The Effects of Cu Powder on the Interface Microstructure Evolution of Hot-Rolled Al 6061/Mg M21/Al 6061 Composite Plates During Annealing
Abstract
1. Introduction
2. Experimental Procedures
2.1. Materials and Preparation of Al-Mg Composite Sheets
2.2. Characterization
3. Results and Discussion
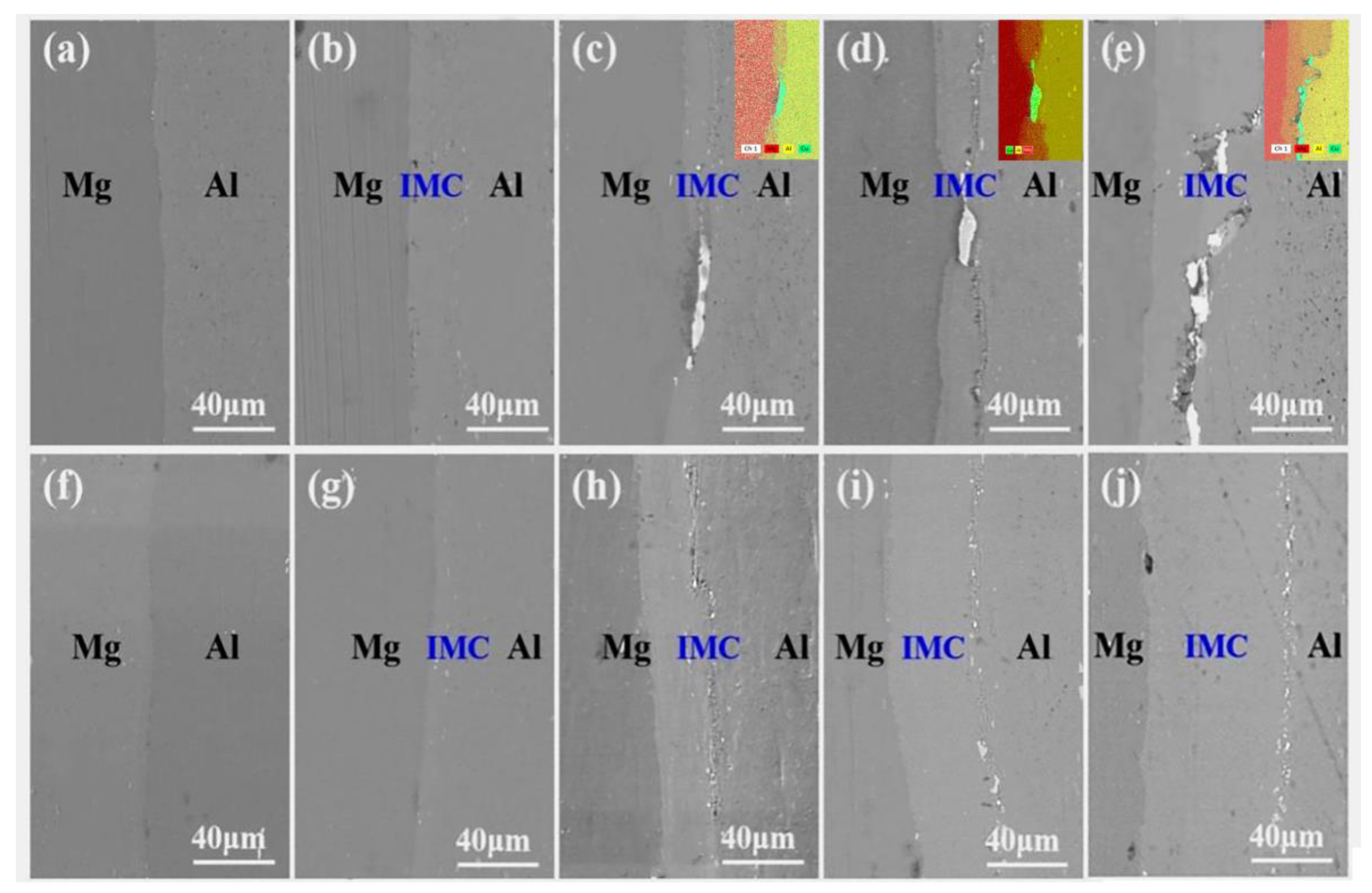
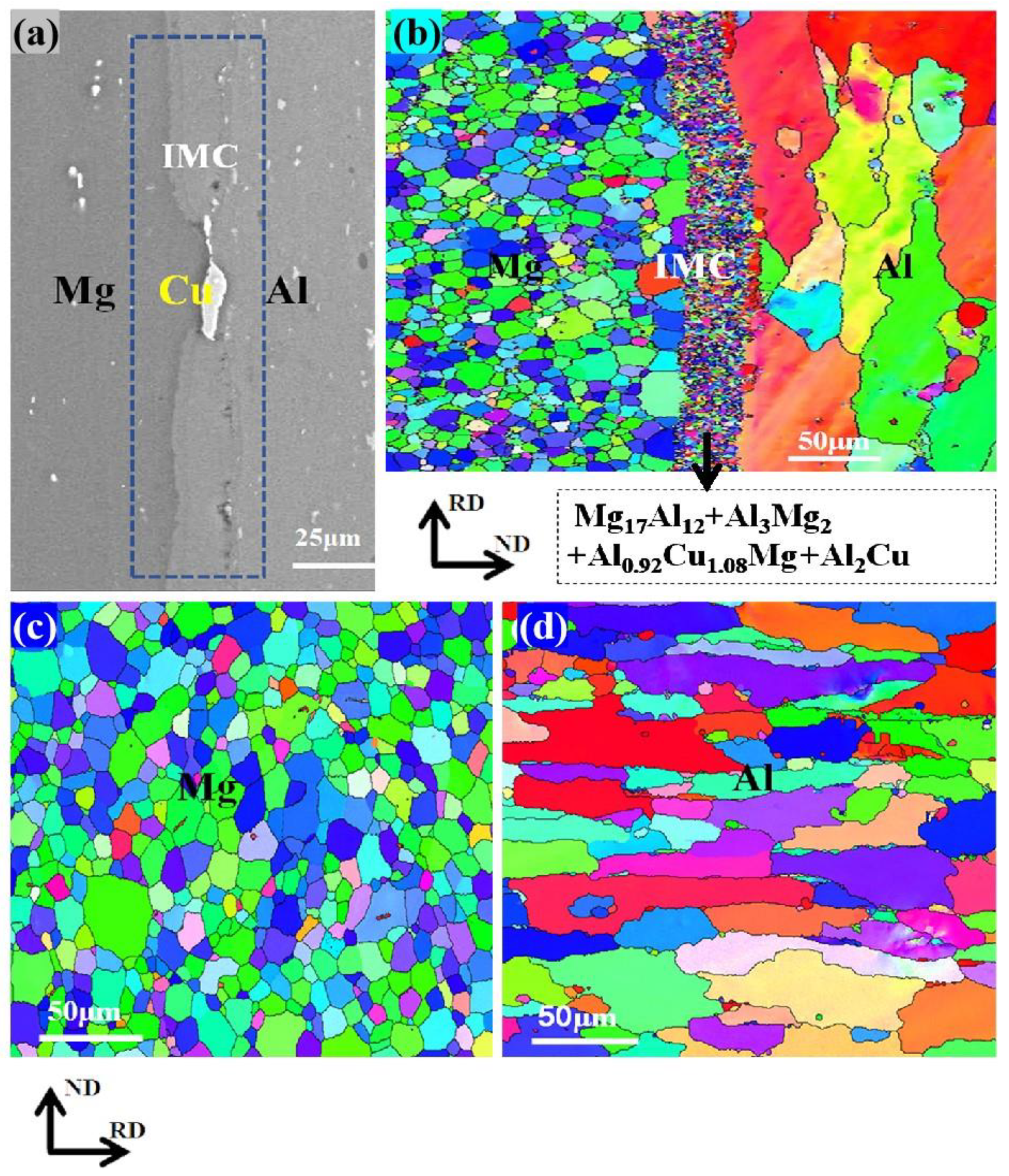
3.1. SEM and XRD Analysis of the Al/Mg Interface
3.2. TEM and EBSD Analysis of IMCs
4. Conclusions
- (1)
- As the annealing temperature increases, the interface near the Mg section within the NP composite plate transitions from a smooth and flat structure to a ‘raised’ interface configuration with varying heights. In contrast, the interfacial microstructure of the AP composite plate evolves from a state characterized by minimal defects, such as pores and cracks, to a discontinuous interface configuration.
- (2)
- The width of Mg-Al IMCs at the Mg-Al interface of the NP composite plate increased from 7.0 µm at 250 °C to 61.2 µm at 400 °C, demonstrating a rapid growth trend. In contrast, in the AP area with Cu powder, when the temperature ranges from 250 °C to 350 °C, the Mg-Al diffusion layer remains thin, varying only between 1 µm and 3.2 µm and, even as the annealing temperature rises to 400 °C, this diffusion layer increases to only 18.8 µm. The incorporation of copper powder significantly suppresses the emergence of Mg-Al IMCs at the junction of Al-Mg composite plates.
- (3)
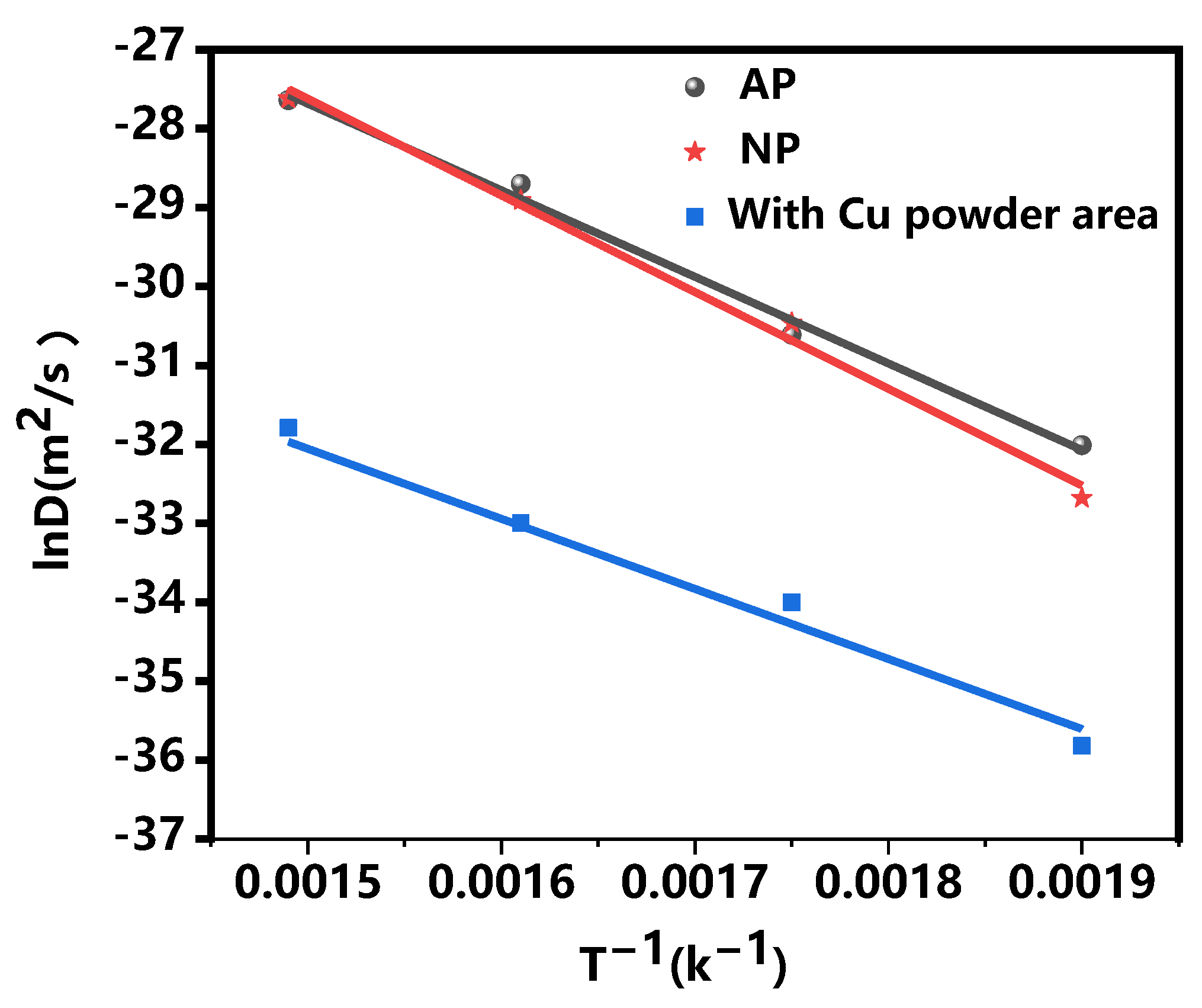
- The diffusion rates of the AP composite plate and NP composite plate increase with rising annealing temperatures. At an identical temperature, the dispersion rate of IMCs in the AP area containing Cu powder is significantly lower than that in the NP composite plate without Cu powder.
- (4)
- As the annealing temperature reaches 350 °C, the phases present at the interface in the NP composite plate devoid of Cu powder are predominantly brittle Al3Mg2 and Mg17Al12 phases. In contrast, the interfacial phases of the AP composite slab containing Cu powder consist mainly of Al3Mg2, Mg17Al12, Al2Cu, and Al0.92Cu1.08Mg phases. Specifically, the total proportion of the Al2Cu phase and Al0.92Cu1.08Mg phase is 6.1%, while the volume ratio of Al3Mg2 to Mg17Al12 is 1.2%. With the addition of Cu powder, new phases such as Al2Cu and Al0.92Cu1.08Mg are generated, which effectively reduce the proportion of brittle phases like Al3Mg2 and Mg17Al12 at the composite plate interface, thereby significantly diminishing IMC diffusion in the Mg-Al interface.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, W.; Wang, X.; Hu, L.; Wang, E. Fabrication of ZK60 magnesium alloy thin sheets with improved ductility by cold rolling and annealing treatment. Mater. Des. 2012, 40, 319–323. [Google Scholar] [CrossRef]
- Pan, H.; Pan, F.; Peng, J.; Gou, J.; Tang, A.; Wu, L.; Dong, H. High-conductivity binary Mg–Zn sheet processed by cold rolling and subsequent aging. J. Alloys Compd. 2013, 578, 493–500. [Google Scholar] [CrossRef]
- Li, G.; Yang, W.; Jiang, W.; Guan, F.; Jiang, H.; Wu, Y.; Fan, Z. The role of vacuum degree in the bonding of Al/Mg bimetal prepared by a compound casting process. J. Mater. Process. Technol. 2019, 265, 112–121. [Google Scholar] [CrossRef]
- Tavassoli, S.; Abbasi, M.; Tahavvori, R. Controlling of IMCs layers formation sequence, bond strength and electrical resistance in AlCu bimetal compound casting process. Mater. Des. 2016, 108, 343–353. [Google Scholar] [CrossRef]
- Md, S.; Birru, A.K. Mechanical and metallurgical properties of friction stir welded dissimilar joints of AZ91 magnesium alloy and AA 6082-T6 aluminium alloy. J. Magnes. Alloys 2019, 7, 264–271. [Google Scholar] [CrossRef]
- Ghalehbandi, S.M.; Malaki, M.; Gupta, M. Accumulative roll bonding—A review. Appl. Sci. 2019, 9, 3627. [Google Scholar] [CrossRef]
- Zhang, Z.; Jiang, W.; Guan, F.; Wang, J.; Li, G.; Fan, Z. Understanding the microstructural evolution and strengthening mechanism of Al/Mg bimetallic interface via the introduction of Y. Mater. Sci. Eng. A 2022, 840, 142974. [Google Scholar] [CrossRef]
- Tsuji, N.; Saito, Y.; Lee, S.; Minamino, Y. ARB (Accumulative Roll-Bonding) and other new techniques to produce bulk ultrafine grained materials. Adv. Eng. Mater. 2003, 5, 338–344. [Google Scholar] [CrossRef]
- Zhao, R.; Yan, M.; Jiao, Z.; Guo, D.; Huang, H. Variation Law of Thickness Fraction of Three-Laminated Aluminum Composite Plate by Solid–Liquid–Solid and Liquid–Solid–Liquid Twin-Roll Casting. Metals 2022, 12, 1710. [Google Scholar] [CrossRef]
- Yang, S.; Li, Y.; Cao, C.; Dong, P.; Li, J.; Li, M. Effect of A356 Covering Plate Temperature on the Microstructure and Mechanical Properties of AZ31/A356 Composite Plate Fabricated by Cast Roll Bonding. Mater. Sci. Eng. A 2019, 759, 128–137. Available online: https://ir.lut.edu.cn/handle/2XXMBERH/113956 (accessed on 9 January 2025).
- Li, S.; Liu, X.; Jia, Y.; Han, J.; Wang, T. Interface characteristics and bonding performance of the corrugated Mg/Al clad plate. Materials 2021, 14, 4412. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Yang, F.; Kong, F.; Wang, X.; Chen, Y. Interface characteristics of Ti6Al4V-TiAl metal-intermetallic laminate (MIL) composites prepared by a novel hot-pack rolling. Mater. Charact. 2018, 144, 173. [Google Scholar] [CrossRef]
- Tian, Y.; She, X.; Yu, J.; Tang, Z.; Zhang, R.; Ai, X.; Dai, H.; Zheng, K.; Pan, F.; Jiang, X. Evolution of interface microstructure of rolled Al-Mg composite plate implanted with Er powder. Surf. Interfaces 2023, 37, 102665. [Google Scholar] [CrossRef]
- Wang, Y.; Prangnell, P.B. The significance of intermetallic compounds formed during interdiffusion in aluminum and magnesium dissimilar welds. Mater. Charact. 2017, 134, 84–95. [Google Scholar] [CrossRef]
- Guo, Y.; Quan, G.; Ren, L.; Liu, B.; Al-Ezzi, S.; Pan, H. Effect of Zn interlayer thickness on the microstructure and mechanical properties of two-step diffusion bonded joint of ZK60Mg and 5083Al. Vacuum 2018, 161, 353–360. [Google Scholar] [CrossRef]
- Liu, N.; Liu, C.; Liang, C.; Zhang, Y. Influence of Ni interlayer on microstructure and mechanical properties of Mg/Al bimetallic castings. Metall. Mater. Trans. A 2018, 49, 3556–3564. [Google Scholar] [CrossRef]
- Jiang, Z.; Fan, Z.; Jiang, W.; Li, G.; Wu, Y.; Guan, F.; Jiang, H. Interfacial microstructures and mechanical properties of Mg/Al bimetal produced by a novel liquid-liquid compound casting process. J. Mech. Work. Technol. 2018, 261, 149–158. [Google Scholar] [CrossRef]
- Wang, X.Y. Influence of Cu-Interlayer Thickness on Microstructures and Mechanical Properties of MIG-Welded Mg-Steel Joints. J. Mater. Eng. Perform. 2016, 25, 910–920. [Google Scholar] [CrossRef]
- Brennan, S.; Bermudez, K.; Kulkarni, N.S.; Sohn, Y. Interdiffusion in the Mg-Al system and intrinsic diffusion in β-Mg2Al3. Met. Mater. Trans. A 2012, 43, 4043–4052. [Google Scholar] [CrossRef]
- Beygi, R.; Akhavan-Safar, A.; Carbas, R.; Barbosa, A.; Marques, E.; da Silva, L. Utilizing a ductile damage criterion for fracture analysis of a dissimilar aluminum/steel joint made by friction stir welding. Eng. Fract. Mech. 2022, 274, 108775. [Google Scholar] [CrossRef]
- Xiao, L.; Wang, N. Growth behavior of intermetallic compounds during reactive diffusion between aluminum alloy 1060 and magnesium at 573-673 K. J. Nucl. Mater. 2015, 456, 389–397. [Google Scholar] [CrossRef]
- Wang, P.; Chen, Z.; Hu, C.; Li, B.; Mo, T.; Liu, Q. Effects of annealing on the interfacial structures and mechanical properties of hot roll bonded Al/Mg clad sheets. Mater. Sci. Eng. A 2020, 792, 139673. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, D.; Cao, X.; Yang, W.; Wang, W. Influence of multi-pass rolling and subsequent annealing on the interface microstructure and mechanical properties of the explosive welding Mg/Al composite plates. Mater. Sci. Eng. A 2018, 723, 97–108. [Google Scholar] [CrossRef]
- Zhu, C.; Xu, S.; Gao, W.; Meng, Y.; Lin, S.; Dai, L. Microstructure characteristics and mechanical properties of Al/Mg joints manufactured by magnetic pulse welding. J. Magnes. Alloys 2021, 11, 2366–2375. [Google Scholar] [CrossRef]
- Wang, T.; Wang, Y.; Bian, L.; Huang, Q. Microstructural evolution and mechanical behavior of Mg/Al laminated composite sheet by novel corrugated rolling and flat rolling. Mater. Sci. Eng. A 2019, 765, 138318. [Google Scholar] [CrossRef]
- Lei, J.; Ma, L.; Cai, Z.; Jia, W.; Zhi, C.; Yuan, Y.; Pan, H.; Xie, H. Interfacial fracture characteristics of Mg/Al composite plates with different thickness ratios by asymmetrical rolling with differential temperature rolls. Mater. Sci. Eng. A Struct. Mater. Prop. Misrostruct. Process. 2023, 869, 144764. [Google Scholar] [CrossRef]
- Chang, Q.; Xie, J.; Mao, A.; Wang, W. Study on Interface Structure of Cu/Al Clad Plates by Roll Casting. Metals 2018, 8, 770. [Google Scholar] [CrossRef]
- Lee, T.H.; Lee, Y.J.; Park, K.T.; Jeong, H.G.; Lee, J.H. Mechanical and asymmetrical thermal properties of Al/Cu composite fabricated by repeated hydrostatic extrusion process. Met. Mater. Int. 2015, 21, 402–407. [Google Scholar] [CrossRef]
- Xu, H.; Liu, C.; Silberschmidt, V.; Pramana, S.; White, T.; Chen, Z.; Acoff, V. Behavior of aluminum oxide, intermetallics and voids in Cu–Al wire bonds. Acta Mater. 2011, 59, 5661–5673. [Google Scholar] [CrossRef]
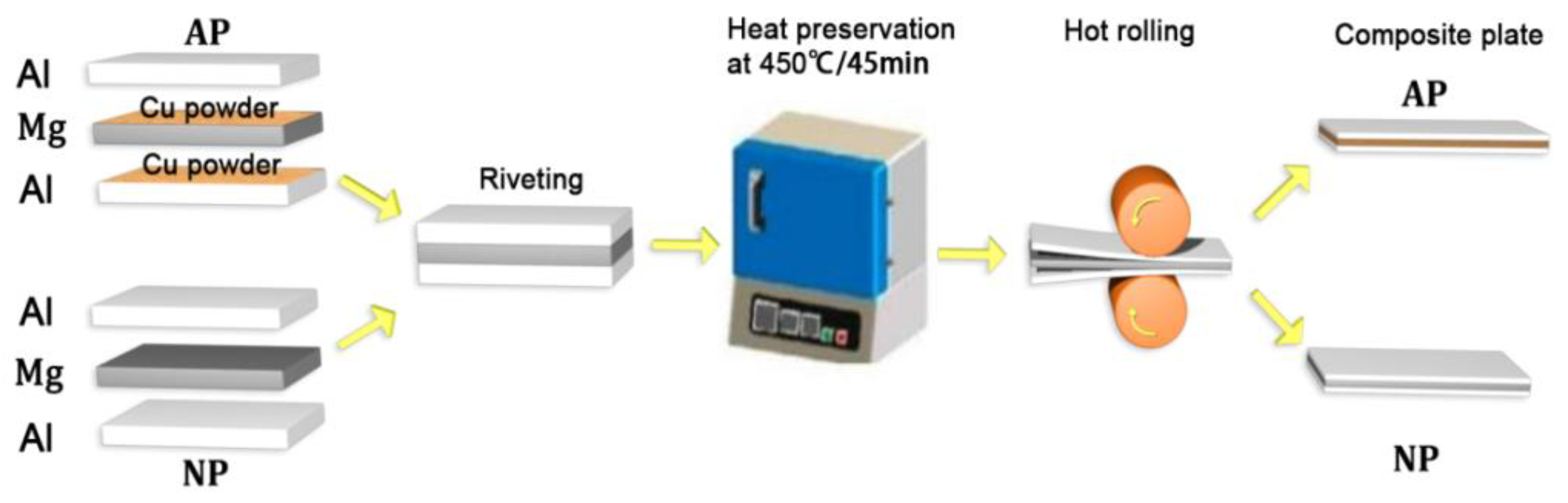
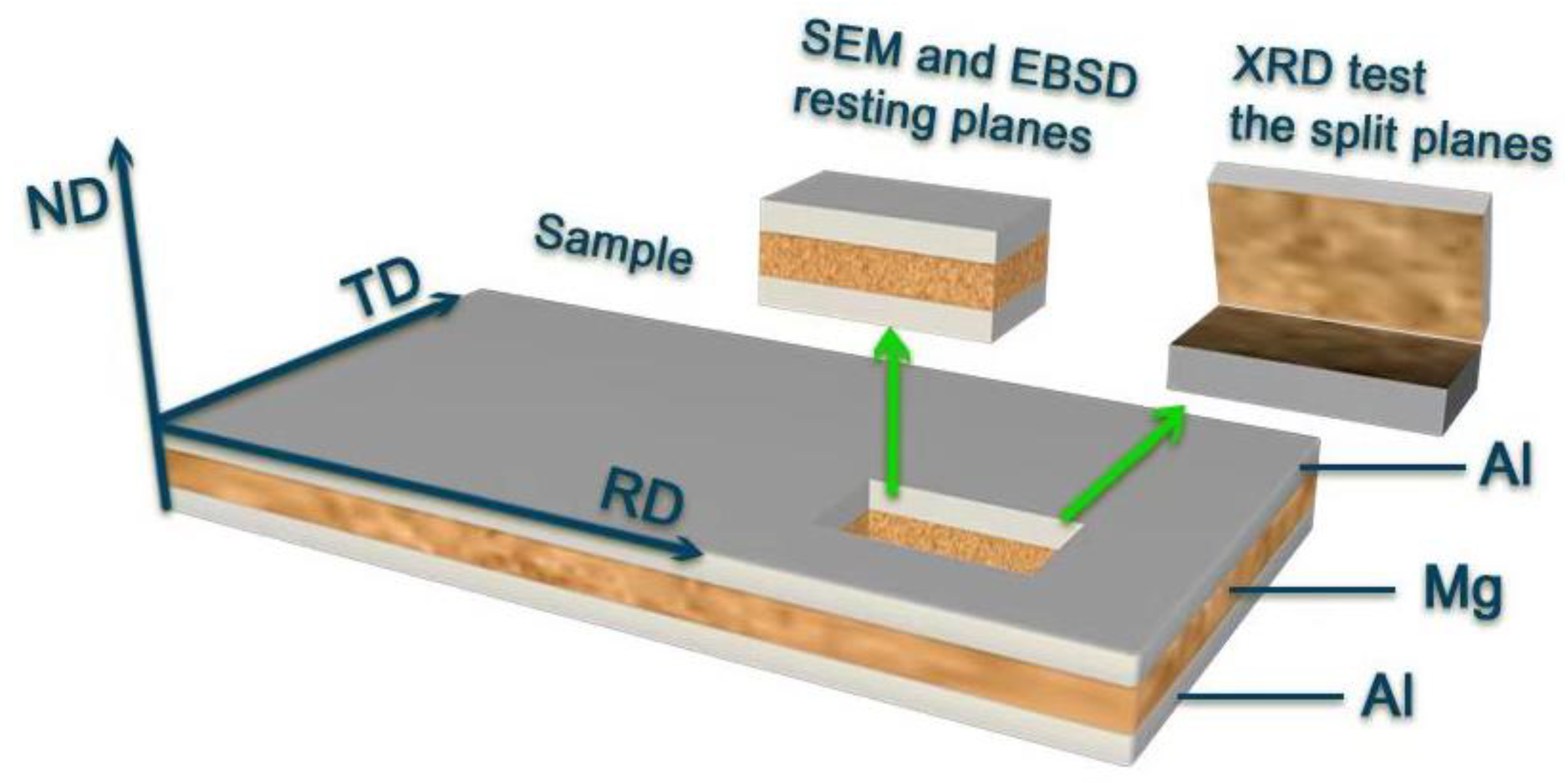




| Material | Al | Mg | Zn | Zr | Si | Fe | Ni | Cu | Be |
|---|---|---|---|---|---|---|---|---|---|
| M21 | - | Bal. | 3.80 | 0.85 | 0 | - | 0.01 | 0.10 | 0.002 |
| 6061 | Bal. | 1.10 | 0.14 | - | 0.69 | 0.56 | - | 0.23 | - |
| Parameters | AP | TC201 | TC251 | TC301 | TC351 | TC401 |
|---|---|---|---|---|---|---|
| NP | TN201 | TN251 | TN301 | TN351 | TN401 | |
| Annealing temperature (°C) | 200 | 250 | 300 | 350 | 400 | |
| Annealing time (h) | 1 | 1 | 1 | 1 | 1 | |
| Style | Interfacial Diffusion Layer Thickness/µM | ||||
|---|---|---|---|---|---|
| Sample Mark | TC251 | TC301 | TC351 | TC401 | |
| AP | AP-without Cu powder area | 5.6 ± 0.8 | 13.7 ± 1.7 | 18.5 ± 1.1 | 57.9 ± 0.8 |
| AP-with Cu powder area | 1.0 ± 0.8 | 3.2 ± 0.9 | 1.87 ± 1.0 | 18.8 ± 2.5 | |
| NP | NP | TN251 | TN301 | TN351 | TN401 |
| 7.0 ± 0.4 | 17.4 ± 1.3 | 29.6 ± 1.6 | 61.2 ± 1.5 | ||
| Temperature/°C | D(AP-Without Cu-Powder Area) m2/s | D(AP-with Cu-Powder Area) m2/s | D(NP) m2/s |
|---|---|---|---|
| 250 | 1.25 × 10−14 | 2.78 × 10−16 | 1.36 × 10−15 |
| 300 | 5.06 × 10−14 | 2.84 × 10−15 | 8.41 × 10−14 |
| 350 | 2.42 × 10−13 | 1.00 × 10−15 | 2.43 × 10−13 |
| 400 | 9.93 × 10−13 | 9.81 × 10−14 | 1.04 × 10−12 |
| Point No. | Chemical Compositions (at.%) | Probable Ingredient | ||
|---|---|---|---|---|
| Mg | Al | Cu | ||
| a1 | 94.8 | 5.2 | 0 | Mg matrix |
| b1 | 56.1 | 43.9 | 0 | Mg17Al12 |
| c1 | 39.3 | 60.7 | 0 | Al3Mg2 |
| d1 | 1.9 | 98.1 | 0 | Al matrix |
| a2 | 87.0 | 10.8 | 2.2 | Mg-Al-Cu intermetallic compounds |
| b2 | 3.5 | 1.0 | 95.5 | Cu |
| c2 | 4.0 | 95.4 | 0.6 | Al matrix |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, N.; Jiang, X.; Zhang, R.; Li, J.; Zheng, K.; Pan, F. The Effects of Cu Powder on the Interface Microstructure Evolution of Hot-Rolled Al 6061/Mg M21/Al 6061 Composite Plates During Annealing. Materials 2025, 18, 655. https://doi.org/10.3390/ma18030655
Yang N, Jiang X, Zhang R, Li J, Zheng K, Pan F. The Effects of Cu Powder on the Interface Microstructure Evolution of Hot-Rolled Al 6061/Mg M21/Al 6061 Composite Plates During Annealing. Materials. 2025; 18(3):655. https://doi.org/10.3390/ma18030655
Chicago/Turabian StyleYang, Na, Xianquan Jiang, Ruihao Zhang, Jian Li, Kaihong Zheng, and Fusheng Pan. 2025. "The Effects of Cu Powder on the Interface Microstructure Evolution of Hot-Rolled Al 6061/Mg M21/Al 6061 Composite Plates During Annealing" Materials 18, no. 3: 655. https://doi.org/10.3390/ma18030655
APA StyleYang, N., Jiang, X., Zhang, R., Li, J., Zheng, K., & Pan, F. (2025). The Effects of Cu Powder on the Interface Microstructure Evolution of Hot-Rolled Al 6061/Mg M21/Al 6061 Composite Plates During Annealing. Materials, 18(3), 655. https://doi.org/10.3390/ma18030655





