Laser-Micro-Annealing of Microcrystalline Ni-Rich NCM Oxide: Towards Micro-Cathodes Integrated on Polyethylene Terephthalate Flexible Substrates
Abstract
1. Introduction
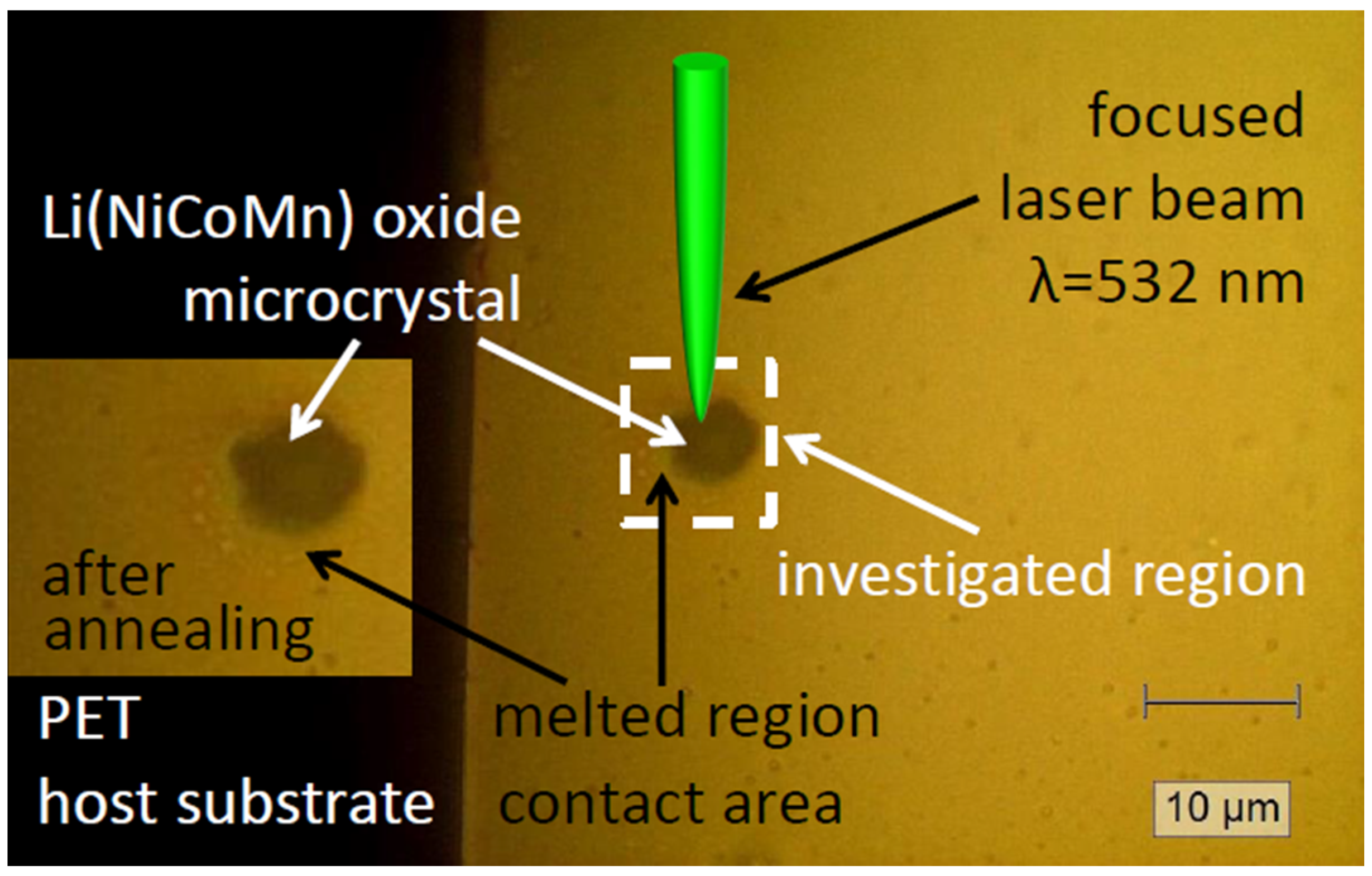
2. Materials and Methods
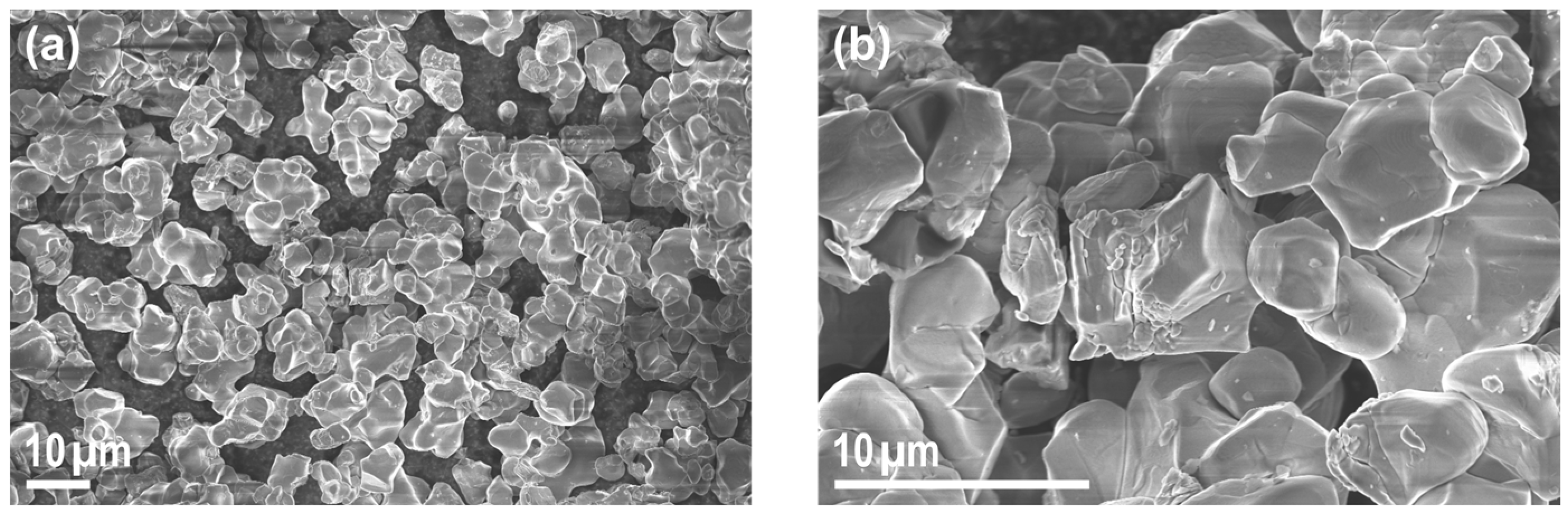
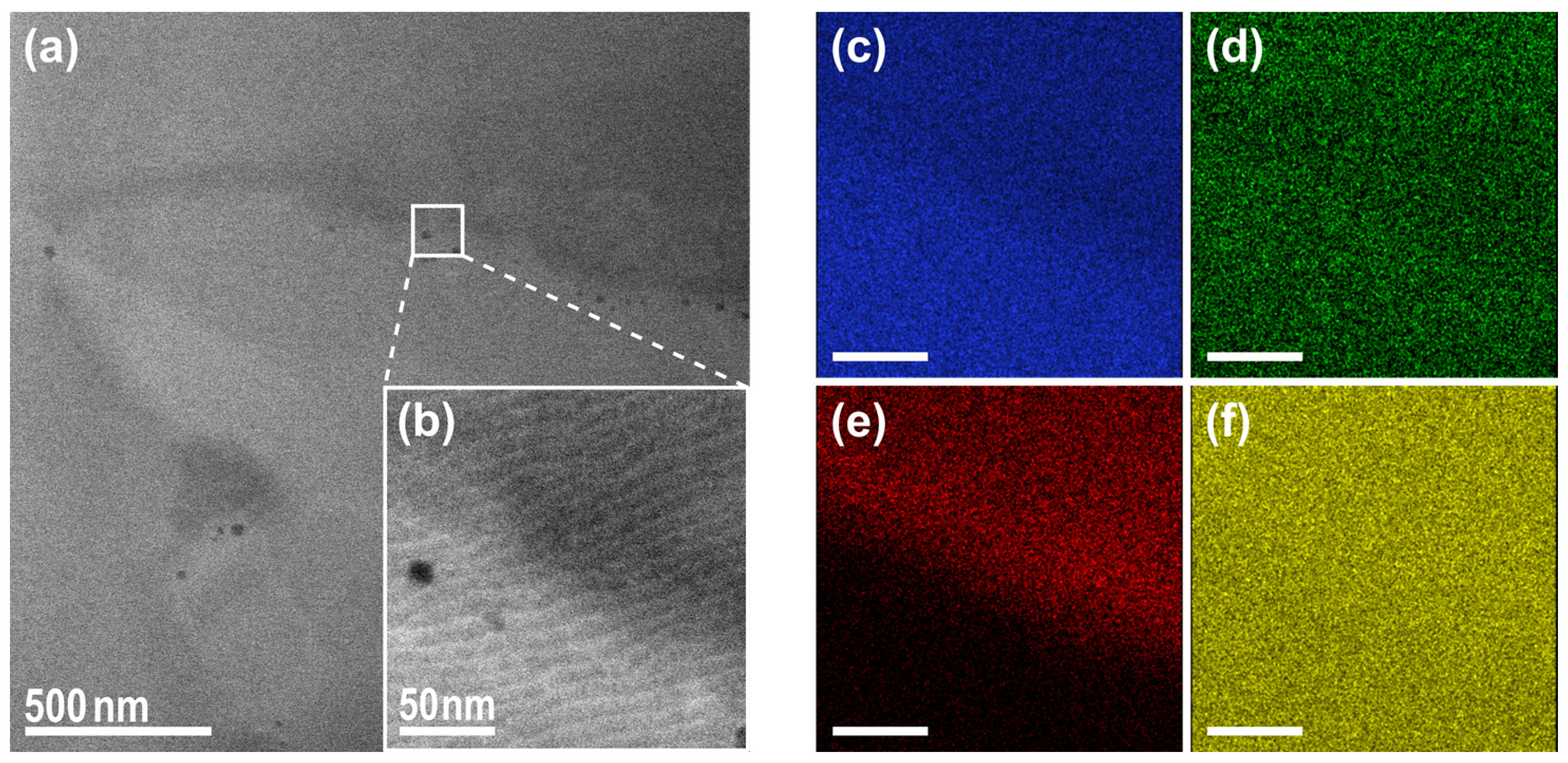
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jiang, L.Z.; Zhou, M.F.; Liu, X.N.; Qin, Q.Z. CeO2 thin film as a lithium ion-storage material fabricated by pulsed laser deposition. Acta Phys.-Chim. Sin. 1999, 15, 752–756. [Google Scholar]
- Kohler, R.; Smyrek, P.; Ulrich, S.; Bruns, M.; Trouillet, V.; Pfleging, W. Patterning and annealing of nanocrystalline LiCoO2 thin films. J. Optoelectron. Adv. Mater. 2010, 12, 547–552. [Google Scholar]
- Pröll, J.; Kohler, R.; Torge, M.; Ulrich, S.; Ziebert, C.; Bruns, M.; Seifert, H.J.; Pfleging, W. Laser microstructuring and annealing processes for lithium manganese oxide cathodes. Appl. Surf. Sci. 2011, 257, 9968–9976. [Google Scholar] [CrossRef]
- Kohler, R.; Besser, H.; Hagen, M.; Ye, J.; Ziebert, C.; Ulrich, S.; Proell, J.; Pfleging, W. Laser micro-structuring of magnetron-sputtered SnOx thin films as anode material for lithium ion batteries. Microsyst. Technol. 2011, 17, 225–232. [Google Scholar] [CrossRef]
- Kim, H. Laser-Printed and Processed LiCoO2 CathodeThick Films for Li-Ion Microbatteries. J. Laser Micro/Nanoeng. 2012, 7, 320–325. [Google Scholar] [CrossRef]
- Pröll, J.; Weidler, P.G.; Kohler, R.; Mangang, A.; Heißler, S.; Seifert, H.J.; Pfleging, W. Comparative studies of laser annealing technique and furnace annealing by X-ray diffraction and Raman analysis of lithium manganese oxide thin films for lithium-ion batteries. Thin Solid Film. 2013, 531, 160–171. [Google Scholar] [CrossRef]
- Kirchhoff, M. Laser applications in battery production—From cutting foils to welding the case 2013. In Proceedings of the 2013, 3rd International Electric Drives Production Conference (EDPC) (IEEE), Nuremberg, Germany, 29–30 October 2013; pp. 1–3. [Google Scholar]
- Pröll, J.; Kim, H.; Piqué, A.; Seifert, H.J.; Pfleging, W. Laser-printing and femtosecond-laser structuring of LiMn2O4 composite cathodes for Li-ion microbatteries. J. Power Source 2014, 255, 116–124. [Google Scholar] [CrossRef]
- Schmidt, P.A.; Schmitz, P.; Zaeh, M.F. Laser beam welding of electrical contacts for the application in stationary energy storage devices. J. Laser Appl. 2016, 28, 022423. [Google Scholar] [CrossRef]
- Lutey AH, A.; Fortunato, A.; Carmignato, S.; Fiorini, M. High speed pulsed laser cutting of LiCoO2 Li-ion battery electrodes. Opt. Laser Technol. 2017, 94, 90–96. [Google Scholar] [CrossRef]
- Habedank, J.B.; Endres, J.; Schmitz, P.; Zaeh, M.F.; Huber, H.P. Femtosecond laser structuring of graphite anodes for improved lithium-ion batteries: Ablation characteristics and process design. J. Laser Appl. 2018, 30, 032205. [Google Scholar] [CrossRef]
- Lee, D.; Oh, B.; Suk, J. The Effect of Compactness on Laser Cutting of Cathode for Lithium-Ion Batteries Using Continuous Fiber Laser. Appl. Sci. 2019, 9, 205. [Google Scholar] [CrossRef]
- Lee, D.; Suk, J. Laser cutting characteristics on uncompressed anode for lithium-ion batteries. Energies 2020, 13, 2630. [Google Scholar] [CrossRef]
- Gebrekiros Berhe, M.; Oh, H.G.; Park, S.-K.; Mondal, M.; Lee, D. Effect of laser-induced groove morphology on the wettability and performance of Lithium-ion batteries. Mater. Des. 2023, 231, 112020. [Google Scholar] [CrossRef]
- Khosla, N.; Narayan, J.; Narayan, R.; Sun, X.-G.; Paranthaman, M.P. Nanosecond Laser Annealing of NMC 811 Cathodes for Enhanced Performance. J. Electrochem. Soc. 2023, 170, 030520. [Google Scholar] [CrossRef]
- Ravesio, E.; Lutey AH, A.; Versaci, D.; Romoli, L.; Bodoardo, S. Nanosecond pulsed laser texturing of Li-ion battery electrode current collectors: Electrochemical characterisation of cathode half-cells. Sustain. Mater. Technol. 2023, 38, e00751. [Google Scholar] [CrossRef]
- Berhe, M.G.; Musse, D.; Oh, H.G.; Park, S.K.; Lee, D. Development of laser structured three-dimensional patterns for improved wettability and performance of electrodes for lithium-ion batteries. Colloids Surf. A Physicochem. Eng. Asp. 2024, 697, 134393. [Google Scholar] [CrossRef]
- Pfleging, W. A review of laser electrode processing for development and manufacturing of lithium-ion batteries. Nanophotonics 2018, 7, 549–573. [Google Scholar] [CrossRef]
- Pfleging, W. Recent progress in laser texturing of battery materials: A review of tuning electrochemical performances, related material development, and prospects for large-scale manufacturing. Int. J. Extrem. Manuf. 2021, 3, 012002. [Google Scholar] [CrossRef]
- Hausbrand, R.; Cherkashinin, G.; Ehrenberg, H.; Gröting, M.; Albe, K.; Hess, C.; Jaegermann, W. Fundamental degradation mechanisms of layered oxide Li-ion battery cathode materials: Methodology, insights and novel approaches. Mater. Sci. Eng. B 2015, 192, 3–25. [Google Scholar] [CrossRef]
- Song, Y.; Cui, Y.; Li, B.; Geng, L.; Yan, J.; Zhu, D.; Zhou, P.; Zhou, J.; Yan, Z.; Xue, Q.; et al. Revealing the origin of high-thermal-stability of single-crystal Ni-rich cathodes toward higher-safety batteries. Nano Energy 2023, 116, 108846. [Google Scholar] [CrossRef]
- Finegan, D.P.; Scheel, M.; Robinson, J.B.; Tjaden, B.; Hunt, I.; Mason, T.J.; Millichamp, J.; Di Michiel, M.; Offer, G.J.; Hinds, G.; et al. In-operando high-speed tomography of lithium-ion batteries during thermal runaway. Nat. Commun. 2015, 6, 6924. [Google Scholar] [CrossRef] [PubMed]
- Smyrek, P.; Pfleging, W. Laser materials processing in manufacturing of lithium-ion batteries. In Processing and Manufacturing of Electrodes for Lithium-Ion Batteries; Institution of Engineering and Technology: Stevenage, UK, 2023; pp. 101–127. [Google Scholar]
- Ehrhart, P. Investigation of radiation damage by X-ray diffraction. J. Nucl. Mater. 1994, 216, 170–198. [Google Scholar] [CrossRef]
- Wood, K.N.; Teeter, G. XPS on Li-Battery-Related Compounds: Analysis of Inorganic SEI Phases and a Methodology for Charge Correction. ACS Appl. Energy Mater. 2018, 1, 4493–4504. [Google Scholar] [CrossRef]
- Huang, R.; Ikuhara, Y. STEM characterization for lithium-ion battery cathode materials. Curr. Opin. Solid State Mater. Sci. 2012, 16, 31–38. [Google Scholar] [CrossRef]
- Meddings, N.; Heinrich, M.; Overney, F.; Lee, J.-S.; Ruiz, V.; Napolitano, E.; Seitz, S.; Hinds, G.; Raccichini, R.; Gaberšček, M.; et al. Application of electrochemical impedance spectroscopy to commercial Li-ion cells: A review. J. Power Source 2020, 480, 228742. [Google Scholar] [CrossRef]
- Yamakawa, N.; Jiang, M.; Key, B.; Grey, C.P. Identifying the Local Structures Formed during Lithiation of the Conversion Material, Iron Fluoride, in a Li Ion Battery: A Solid-State NMR, X-ray Diffraction, and Pair Distribution Function Analysis Study. J. Am. Chem. Soc. 2009, 131, 10525–10536. [Google Scholar] [CrossRef]
- Lanz, M.; Lehmann, E.; Imhof, R.; Exnar, I.; Novák, P. In situ neutron radiography of lithium-ion batteries during charge/discharge cycling. J. Power Source 2001, 101, 177–181. [Google Scholar] [CrossRef]
- Heber, M.; Hofmann, K.; Hess, C. Raman Diagnostics of Cathode Materials for Li-Ion Batteries Using Multi-Wavelength Excitation. Batteries 2022, 8, 10. [Google Scholar] [CrossRef]
- Flores, E.; Novák, P.; Aschauer, U.; Berg, E.J. Cation Ordering and Redox Chemistry of Layered Ni-Rich Ni-Rich LixNi1–2yCoyMnO2: An Operando Raman Spectroscopy Study. Chem. Mater. 2020, 32, 186–194. [Google Scholar] [CrossRef]
- Zhang, B.; Tekle, H.; O’Malley, R.J.; Sander, T.; Smith, J.D.; Gerald, R.E.; Huang, J. In Situ and Real-Time Mold Flux Analysis Using a High-Temperature Fiber-Optic Raman Sensor for Steel Manufacturing Applications. J. Light. Technol. 2023, 41, 4419–4429. [Google Scholar] [CrossRef]
- Ferreira, A.N.C.; Ferreira, W.C.; Duarte, A.V.; Santos, C.C.; Freire, P.T.C.; Luz-Lima, C.; Moura, J.V.B. In situ high-temperature Raman scattering study of monoclinic Ag2Mo2O7 microrods. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 295, 122632. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Tekle, H.; O’Malley, R.J.; Smith, J.D.; Gerald, R.E.; Huang, J. In Situ High-Temperature Raman Spectroscopy via a Remote Fiber-Optic Raman Probe. IEEE Trans. Instrum. Meas. 2023, 72, 1–8. [Google Scholar] [CrossRef]
- Branch, S.D.; Felmy, H.M.; Schafer Medina, A.; Bryan, S.A.; Lines, A.M. Exploring the Complex Chemistry of Uranium within Molten Chloride Salts. Ind. Eng. Chem. Res. 2023, 62, 14901–14909. [Google Scholar] [CrossRef]
- Templier, F. GaN-based emissive microdisplays: A very promising technology for compact, ultra-high brightness display systems. J. Soc. Inf. Disp. 2016, 24, 669–675. [Google Scholar] [CrossRef]
- Mikulics, M.; Arango, Y.C.; Winden, A.; Adam, R.; Hardtdegen, A.; Grützmacher, D.; Plinski, E.; Gregušová, D.; Novák, J.; Kordoš, P.; et al. Direct electro-optical pumping for hybrid CdSe nanocrystal/III-nitride based nano-light-emitting diodes. Appl. Phys. Lett. 2016, 108, 061107. [Google Scholar] [CrossRef]
- Ra, Y.-H.; Wang, R.; Woo, S.Y.; Djavid, M.; Sadaf, S.M.; Lee, J.; Botton, G.A.; Mi, Z. Full-Color Single Nanowire Pixels for Projection Displays. Nano Lett. 2016, 16, 4608–4615. [Google Scholar] [CrossRef] [PubMed]
- Krause, T.; Hanke, M.; Nicolai, L.; Cheng, Z.; Niehle, M.; Trampert, A.; Kahnt, M.; Falkenberg, G.; Schroer, C.G.; Hartmann, J.; et al. Structure and Composition of Isolated Core-Shell (In,Ga)N/GaN Rods Based on Nanofocus X-Ray Diffraction and Scanning Transmission Electron Microscopy. Phys. Rev. Appl. 2017, 7, 024033. [Google Scholar] [CrossRef]
- Wierer, J.J.; Tansu, N. III-Nitride Micro-LEDs for Efficient Emissive Displays. Laser Photon. Rev. 2019, 13, 1900141. [Google Scholar] [CrossRef]
- Franch, N.; Canals, J.; Moro, V.; Alonso, O.; Moreno, S.; Vilà, A.; Prades, J.D.; Gülink, J.; Wasisto, H.S.; Waag, A.; et al. Towards a super-resolution structured illumination microscope based on an array of nanoLEDs. In Novel Optical Systems, Methods, and Applications XXII; Hahlweg, C.F., Mulley, J.R., Eds.; SPIE: Bellingham, DC, USA, 2019; p. 23. [Google Scholar]
- Ding, K.; Avrutin, V.; Izyumskaya, N.; Özgür, Ü.; Morkoç, H. Micro-LEDs, a Manufacturability Perspective. Appl. Sci. 2019, 9, 1206. [Google Scholar] [CrossRef]
- Mikulics, M.; Hardtdegen, H.H. Fully photon operated transmistor/all-optical switch based on a layered Ge1Sb2Te4 phase change medium. FlatChem 2020, 23, 100186. [Google Scholar] [CrossRef]
- Mikulics, M.; Sofer, Z.; Winden, A.; Trellenkamp, S.; Förster, B.; Mayer, J.; Hardtdegen, H.H. Nano-LED induced chemical reactions for structuring processes. Nanoscale Adv. 2020, 2, 5421–5427. [Google Scholar] [CrossRef]
- Kishino, K.; Sakakibara, N.; Narita, K.; Oto, T. Two-dimensional multicolor (RGBY) integrated nanocolumn micro-LEDs as a fundamental technology of micro-LED display. Appl. Phys. Express 2020, 13, 014003. [Google Scholar] [CrossRef]
- Zhou, G.; Lin, R.; Qian, Z.; Zhou, X.; Shan, X.; Cui, X.; Tian, P. GaN-based micro-LEDs and detectors defined by current spreading layer: Size-dependent characteristics and their multifunctional applications. J. Phys. D. Appl. Phys. 2021, 54, 335104. [Google Scholar] [CrossRef]
- Wu, M.-C.; Chen, I.-T. High-Resolution 960 × 540 and 1920 × 1080 UV Micro Light-Emitting Diode Displays with the Application of Maskless Photolithography. Adv. Photonics Res. 2021, 2, 1–8. [Google Scholar] [CrossRef]
- Mikulics, M.; Mayer, J.; Hardtdegen, H.H. Cutting-edge nano-LED technology. J. Appl. Phys. 2022, 131, 110903. [Google Scholar] [CrossRef]
- Mikulics, M.; Adam, R.; Sobolewski, R.; Heidtfeld, S.; Cao, D.; Bürgler, D.E.; Schneider, C.M.; Mayer, J.; Hardtdegen, H.H. Nano-LED driven phase change evolution of layered chalcogenides for Raman spectroscopy investigations. FlatChem 2022, 36, 100447. [Google Scholar] [CrossRef]
- Chen, D.; Chen, Y.-C.; Zeng, G.; Zhang, D.W.; Lu, H.-L. Integration Technology of Micro-LED for Next-Generation Display. Research 2023, 6, 47. [Google Scholar] [CrossRef]
- Mikulics, M.; Kordoš, P.; Gregušová, D.; Sofer, Z.; Winden, A.; Trellenkamp, S.; Moers, J.; Mayer, J.; Hardtdegen, H. Conditioning nano-LEDs in arrays by laser-micro-annealing: The key to their performance improvement. Appl. Phys. Lett. 2021, 118, 043101. [Google Scholar] [CrossRef]
- Ruess, R.; Ulherr, M.A.; Trevisanello, E.; Schröder, S.; Henss, A.; Janek, J. Transition Metal Oxides and Li2CO3 as Precursors for the Synthesis of Ni-Rich Single-Crystalline NCM for Sustainable Lithium-Ion Battery Production. J. Electrochem. Soc. 2022, 169, 070531. [Google Scholar] [CrossRef]
- Gardner, B.; Matousek, P.; Stone, N. Direct monitoring of light mediated hyperthermia induced within mammalian tissues using surface enhanced spatially offset Raman spectroscopy (T-SESORS). Analyst 2019, 144, 3552–3555. [Google Scholar] [CrossRef]
- Bowie, B.T.; Griffiths, P.R. Determination of the Resolution of a Multichannel Raman Spectrometer Using Fourier Transform Raman Spectra. Appl. Spectrosc. 2003, 57, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Brooker, M.H.; Bates, J.B. Raman and Infrared Spectral Studies of Anhydrous Li2CO3 and Na2CO3. J. Chem. Phys. 1971, 54, 4788–4796. [Google Scholar] [CrossRef]
- Hase, Y.; Yoshida IV, P. Low frequency bands of Li2CO3 crystal. Spectrochim. Acta Part. A Mol. Spectrosc. 1979, 35, 379. [Google Scholar] [CrossRef]
- Brooker, M.H.; Wang, J. Raman and infrared studies of lithium and cesium carbonates. Spectrochim. Acta Part. A Mol. Spectrosc. 1992, 48, 999–1008. [Google Scholar] [CrossRef]
- Pasierb, P.; Komornicki, S.; Rokita, M.; Rȩkas, M. Structural properties of Li2CO3–BaCO3 system derived from IR and Raman spectroscopy. J. Mol. Struct. 2001, 596, 151–156. [Google Scholar] [CrossRef]
- Kerlau, M.; Marcinek, M.; Srinivasan, V.; Kostecki, R.M. Studies of local degradation phenomena in composite cathodes for lithium-ion batteries. Electrochim. Acta 2007, 52, 5422–5429. [Google Scholar] [CrossRef]
- Zhang, X.; Mauger, A.; Lu, Q.; Groult, H.; Perrigaud, L.; Gendron, F.; Julien, C.M. Synthesis and characterization of LiNi1/3Mn1/3Co1/3O2 by wet-chemical method. Electrochim. Acta 2010, 55, 6440–6449. [Google Scholar] [CrossRef]
- Zhao, H.; Bai, Y.; Jin, H.; Zhou, J.; Wang, X.; Wu, C. Unveiling thermal decomposition kinetics of Single-Crystalline Ni-Rich LiNi0.88Co0.07Mn0.05O2 cathode for safe Lithium-Ion batteries. Chem. Eng. J. 2022, 435, 134927. [Google Scholar] [CrossRef]
- Ruther, R.E.; Callender, A.F.; Zhou, H.; Martha, S.K.; Nanda, J. Raman Microscopy of Lithium-Manganese-Rich Transition Metal Oxide Cathodes. J. Electrochem. Soc. 2015, 162, A98–A102. [Google Scholar] [CrossRef]
- Kim, A.-Y.; Strauss, F.; Bartsch, T.; Teo, J.H.; Hatsukade, T.; Mazilkin, A.; Janek, J.; Hartmann, P.; Brezesinski, T. Stabilizing Effect of a Hybrid Surface Coating on a Ni-Rich NCM Cathode Material in All-Solid-State Batteries. Chem. Mater. 2019, 31, 9664–9672. [Google Scholar] [CrossRef]
- Mikulics, M.; Hardtdegen, H.; Adam, R.; Grützmacher, D.; Gregušová, D.; Novák, J.; Kordoš, P.; Sofer, Z.; Serafini, J.; Zhang, J.; et al. Impact of thermal annealing on nonequilibrium carrier dynamics in single-crystal, freestanding GaAs mesostructures. Semicond. Sci. Technol. 2014, 29, 045022. [Google Scholar] [CrossRef]
- Mikulics, M.; Lu, J.G.; Huang, L.; Tse, P.L.; Zhang, J.Z.; Mayer, J.; Hardtdegen, H. Laser micro annealing conditioning for the suppression of statistical scatter in freestanding Sb2Te3 nanowire resistance. FlatChem 2020, 21, 100164. [Google Scholar] [CrossRef]
- Conde, J.P.; Alpuim, P.; Chu, V. Thin-Film Transistors on PET at 100 °C. MRS Proc. 2002, 715, A3.1. [Google Scholar] [CrossRef]
- Paetzold, R.; Heuser, K.; Henseler, D.; Roeger, S.; Wittmann, G.; Winnacker, A. Performance of flexible polymeric light-emitting diodes under bending conditions. Appl. Phys. Lett. 2003, 82, 3342–3344. [Google Scholar] [CrossRef]
- Chwang, A.B.; Rothman, M.A.; Mao, S.Y.; Hewitt, R.H.; Weaver, M.S.; Silvernail, J.A.; Rajan, K.; Hack, M.; Brown, J.J.; Chu, X.; et al. Thin film encapsulated flexible organic electroluminescent displays. Appl. Phys. Lett. 2003, 83, 413–415. [Google Scholar] [CrossRef]
- Ichikawa, Y.; Yoshida, T.; Hama, T.; Sakai, H.; Harashima, K. Production technology for amorphous silicon-based flexible solar cells. Sol. Energy Mater. Sol. Cells 2001, 66, 107–115. [Google Scholar] [CrossRef]
- Jinhua, L.; Ningyi, Y.; Chan HL, W. Preparation of PCLT/P(VDF-TrFE) pyroelectric sensor based on plastic film substrate. Sens. Actuators A Phys. 2002, 100, 231–235. [Google Scholar] [CrossRef]
- Mikulics, M.; Adam, R.; Marso, M.; Forster, A.; Kordos, P.; Luth, H.; Wu, S.; Zheng, X.; Sobolewski, R. Ultrafast low-temperature-grown epitaxial GaAs photodetectors transferred on flexible plastic substrates. IEEE Photonics Technol. Lett. 2005, 17, 1725–1727. [Google Scholar] [CrossRef]
- Jia, H.; Veldeman, J.; Burgelman, M. Magnetic properties and microstructure of CoCr and CoCrTa thin films sputtered at high pressure on a PET substrate. J. Magn. Magn. Mater. 2001, 223, 73–80. [Google Scholar] [CrossRef]
- Jung, R.; Morasch, R.; Karayaylali, P.; Phillips, K.; Maglia, F.; Stinner, C.; Shao-Horn, Y.; Gasteiger, H.A. Effect of Ambient Storage on the Degradation of Ni-Rich Positive Electrode Materials (NMC811) for Li-Ion Batteries. J. Electrochem. Soc. 2018, 165, A132–A141. [Google Scholar] [CrossRef]
- Terlemezoglu, M.; Surucu, O.; Isik, M.; Gasanly, N.M.; Parlak, M. Temperature-dependent optical characteristics of sputtered NiO thin films. Appl. Phys. A 2022, 128, 50. [Google Scholar] [CrossRef]
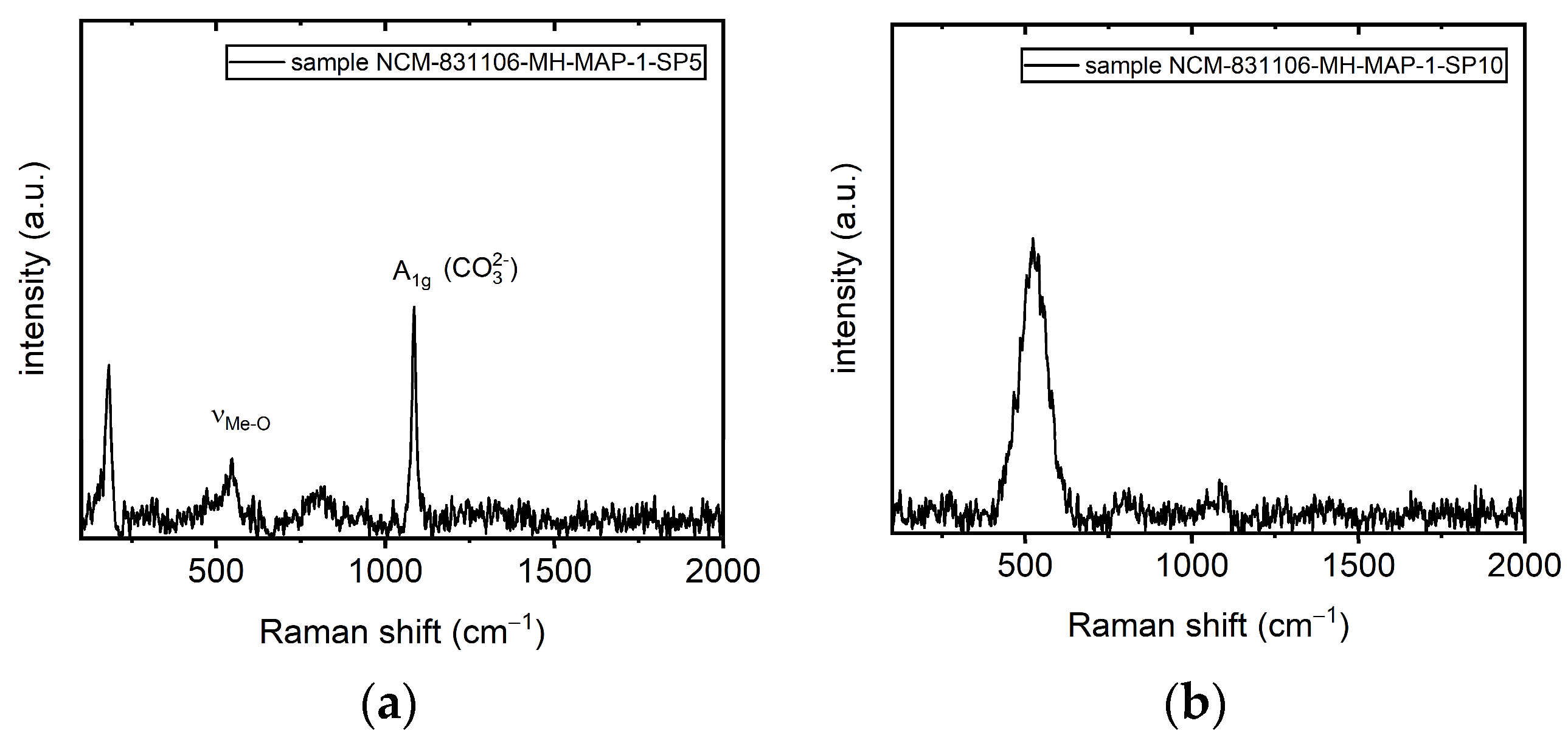
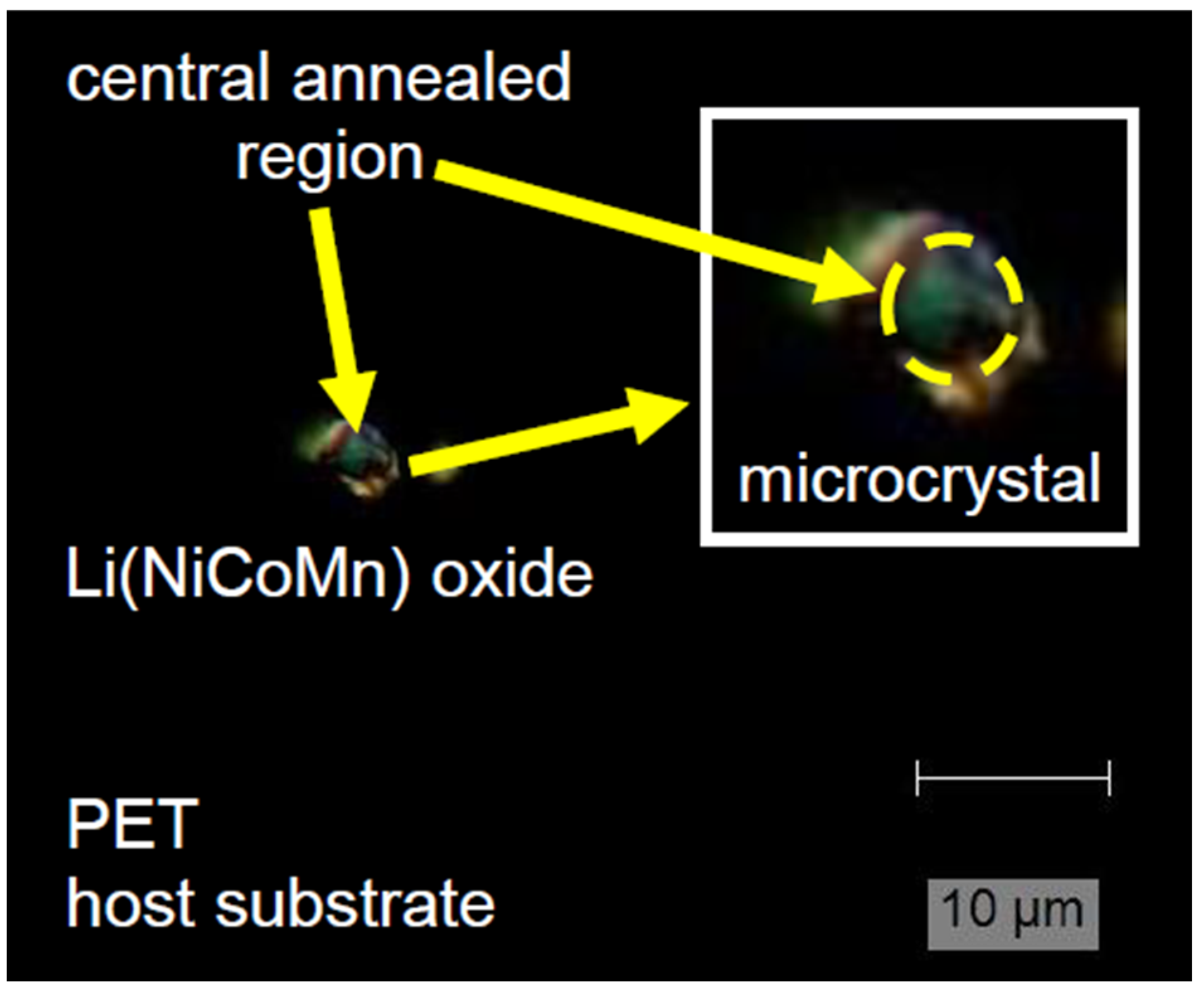
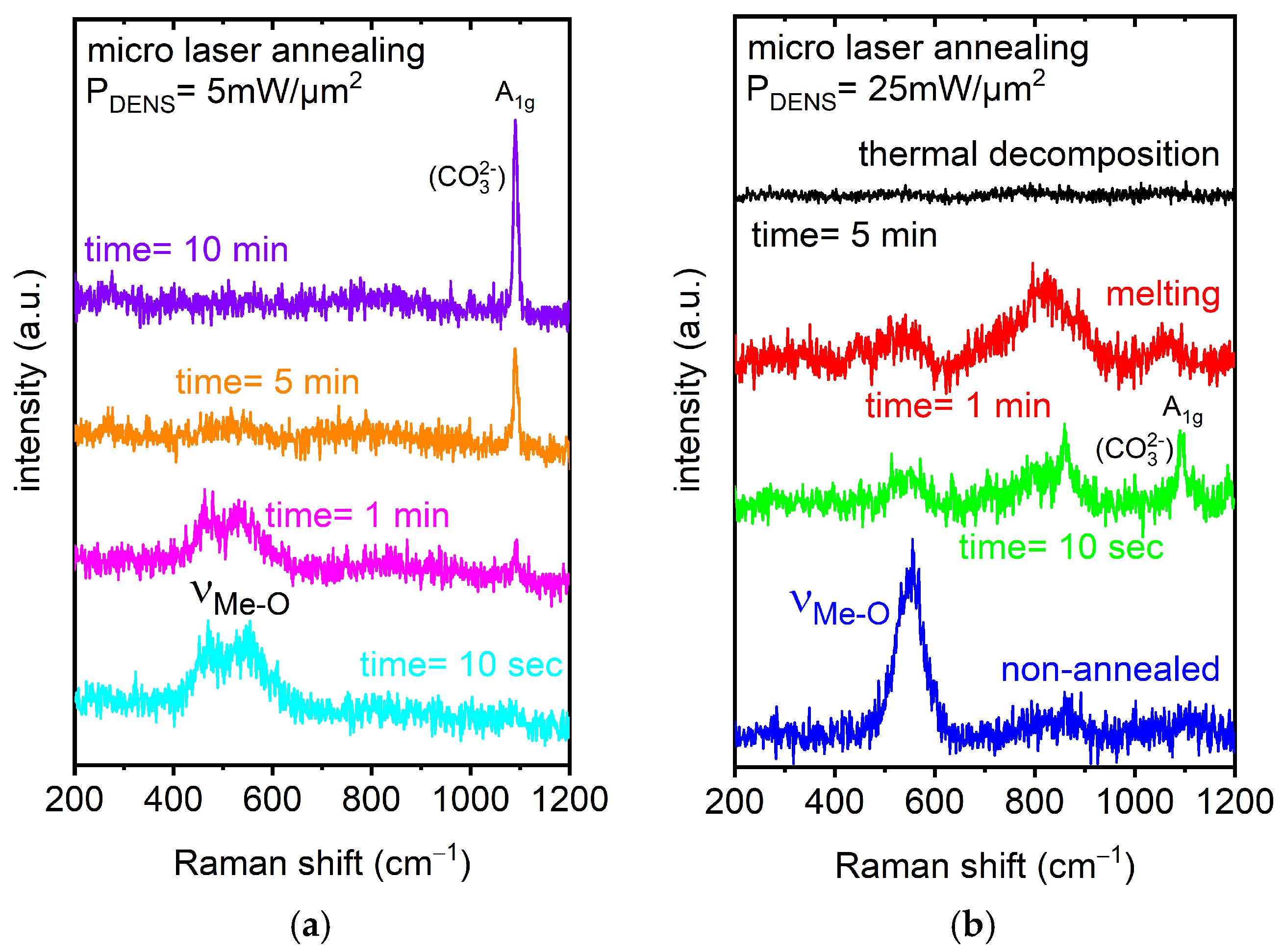
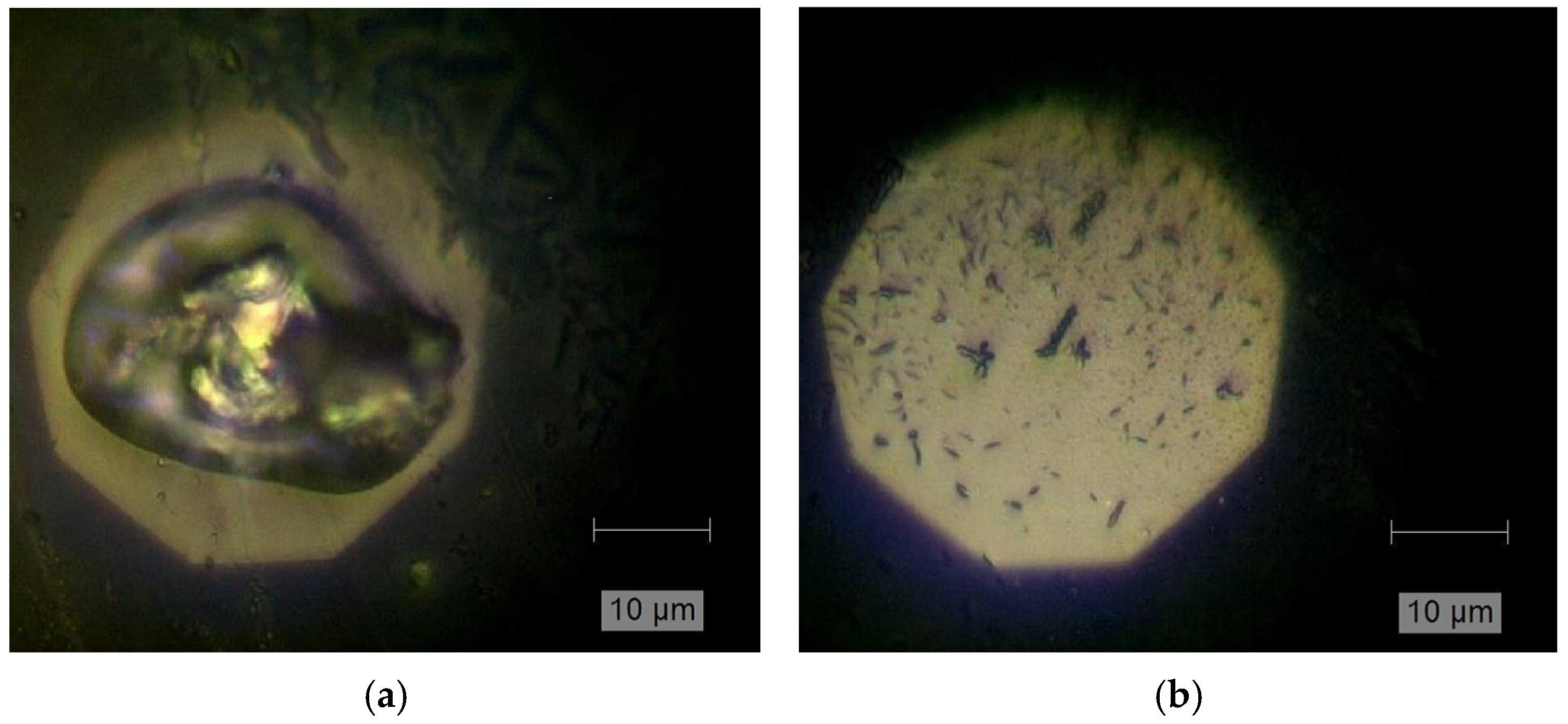
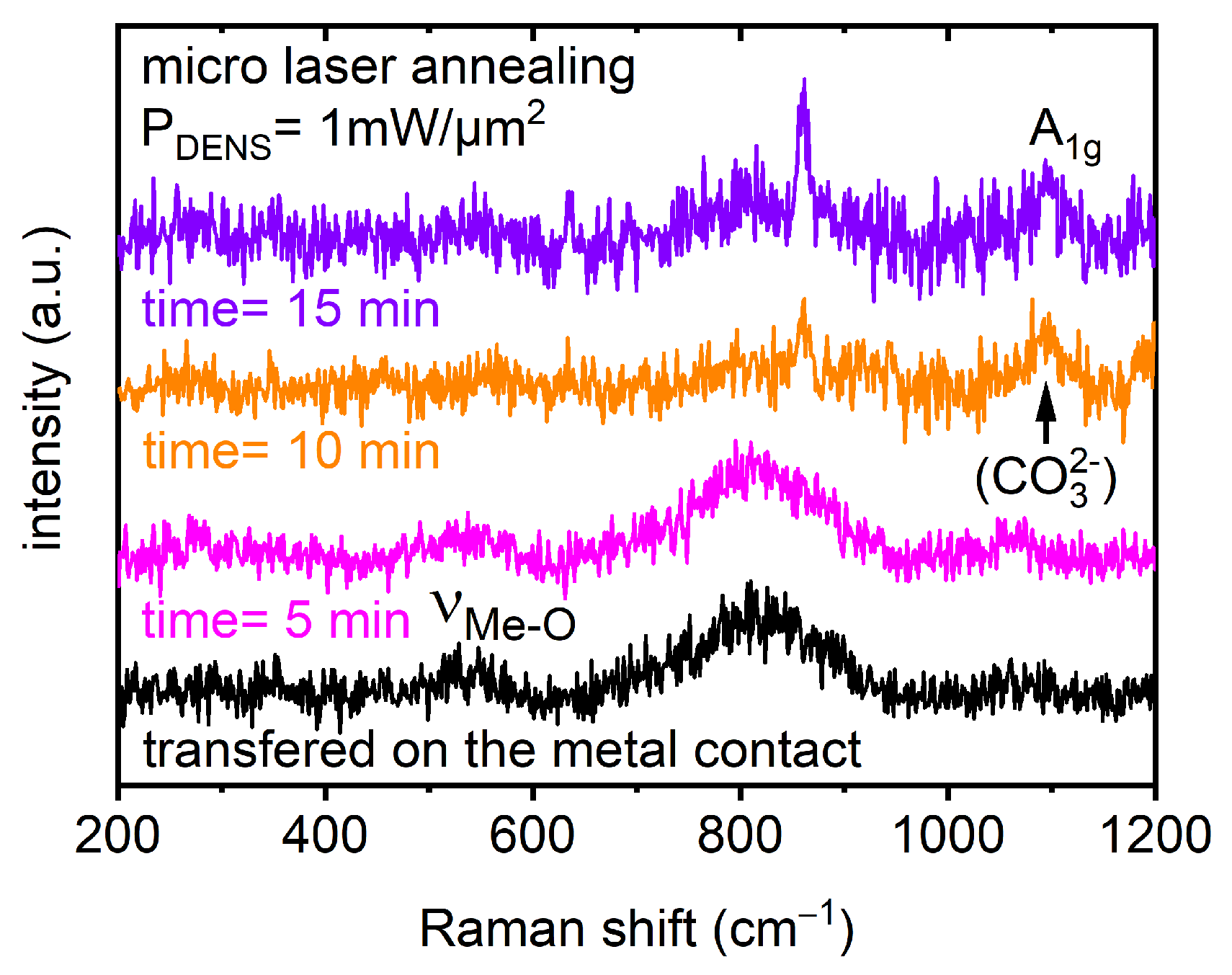
| Optical Power Density (mW/µm2) | Annealing Time | Raman RT Study 200–1200 (cm−1) | Notice | Interpretation | |
|---|---|---|---|---|---|
| Mode | Assignment | ||||
| pristine | pristine | 550 | νMe-O | broad band FWHM ~70cm−1 | overlapping Me-O stretching and bending vibrations related to Ni, Co, and Mn as the metal (Me) [59,60] |
| 5 | 10 s | 470 | Eg | overlapping O-Me-O bending vibrations related to Ni, Co, and Mn as the metal (Me) [59,60] | |
| 550 | A1g | overlapping Me-O symmetric stretching vibrations related to Ni, Co, and Mn as the metal (Me) [59,60] | |||
| 1 min | 470 | Eg | the intensity began to decrease | ||
| 550 | A1g | the intensity began to decrease | |||
| 1085 | A1g | appeared | CO32− symmetric stretching vibrations [55,57,58] | ||
| 5 min | 470, 550 | Eg and A1g | both bands: the intensity dramatically decreased | beginning phase transition | |
| 1085 | A1g | intensity increase | |||
| 10 min | 1085 | A1g | intensity increase | recrystallization | |
| 25 | 10 s | 520–580 | several overlapping modes detected | ||
| 860 | 2TO | intensity increase | Ni oxide-related [74] | ||
| 1085 | A1g | intensity decrease | CO32− symmetric stretching vibrations [55,57,58] | ||
| 1 min | 520–580 | several overlapping modes detected | |||
| 770–890 | several overlapping modes detected | Ni oxide-related [74] melting process confirmed by optical inspection | |||
| 1050–1090 | A1g | the intensity began to dramatically decrease | |||
| 5 min | bands not detected anymore | thermal decomposition | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahrens, L.; Mikulics, M.; Schröder, S.; Mayer, J.; Hardtdegen, H.H. Laser-Micro-Annealing of Microcrystalline Ni-Rich NCM Oxide: Towards Micro-Cathodes Integrated on Polyethylene Terephthalate Flexible Substrates. Materials 2025, 18, 680. https://doi.org/10.3390/ma18030680
Ahrens L, Mikulics M, Schröder S, Mayer J, Hardtdegen HH. Laser-Micro-Annealing of Microcrystalline Ni-Rich NCM Oxide: Towards Micro-Cathodes Integrated on Polyethylene Terephthalate Flexible Substrates. Materials. 2025; 18(3):680. https://doi.org/10.3390/ma18030680
Chicago/Turabian StyleAhrens, Lara, Martin Mikulics, Steffen Schröder, Joachim Mayer, and Hilde Helen Hardtdegen. 2025. "Laser-Micro-Annealing of Microcrystalline Ni-Rich NCM Oxide: Towards Micro-Cathodes Integrated on Polyethylene Terephthalate Flexible Substrates" Materials 18, no. 3: 680. https://doi.org/10.3390/ma18030680
APA StyleAhrens, L., Mikulics, M., Schröder, S., Mayer, J., & Hardtdegen, H. H. (2025). Laser-Micro-Annealing of Microcrystalline Ni-Rich NCM Oxide: Towards Micro-Cathodes Integrated on Polyethylene Terephthalate Flexible Substrates. Materials, 18(3), 680. https://doi.org/10.3390/ma18030680





