Thermal Degradation of Polymer Composites Based on Unsaturated-Polyester-Resin- and Vinyl-Ester-Resin- Filled Kraft Lignin
Abstract
1. Introduction
2. Materials and Methods
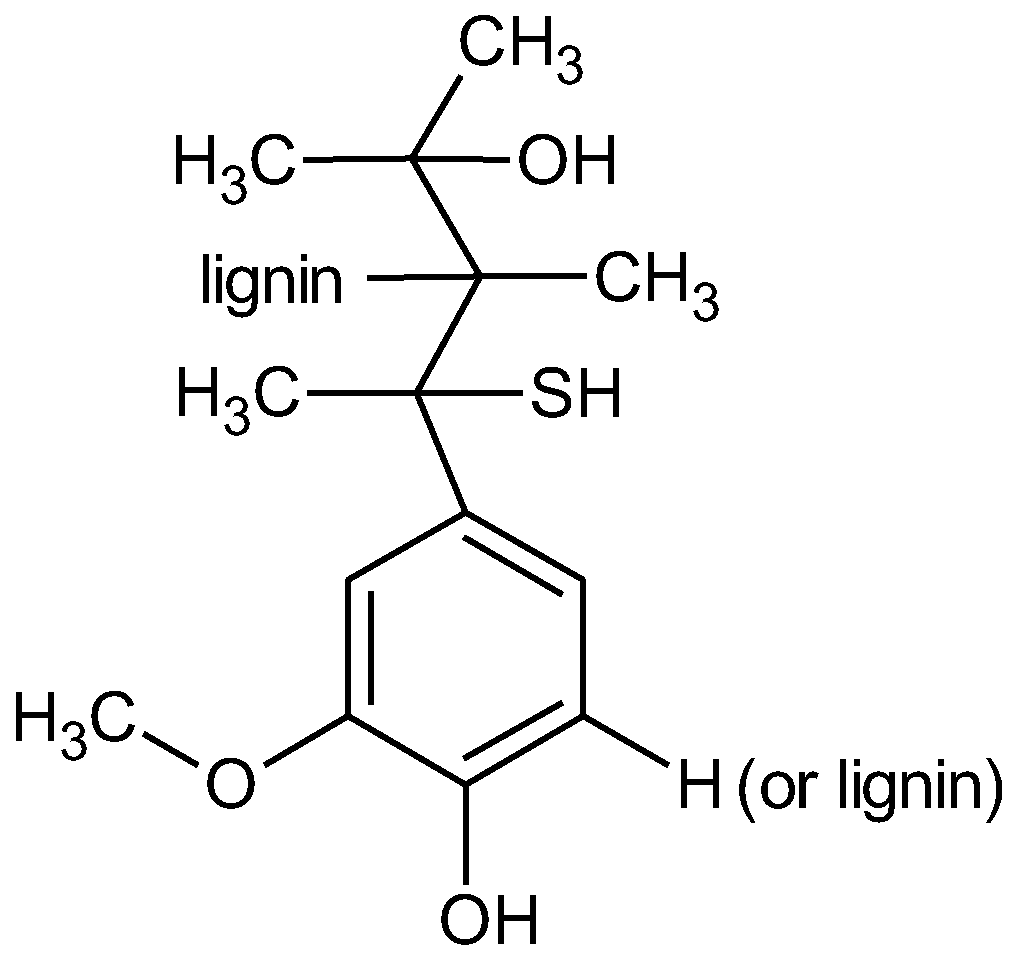
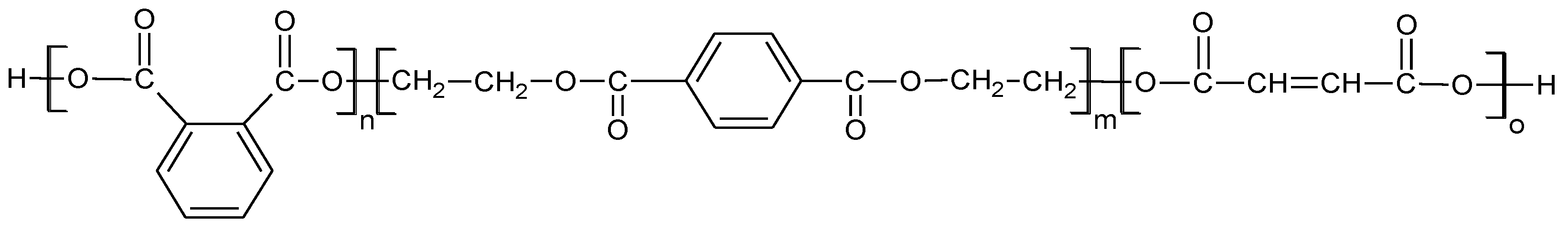
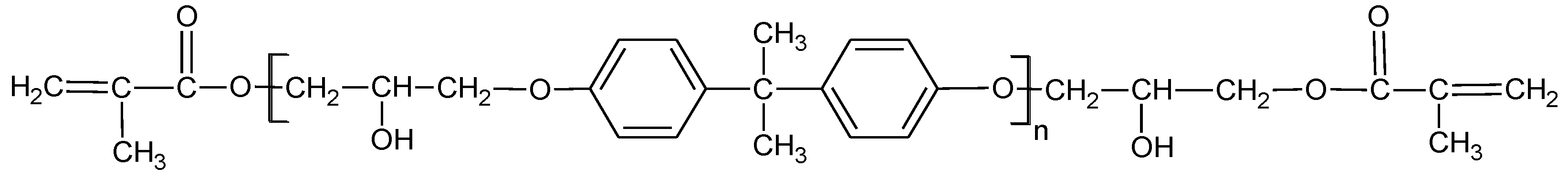
2.1. Materials
2.2. Methods
2.2.1. Thermal Properties
2.2.2. Calculation of Activation Energy of Desorption
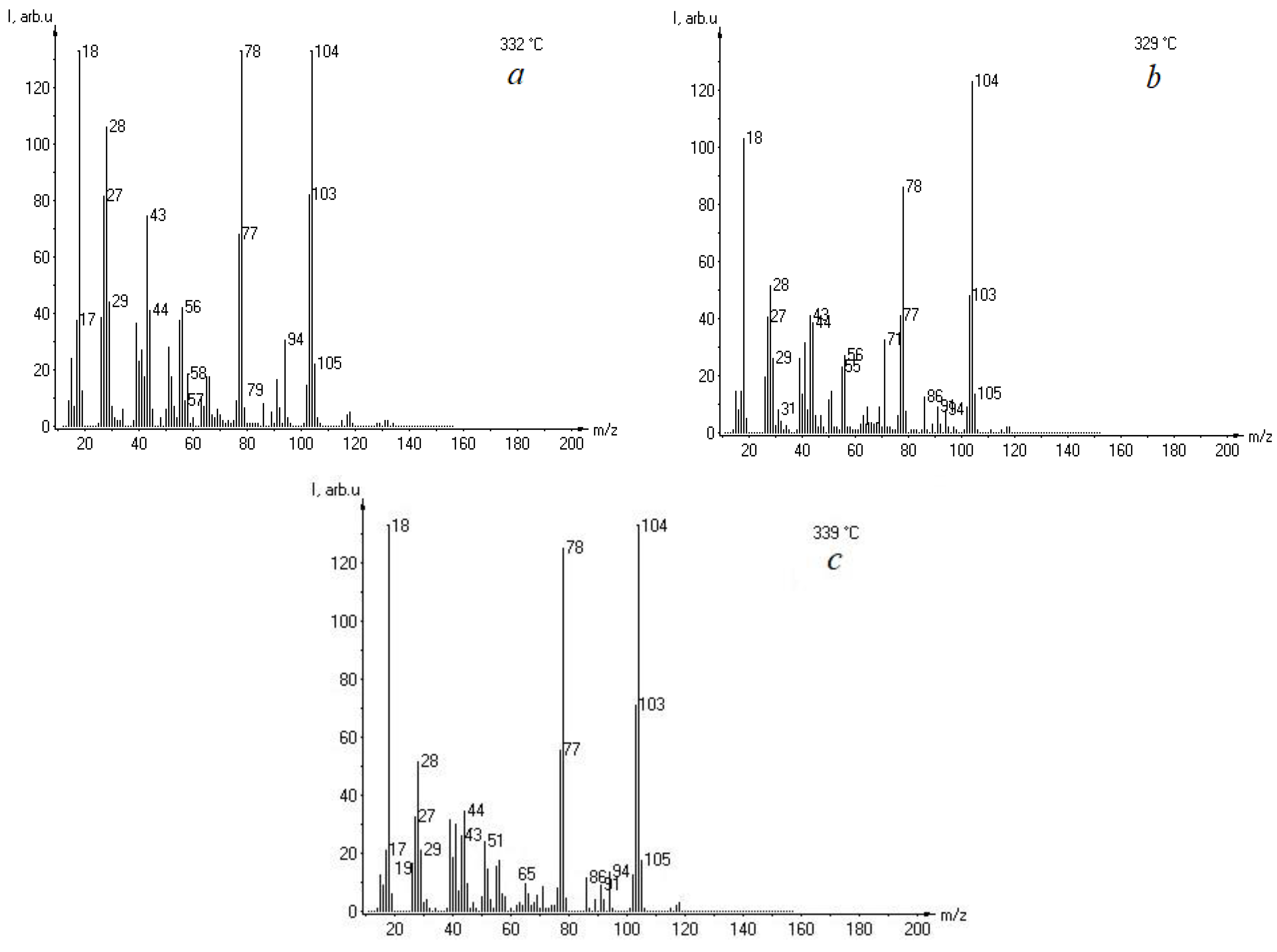
3. Results and Discussion
- (1)
- for the native resin with a temperature of Tmax1 = 456 °C, Tmax2 = 540 °C, and Tmax3 = 598 °C;
- (2)
- for a resin with 5 wt% lignin with a temperature of Tmax1 = 455 °C, Tmax2 = 517 °C, and Tmax3 = 645 °C;
- (3)
- for a resin with 15 wt% lignin with a temperature of Tmax1 = 445 °C and Tmax2 = 510 °C.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Attia-Essaies, S.; Barhoumi, N.; Ayachi, H.; Bouraoui, H.; Srasra, E. Thermal and Mechanical Performances of Unsaturated Polyester Composites Reinforced by Natural Fillers. Chem. Afr. 2024, 7, 1523–1533. [Google Scholar] [CrossRef]
- Nodehi, M. Epoxy, polyester and vinyl ester based polymer concrete: A review. Innov. Infrastruct. Solut. 2022, 7, 64. [Google Scholar] [CrossRef]
- Chabros, A.; Gawdzik, B.; Podkościelna, B.; Goliszek, M.; Pączkowski, P. Composites of Unsaturated Polyester Resins with Microcrystalline Cellulose and Its Derivatives. Materials 2020, 13, 62. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Chung, H. Biodegradable polymers: From synthesis methods to applications of lignin-graft-polyester. Green Chem. 2024, 26, 10774–10803. [Google Scholar] [CrossRef]
- Ma, C.; Kim, T.-H.; Liu, K.; Ma, M.-G.; Choi, S.-E.; Si, C. Multifunctional Lignin-Based Composite Materials for Emerging Applications. Front. Bioeng. Biotechnol. 2021, 9, 708976. [Google Scholar] [CrossRef] [PubMed]
- Winterowd, J.G. Liquid Kraft Lignin Compositions. U.S. Patent 9,090,731, 28 July 2015. [Google Scholar]
- Bass, G.F.; Epps, T.H., III. Recent developments towards performance-enhancing lignin-based polymers. Polym. Chem. 2021, 12, 4130–4158. [Google Scholar] [CrossRef]
- Karaoki, P.K.; Zhang, S.; Cai, C.M.; Dim, P.E.; Ragauskas, A.J. Thermally stable and self-healable lignin-based polyester. Polym. Test. 2024, 137, 108515. [Google Scholar] [CrossRef]
- Heldt, H.-W. Plant Biochemistry, 3rd ed.; Elsevier Academic Press: San Diego, CA, USA, 2005; ISBN 0-12-088391-033. [Google Scholar]
- Jędrzejczak, P.; Puszka, A.; Kubiak, A.; Podkościelna, B.; Klapiszewski, Ł. New lignin-based hybrid materials as functional additives for polymer biocomposites: From design to application. Int. J. Biol. Macromol. 2021, 190, 624–635. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, G.; Antorrena, G.; González, J.; Freire, S. The Influence of Pulping Conditions on the Structure of Acetosolv Eucalyptus Lignins. J. Wood Chem. Technol. 1997, 17, 147–162. [Google Scholar] [CrossRef]
- Olivares, M.; Guzmán, J.; Natho, A.; Saavedra, A. Kraft lignin utilization in adhesives. Wood Sci. Technol. 1988, 22, 157–165. [Google Scholar] [CrossRef]
- Laurichesse, S.; Avérous, L. Chemical modification of lignins: Towards biobased polymers. Prog. Polym. Sci. 2014, 39, 1266–1290. [Google Scholar] [CrossRef]
- Sen, S.; Patil, S.; Argyropoulos, D.S. Thermal properties of lignin in copolymers, blends, and composites: A review. Green Chem. 2015, 17, 4862–4887. [Google Scholar] [CrossRef]
- Ahmaruzzaman, M. Industrial wastes as low-cost potential adsorbents for the treatment of wastewater laden with heavy metals. Adv. Colloid Interface Sci. 2011, 166, 36–59. [Google Scholar] [CrossRef] [PubMed]
- Vishtal, A.G.; Kraslawski, A. Challenges in industrial applications of technical lignins. BioResources 2011, 6, 3547–3568. [Google Scholar] [CrossRef]
- Thakur, V.K.; Thakur, M.K.; Raghavan, P.; Kessler, M.R. Progress in green polymer composites from lignin for multifunctional applications: A review. ACS Sustain. Chem. Eng. 2014, 2, 1072–1092. [Google Scholar] [CrossRef]
- Windeisen, E.; Wegener, G. Lignin as building unit for polymers. In Polymer Science: A Comprehensive Reference. Polymers for a Sustainable Environment and Green Energy; McGrath, J.E., Hickner, M.A., Höfer, R., Eds.; Elsevier B.V.: Amsterdam, The Netherlands, 2012; pp. 255–265. [Google Scholar]
- Yeasmin, F.; Ara Masud, R.; Chisty, A.H.; Hossain, M.A.; Mallik, A.K.; Rahman, M.M. Lignin-metal oxide composite for photocatalysis and photovoltaics. In Renewable Polymers and Polymer-Metal Oxide Composites: Synthesis, Properties, and Applications; Haider, S., Haider, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 447–476. [Google Scholar] [CrossRef]
- Khan, T.A.; Lee, J.-H.; Kim, H.-J. Lignin-Based Adhesives and Coatings. In Lignocellulose for Future Bioeconomy; Ariffin, H., Sapuan, S.M., Hassan, M.A., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 153–206. [Google Scholar] [CrossRef]
- Nasrullah, A.; Bhat, A.H.; Sada Khan, A.; Ajab, H. Comprehensive approach on the structure, production, processing, and application of lignin. In Lignocellulosic Fibre and Biomass-Based Composite Materials; Jawaid, M., Tahir, P.M., Saba, N., Eds.; Woodhead Publishing: Cambridge, UK, 2017; pp. 165–178. [Google Scholar] [CrossRef]
- Wypych, A. Lignin. In Databook of Adhesion Promoters, 2nd ed.; ChemTec Publishing: Toronto, ON, Canada, 2018; p. 149. [Google Scholar] [CrossRef]
- Seitz, J.; Buschalsky, A.; Reinders, P.M.; Mai, C.; Viöl, W.; Köhler, R. Generation of a lignin-polyester coating for the corrosion protection of steel surfaces applied by atmospheric plasma spraying. Prog. Org. Coat. 2024, 194, 108598. [Google Scholar] [CrossRef]
- Zaghloul, M.Y.M.; Zaghloul, M.M.Y.; Zaghloul, M.M.Y. Developments in polyester composite materials—An in-depth review on natural fibres and nano fillers. Compos. Struct. 2021, 278, 114698. [Google Scholar] [CrossRef]
- Hodásová, L.; Jablonský, M.; Škulcová, A.; Ház, A. Lignin, potential products and their market value. Wood Res. 2015, 60, 973–986. [Google Scholar]
- Yeo, J.-S.; Lee, J.-H.; Hwang, S.-H. Effects of lignin on the volume shrinkage and mechanical properties of a styrene/unsaturated polyester/lignin ternary composite system. Compos. Part B Eng. 2017, 130, 167–173. [Google Scholar] [CrossRef]
- Yeo, J.-S.; Seong, D.-W.; Hwang, S.-H. Chemical surface modification of lignin particle and its application as filler in the polypropylene composites. J. Ind. Eng. Chem. 2015, 31, 80–85. [Google Scholar] [CrossRef]
- Machado, M.; Hofmann, M.; Garrido, M.; Correia, J.R.; Bordado, J.C.; Rosa, I.C. Incorporation of Lignin in Bio-Based Resins for Potential Application in Fiber–Polymer Composites. Appl. Sci. 2023, 13, 8342. [Google Scholar] [CrossRef]
- Rogers, D.P. Lignin-Derived Thermosetting Vinyl Ester Resins for High Performance Applications. Master’s Thesis, Rowan University, Glassboro, NJ, USA, 2015. [Google Scholar]
- Miyamoto, M. Vinyl Ester Resin Composition That Contains Minute Polymer Particles, Process for Production of Same, and Cured Products of Same. U.S. Patent 9,109,069, 18 August 2015. [Google Scholar]
- Thielemans, W.; Can, E.; Morye, S.S.; Wool, R.P. Novel applications of lignin in composite materials. J. Appl. Polym. Sci. 2002, 83, 323–331. [Google Scholar] [CrossRef]
- Laleg, M.; Jemaa, N.; Wafa Al Dajani, W.; Zhang, Y.; Paleologou, M. Meltable Lignin Compositions, Methods for Producing Them and Their Uses. U.S. Patent 2021/0040273, 11 February 2021. [Google Scholar]
- Vinardell, M.P.; Mitjans, M. Lignins and Their Derivatives with Beneficial Effects on Human Health. Int. J. Mol. Sci. 2017, 18, 1219. [Google Scholar] [CrossRef]
- Nan, N.; Hu, W.; Wang, J. Lignin-Based Porous Biomaterials for Medical and Pharmaceutical Applications. Biomedicines 2022, 10, 747. [Google Scholar] [CrossRef]
- Karagoz, P.; Khiawjan, S.; Marques, M.P.C.; Santzouk, S.; Bugg, T.D.H.; Lye, G.J. Pharmaceutical applications of lignin-derived chemicals and lignin-based materials: Linking lignin source and processing with clinical indication. Biomass Convers. Biorefinery 2024, 14, 26553–26574. [Google Scholar] [CrossRef]
- Pagonis, V.; Kitis, G.; Furetta, C. Numerical and Practical Exercises in Thermoluminescence; Springer Science: New York, NY, USA, 2006. [Google Scholar]
- Redkina, A.V.; Konovalova, N.D.; Khomenko, K.N.; Belokopytov, J.V. Synthesis from titanaerosil mesoporous of systems of TiO2–SiO2 with supported V2O5, their physical-chemical and catalytic properties. I. Hydrothermal synthesis, acidic and catalytic properties of Ti–MCM-41 in the process of dehydrogenation of propane. Catal. Petrochem. 2012, 21, 1–10. (In Russian) [Google Scholar]
- Pączkowski, P.; Puszka, A.; Gawdzik, B. Investigation of Degradation of Composites Based on Unsaturated Polyester Resin and Vinyl Ester Resin. Materials 2022, 15, 1286. [Google Scholar] [CrossRef]
- Pączkowski, P.; Puszka, A.; Gawdzik, B. Effect of Eco-Friendly Peanut Shell Powder on the Chemical Resistance, Physical, Thermal and Thermomechanical Properties of Unsaturated Polyester Resin Composites. Polymers 2021, 13, 3690. [Google Scholar] [CrossRef] [PubMed]
- Pączkowski, P.; Puszka, A.; Gawdzik, B. Green Composites Based on Unsaturated Polyester Resin from Recycled Poly(Ethylene Terephthalate) with Wood Flour as Filler—Synthesis, Characterization and Aging Effect. Polymers 2020, 12, 2966. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Li, J.; Zhang, J.; Cai, Z. Thermal Decomposition of Kraft Lignin under Gas Atmospheres of Argon, Hydrogen, and Carbon Dioxide. Polymers 2018, 10, 729. [Google Scholar] [CrossRef] [PubMed]
- Ferdosian, F.; Yuan, Z.; Anderson, M.; Xu, C.C. Thermal performance and thermal decomposition kinetics of lignin-based epoxy resins. J. Anal. Appl. Pyrolysis 2016, 119, 124–132. [Google Scholar] [CrossRef]
- Brebu, M.; Vasile, C. Thermal degradation of lignin—A Review. Cellul. Chem. Technol. 2010, 44, 353–363. [Google Scholar]
- Arza, C.R.; Wang, P.; Linares-Pastén, J.; Zhang, B. Synthesis, thermal, rheological characteristics, and enzymatic degradation of aliphatic polyesters with lignin-based aromatic pendant groups. J. Polym. Sci. Part A Polym. Chem. 2019, 57, 2314–2323. [Google Scholar] [CrossRef]
- Xanthopoulou, E.; Zamboulis, A.; Terzopoulou, Z.; Bikiaris, D.N.; Kourtidou, D.; Tarani, E.; Chrissafis, C.; Papageorgiou, G.Z. Towards novel lignin-based aromatic polyesters: In-depth study of the thermal degradation and crystallization of poly(propylene vanillate). Thermochim. Acta 2022, 709, 179145. [Google Scholar] [CrossRef]
- Siharova, N.V.; Sementsov, Y.I.; Zhuravsky, S.V.; Borysenko, M.V.; Starokadomsky, D.L.; Yurieva, K.A.; Terets, A.D.; Mistchanchuk, O.V.; Pączkowski, P.; Gawdzik, B. Thermal destruction and thermophysical properties of polymer composites based on polyester resin with different content of carbon nanotubes. Him. Fiz. Tehnol. Poverhni 2024, 15, 488–499. [Google Scholar] [CrossRef]

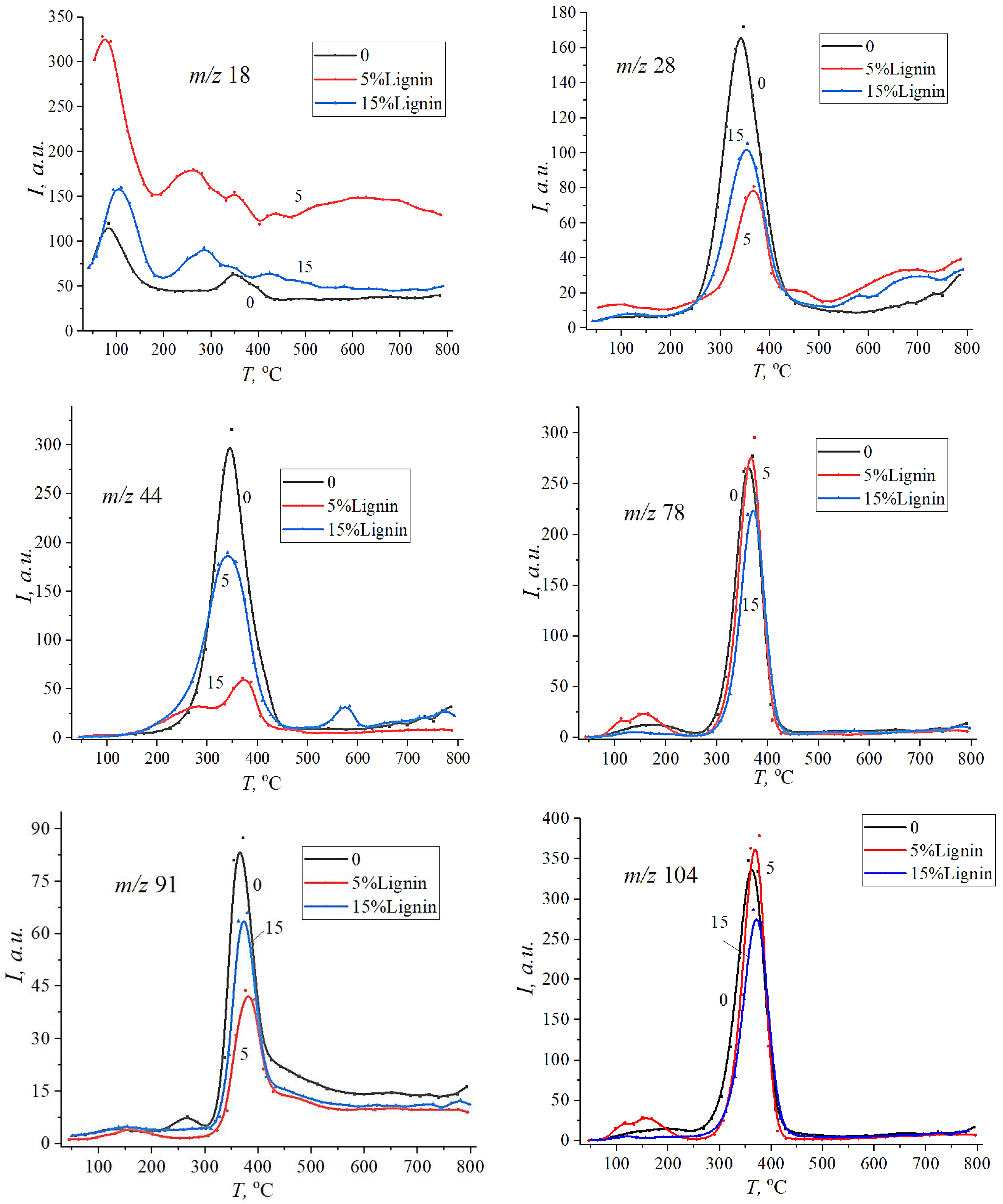
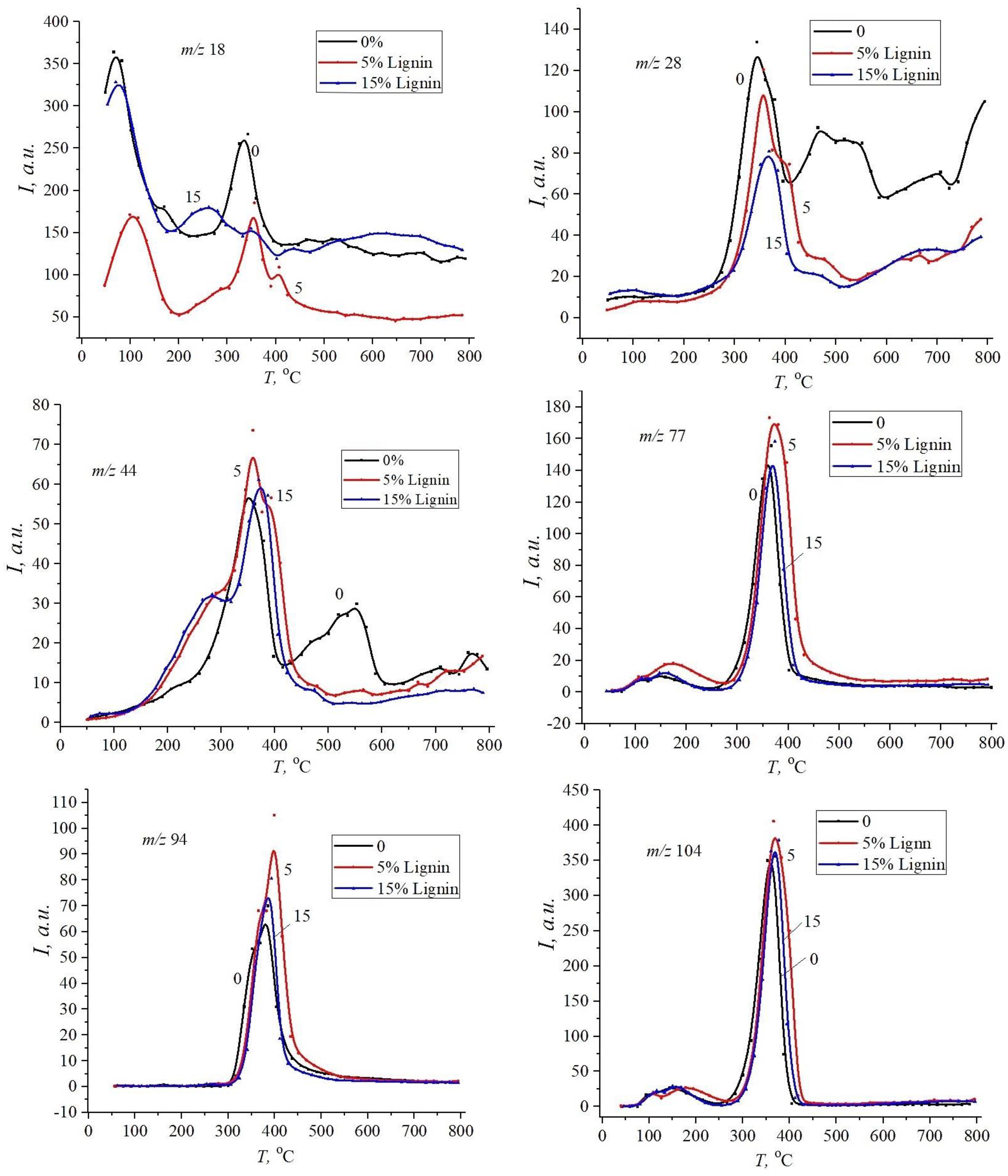
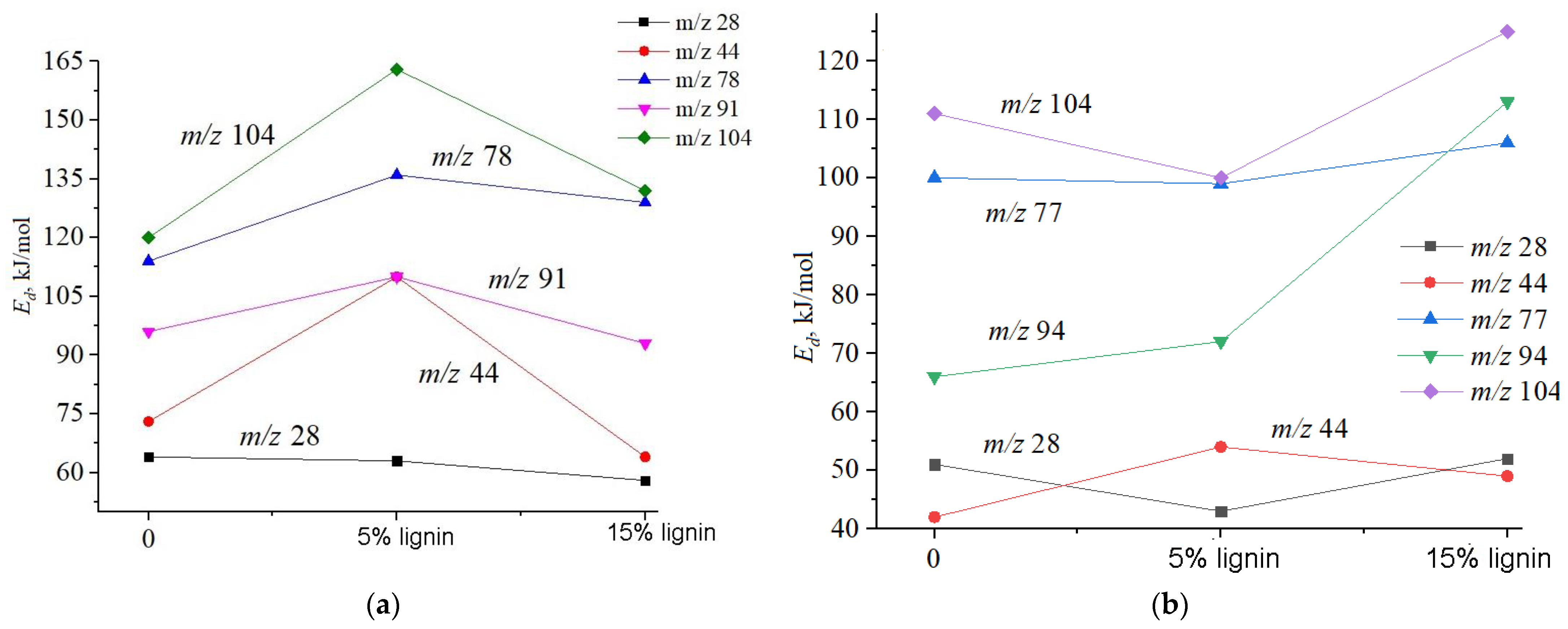
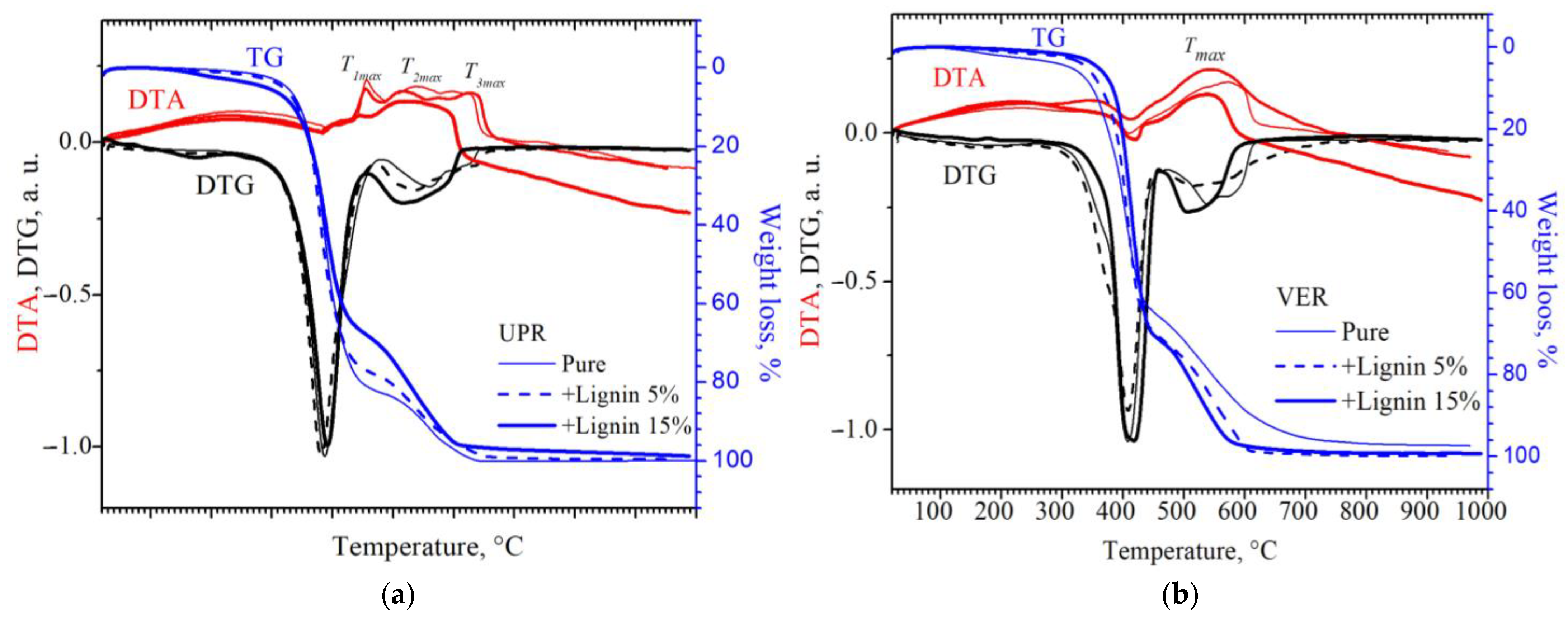
| Sample | , J·mol−1·K−1 | , eB | , J·mol−1·K−1 | , eB | , J·mol−1·K−1 | , eB |
|---|---|---|---|---|---|---|
| pure UPR | 90.8 | 0.94 | 102.7 | 1.06 | 110.0 | 1.14 |
| UPR + 5 wt% lignin | 90.6 | 0.94 | 99.4 | 1.03 | 107.7 | 1.11 |
| UPR + 15 wt% lignin | 89.2 | 0.92 | 98.4 | 1.03 | - | - |
| Sample | , J·mol−1·K−1 | , eB |
|---|---|---|
| pure VER | 108.5 | 1.12 |
| VER + 5 wt% lignin | 103.4 | 1.07 |
| VER + 15 wt% lignin | 102.7 | 1.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siharova, N.V.; Pączkowski, P.; Sementsov, Y.I.; Zhuravsky, S.V.; Borysenko, M.V.; Terets, A.D.; Mischanchuk, O.V.; Terets, M.I.; Hrebelna, Y.V.; Gawdzik, B. Thermal Degradation of Polymer Composites Based on Unsaturated-Polyester-Resin- and Vinyl-Ester-Resin- Filled Kraft Lignin. Materials 2025, 18, 524. https://doi.org/10.3390/ma18030524
Siharova NV, Pączkowski P, Sementsov YI, Zhuravsky SV, Borysenko MV, Terets AD, Mischanchuk OV, Terets MI, Hrebelna YV, Gawdzik B. Thermal Degradation of Polymer Composites Based on Unsaturated-Polyester-Resin- and Vinyl-Ester-Resin- Filled Kraft Lignin. Materials. 2025; 18(3):524. https://doi.org/10.3390/ma18030524
Chicago/Turabian StyleSiharova, Nadiia V., Przemysław Pączkowski, Yuriy I. Sementsov, Serhiy V. Zhuravsky, Mykola V. Borysenko, Andriy D. Terets, Olexandr V. Mischanchuk, Mariia I. Terets, Yulia V. Hrebelna, and Barbara Gawdzik. 2025. "Thermal Degradation of Polymer Composites Based on Unsaturated-Polyester-Resin- and Vinyl-Ester-Resin- Filled Kraft Lignin" Materials 18, no. 3: 524. https://doi.org/10.3390/ma18030524
APA StyleSiharova, N. V., Pączkowski, P., Sementsov, Y. I., Zhuravsky, S. V., Borysenko, M. V., Terets, A. D., Mischanchuk, O. V., Terets, M. I., Hrebelna, Y. V., & Gawdzik, B. (2025). Thermal Degradation of Polymer Composites Based on Unsaturated-Polyester-Resin- and Vinyl-Ester-Resin- Filled Kraft Lignin. Materials, 18(3), 524. https://doi.org/10.3390/ma18030524








