Design and Characterization of Silane-Modified Bio-Based Non-Isocyanate Polyurethane Coatings for Advanced Surface Applications
Abstract
1. Introduction
2. Experimental Details
2.1. Materials
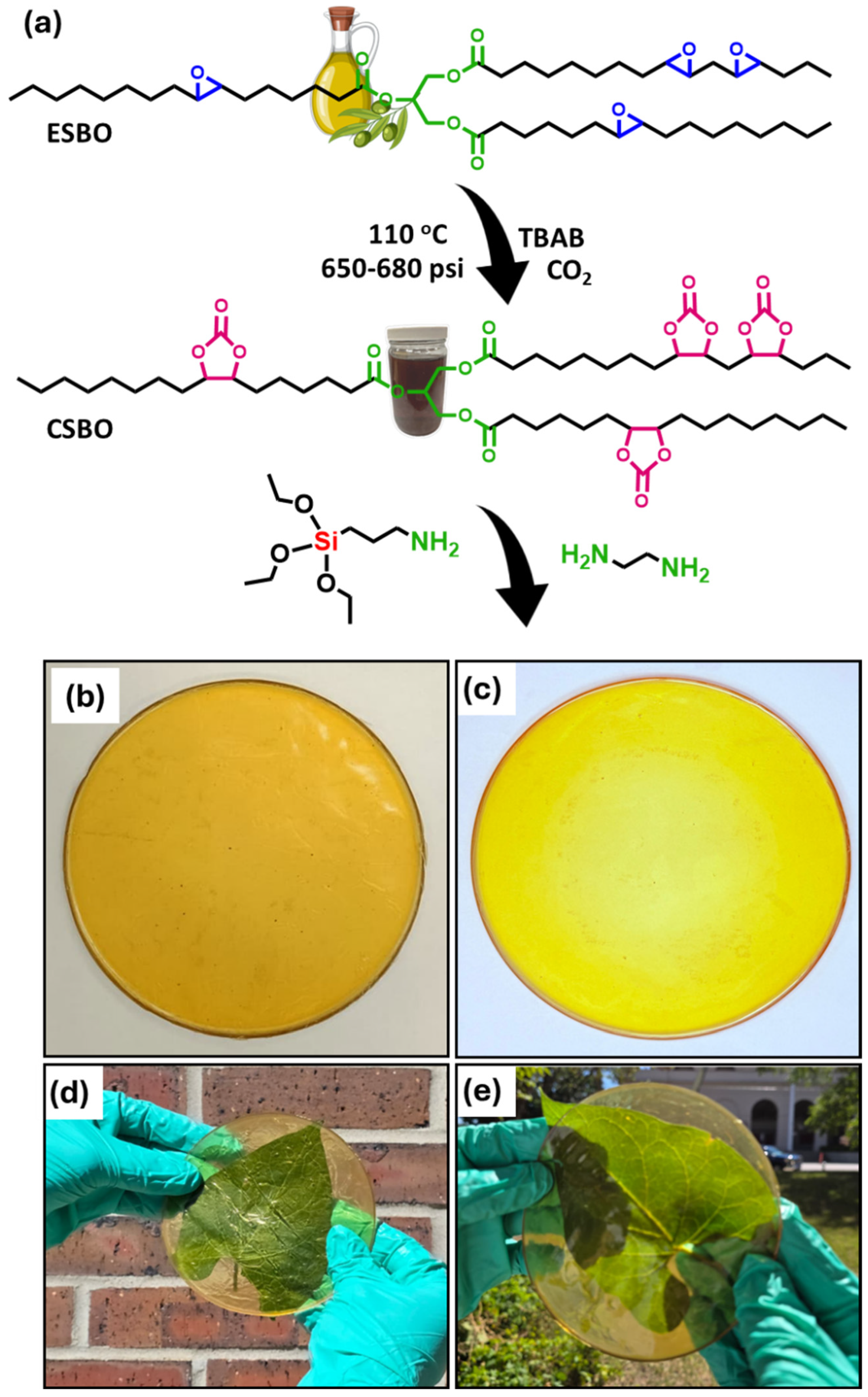
2.2. Synthesis of Carbonated Soybean Oil
2.3. Synthesis of Non-Isocyanate Polyurethane Coating Materials
2.4. Mechanism of the Synthesis of Non-Isocyanate Polyurethane
2.5. Characterizations
3. Characterization Results and Discussion
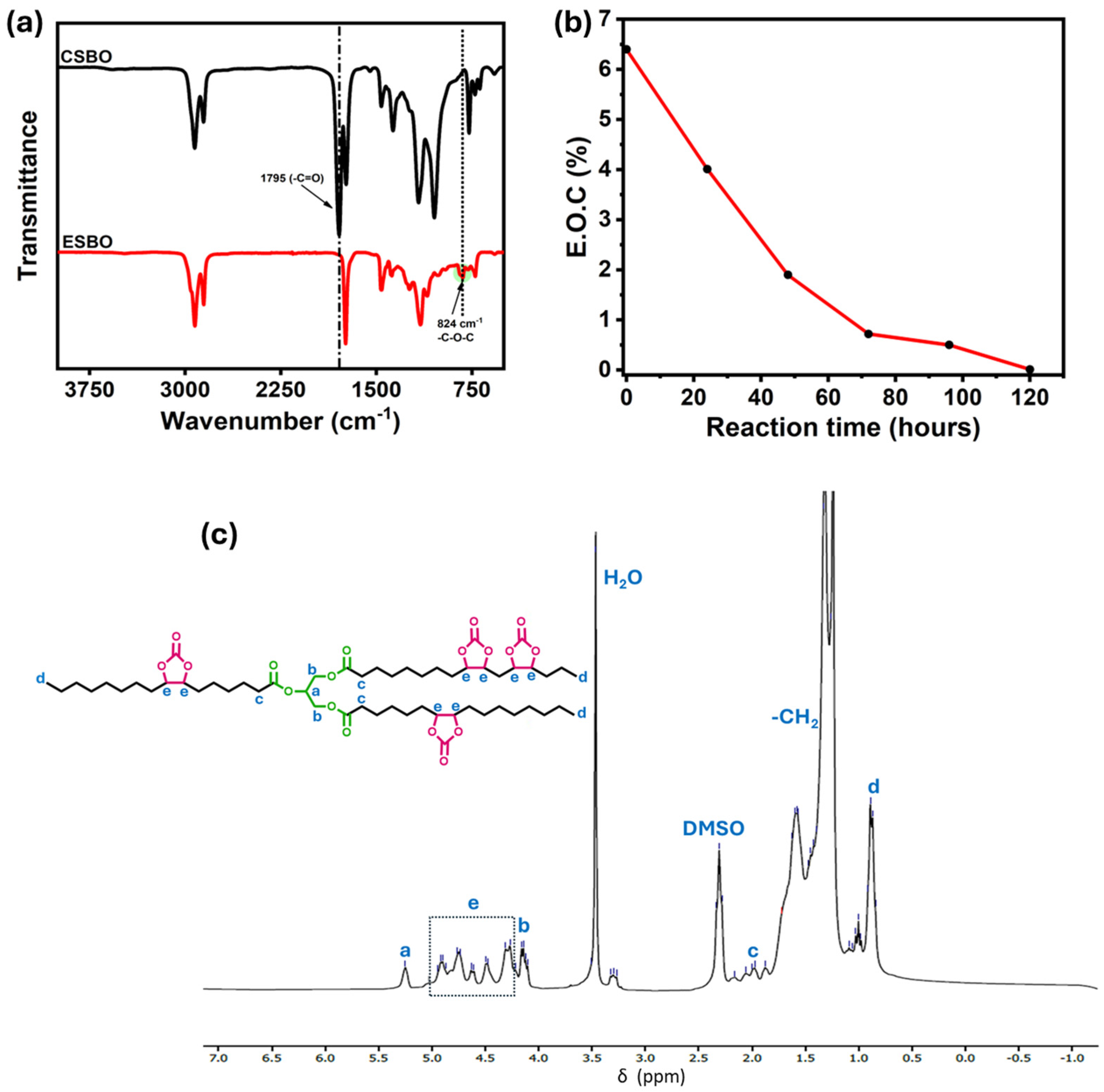
3.1. Characterizations of the Synthesis of Carbonated Soybean Oil
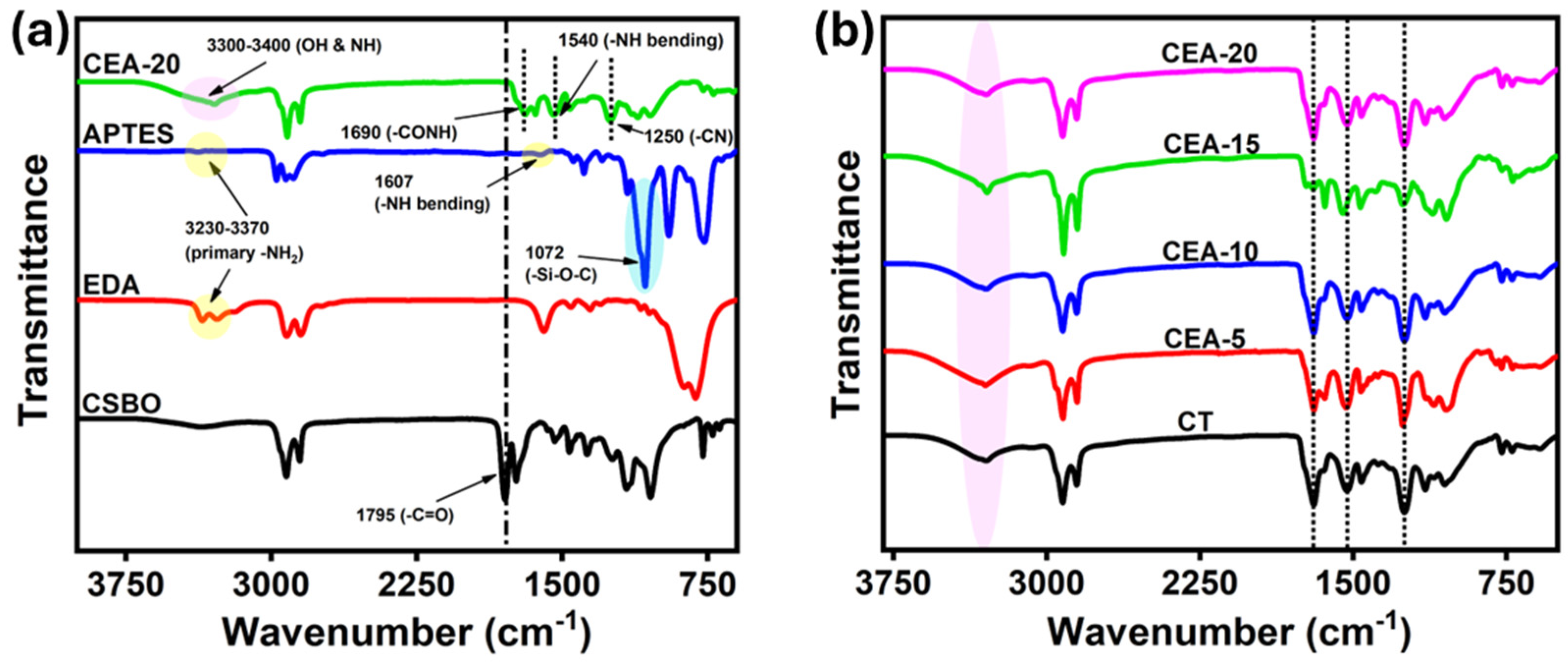
3.2. Structural Characterizations of Non-Isocyanate Polyurethane Coating Materials
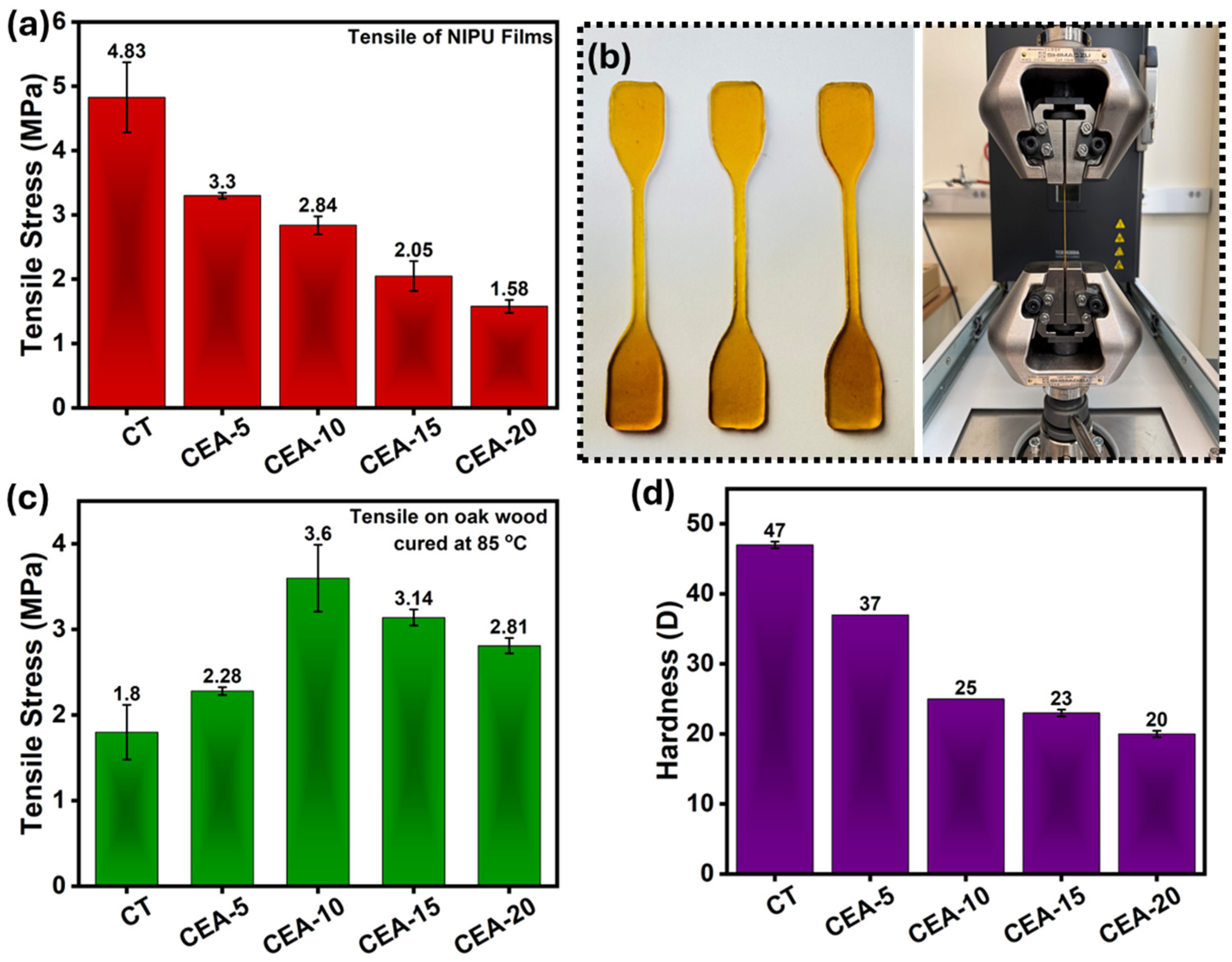
3.3. Mechanical Property of Non-Isocyanate Polyurethane Coating Materials
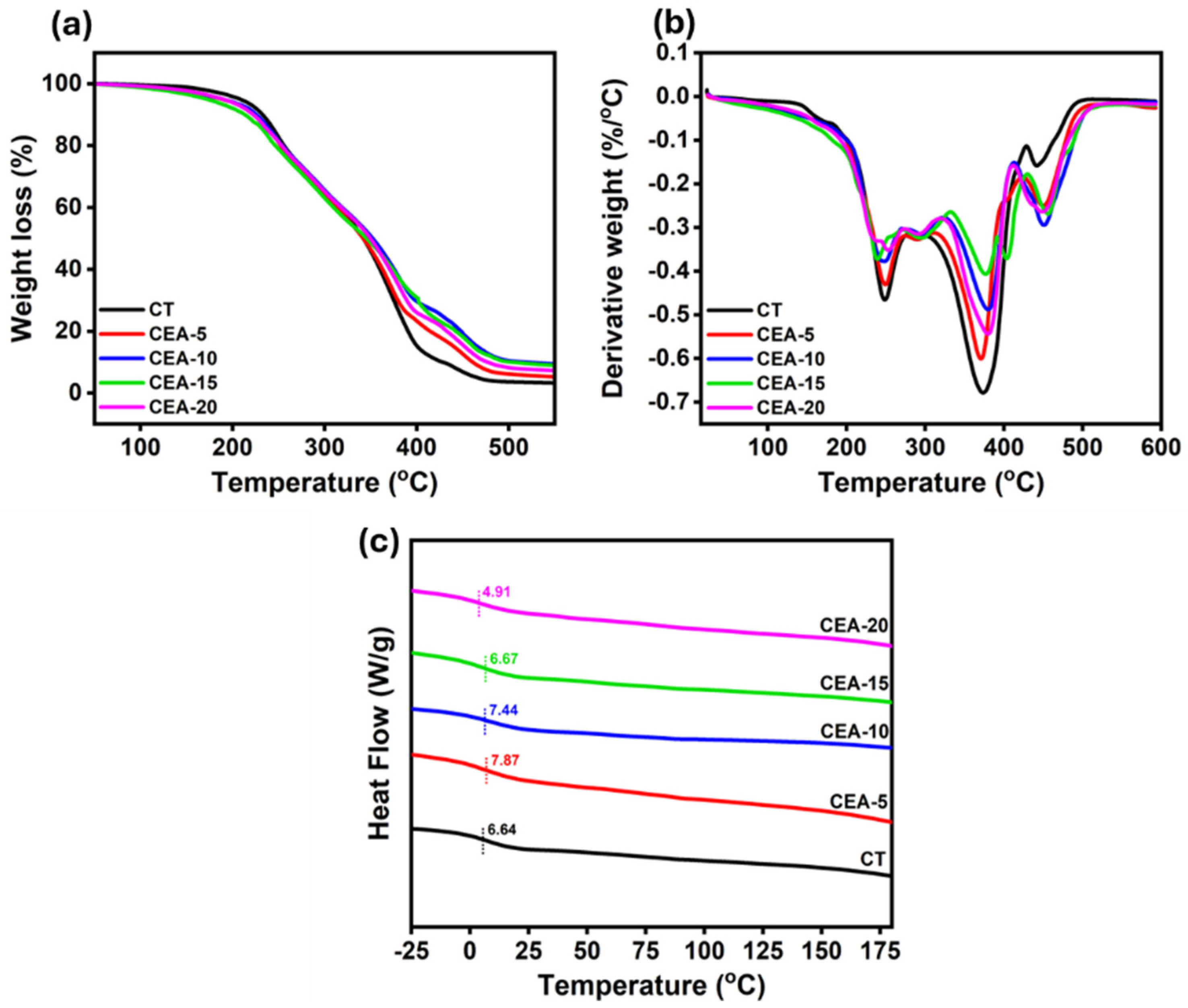
3.4. Thermal Property of Non-Isocyanate Polyurethane Coating Materials
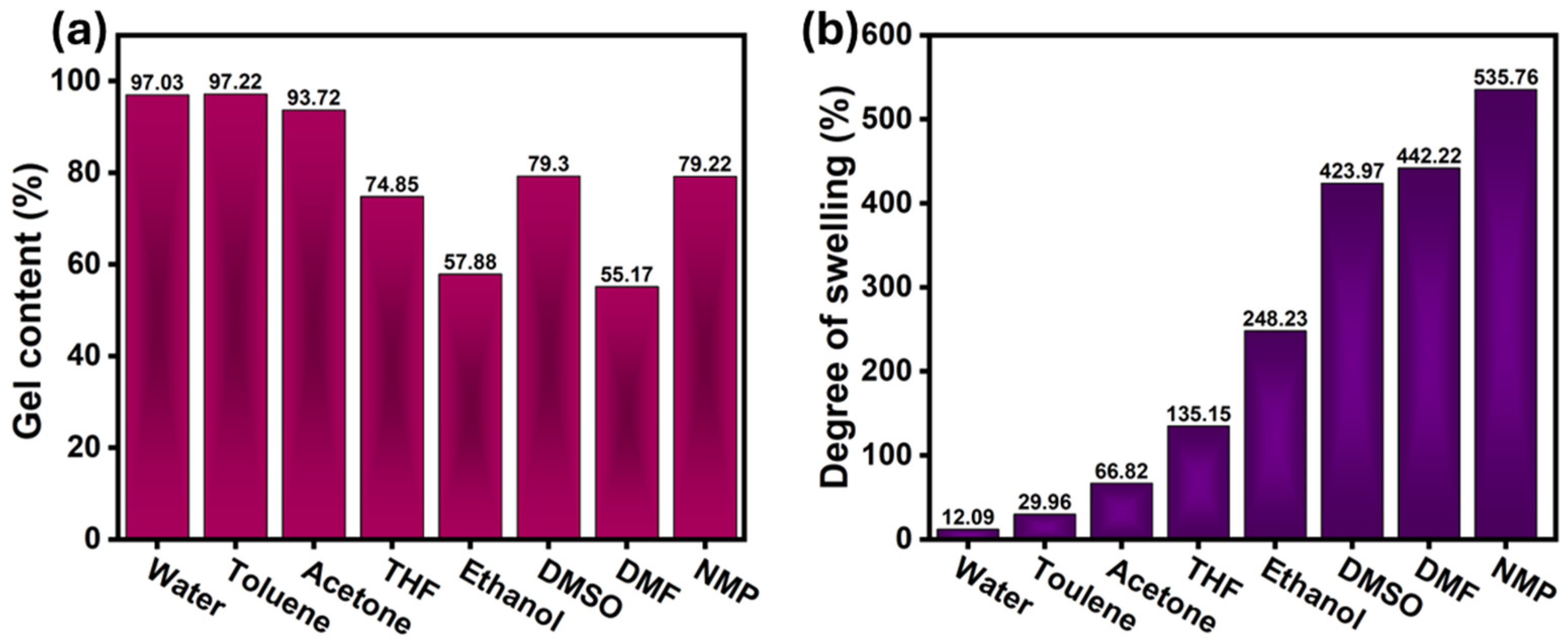
3.5. Gel Fraction and Degree of Swelling Test
3.6. Contact Angle Measurement
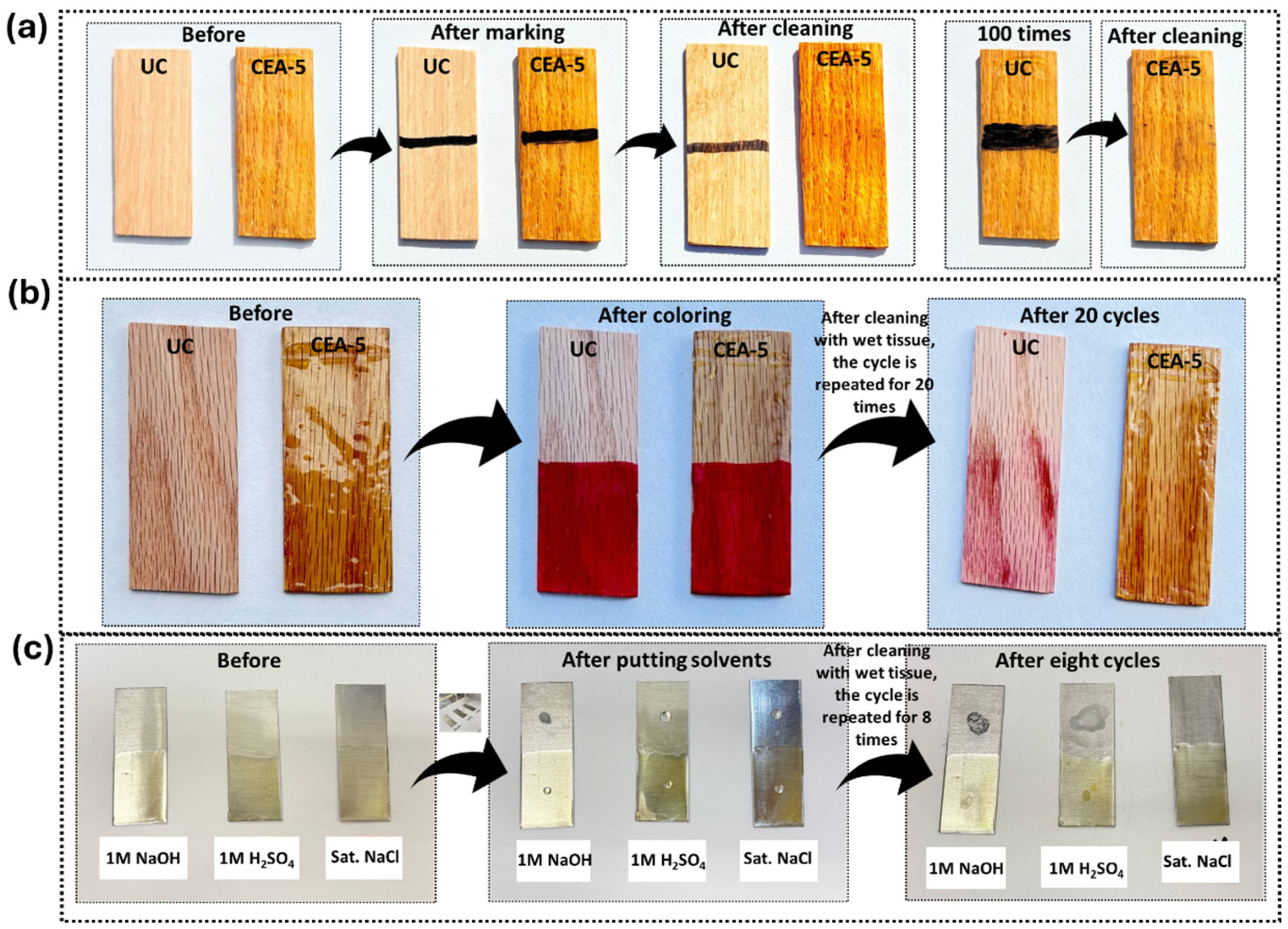
3.7. Ink Contraction Behavior/Color Repellency Test
3.8. Chemical Resistance Test
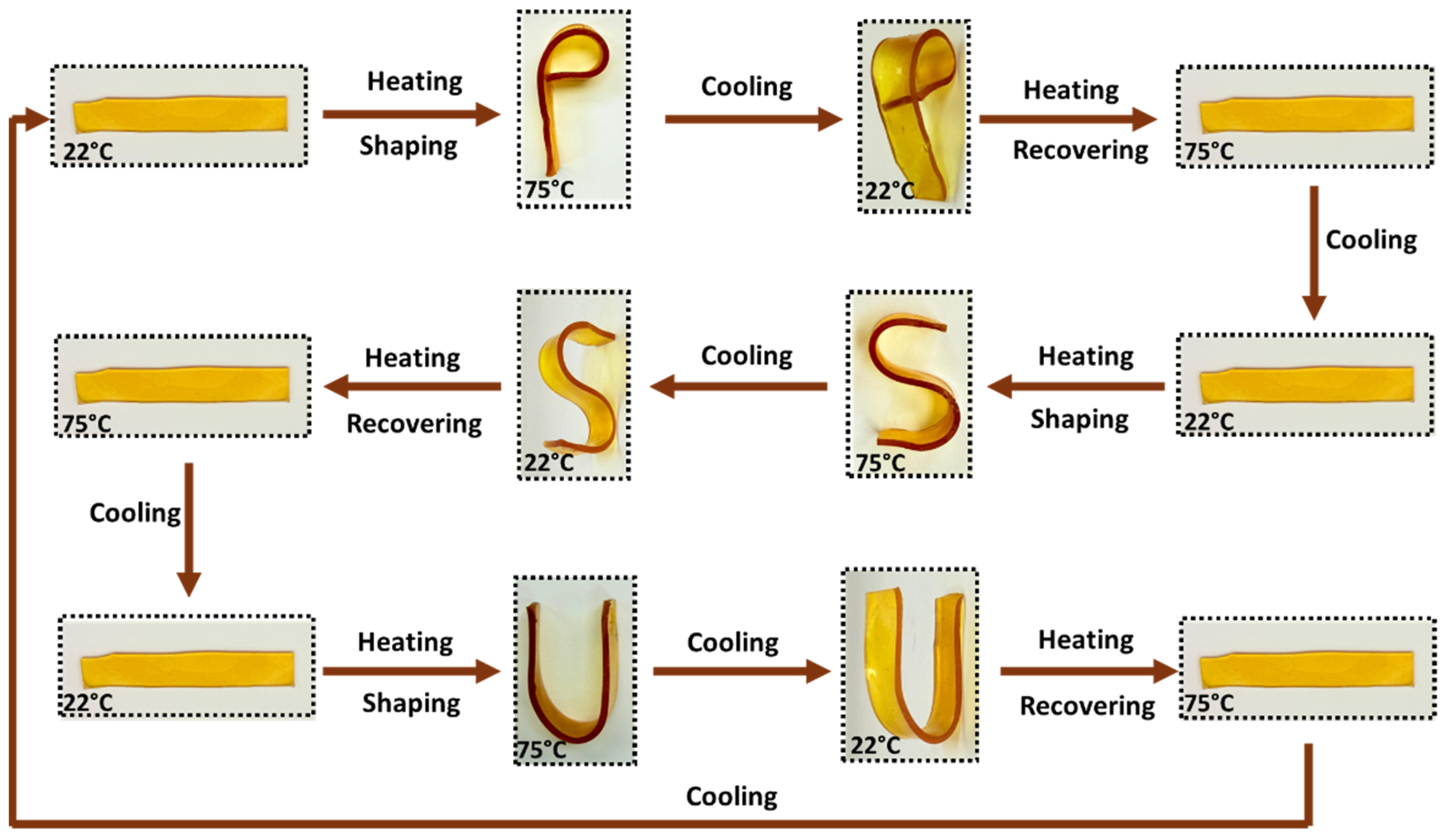
3.9. Shape Memory Observation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chaudhary, M.L.; Patel, R.; Gupta, R.K. Beyond Isocyanates: Advances in Non-Isocyanate Polyurethane Chemistry and Applications. Polymer 2025, 332, 128553. [Google Scholar] [CrossRef]
- Chaudhary, M.L.; Gupta, R.K. Environmental and Health Concerns in Polyurethane. In Non-Isocyanate Polyurethanes: Chemistry, Progress, and Challenges; Mayankkumar, C., Ram, G., Eds.; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2025; pp. 15–30. [Google Scholar]
- Patel, R.; Chaudhary, M.L.; Chaudhary, S.; Gupta, R.K. Effect of distinct molecular structure of diols on the properties of bio-based wood adhesive. Int. J. Adhes. Adhes. 2025, 138, 103936. [Google Scholar] [CrossRef]
- Chaudhary, M.L.; Patel, R.; Chaudhari, S.; Gupta, R.K. Impact of diverse diols and diisocyanates on thermosetting bio-based polyurethane films. Next Mater. 2025, 8, 100609. [Google Scholar] [CrossRef]
- Chaudhary, M.L.; Patel, Y.N.; Chaudhari, S.; Patel, R.; Gupta, R.K. Enhancing the mechanical strength of soy-based polyurethane adhesives through incorporation of schiff base diols. Int. J. Adhes. Adhes. 2025, 142, 104118. [Google Scholar] [CrossRef]
- Wang, J.; Seidi, F.; Huang, Y.; Xiao, H. Smart lignin-based polyurethane conjugated with corrosion inhibitor as bio-based anticorrosive sublayer coating. Ind. Crops Prod. 2022, 188, 115719. [Google Scholar] [CrossRef]
- Ha, Z.; Lei, L.; Zhou, M.; Xia, Y.; Chen, X.; Mao, P.; Fan, B.; Shi, S. Bio-Based Waterborne Polyurethane Coatings with High Transparency, Antismudge and Anticorrosive Properties. ACS Appl. Mater. Interfaces 2023, 15, 7427–7441. [Google Scholar] [CrossRef]
- Yao, L.; Baharum, A.; Yu, L.J.; Yan, Z.; Haji Badri, K. Bio-based polyurethane as a sustainable coating material for controlled-release fertilizer. Discov. Sustain. 2025, 6, 828. [Google Scholar] [CrossRef]
- Kashyap, S.S.; Borah, K.; Shailesh Kumar, J.S.; Sheerazi, Z.; Narayan, R.; Ahmed, M. Role of Silicoaluminophosphate as a Corrosion Inhibiting Nanocontainer and Filler in Environmentally Benign Bio-Based Hybrid Coatings. ACS Sustain. Resour. Manag. 2024, 1, 1211–1224. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, G.; Wang, L.; Zhou, Y.; Lei, Z.; Wang, X. A Biomass-Derived Imine-Linked Waterborne Polyurethane for Self-Healable Anticorrosive Coating. ACS Appl. Polym. Mater. 2025, 7, 11998–12006. [Google Scholar] [CrossRef]
- Chen, B.; Fang, H.; Cheng, F.; Sun, J.; Jiang, T. A Biomass Graphene Composite Fluorescent Coating for Encryption of Anti-Counterfeit Information. ACS Appl. Polym. Mater. 2025, 7, 3409–3419. [Google Scholar] [CrossRef]
- Wang, H.; Min, J.; Qu, J. High strength, self-healing, and highly hydrophobic bio-based non-isocyanate polyurethane. Prog. Org. Coat. 2025, 208, 109435. [Google Scholar] [CrossRef]
- Narducci, L.; Habets, T.; Grossman, Q.; Grignard, B.; Meier, M.A.R.; Detrembleur, C. Solvent-free Synthesis of Room-Temperature Curable Nonisocyanate Polyurethane Adhesives and Coatings. Macromolecules 2025, 58, 6452–6465. [Google Scholar] [CrossRef]
- Qin, X.; Wang, X.; Zhang, Z. Toward Non-Isocyanate Polyurethanes: Green Synthesis Routes and Recycling Technologies. Chem. Eur. J. 2025, 31, e02319. [Google Scholar] [CrossRef]
- Haniffa, M.M.A.C.; Munawar, K.; Ching, Y.C.; Illias, H.A.; Chuah, C.H. Bio-based Poly(hydroxy urethane)s: Synthesis and Pre/Post-Functionalization. Chem. Asian J. 2021, 16, 1281–1297. [Google Scholar] [CrossRef]
- Schimpf, V.; Ritter, B.S.; Weis, P.; Parison, K.; Mülhaupt, R. High Purity Limonene Dicarbonate as Versatile Building Block for Sustainable Non-Isocyanate Polyhydroxyurethane Thermosets and Thermoplastics. Macromolecules 2017, 50, 944–955. [Google Scholar] [CrossRef]
- Balla, E.; Bikiaris, D.N.; Pardalis, N.; Bikiaris, N.D. Toward Sustainable Polyurethane Alternatives: A Review of the Synthesis, Applications, and Lifecycle of Non-Isocyanate Polyurethanes (NIPUs). Polymers 2025, 17, 1364. [Google Scholar] [CrossRef]
- Gomez-Lopez, A.; Grignard, B.; Calvo, I.; Detrembleur, C.; Sardon, H. Synergetic Effect of Dopamine and Alkoxysilanes in Sustainable Non-Isocyanate Polyurethane Adhesives. Macromol. Rapid Commun. 2021, 42, 2000538. [Google Scholar] [CrossRef]
- Dong, J.; Liu, B.; Ding, H.; Shi, J.; Liu, N.; Dai, B.; Kim, I. Bio-based healable non-isocyanate polyurethanes driven by the cooperation of disulfide and hydrogen bonds. Polym. Chem. 2020, 11, 7524–7532. [Google Scholar] [CrossRef]
- Choong, P.S.; Chong, N.X.; Wai Tam, E.K.; Seayad, A.M.; Seayad, J.; Jana, S. Biobased Nonisocyanate Polyurethanes as Recyclable and Intrinsic Self-Healing Coating with Triple Healing Sites. ACS Macro Lett. 2021, 10, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Pichon, E.; De Smet, D.; Rouster, P.; Freulings, K.; Pich, A.; Bernaerts, K. V Bio-based non-isocyanate polyurethane(urea) waterborne dispersions for water resistant textile coatings. Mater. Today Chem. 2023, 34, 101822. [Google Scholar] [CrossRef]
- Al Ghatta, A.; Hallett, J.P. Bioderived furanic compounds as replacements for BTX in chemical intermediate applications. RSC Sustain. 2023, 1, 698–745. [Google Scholar] [CrossRef]
- Ling, Z.; Gu, L.; Liu, S.; Su, Y.; Zhou, Q. Non-isocyanate polyurethane from bio-based feedstocks and their interface applications. RSC Appl. Interfaces 2025, 2, 1123–1142. [Google Scholar]
- Patel, P.; Patel, R.; Chaudhary, M.L.; Chaudhary, N.; Gupta, R.K. High-Performance Bio-Based Non-Isocyanate Polyurethane Adhesive: A Solvent and Catalyst-Free Synthesis Approach. J. Polym. Environ. 2024, 32, 5024–5035. [Google Scholar]
- Peña-Alonso, R.; Rubio, F.; Rubio, J.; Oteo, J.L. Study of the hydrolysis and condensation of γ-Aminopropyltriethoxysilane by FT-IR spectroscopy. J. Mater. Sci. 2007, 42, 595–603. [Google Scholar]
- Livage, J.; Henry, M.; Sanchez, C. Sol-gel chemistry of transition metal oxides. Prog. Solid State Chem. 1988, 18, 259–341. [Google Scholar] [CrossRef]
- Tao, Q.; He, H.; Li, T.; Frost, R.L.; Zhang, D.; He, Z. Tailoring surface properties and structure of layered double hydroxides using silanes with different number of functional groups. J. Solid State Chem. 2014, 213, 176–181. [Google Scholar] [CrossRef]
- Innocenzi, P. Infrared spectroscopy of sol–gel derived silica-based films: A spectra-microstructure overview. J. Non-Cryst. Solids 2003, 316, 309–319. [Google Scholar]
- Liu, G.; Guan, C.; Xia, H.; Guo, F.; Ding, X.; Peng, Y. Novel Shape-Memory Polymer Based on Hydrogen Bonding. Macromol. Rapid Commun. 2006, 27, 1100–1104. [Google Scholar]
- Zhang, S.; Ran, Q.; Fu, Q.; Gu, Y. Preparation of Transparent and Flexible Shape Memory Polybenzoxazine Film through Chemical Structure Manipulation and Hydrogen Bonding Control. Macromolecules 2018, 51, 6561–6570. [Google Scholar] [CrossRef]
- Xu, J.; Shi, W.; Pang, W. Synthesis and shape memory effects of Si–O–Si cross-linked hybrid polyurethanes. Polymer 2006, 47, 457–465. [Google Scholar]
- ASTM D882; Standard Test Method for Tensile Properties of Thin Plastic Sheeting. ASTM International: West Conshohocken, PA, USA, 2018.
- ASTM D2240; Standard Test Method for Rubber Property—Durometer Hardness. ASTM International: West Conshohocken, PA, USA, 2021.
- Dhore, N.; Prasad, E.; Narayan, R.; Rao, C.R.K.; Palanisamy, A. Studies on biobased non-isocyanate polyurethane coatings with potential corrosion resistance. Sustain. Chem. 2023, 4, 95–109. [Google Scholar] [CrossRef]
- Patel, P.; de Souza, F.M.; Gupta, R.K. Study of Soybean Oil-Based Non-Isocyanate Polyurethane Films via a Solvent and Catalyst-Free Approach. ACS Omega 2024, 9, 5862–5875. [Google Scholar]
- Doley, S.; Dolui, S.K. Solvent and catalyst-free synthesis of sunflower oil based polyurethane through non-isocyanate route and its coatings properties. Eur. Polym. J. 2018, 102, 161–168. [Google Scholar]
- Li, Z.; Zhao, Y.; Yan, S.; Wang, X.; Kang, M.; Wang, J.; Xiang, H. Catalytic Synthesis of Carbonated Soybean Oil. Catal. Lett. 2008, 123, 246–251. [Google Scholar] [CrossRef]
- Javni, I.; Hong, D.P.; Petrović, Z.S. Soy-based polyurethanes by nonisocyanate route. J. Appl. Polym. Sci. 2008, 108, 3867–3875. [Google Scholar]
- Didovets, Y.; Brela, M.Z. Structure–Property Relationship between Hard Segments of Shape Memory Polyurethane Copolymers and Interchain Hydrogen Bonds: A Comprehensive Theoretical Study. J. Phys. Chem. B 2025, 129, 10504–10520. [Google Scholar] [CrossRef]
- Saha, J.K.; Rahman, M.M.; Haq, M.B.; Al Shehri, D.A.; Jang, J. Theoretical and Experimental Studies of Hydrogen Bonded Dihydroxybenzene Isomers Polyurethane Adhesive Material. Polymers 2022, 14, 1701. [Google Scholar] [CrossRef]
- Liu, J.; Ma, R.; Zheng, W.; Wang, M.; Sun, T.; Zhu, J.; Tang, Y.; Wang, J. Cross-Linking Network of Soft–Rigid Dual Chains to Effectively Suppress Volume Change of Silicon Anode. J. Phys. Chem. Lett. 2022, 13, 7712–7721. [Google Scholar] [PubMed]
- Chuang, F.S.; Tsen, W.C.; Shu, Y.C. The effect of different siloxane chain-extenders on the thermal degradation and stability of segmented polyurethanes. Polym. Degrad. Stab. 2004, 84, 69–77. [Google Scholar] [CrossRef]
- Adhikari, R.; Gunatillake, P.A.; McCarthy, S.J.; Bown, M.; Meijs, G.F. Low-modulus siloxane–polyurethanes. Part II. Effect of chain extender structure on properties and morphology. J. Appl. Polym. Sci. 2003, 87, 1092–1100. [Google Scholar]
- Kowalczyk, S.; Dębowski, M.; Iuliano, A.; Brzeski, S.; Plichta, A. Synthesis of (Hyper)Branched Monohydroxyl Alkoxysilane Oligomers toward Silanized Urethane Prepolymers. Molecules 2022, 27, 2790. [Google Scholar] [CrossRef]
- Jena, K.K.; Narayan, R.; Alhassan, S.M. Highly branched graphene siloxane−polyurethane-urea (PU-urea) hybrid coatings. Prog. Org. Coat. 2017, 111, 343–353. [Google Scholar] [CrossRef]
- Ramalho, L.D.C.; Sánchez-Arce, I.J.; Campilho, R.D.S.G.; Belinha, J. Strength prediction of composite single lap joints using the critical longitudinal strain criterion and a meshless method. Int. J. Adhes. Adhes. 2021, 108, 102884. [Google Scholar] [CrossRef]
- Tenorio-Alfonso, A.; Sánchez, M.C.; Franco, J.M. Preparation, Characterization and Mechanical Properties of Bio-Based Polyurethane Adhesives from Isocyanate-Functionalized Cellulose Acetate and Castor Oil for Bonding Wood. Polymers 2017, 9, 132. [Google Scholar] [CrossRef]
- Borrero-López, A.M.; Guzmán, D.B.; González-Delgado, J.A.; Arteaga, J.F.; Valencia, C.; Pischel, U.; Franco, J.M. Toward UV-Triggered Curing of Solvent-Free Polyurethane Adhesives Based on Castor Oil. ACS Sustain. Chem. Eng. 2021, 9, 11032–11040. [Google Scholar] [CrossRef]
- Khoon Poh, A.; Choy Sin, L.; Sit Foon, C.; Cheng Hock, C. Polyurethane wood adhesive from palm oil-based polyester polyol. J. Adhes. Sci. Technol. 2014, 28, 1020–1033. [Google Scholar] [CrossRef]
- Aung, M.M.; Yaakob, Z.; Kamarudin, S.; Abdullah, L.C. Synthesis and characterization of Jatropha (Jatropha curcas L.) oil-based polyurethane wood adhesive. Ind. Crops Prod. 2014, 60, 177–185. [Google Scholar] [CrossRef]
- Kong, X.; Liu, G.; Curtis, J.M. Characterization of canola oil based polyurethane wood adhesives. Int. J. Adhes. Adhes. 2011, 31, 559–564. [Google Scholar] [CrossRef]
- Silva, B.B.R.; Santana, R.M.C.; Forte, M.M.C. A solventless castor oil-based PU adhesive for wood and foam substrates. Int. J. Adhes. Adhes. 2010, 30, 559–565. [Google Scholar] [CrossRef]
- Gama, N.; Ferreira, A.; Barros-Timmons, A. Cure and performance of castor oil polyurethane adhesive. Int. J. Adhes. Adhes. 2019, 95, 102413. [Google Scholar] [CrossRef]
- Chaudhary, M.L.; Patel, P.; Patel, R.; Gupta, R.K. Castor oil-based polyurethane adhesives: Effect of cross-linker on the bond strength. Mater. Today Commun. 2024, 39, 109172. [Google Scholar] [CrossRef]
- Gomez-Lopez, A.; Grignard, B.; Calvo, I.; Detrembleur, C.; Sardon, H. Monocomponent non-isocyanate polyurethane adhesives based on a sol–gel process. ACS Appl. Polym. Mater. 2020, 2, 1839–1847. [Google Scholar] [CrossRef]
- Dong, T.; Dheressa, E.; Wiatrowski, M.; Pereira, A.P.; Zeller, A.; Laurens, L.M.L.; Pienkos, P.T. Assessment of Plant and Microalgal Oil-Derived Nonisocyanate Polyurethane Products for Potential Commercialization. ACS Sustain. Chem. Eng. 2021, 9, 12858–12869. [Google Scholar] [CrossRef]
- Pagacz, J.; Hebda, E.; Janowski, B.; Sternik, D.; Jancia, M.; Pielichowski, K. Thermal decomposition studies on polyurethane elastomers reinforced with polyhedral silsesquioxanes by evolved gas analysis. Polym. Degrad. Stab. 2018, 149, 129–142. [Google Scholar] [CrossRef]
- Bhuvaneswari, G.H. 3-Degradability of Polymers. In Plastics Design Library; William Andrew Publishing: Norwich, NY, USA, 2018; pp. 29–44. [Google Scholar]
- Caussé, N.; Bonin, P.; Thierry, D.; Le Bozec, N.; Roggero, A.; Pébère, N. Ageing processes of coil-coated materials: Temperature-controlled electrochemical impedance analysis. Prog. Org. Coat. 2023, 183, 107682. [Google Scholar] [CrossRef]
- Bukowczan, A.; Łukaszewska, I.; Pielichowski, K. Thermal degradation of non-isocyanate polyurethanes. J. Therm. Anal. Calorim. 2024, 149, 10885–10899. [Google Scholar] [CrossRef]
- Parcheta-Szwindowska, P.; Rohde, K.; Datta, J. Bio-derived polyurethanes obtained by non-isocyanate route using polyol-based bis (cyclic carbonate) s—Studies on thermal decomposition behavior. J. Therm. Anal. Calorim. 2022, 147, 13329–13339. [Google Scholar] [CrossRef]
- Shang, Q.; Hu, L.; Yang, X.; Hu, Y.; Bo, C.; Pan, Z.; Ren, X.; Liu, C.; Zhou, Y. Superhydrophobic cotton fabric coated with tannic acid/polyhedral oligomeric silsesquioxane for highly effective oil/water separation. Prog. Org. Coat. 2021, 154, 106191. [Google Scholar] [CrossRef]
- Andrady, A.L.; Heikkilä, A.M.; Pandey, K.K.; Bruckman, L.S.; White, C.C.; Zhu, M.; Zhu, L. Effects of UV radiation on natural and synthetic materials. Photochem. Photobiol. Sci. 2023, 22, 1177–1202. [Google Scholar] [CrossRef]
- Tryznowski, M.; Izdebska-Podsiadły, J.; Żołek-Tryznowska, Z. Wettability and surface free energy of NIPU coatings based on bis(2,3-dihydroxypropyl)ether dicarbonate. Prog. Org. Coat. 2017, 109, 55–60. [Google Scholar] [CrossRef]
- Zhong, X.; Lv, L.; Hu, H.; Jiang, X.; Fu, H. Bio-based coatings with liquid repellency for various applications. Chem. Eng. J. 2020, 382, 123042. [Google Scholar] [CrossRef]
- Patel, R.; Patel, P.; Chaudhary, M.L.; Gupta, R.K. Fluorine-Free, Biobased Antismudge Polyurethane Coating with Enhanced Flame Retardancy. ACS Appl. Polym. Mater. 2024, 6, 7278–7287. [Google Scholar] [CrossRef]
- Bodhak, C.; Patel, D.; Gupta, R.K. Mechanically Robust, Self-Healable, and Reprocessable Geraniol-Based Epoxy Vitrimer by Dynamic Boronic Ester Bonds. ACS Appl. Eng. Mater. 2025, 3, 1599–1612. [Google Scholar] [CrossRef]
| Sr. No. | Bio-Based Material | Fillers | Substrate | Tensile Strength (MPa) | Reference DOI |
|---|---|---|---|---|---|
| 1 | Castor oil (50%) | HDI, cellulose Acetate | Poplar wood | 2.84 | [47] |
| 2 | Castor oil (20%) | HDI, cellulose Acetate | Poplar wood | 2.37 | [47] |
| 3 | Castor oil (70%) | HDI, cellulose Acetate | Poplar wood | 0.94 | [47] |
| 4 | Castor oil | Cadaverine + Nvoc-Cl | Polyethylene | 4.6 | [48] |
| 5 | Castor Oil | Cadaverine + Nvoc-Cl | Oak wood | 2 | [48] |
| 6 | Palm oil-based polyester polyol with MDI | Glycerol | Teak wood | 5.3 | [49] |
| 7 | Palm oil-based polyester polyol with toluene diisocyanate | Glycerol | Teak wood | 4.7 | [49] |
| 8 | Palm oil polyol | - | Hard wood | 2.6 | [50] |
| 9 | Jatropha oil polyol | - | Hard wood | 4.9 | [50] |
| 10 | Canola polyol | - | Birch wood | 5.7 | [51] |
| 11 | Castor Oil | - | Wood | 2.19 | [52] |
| 12 | Castor Oil | - | Pinewood | 1.9 | [53] |
| 13 | COP | N, N-bis(2-hydroxyethyl) thiophene-2,5-dicarboxamide | Oak wood | 7.22 | [54] |
| 14 | COP + ETAM | N, N-Bis(2-hydroxyethyl)-terephthalamid | Oak wood | 9.68 | [54] |
| 15 | poly(propylene glycol dicyclic carbonate) | APTES | Stainless steel | 2.7 | [55] |
| 16 | CSBO | EDA | Oak wood | 6.23 | [24] |
| 17 | CSBO | Butane diamine | Oak wood | 6.23 | [24] |
| 18 | CSBO | Hexamethylene diamine | Oak wood | 6.23 | [24] |
| 19 | CSBO | Butane diamine | Film | 1.1 | [56] |
| 20 | CSBO | Pentane diamine | Film | 1.0 | [56] |
| 21 | CSBO | Octane diamine | Film | 1.2 | [56] |
| 22 | Carbonated algae oil | Butane diamine | Film | 0.5 | [56] |
| 23 | Carbonated algae oil | Pentane diamine | Film | 0.3 | [56] |
| 24 | CSBO | EDA | Film | 1.97 | [24] |
| 25 | CSBO | Butane diamine | Film | 0.59 | [24] |
| 26 | CSBO | Hexamethylene diamine | Film | 0.22 | [24] |
| 27 | CSBO | EDA | Film | 4.83 | [This work] |
| 28 | CSBO | EDA + APTES | Film | 3.3 | [This work] |
| 29 | CSBO | EDA | Oak wood | 1.8 | [This work] |
| 30 | CSBO | EDA + APTES | Oak wood | 3.6 | [This work] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patel, R.; Kumar, A.; Chaudhary, M.L.; Gupta, R.K. Design and Characterization of Silane-Modified Bio-Based Non-Isocyanate Polyurethane Coatings for Advanced Surface Applications. Materials 2025, 18, 5551. https://doi.org/10.3390/ma18245551
Patel R, Kumar A, Chaudhary ML, Gupta RK. Design and Characterization of Silane-Modified Bio-Based Non-Isocyanate Polyurethane Coatings for Advanced Surface Applications. Materials. 2025; 18(24):5551. https://doi.org/10.3390/ma18245551
Chicago/Turabian StylePatel, Rutu, Ajay Kumar, Mayankkumar L. Chaudhary, and Ram K. Gupta. 2025. "Design and Characterization of Silane-Modified Bio-Based Non-Isocyanate Polyurethane Coatings for Advanced Surface Applications" Materials 18, no. 24: 5551. https://doi.org/10.3390/ma18245551
APA StylePatel, R., Kumar, A., Chaudhary, M. L., & Gupta, R. K. (2025). Design and Characterization of Silane-Modified Bio-Based Non-Isocyanate Polyurethane Coatings for Advanced Surface Applications. Materials, 18(24), 5551. https://doi.org/10.3390/ma18245551








