Effect of Asphaltenes on the Stability of Water in Crude Oil Emulsions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Emulsion Preparation and Characterization
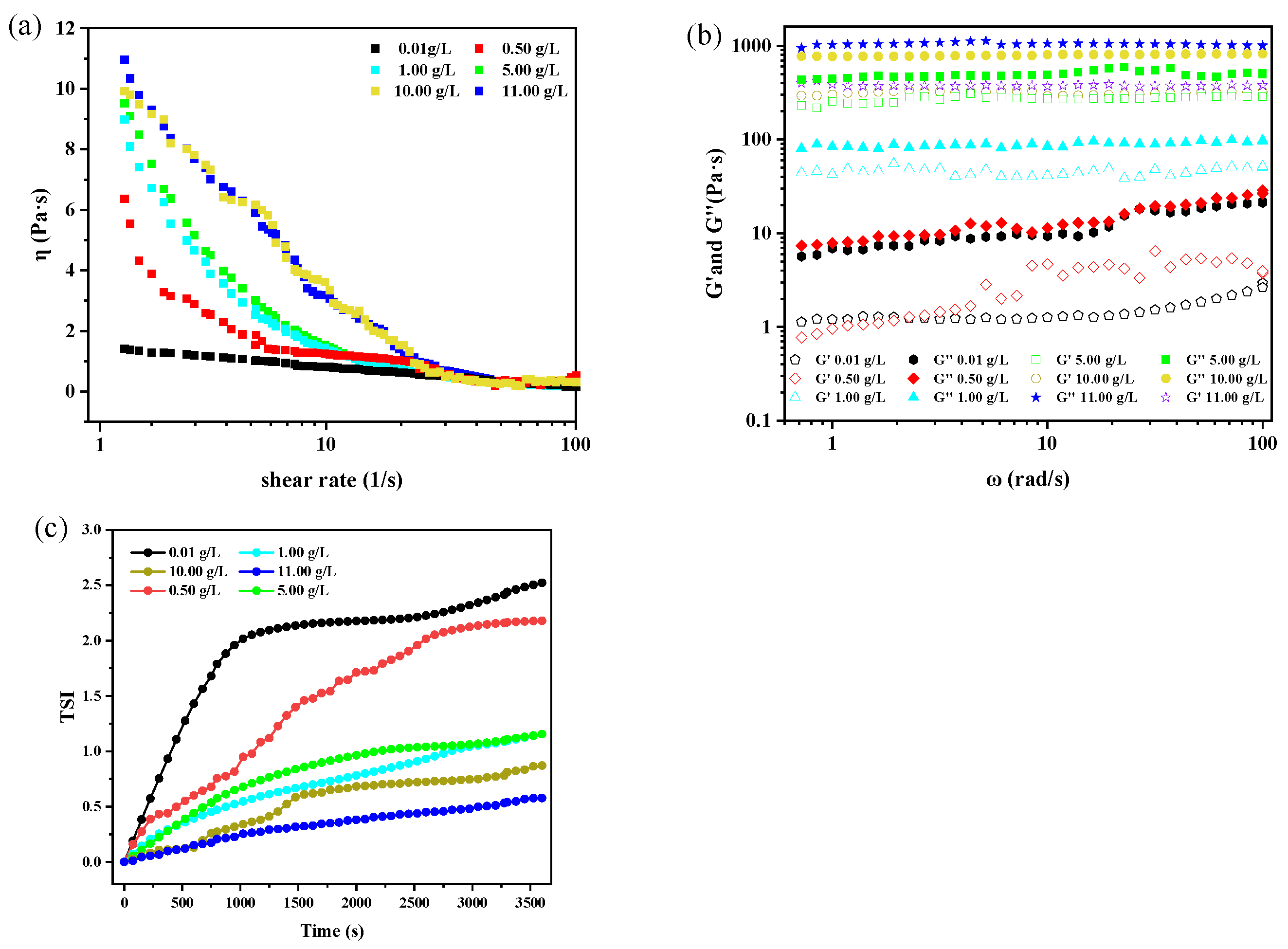
2.3. Rheological Performance Test
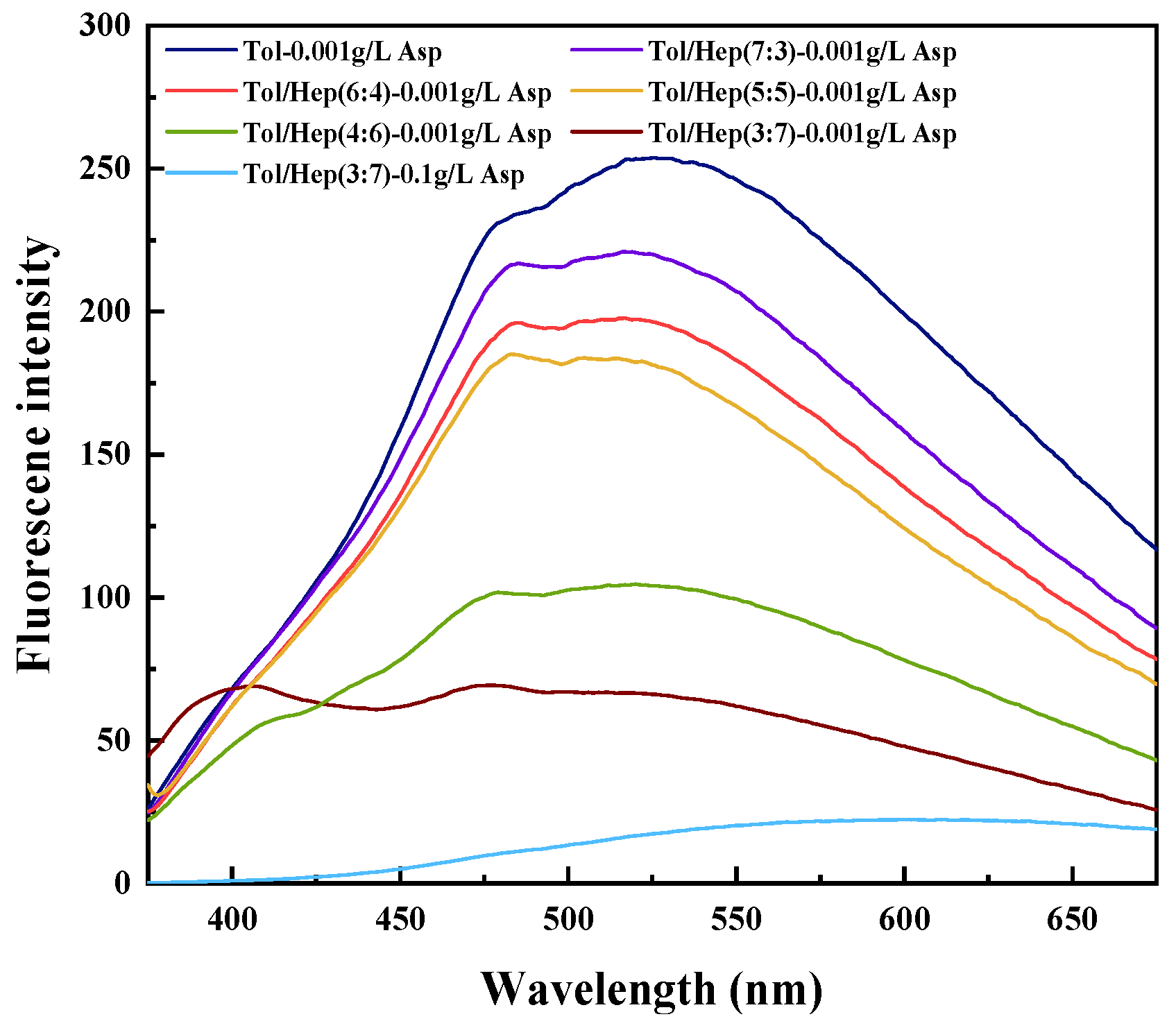
2.4. Fluorescence Spectrometry
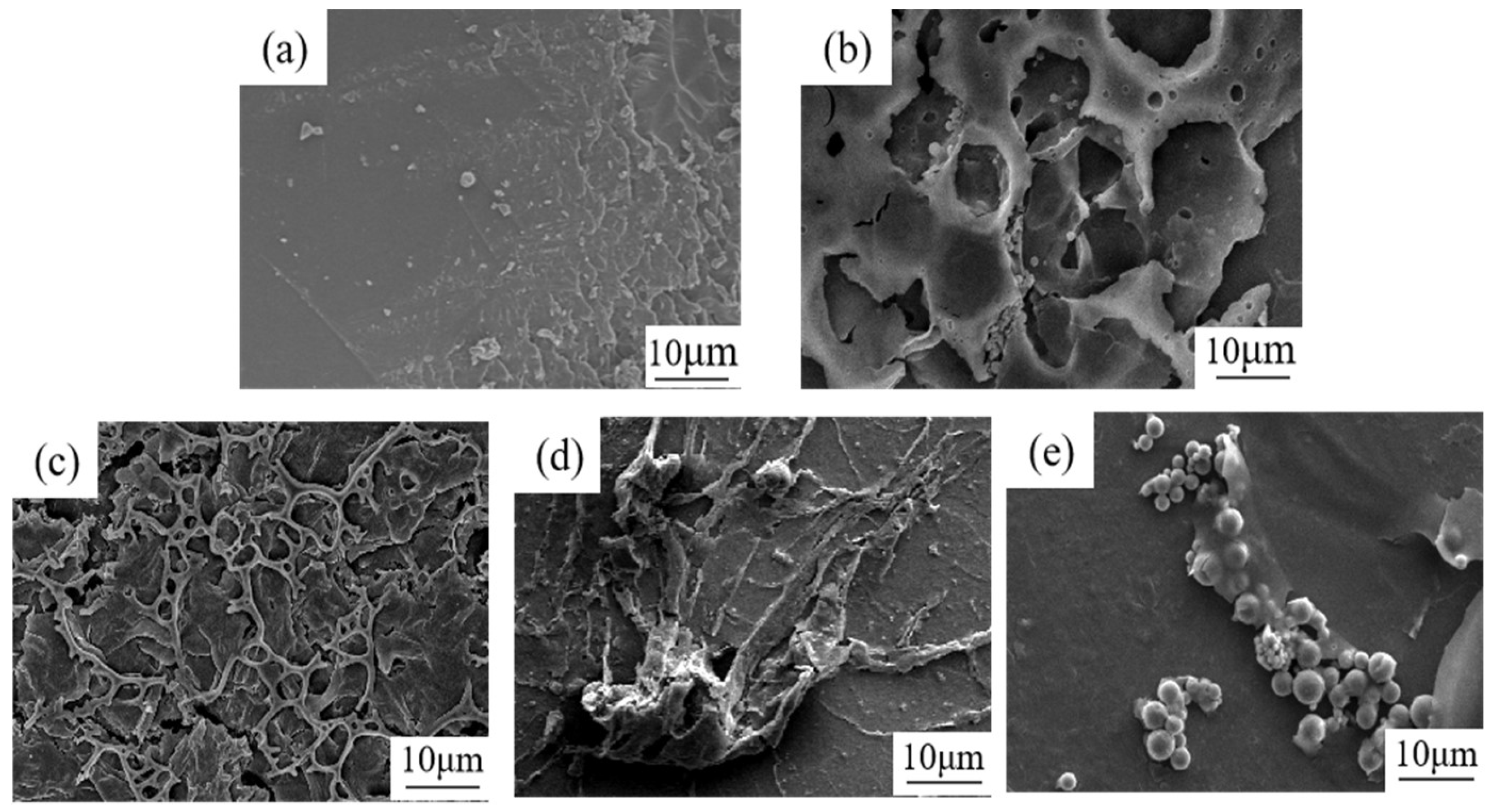
2.5. Characterization of the Water/Oil Interface
3. Results
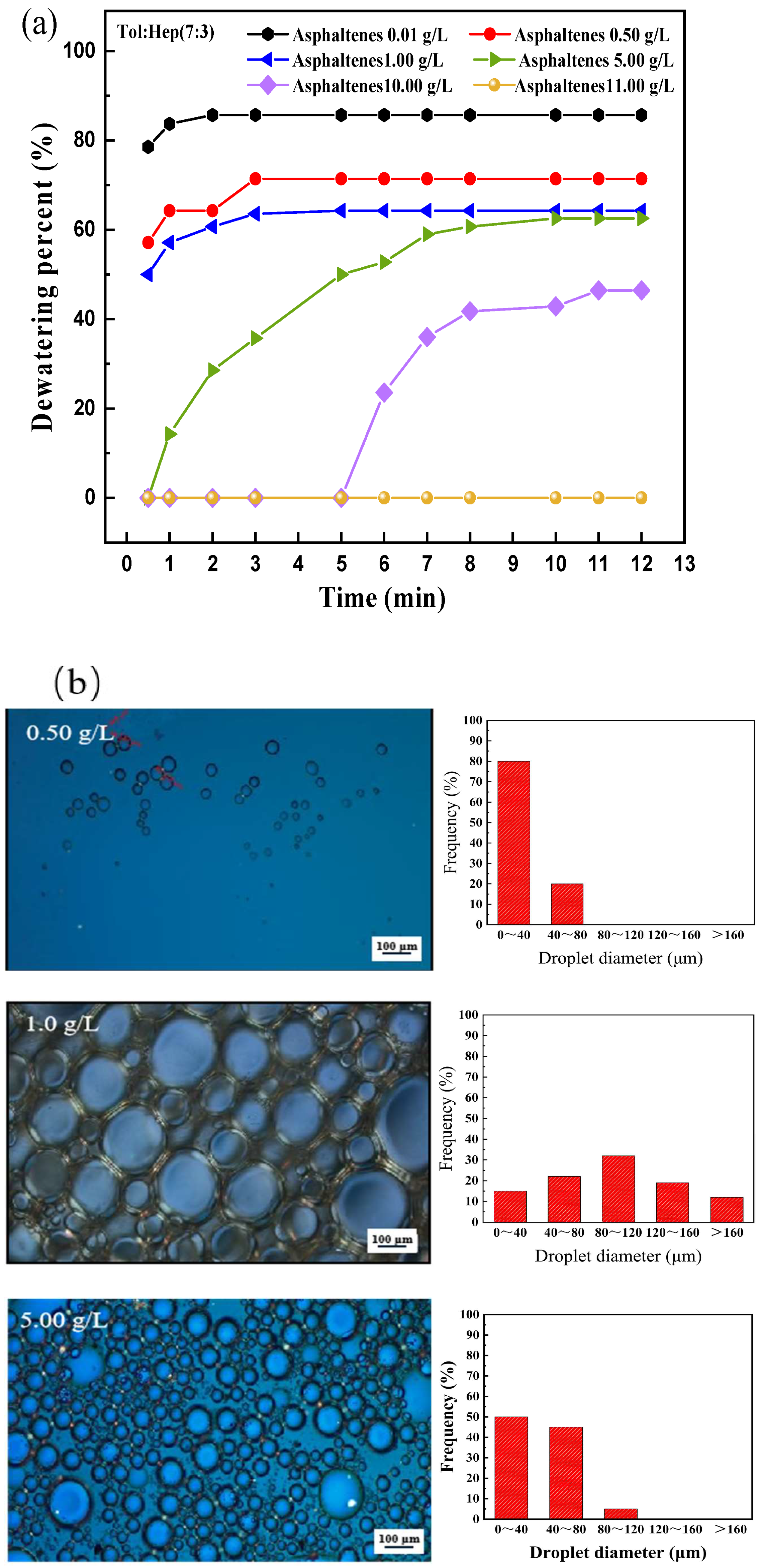
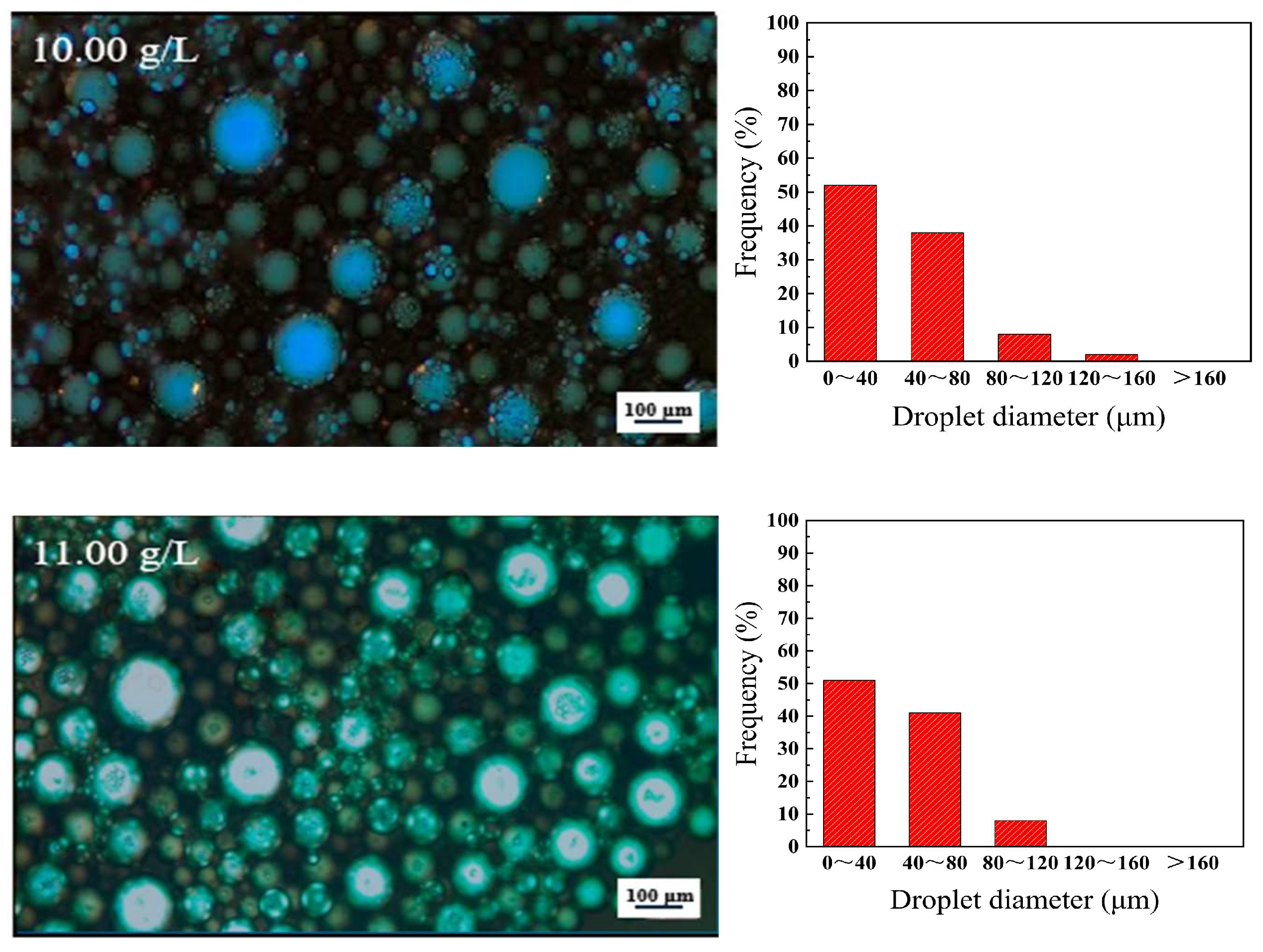
3.1. Effect of Asphaltene Content on the Stability of W/O Emulsions
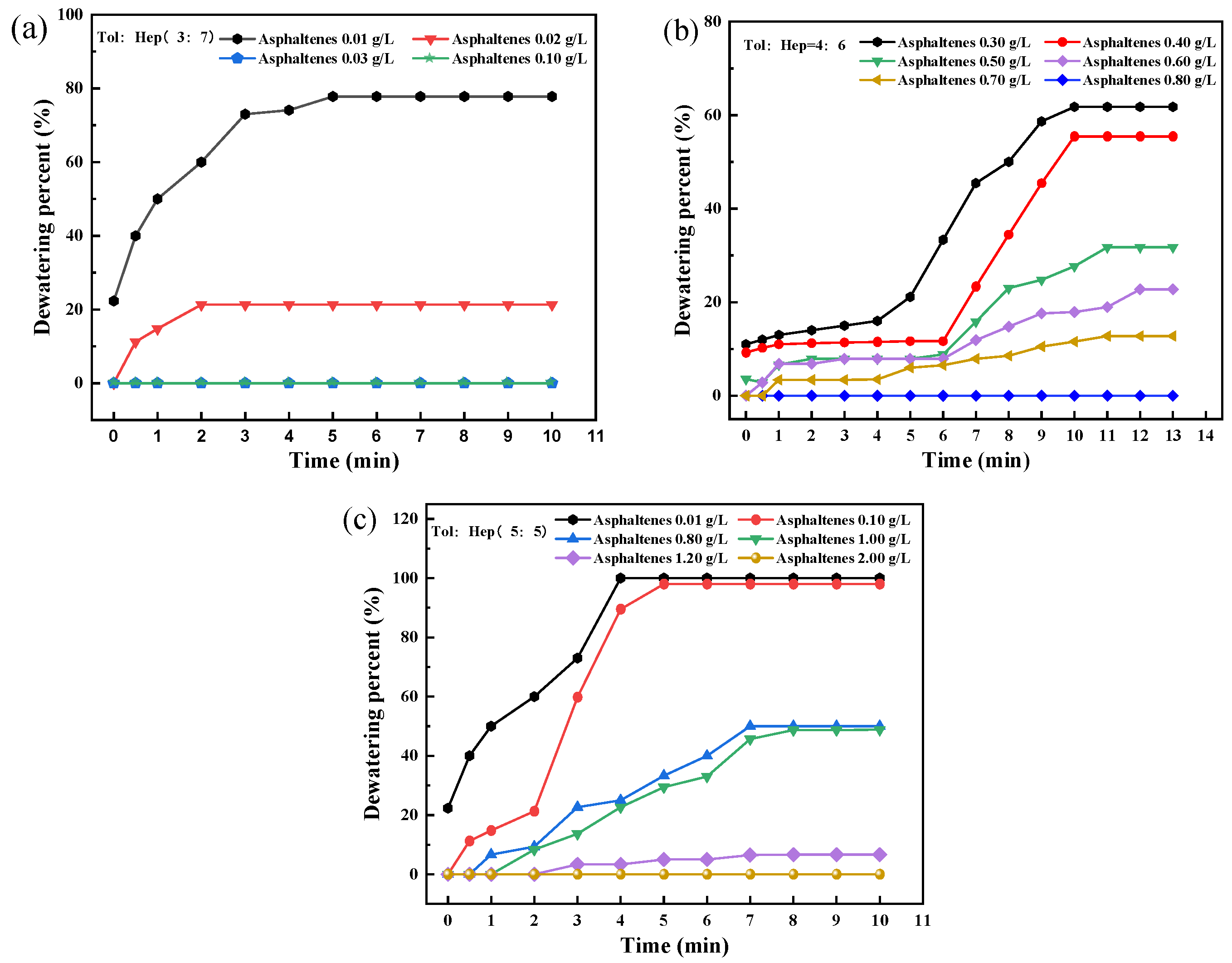
3.2. Effect of Aromaticity of Simulated Oil on the Stability of Asphaltene-Containing Emulsions
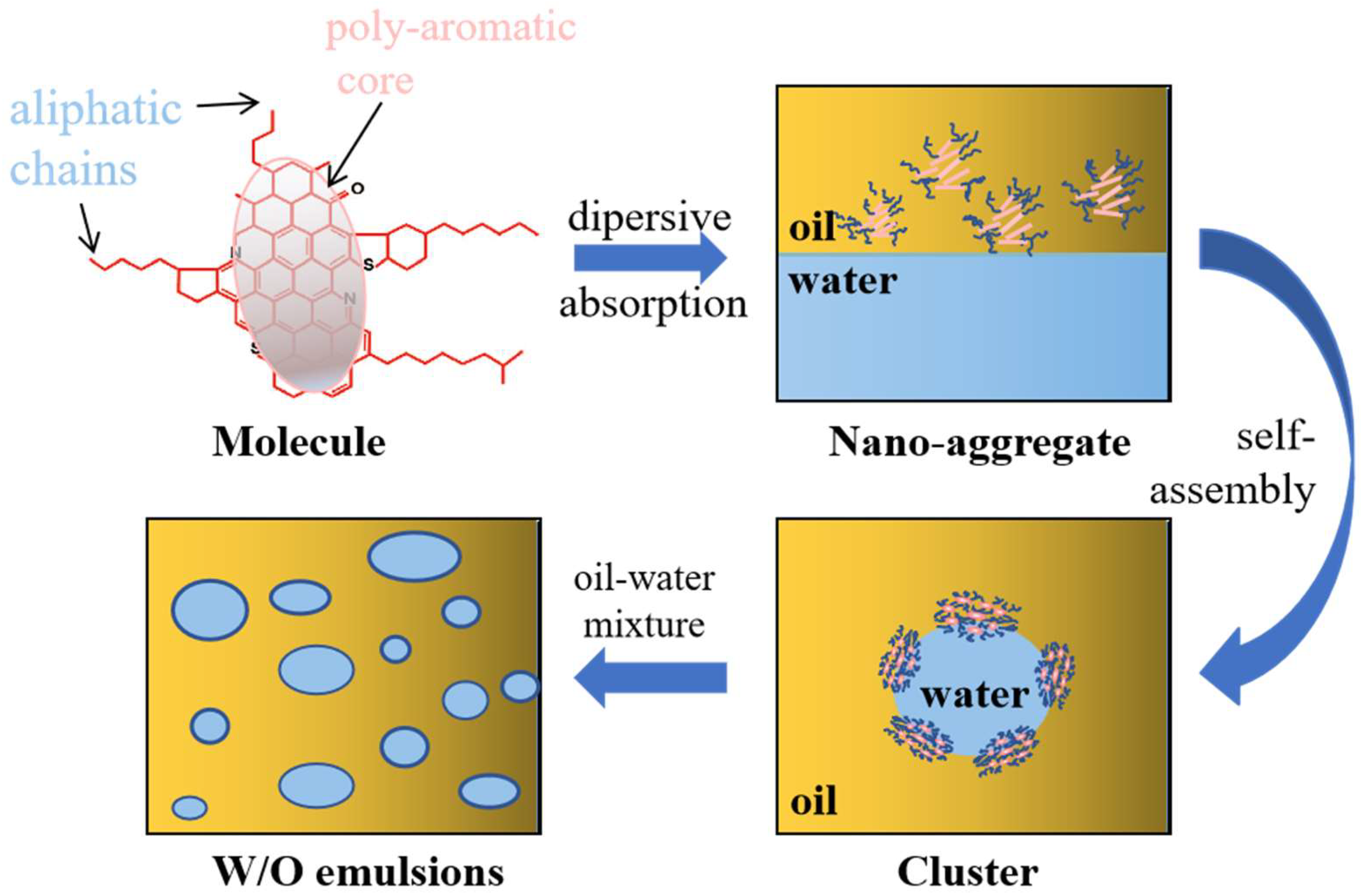
3.3. Mechanism of Asphaltene at the Oil/Water Interface
4. Conclusions
- The presence of high contents of asphaltene in heavy crude oil is a critical factor that impacts the dewatering process. At an asphaltene concentration of 0.5 g/L, the dehydration rate is 67%, with fewer droplets and a thinner interface film, resulting in poor stability. As the asphaltene concentration increases, the dehydration rate decreases. And at 11.0 g/L, the rate drops to zero. The droplet size becomes more uniform, and the interface film thickens, significantly improving emulsion stability. The asphaltene concentration also increases emulsion viscosity, along with both the storage modulus (G′) and loss modulus (G″). When the asphaltene concentration was 11 g/L, G′ and G″ reaches 1033 Pa·s and G″ 416 Pa·s, with G′ exceeding G″, suggesting elastic-dominated behavior. The average TSI value of 0.36 further confirms that the emulsion is more stable and very difficult to break;
- The emulsifying ability of asphaltenes decreases significantly with increased aromaticity in the oil phase. In the toluene–n-heptane emulsion system, the critical aggregation concentrations (CACs) of asphaltenes are 0.05 g/L, 0.8 g/L, and 1.2 g/L for emulsions containing 30%, 40%, and 50% toluene, respectively, which indicates that the critical aggregation concentration (CAC) of asphaltenes increases with the toluene concentrations. The enhanced aromaticity of the emulsion increases asphaltene solubility, reducing its adsorption at the oil–water interface, which decreases emulsion stability and promotes emulsion breakdown and dehydration. Therefore, for crude oils with high asphaltene content prone to emulsification, blending with aromatic-rich oils can optimize the emulsion-breaking and dehydration processes. This work provides theoretical and practical guidance for breaking emulsions during crude oil dewatering pretreatment.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kooti, G.; Dabir, B.; Taherdangkoo, R.; Butscher, C. Modelling Droplet Size Distribution in Inline Electrostatic Coalescers for Improved Crude Oil Processing. Sci. Rep. 2023, 13, 20209. [Google Scholar] [CrossRef] [PubMed]
- McLean, J.D.; Kilpatrick, P.K. Effects of Asphaltene Aggregation in Model Heptane–Toluene Mixtures on Stability of Water-in-Oil Emulsions. J. Colloid Interface Sci. 1997, 196, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Navas-Cáceres, O.D.; Parada, M.; Zafra, G. Development of a Highly Tolerant Bacterial Consortium for Asphaltene Biodegradation in Soils. Environ. Sci. Pollut. Res. 2023, 30, 123439–123451. [Google Scholar] [CrossRef] [PubMed]
- Tchoukov, P.; Yang, F.; Xu, Z.; Dabros, T.; Czarnecki, J.; Sjöblom, J. Role of Asphaltenes in Stabilizing Thin Liquid Emulsion Films. Langmuir 2014, 30, 3024–3033. [Google Scholar] [CrossRef]
- Shi, C.; Zhang, L.; Xie, L.; Lu, X.; Liu, Q.; He, J.; Mantilla, C.A.; Van den berg, F.G.A.; Zeng, H. Surface Interaction of Water-in-Oil Emulsion Droplets with Interfacially Active Asphaltenes. Langmuir 2017, 33, 1265–1274. [Google Scholar] [CrossRef]
- Ashoorian, S.; Javadi, A.; Hosseinpour, N.; Husein, M. Evolution of Adsorbed Layers of Asphaltenes at Oil-Water Interfaces: A Novel Experimental Protocol. J. Colloid Interface Sci. 2021, 594, 80–91. [Google Scholar] [CrossRef]
- Nguele, R.; Sasaki, K. Asphaltene Behavior at the Interface Oil-Nanofluids: Implications to Adsorption. Colloids Surf. A Physicochem. Eng. Asp. 2021, 622, 126630. [Google Scholar] [CrossRef]
- Alimohammadi, S.; Zendehboudi, S.; James, L. A Comprehensive Review of Asphaltene Deposition in Petroleum Reservoirs: Theory, Challenges, and Tips. Fuel 2019, 252, 753–791. [Google Scholar] [CrossRef]
- Alhreez, M.; Wen, D. Controlled Releases of Asphaltene Inhibitors by Nanoemulsions. Fuel 2018, 234, 538–548. [Google Scholar] [CrossRef]
- Liu, Y.; Bai, J.; Guo, P.; Zhang, W.; Zhong, L.G.; Lyu, C.H.; Hao, Y.; Zhang, M.Q.; Han, X.D.; Bi, P.D. Experimental Study on Water-in-Heavy-Oil Droplets Stability and Viscosity Variations in the Dilution Process of Water-in-Heavy-Oil Emulsions by Light Crude Oil. Energies 2024, 17, 332. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, W.; Lun, Z.; Zhang, Y.H.; Zhang, Q.X.; Yang, P.J.; Li, Y.; Sun, C.D. Factors and kinetics related to the formation of heavy oil-in-water emulsions. Energies 2023, 16, 5499. [Google Scholar] [CrossRef]
- Li, X.; Lu, S.; Niu, M.; Cheng, R.Z.; Gong, Y.J.; Xu, J. Asphaltene inhibition and flow improvement of crude oil with a high content of asphaltene and wax by polymers bearing ultra-long side chain. Energies 2021, 14, 8243. [Google Scholar] [CrossRef]
- Vladimir, A.; Xiu, W.; Mehrnoosh, M. Stability Proxies for Water-in-Oil Emulsions and Implications in Aqueous-based Enhanced Oil Recovery. Energies 2011, 4, 1058. [Google Scholar] [CrossRef]
- Morimoto, M.; Imamura, H.; Shibuta, S.; Morita, T.; Nishikawa, K.; Yamamoto, H.; Tanaka, R.; Takanohashi, T. Asphaltene Aggregation Behavior in Bromobenzene Determined By Small-Angle X-Ray Scattering. Energy Fuels 2015, 29, 5737–5743. [Google Scholar] [CrossRef]
- Mullins, O.C.; Sabbah, H.; Eyssautier, J.; Pomerantz, A.E.; Barré, L.; Andrews, A.B.; Ruiz-Morales, Y.; Mostowfi, F.; McFarlane, R.; Goual, L.; et al. Advances in Asphaltene Science and the Yen–Mullins Model. Energy Fuels 2012, 26, 3986–4003. [Google Scholar] [CrossRef]
- Alhreez, M.; Wen, D. Molecular Structure Characterization of Asphaltene in the Presence of Inhibitors with Nanoemulsions. RSC Adv. 2019, 9, 19560–19570. [Google Scholar] [CrossRef]
- Subramanian, D.; Wu, K.; Firoozabadi, A. Ionic Liquids as Viscosity Modifiers for Heavy and Extra-Heavy Crude Oils. Fuel 2015, 143, 519–526. [Google Scholar] [CrossRef]
- Andreatta, G.; Bostrom, N.; Mullins, O.C. High-Q Ultrasonic Determination of the Critical Nanoaggregate Concentration of Asphaltenes and the Critical Micelle Concentration of Standard Surfactants. Langmuir 2005, 21, 2728–2736. [Google Scholar] [CrossRef]
- Deng, M.; Cao, X.; Tang, B.; Yuan, Y. Revealing Self-Aggregation Mechanism of Asphaltenes during Oxidative Aging Using Quantum Mechanical Calculations. J. Mol. Liq. 2023, 371, 121063. [Google Scholar] [CrossRef]
- Horeh, N.B.; Hosseinpour, N.; Bahramian, A. Asphaltene Inhibitor Performance as a Function of the Asphaltene Molecular/Aggregate Characteristics: Evaluation by Interfacial Rheology Measurement and Bulk Methods. Fuel 2023, 339, 127420. [Google Scholar] [CrossRef]
- Dastjerdi, M.V.; Sayahi, H.; Koochaki, A.; Jamshidi, Z. Asphaltene Aggregation under the Influence of Structural Features and Interaction Energies: Combination of Quantum Mechanical and Molecular Dynamics Approaches. Colloids Surf. A Physicochem. Eng. Asp. 2023, 671, 131660. [Google Scholar] [CrossRef]
- ASTM D1217-20; Standard Test Method for Density and Relative Density (Specific Gravity) of Liquids by Bingham Pycnometer, Annual Book of ASTM Standards. ASTM International: West Conshohocken, PA, USA, 2020.
- ASTM D1217-24; Standard Test Method for Kinematic Viscosity of Transparent and Opaque Liquids (and Calculation of Dynamic Viscosity), Annual Book of ASTM Standards. ASTM International: West Conshohocken, PA, USA, 2024.
- ASTM D6470-99; Standard Test Method for Salt in Crude Oils (Potentiometric Method), Annual Book of ASTM Standards. ASTM International: West Conshohocken, PA, USA, 2020.
- ASTM D5453-24; Standard Test Method for Determination of Total Sulfur in Light Hydrocarbons, Spark Ignition Engine Fuel, Diesel Engine Fuel, and Engine Oil by Ultraviolet Fluorescence, Annual Book of ASTM Standards. ASTM International: West Conshohocken, PA, USA, 2024.
- ASTM D4124-09; Standard Test Method for Separation of Asphalt into Four Fractions, Annual Book of ASTM Standards. ASTM International: West Conshohocken, PA, USA, 2018.
- ASTM D7996-15; Standard Test Method for Measuring Visible Spectrum of Asphaltenes in Heavy Fuel Oils and Crude Oils by Spectroscopy in a Microfluidic Platform, Annual Book of ASTM Standards. ASTM International: West Conshohocken, PA, USA, 2015.
- Yanes, J.F.R.; Feitosa, F.X.; do Carmo, F.R.; de Sant’Ana, H.B. Paraffin Effects on the Stability and Precipitation of Crude Oil Asphaltenes: Experimental Onset Determination and Phase Behavior Approach. Fluid Phase Equilibria 2018, 474, 116–125. [Google Scholar] [CrossRef]
- Jiang, H.; Liu, X.; Liang, C.; Xu, Y.; Jia, Y. Effect of Asphaltenes Structure on Interfacial Properties: A Dissipative Particle Dynamics Study. Colloids Surf. A Physicochem. Eng. Asp. 2023, 673, 131849. [Google Scholar] [CrossRef]
- Gorbacheva, S.N.; Ilyin, S.O. Structure, Rheology and Possible Application of Water-in-Oil Emulsions Stabilized by Asphaltenes. Colloids Surf. A Physicochem. Eng. Asp. 2021, 618, 126442. [Google Scholar] [CrossRef]
- Salehi, N.; Dehaghani, A.S.; Haghighi, M. Investigation of Fluid-Fluid Interaction between Surfactant-Ion-Tuned Water and Crude Oil: A New Insight into Asphaltene Behavior in the Emulsion Interface. J. Mol. Liq. 2023, 376, 121311. [Google Scholar] [CrossRef]
- Vinogradov, G.V. Ultimate Regimes of Deformation of Linear Flexible Chain Fluid Polymers. Polymer 1977, 18, 1275–1285. [Google Scholar] [CrossRef]
- Jansen, K.M.B.; Agterof, W.G.M.; Mellema, J. Viscosity of Surfactant Stabilized Emulsions. J. Rheol. 2001, 45, 1359–1371. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, X.; Guo, S. Comb Polybutadiene with Long Polystyrene Side Chains: A Solution for Tunable Flowability and Enhancing Dielectric Properties in High-Frequency Printed Board Adhesive Films. ACS Appl. Mater. Interfaces 2023, 15, 41019–41030. [Google Scholar] [CrossRef]
- Ilyin, S.O.; Makarova, V.V.; Polyakova, M.Y.; Kulichikhin, V.G. Phase State and Rheology of Polyisobutylene Blends with Silicone Resin. Rheol. Acta 2020, 59, 375–386. [Google Scholar] [CrossRef]
- Malkin, A.Y.; Ilyin, S.O.; Arinina, M.P.; Kulichikhin, V.G. The Rheological State of Suspensions in Varying the Surface Area of Nano-Silica Particles and Molecular Weight of the Poly (Ethylene Oxide) Matrix. Colloid Polym. Sci. 2017, 295, 555–563. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, L.; Lu, X.; Shi, C.; Tang, T.; Wang, X.; Huang, Q.; Zeng, H. Adsorption Kinetics of Asphaltenes at Oil/Water Interface: Effects of Concentration and Temperature. Fuel 2018, 212, 387–394. [Google Scholar] [CrossRef]
- Parra-Barraza, H.; Hernández-Montiel, D.; Lizardi, J.; Hernández, J.; Urbina, R.H.; Valdez, M.A. The Zeta Potential and Surface Properties of Asphaltenes Obtained with Different Crude Oil/n-Heptane Proportions☆. Fuel 2003, 82, 869–874. [Google Scholar] [CrossRef]
- Soleymani, H.; Hosseinpour, N.; Bahaloo, M.H.; Taghipour, M. Optimizing Oil-Water Interfacial Rheology for in-Situ Synthesis of Nanoparticles and Asphaltene Control during Oil Production. Colloids Surf. A Physicochem. Eng. Asp. 2024, 682, 132888. [Google Scholar] [CrossRef]
- Desai, V.R.; Sidarai, A.H.; Hunagund, S.M.; Basanagouda, M.; Melavanki, R.M.; Fattepur, R.H.; Kadadevarmath, J.S. Steady State Absorption and Fluorescence Study: Estimation of Ground and Excited State Dipole Moments of Newly Synthesized Pyridazin-3(2H)-One Derivatives. J. Mol. Liq. 2016, 223, 141–149. [Google Scholar] [CrossRef]
- Shirai, K.; Matsuoka, M.; Fukunishi, K. Fluorescence Quenching by Intermolecular π–π Interactions of 2,5-Bis(N,N-Dialkylamino)-3,6-Dicyanopyrazines. Dye. Pigment. 1999, 42, 95–101. [Google Scholar] [CrossRef]
- Zeng, H.; Song, Y.-Q.; Johnson, D.L.; Mullins, O.C. Critical Nanoaggregate Concentration of Asphaltenes by Direct-Current (DC) Electrical Conductivity. Energy Fuels 2009, 23, 1201–1208. [Google Scholar] [CrossRef]
- Mullins, O.C.; Seifert, D.J.; Zuo, J.Y.; Zeybek, M. Clusters of Asphaltene Nanoaggregates Observed in Oilfield Reservoirs. Energy Fuels 2013, 27, 1752–1761. [Google Scholar] [CrossRef]
- Hristova, E.; Tchoukov, P.; Stoyanov, S.R. Coalescence Inhibition and Agglomeration Initiation near the Critical Dilution of Asphaltene Precipitation. Colloids Surf. A Physicochem. Eng. Asp. 2021, 629, 127400. [Google Scholar] [CrossRef]
- Mullins, O.C. The Modified Yen Model. Energy Fuels 2010, 24, 2179–2207. [Google Scholar] [CrossRef]
- Li, X.; Chi, P.; Guo, X.; Sun, Q. Effects of Asphaltene Concentration and Asphaltene Agglomeration on Viscosity. Fuel 2019, 255, 115825. [Google Scholar] [CrossRef]


| Parameters | Value | Method |
|---|---|---|
| Density (20 °C)/g cm−3 | 0.93 | ASTM D1217 [22] |
| Viscosity (50 °C)/mm2 s−1 | 59.00 | ASTM D445 [23] |
| Salt/mg Cl−1 kg−1 | 101.56 | ASTM D6470 [24] |
| Sulfur content/wt% | 0.88 | ASTM D5453 [25] |
| Resins/wt% | 24.90 | ASTM D4124 [26] |
| Asphlatenes/wt% | 11.40 | ASTM D4124 [26] |
| Saturates/wt% | 29.32 | ASTM D4124 [26] |
| Aromatics/wt% | 34.38 | ASTM D4124 [26] |
| Asphaltene sample | Composition (wt%) | ||||
| C | H | N | S | O (by difference) | |
| 83.31 | 7.21 | 1.55 | 2.35 | 2.32 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, Y.; Zhang, X.; Cheng, L.; Zhang, H.; Tang, J.; Chen, H.; Fan, Q.; Ouyang, X. Effect of Asphaltenes on the Stability of Water in Crude Oil Emulsions. Materials 2025, 18, 630. https://doi.org/10.3390/ma18030630
Peng Y, Zhang X, Cheng L, Zhang H, Tang J, Chen H, Fan Q, Ouyang X. Effect of Asphaltenes on the Stability of Water in Crude Oil Emulsions. Materials. 2025; 18(3):630. https://doi.org/10.3390/ma18030630
Chicago/Turabian StylePeng, Yan, Xiangyu Zhang, Lihua Cheng, Hong Zhang, Jieyun Tang, Hong Chen, Qinzhen Fan, and Xinping Ouyang. 2025. "Effect of Asphaltenes on the Stability of Water in Crude Oil Emulsions" Materials 18, no. 3: 630. https://doi.org/10.3390/ma18030630
APA StylePeng, Y., Zhang, X., Cheng, L., Zhang, H., Tang, J., Chen, H., Fan, Q., & Ouyang, X. (2025). Effect of Asphaltenes on the Stability of Water in Crude Oil Emulsions. Materials, 18(3), 630. https://doi.org/10.3390/ma18030630





