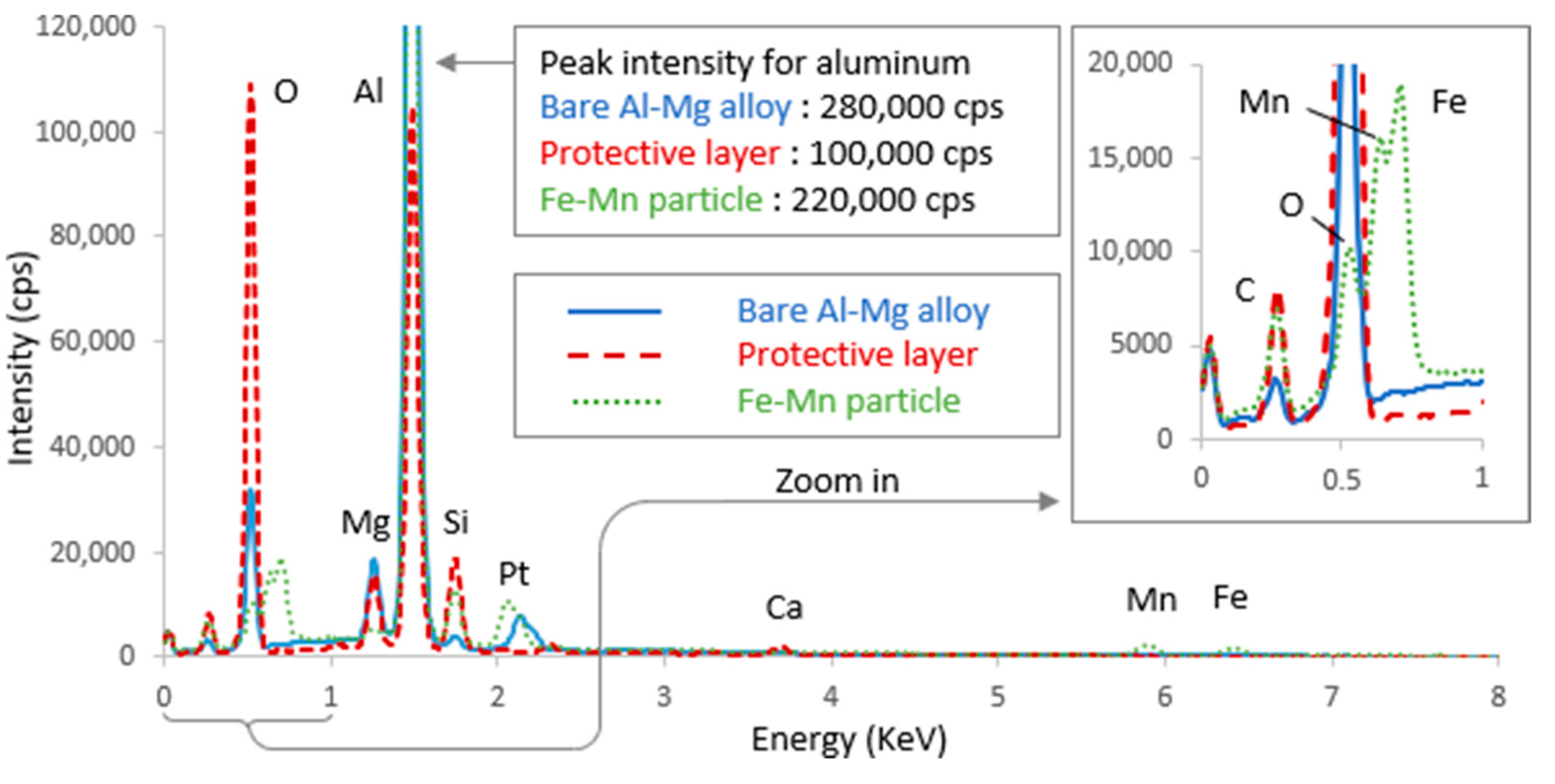
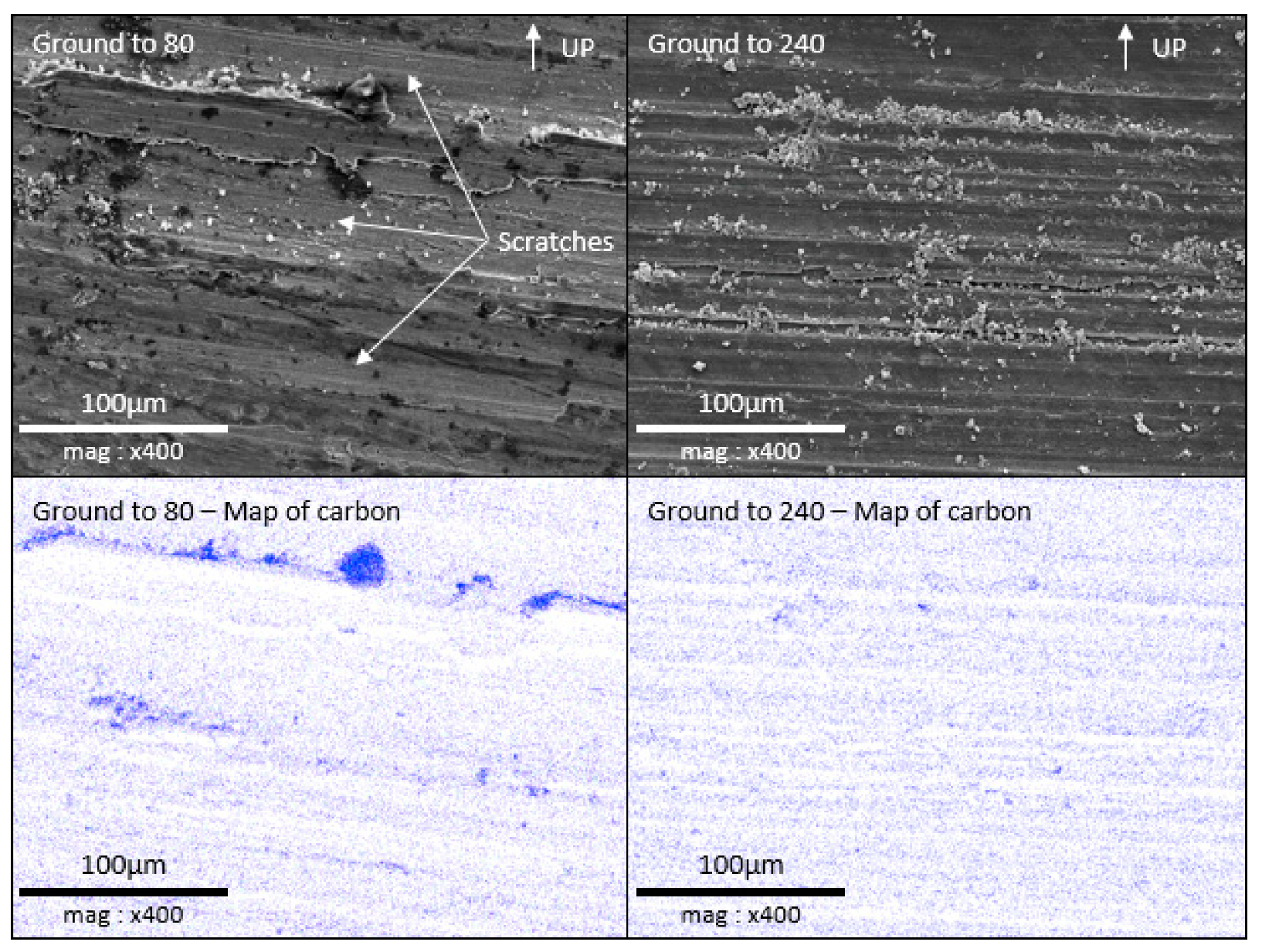
3.3. Surface SEM/EDX Characterization
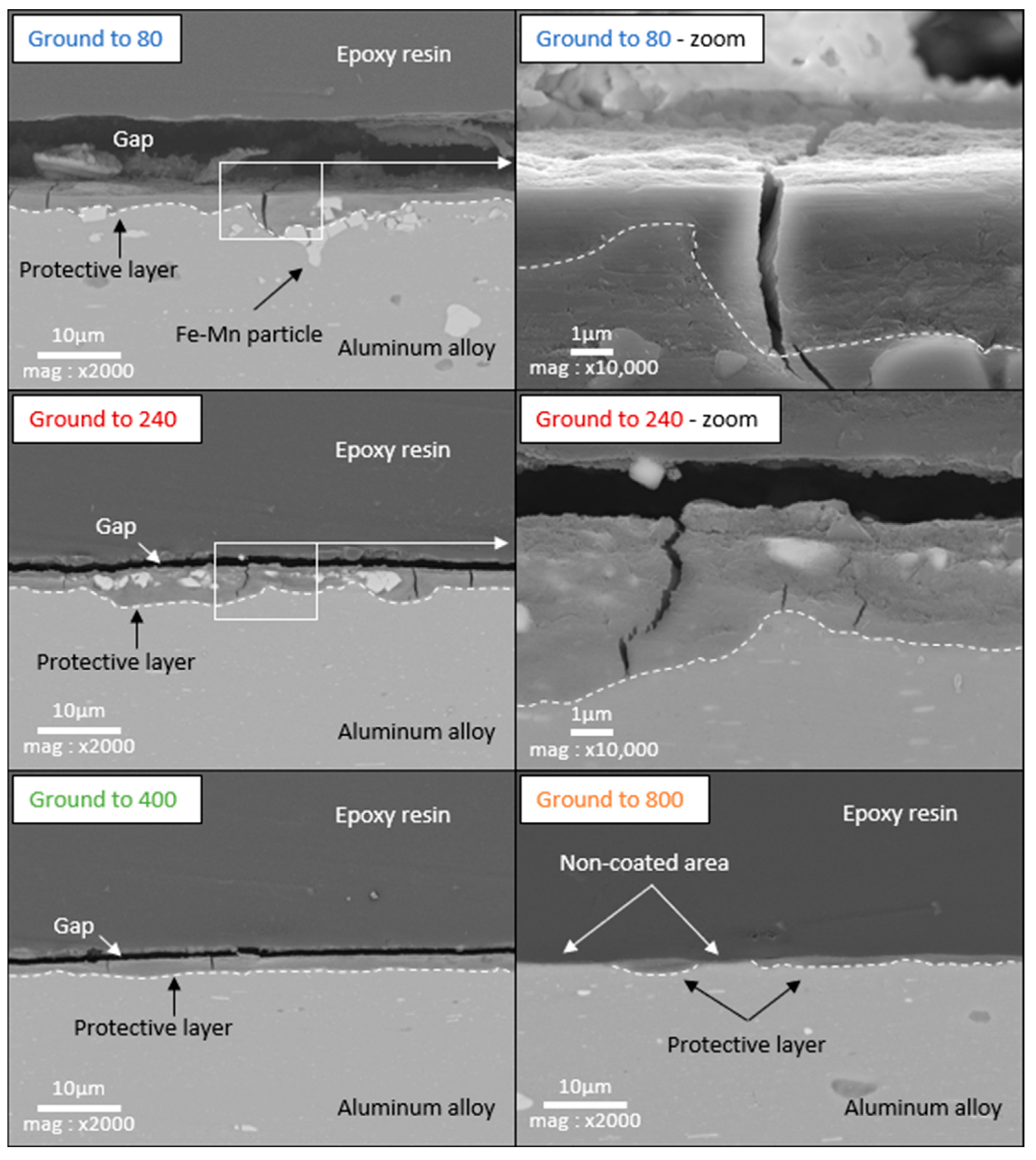
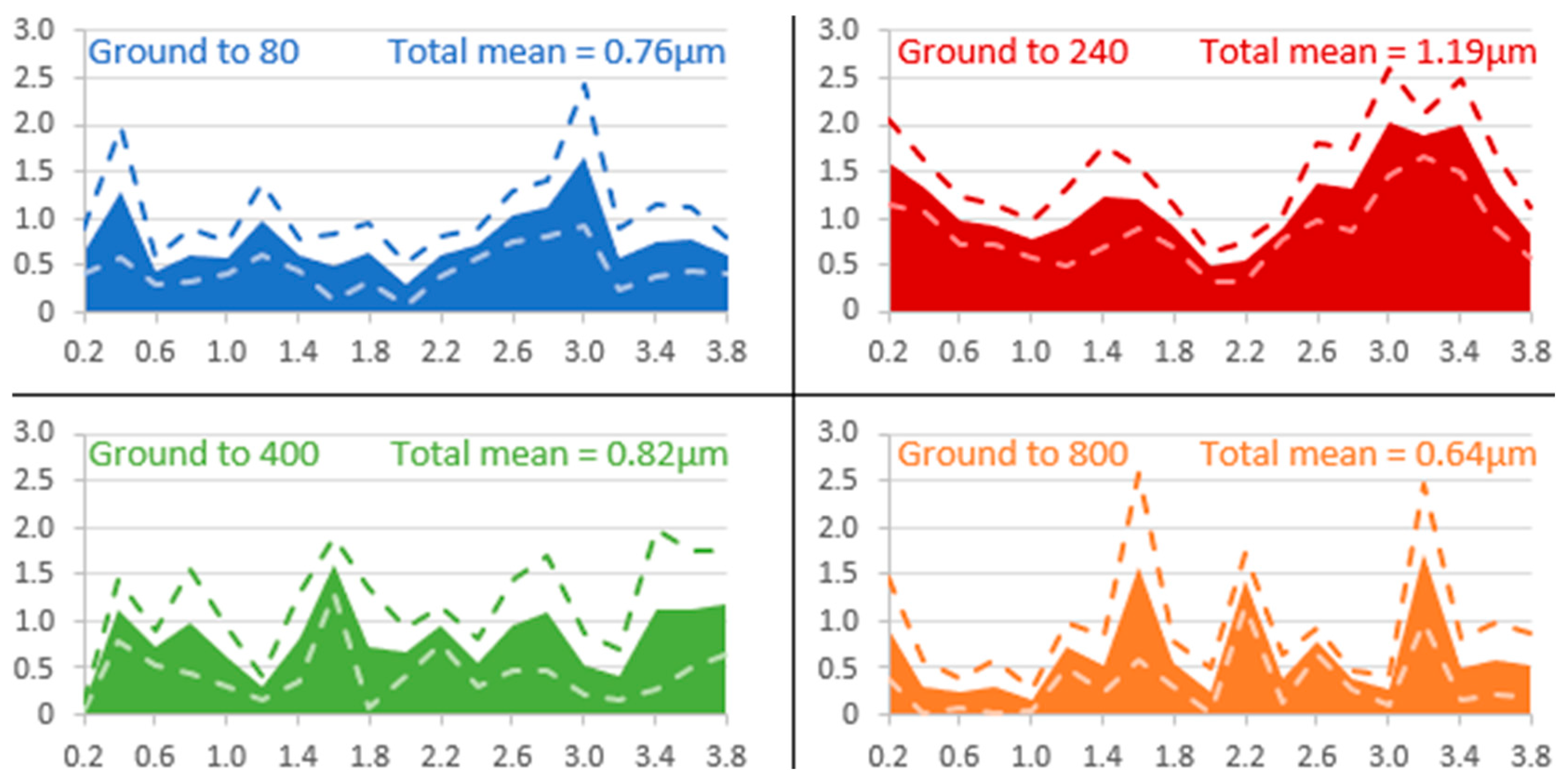
3.4. Cross-Section SEM/EDX Characterization and Thickness of the Layer
3.5. TEM Observation of the Layer
3.6. Assesment of the Protective Properties of the Formed Layer
3.7. Assessment of Surface Colonization by Microorganisms Using SEM/EDX
4. Discussion
- -
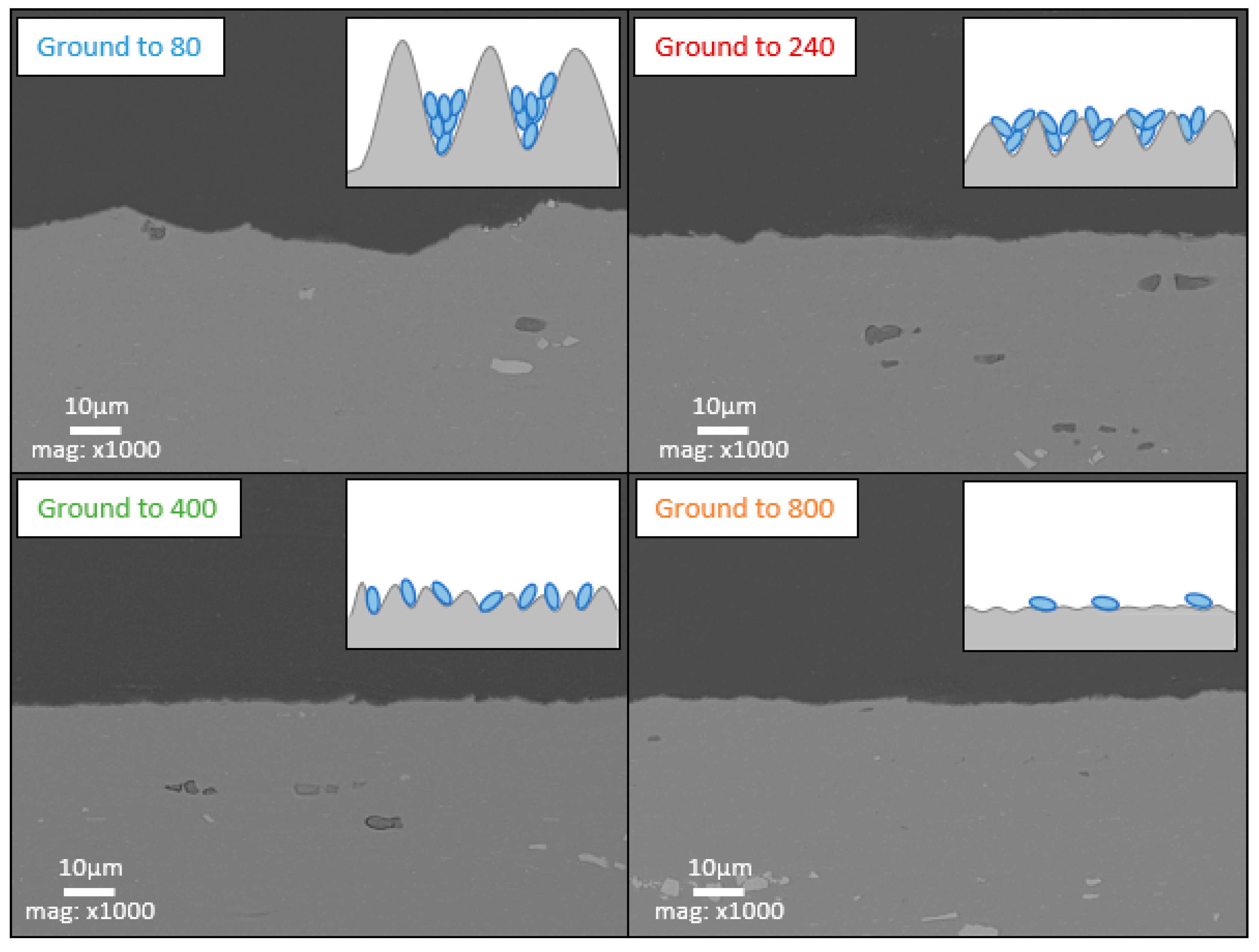
- Low roughness, ground to 800: photos taken after the immersion phase show that the 800-ground electrode presents uncoated areas (Figure 4). Indeed, after immersion, the surface is not fully covered by the layer, according to SEM observations of the surface (Figure 5) and cross-section (Figure 7). Moreover, the layer has a significantly lower average thickness than for electrodes ground to other grades (Figure 8). The low roughness obtained with the 800 grinding is therefore unfavorable for the formation of an evenly distributed protective layer.
- -
- High roughness, ground to 80: SEM observation of the surface reveals that a few areas are not covered by the layer, but these areas are much less numerous than with the 800 grinding. The average thickness is significantly lower than with the 240 grinding. Therefore, the 80 grinding does not produce the optimum roughness.
- -
- Intermediate roughness, ground to 240 and 400: according to photos and SEM observations, electrodes ground to these grades have a layer that covers their entire surface, contrary to the 80- and 800-ground electrodes. However, the thickness of the layer obtained on the 240-ground electrode is significantly higher and more homogeneously distributed (lower standard deviation) than the layer obtained on the 400-ground electrode. Moreover, the thickness of this layer is higher with the 240-ground electrode than any layer obtained with any other grinding process. Therefore, grinding to 240 provides the best initial surface condition to promote the development of the layer on the surface of the aluminum alloy. The protective nature of the layer was clearly demonstrated (Figure 10) by a twofold increase in the passive domain (both perfect and imperfect). The layer is thought to act as an anti-corrosion coating by limiting the penetration of chloride ions to the substrate below. A layer that is homogeneous in thickness and composition will be more effective in protecting the substrate underneath, as observed with the 240 ground electrodes.
4.1. Influence of Surface Roughnees on Electrochemical Behavior
4.2. Role of Microorganisms in Protective Layer Formation: Biotic vs. Abiotic Conditions
4.3. Mechanisms of Dissolution-Precipitation in Layer Formation
4.4. Bacterial Adhesion and Surface Roughness
4.5. Optimal Grinding Conditions for Protective Layer Development
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Develay, R. Propriétés de l’Aluminium et des Alliages d’Aluminium Corroyés. Techniques de l’Ingénieur, vol. Base Documentaire: TIB357DUO. In Métaux et Alliages non Ferreux. Editions T.I., 1992. [En ligne]. Available online: https://www.techniques-ingenieur.fr/base-documentaire/materiaux-th11/metaux-et-alliages-non-ferreux-42357210/proprietes-de-l-aluminium-et-des-alliages-d-aluminium-corroyes-m440/ (accessed on 20 August 2020).
- Liengen, T.; Féron, D.; Basséguy, R.; Beech, I. Understanding Biocorrosion: Fundamentals and Applications; Woodhead Publishing: Cambridge, UK, 2015; ISBN 978-1-78242-120-7. [Google Scholar] [CrossRef]
- Javaherdashti, R. Microbiologically Influenced Corrosion. In Engineering Materials and Processes; Springer: London, UK, 2008; ISBN 978-1-84800-073-5. [Google Scholar]
- Little, B.J.; Blackwood, D.J.; Hinks, J.; Lauro, F.M.; Marsili, E.; Okamoto, A.; Rice, S.A.; Wade, S.A.; Flemming, H.-C. Microbially influenced corrosion—Any progress? Corros. Sci. 2020, 170, 108641. [Google Scholar] [CrossRef]
- Telegdi, J.; Shaban, A.; Trif, L. Review on the microbiologically influenced corrosion and the function of biofilms. Int. J. Corros. Scale Inhib. 2020, 9, 1–33. [Google Scholar] [CrossRef]
- Vargel, C. Corrosion of Aluminium, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2020; ISBN 978-0-08-099925-8. [Google Scholar]
- Wahid, M.A.; Siddiquee, A.N.; Khan, Z.A. Aluminum alloys in marine construction: Characteristics, application, and problems from a fabrication viewpoint. Mar. Syst. Ocean Technol. 2020, 15, 70–80. [Google Scholar] [CrossRef]
- Yang, Y.J.; Kim, S.J. Electrochemical Characteristics of Aluminum Alloys in Sea Water for Marine Environment. Acta Phys. Pol. A 2019, 135, 1005–1011. [Google Scholar] [CrossRef]
- Hagans, P.L.; Haas, C.M. Chromate Conversion Coatings. ASM Handb. 1994, 5, 7. [Google Scholar] [CrossRef]
- Gigandet, M.-P.; Thiery, L. Traitements de Conversion sans Chrome Hexavalent. Techniques de l’Ingénieur. In Traitements de Surface des Métaux en Milieu Aqueux. Editions T.I. 2004. Available online: https://www.techniques-ingenieur.fr/base-documentaire/materiaux-th11/traitements-de-surface-des-metaux-en-milieu-aqueux-42359210/traitements-de-conversion-sans-chrome-hexavalent-m1559/ (accessed on 22 July 2021).
- Zhao, J.; Frankel, G.; McCreery, R.L. Corrosion Protection of Untreated AA-2024-T3 in Chloride Solution by a Chromate Conversion Coating Monitored with Raman Spectroscopy. J. Electrochem. Soc. 1998, 145, 2258–2264. [Google Scholar] [CrossRef]
- Commission Regulation (EU) No 301/2014 of 25 March 2014 Amending Annex XVII to Regulation (EC) No 1907/2006 of the European Parliament and of the Council on the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) as Regards Chromium VI Compounds. Mars 2014. Available online: https://eur-lex.europa.eu/eli/reg/2014/301/oj (accessed on 25 May 2021).
- Meng, Q.; Frankel, G.S. Effect of Copper Content on Chromate Conversion Coating Protection of 7xxx-T6 Aluminum Alloys. Corrosion 2004, 60, 897–905. [Google Scholar] [CrossRef]
- McCafferty, E. Sequence of steps in the pitting of aluminum by chloride ions. Corros. Sci. 2003, 45, 1421–1438. [Google Scholar] [CrossRef]
- Jaume, J.; Marques, M.J.F.; Délia, M.L.; Basséguy, R. Surface modification of 5083 Aluminum-Magnesium induced by marine microorganisms. Corros. Sci. 2021, 194, 109934. [Google Scholar] [CrossRef]
- Rimboud, M.; Barakat, M.; Achouak, W.; Bergel, A.; Délia, M.-L. Oxygen-reducing microbial cathodes in hypersaline electrolyte. Bioresour. Technol. 2020, 319, 124165. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, R.; Santaella, C.; Achouak, W.; Godon, J.-J.; Bonnafous, A.; Bergel, A.; Délia, M.-L. Correlation of the Electrochemical Kinetics of High-Salinity-Tolerant Bioanodes with the Structure and Microbial Composition of the Biofilm. ChemElectroChem 2014, 1, 1966–1975. [Google Scholar] [CrossRef]
- Nkoua, C.; Josse, C.; Proietti, A.; Basseguy, R.; Blanc, C. Corrosion behaviour of the microbially modified surface of 5083 aluminium alloy. Corros. Sci. 2023, 210, 110812. [Google Scholar] [CrossRef]
- Marques, M.J.; Jaume, J.; Diderot, A.; Délia, M.-L.; Basséguy, R. Interactions between marine microorganisms and metal: The start point of a new bioinspired solution for corrosion protection. Matériaux Tech. 2022, 110, 603. [Google Scholar] [CrossRef]
- Marques, M.J.F.; Jaume, J.; Mercier, D.; Seyeux, A.; Zanna, S.; Basseguy, R.; Marcus, P. The Positive impact of biomineralization for marine corrosion protection of AA5083 alloy. Corros. Sci. 2024, 233, 112053. [Google Scholar] [CrossRef]
- Marques, M.J.F.; Alves, I.N.; Gonçalves, R.P.; Diamantino, T.C. AA 5083 Al Alloy Corrosion in Estuarine Environment. In Proceedings of the Poster at Eurocorr 2013, Estoril, Portugal, 1–5 September 2013. [Google Scholar]
- Marques, M.J.F.; Benedetti, A.; Castelli, F.; Delucchi, M.; Faimali, M.; Delsante, S.; Valenza, F.; Garaventa, F.; Pavanello, G.; Basseguy, R. Influence of natural seawater variables on the corrosion behaviour of aluminium-magnesium alloy. Bioelectrochemistry 2023, 149, 108321. [Google Scholar] [CrossRef] [PubMed]
- Almansour, A.; Azizi, M.; Jesri, A.; Entakly, S. Effect of Surface Roughness on Corrosion behavior of Aluminum Alloy 6061 in Salt Solution (3.5%NaCl). Int. J. Acad. Sci. Res. 2015, 3, 37–45. [Google Scholar]
- Champigneux, P.; Delia, M.-L.; Bergel, A. Impact of electrode micro- and nano-scale topography on the formation and performance of microbial electrodes. Biosens. Bioelectron. 2018, 118, 231–246. [Google Scholar] [CrossRef]
- Dumas, C.; Basseguy, R.; Bergel, A. DSA to grow electrochemically active biofilms of Geobacter sulfurreducens. Electrochimica Acta 2008, 53, 3200–3209. [Google Scholar] [CrossRef]
- Characklis, W.G.; Marshall, K.C. (Eds.) Biofilms; Wiley series in ecological and applied microbiology; Wiley: New York, NY, USA, 1990; ISBN 978-0-471-82663-7. [Google Scholar]
- Flint, S.H.; Brooks, J.D.; Bremer, P.J. Properties of the stainless steel substrate, influencing the adhesion of thermo-resistant streptococci. J. Food Eng. 2000, 43, 235–242. [Google Scholar] [CrossRef]
- Helbig, R.; Günther, D.; Friedrichs, J.; Rößler, F.; Lasagni, A.; Werner, C. The impact of structure dimensions on initial bacterial adhesion. Biomater. Sci. 2016, 4, 1074–1078. [Google Scholar] [CrossRef]
- Medilanski, E.; Kaufmann, K.; Wick, L.Y.; Wanner, O.; Harms, H. Influence of the Surface Topography of Stainless Steel on Bacterial Adhesion. Biofouling 2002, 18, 193–203. [Google Scholar] [CrossRef]
- Ivanova, E.P.; Truong, V.K.; Webb, H.K.; Baulin, V.A.; Wang, J.Y.; Mohammodi, N.; Wang, F.; Fluke, C.; Crawford, R.J. Differential attraction and repulsion of Staphylococcus aureus and Pseudomonas aeruginosa on molecularly smooth titanium films. Sci. Rep. 2011, 1, 165. [Google Scholar] [CrossRef] [PubMed]





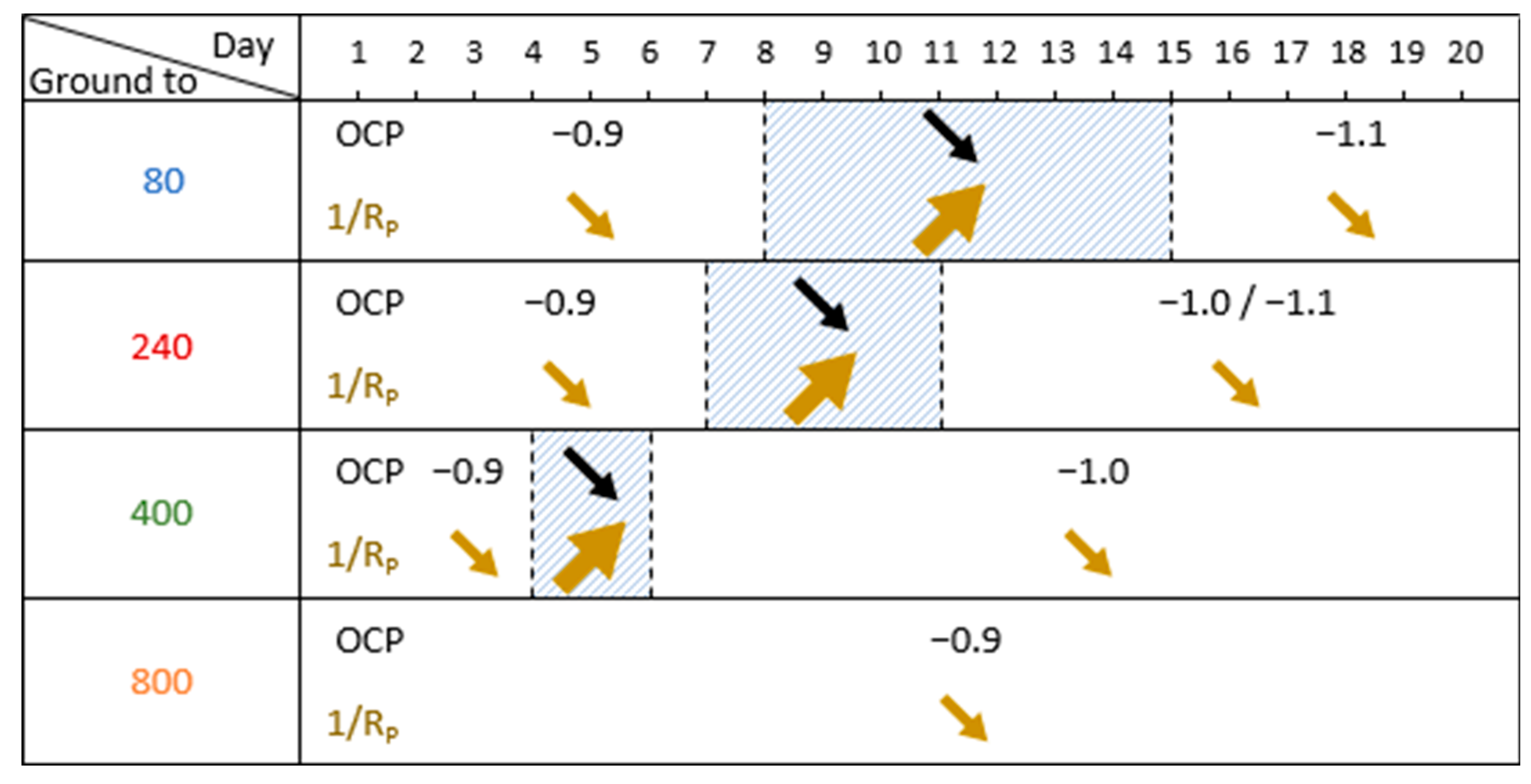
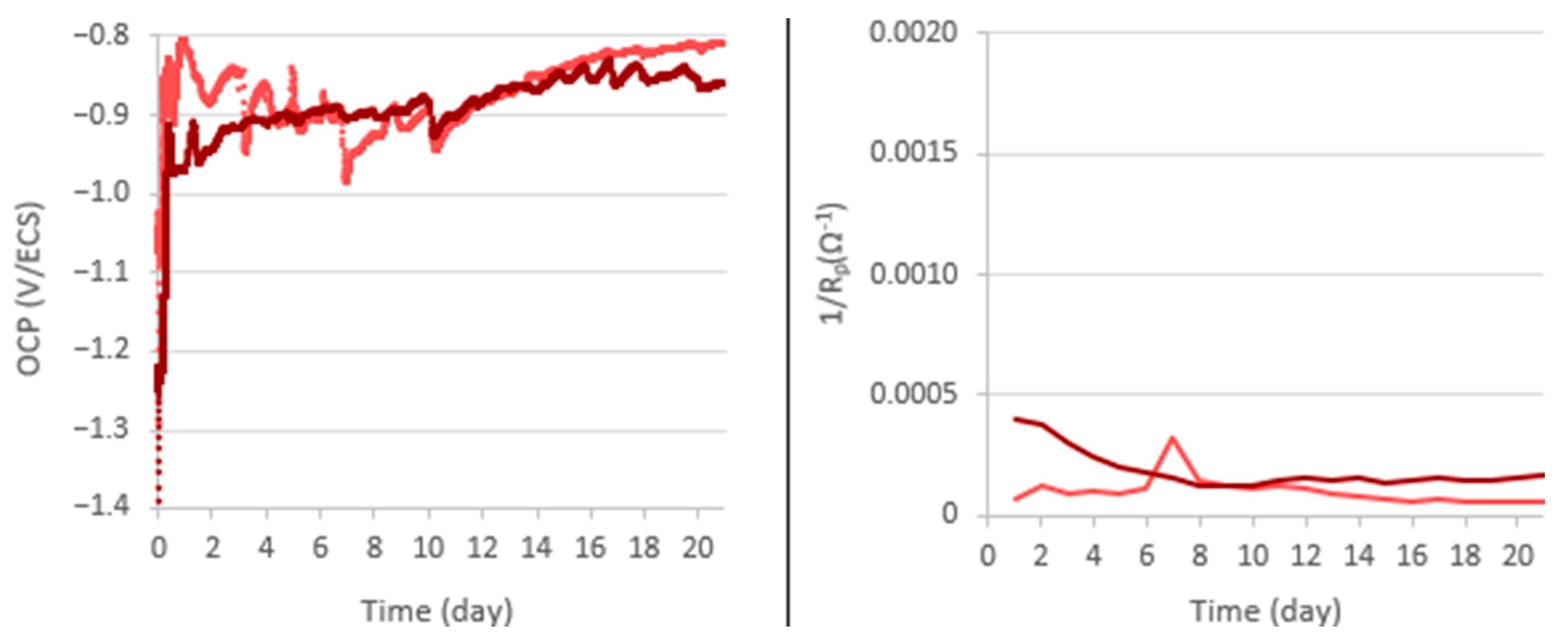
| Electrode Ground to | Transition Phase | OCP After Transition Phase (V/SCE) | |
|---|---|---|---|
| Start at | Duration (day) | ||
| 80 | 8th day | 7 | −1.1 |
| 240 | 7th day | 4 | −1.1 to −1.0 |
| 400 | 4th day | 2 | −1.0 |
| Sample | OCP (V/SCE) | Epitting (V/SCE) | Erepassivation (V/SCE) | Passivity (V) | Perfect Passivity (V) | Imperfect Passivity (V) |
|---|---|---|---|---|---|---|
| Sample 1 | −1.15 ± 0.03 | −0.31 ± 0.00 | −0.72 ± 0.04 | 0.84 ± 0.03 | 0.43 ± 0.07 | 0.41 ± 0.04 |
| Sample 2 | −1.13 ± 0.01 | −0.33 ± 0.03 | −0.73 ± 0.03 | 0.80 ± 0.04 | 0.40 ± 0.04 | 0.40 ± 0.06 |
| Sample abiotic condition | −0.99 ± 0.04 | −0.53 ± 0.01 | −0.77 ± 0.04 | 0.46 ± 0.04 | 0.22 ± 0.08 | 0.24 ± 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaume, J.; Délia, M.-L.; Basséguy, R. The Influence of Roughness on the Protective Layer Formation Induced by Marine Microorganisms on 5083 Aluminum Alloy. Materials 2025, 18, 708. https://doi.org/10.3390/ma18030708
Jaume J, Délia M-L, Basséguy R. The Influence of Roughness on the Protective Layer Formation Induced by Marine Microorganisms on 5083 Aluminum Alloy. Materials. 2025; 18(3):708. https://doi.org/10.3390/ma18030708
Chicago/Turabian StyleJaume, Julien, Marie-Line Délia, and Régine Basséguy. 2025. "The Influence of Roughness on the Protective Layer Formation Induced by Marine Microorganisms on 5083 Aluminum Alloy" Materials 18, no. 3: 708. https://doi.org/10.3390/ma18030708
APA StyleJaume, J., Délia, M.-L., & Basséguy, R. (2025). The Influence of Roughness on the Protective Layer Formation Induced by Marine Microorganisms on 5083 Aluminum Alloy. Materials, 18(3), 708. https://doi.org/10.3390/ma18030708






