Microstructural Evolution of Al-Cu/TiC In Situ Composites via Solid–Liquid Titanium–Carbon Reactions
Abstract
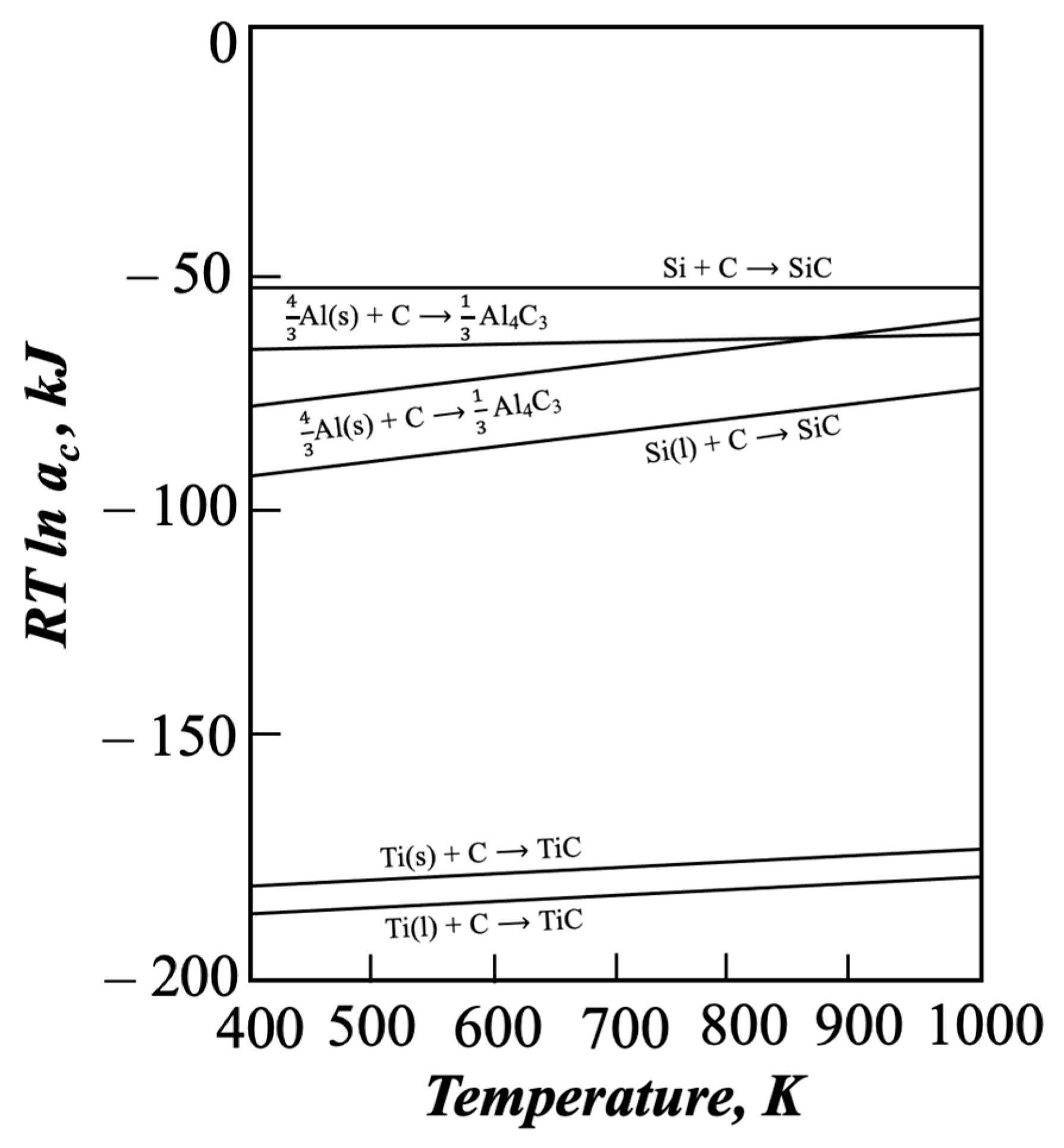
1. Introduction
2. Experimental Procedure
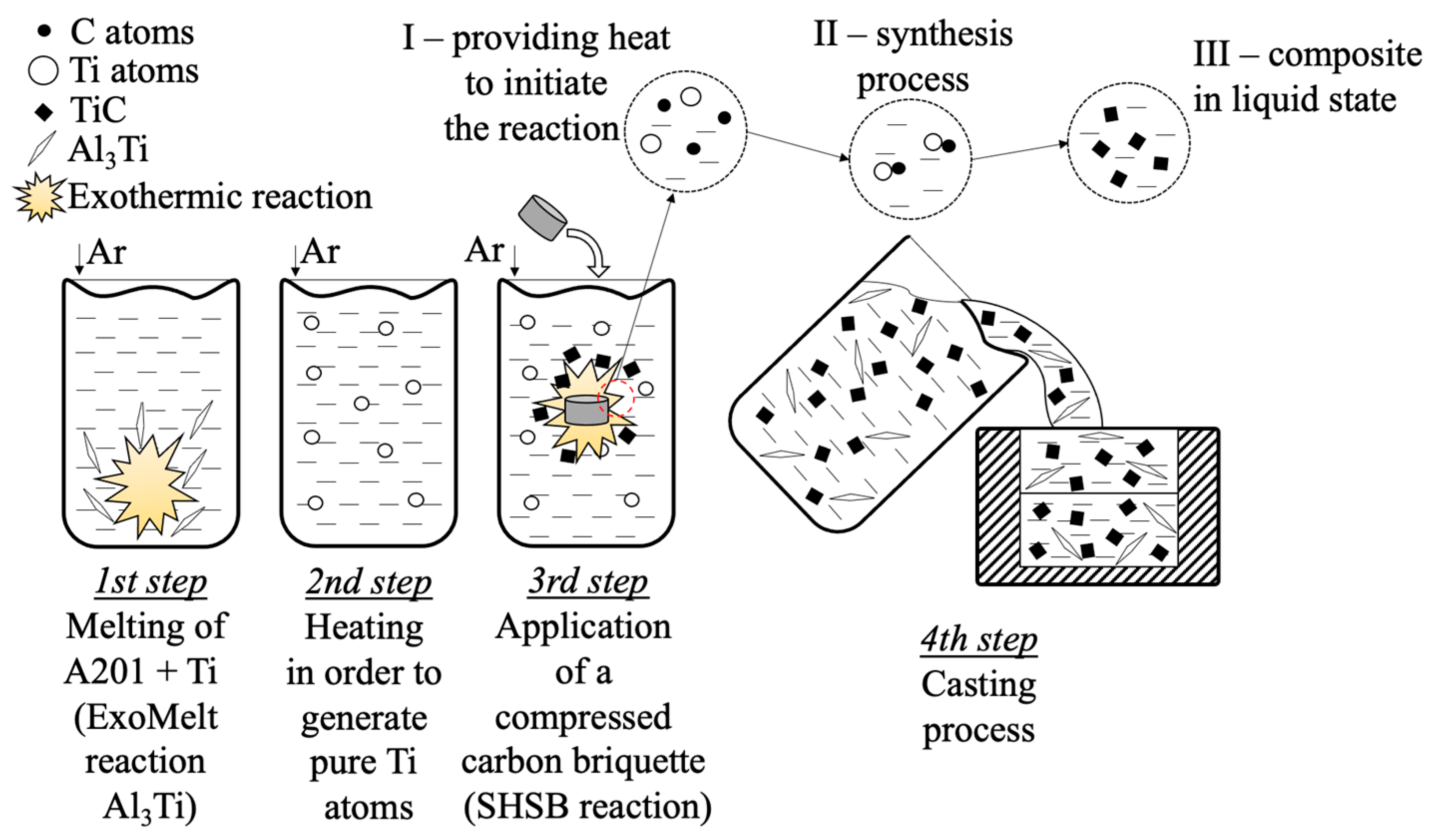
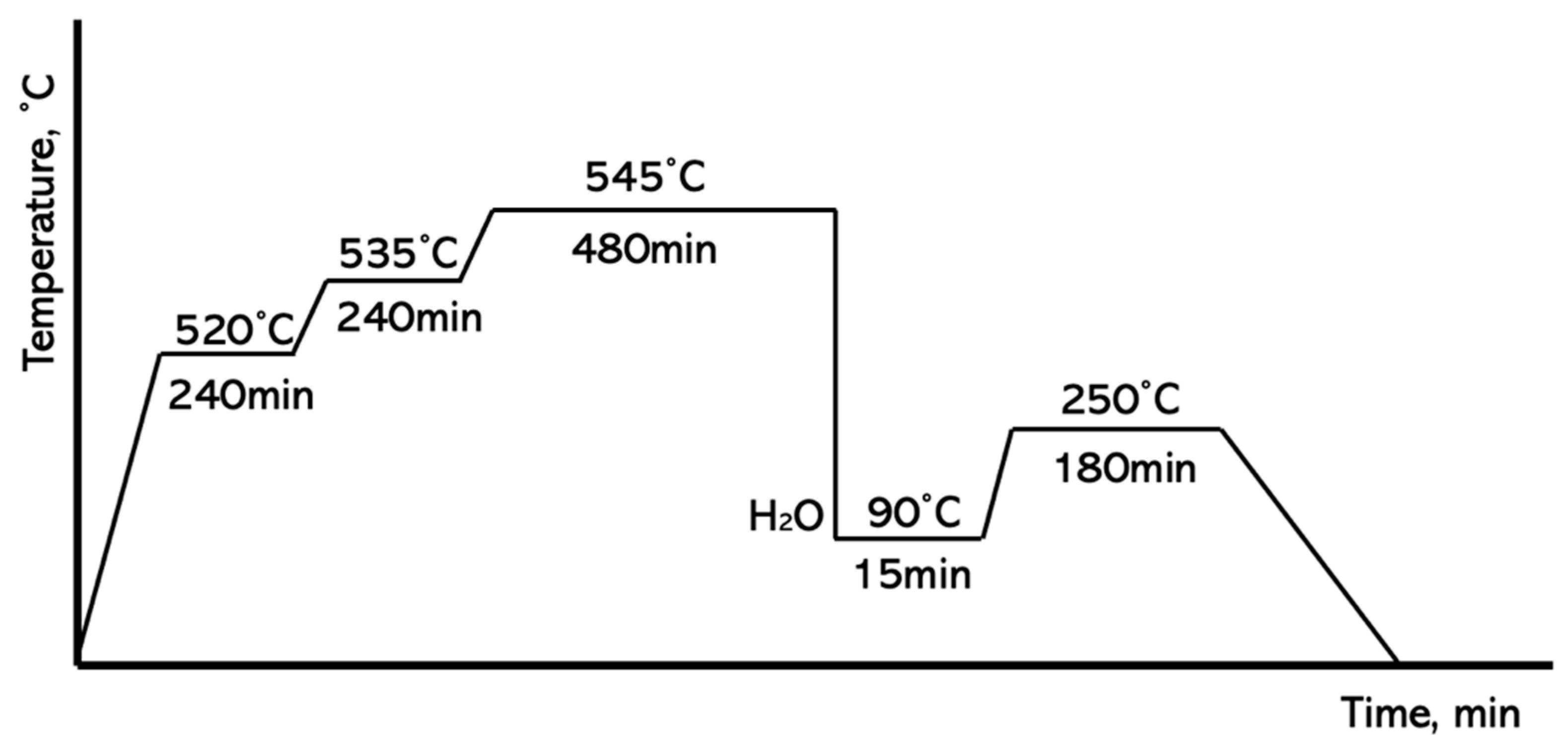
2.1. Melting Procedure
- ○
- reference material: cast A201 alloy (reference sample);
- ○
- A201 alloy enriched with the intermetallic Al3Ti phase;
- ○
- A201/TiC composite material.
2.2. Metallography Procedure
3. Results
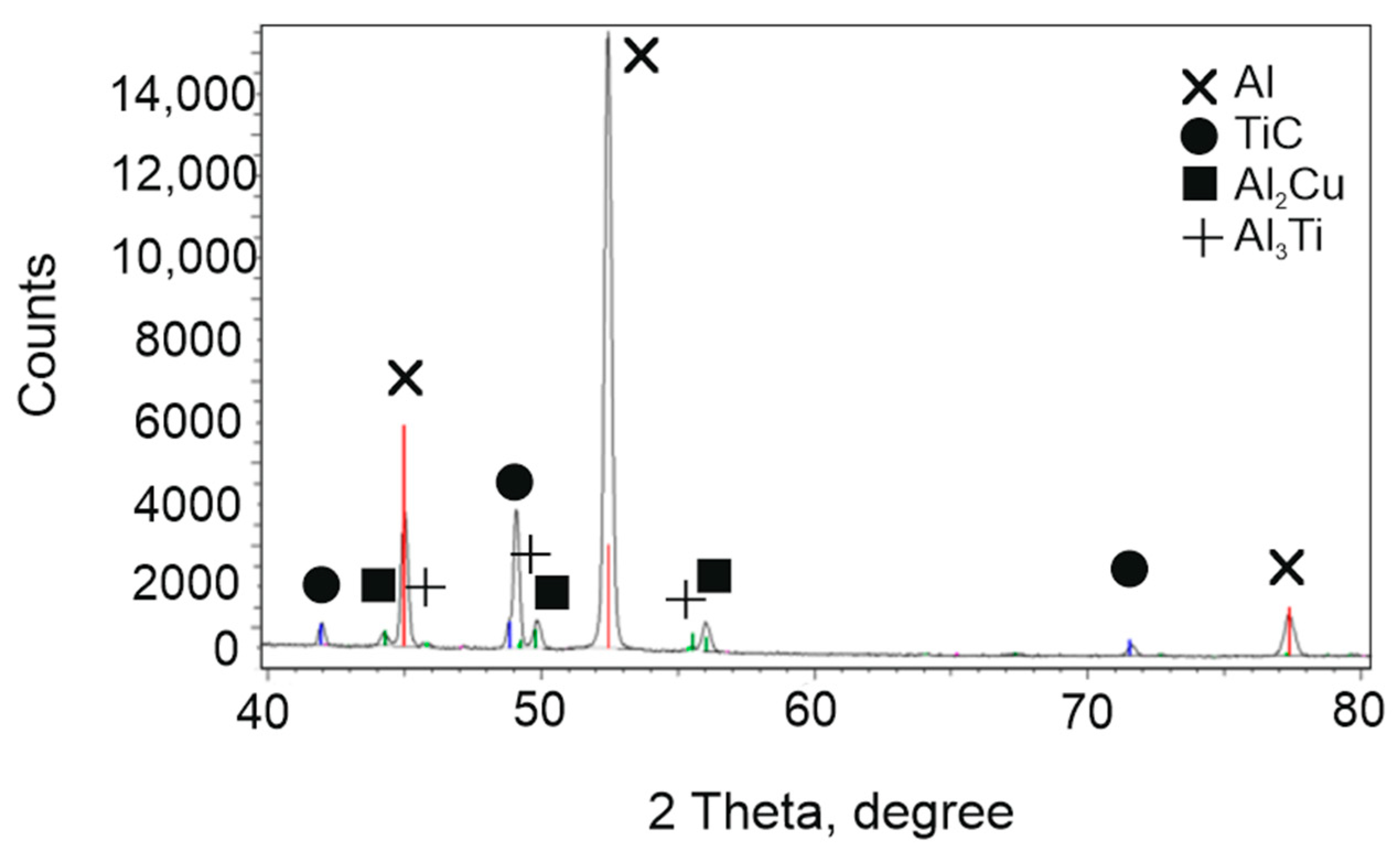
3.1. Chemical Composition
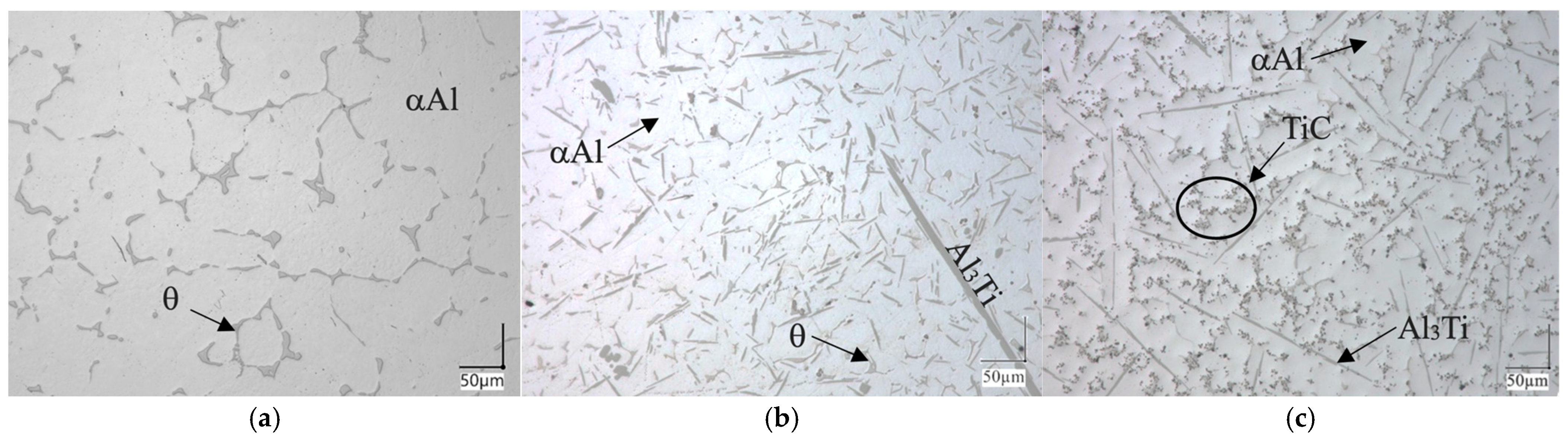
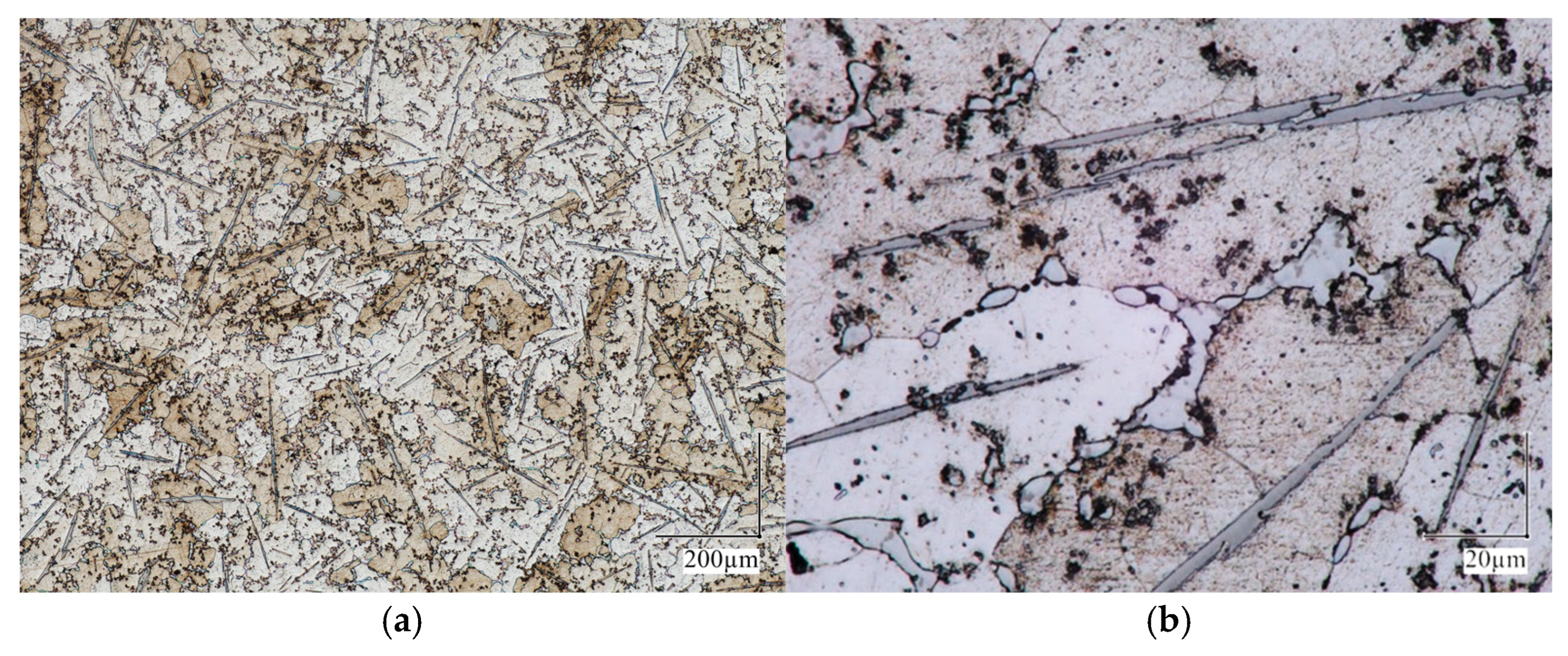
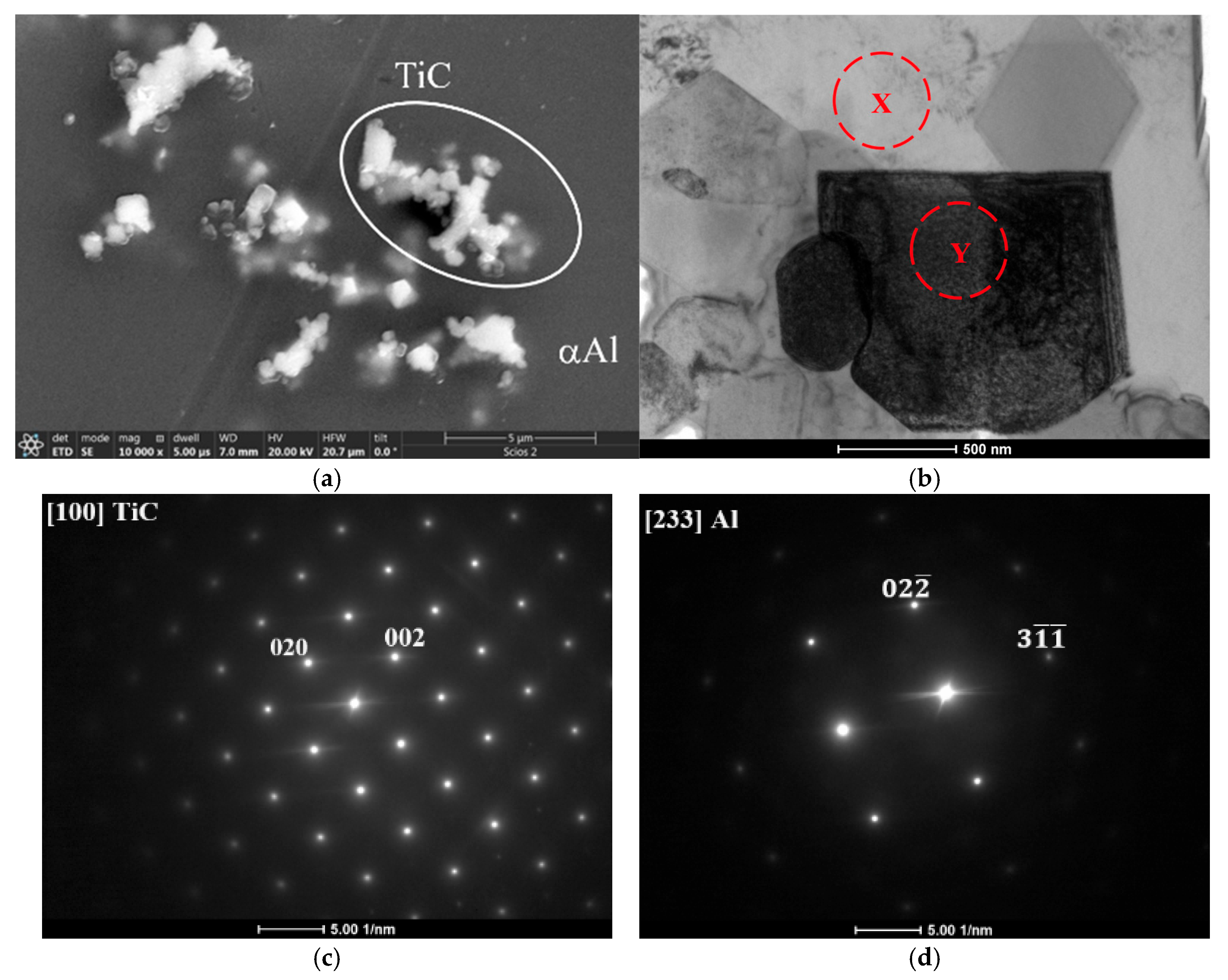
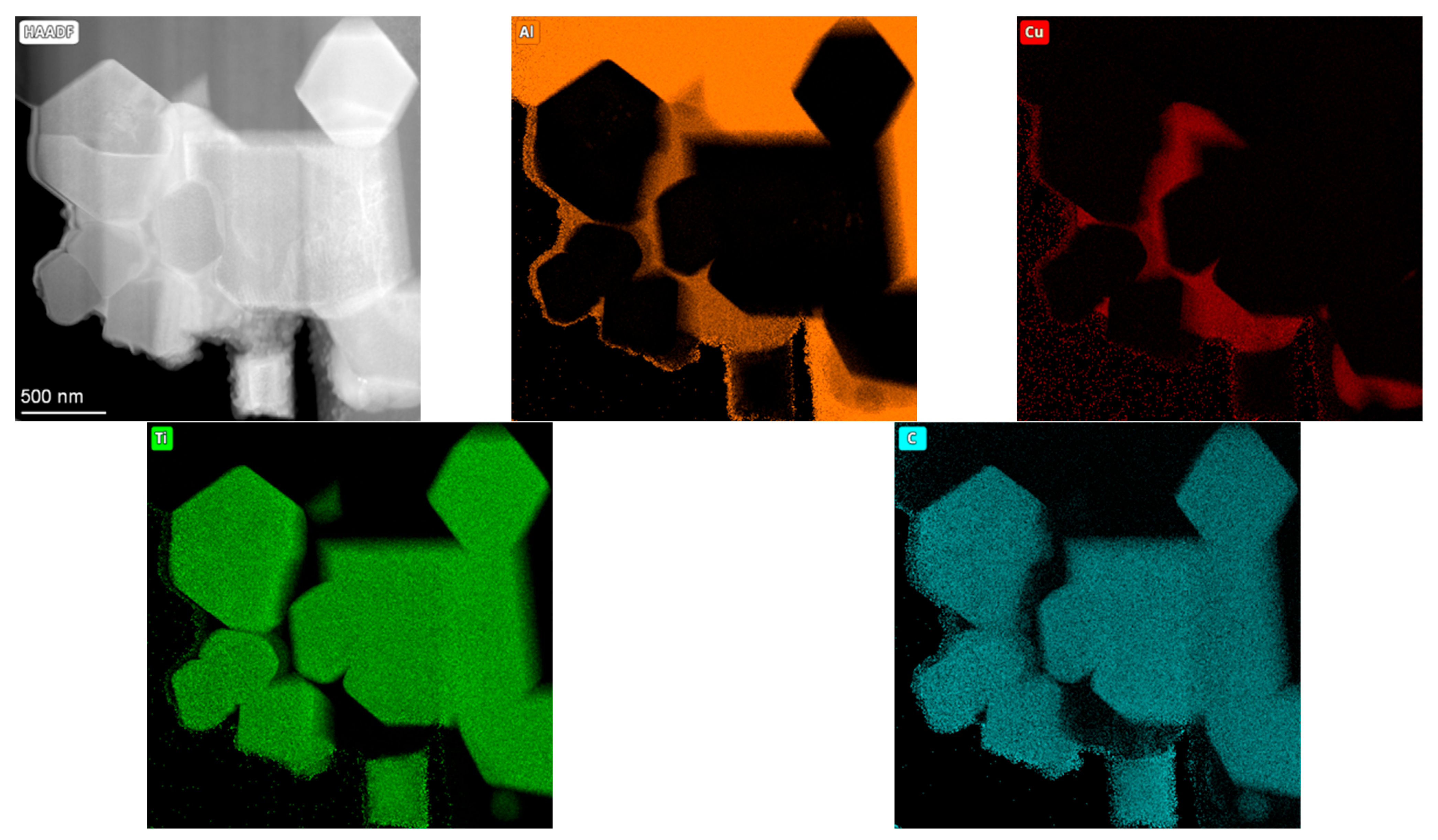
3.2. Metallography
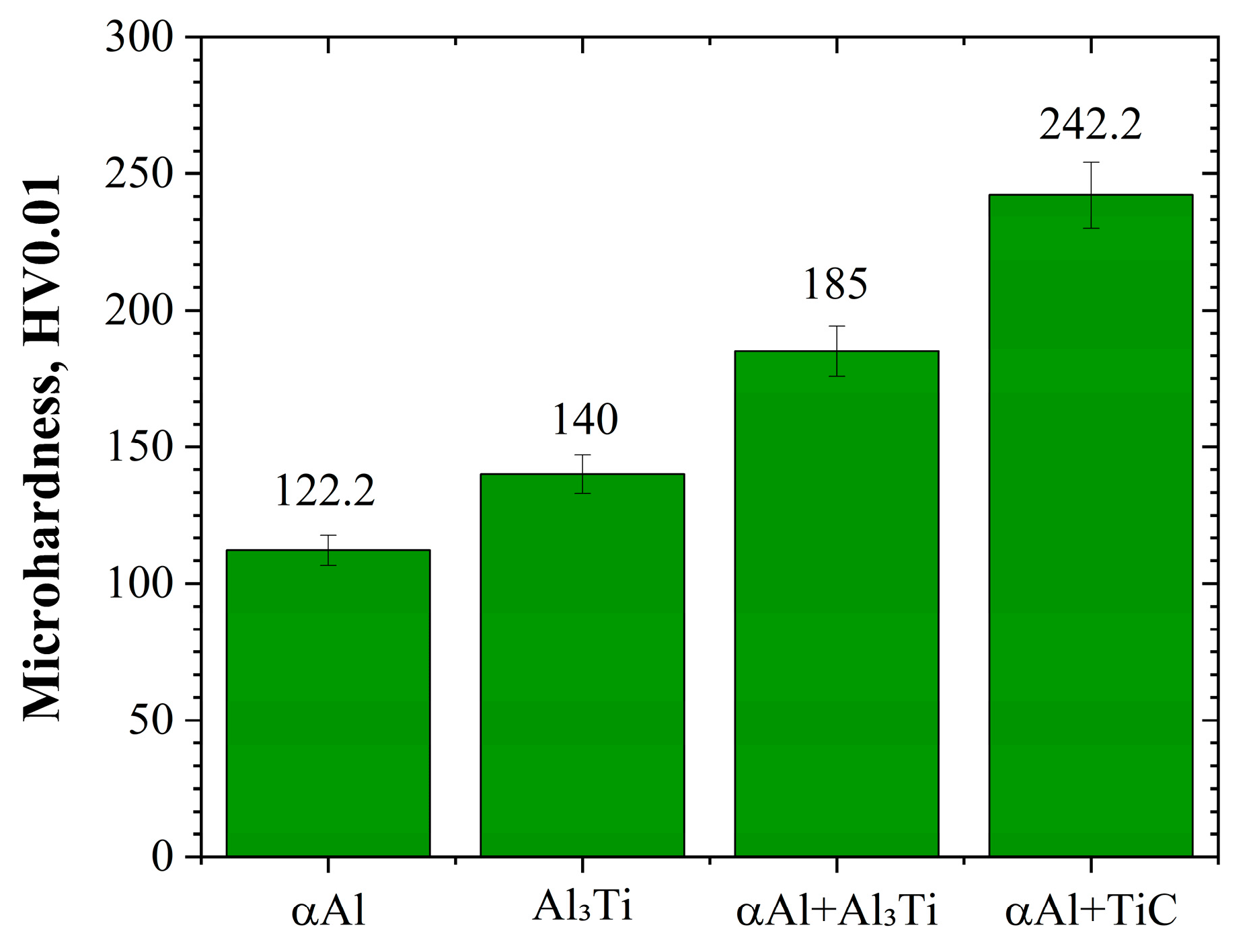
3.3. Microhardness
4. Discussion
5. Summary
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviation
| A201 base | Al-Cu (A201) alloy as cast |
| A201 Al3Ti | Al-Cu (A201) with Ti3Al particles |
| MMCs | Metal Matrix Composites |
| SHSB | Self-propagating High-temperature Synthesis in Bath |
| A201 TiC | Al-Cu (A201) reinforced with titanium carbides by SHSB method |
References
- Li, B.; Wang, Y.; Zheng, Y.; Xu, H.; Xie, B.; Qin, H.; Mao, H. Cooling rate effects on microstructure and properties of Al–Cu–Mg–Ag alloys: A comparative study of freeze-casting and resin sand mold casting. Intermetallics 2025, 185, 108898. [Google Scholar] [CrossRef]
- Eskin, D.; Du, Q.; Ruvalcaba, D.; Katgerman, L. Experimental study of structure formation in binary Al–Cu alloys at different cooling rates. Mater. Sci. Eng. A 2005, 405, 1–10. [Google Scholar] [CrossRef]
- Kim, K.; Zhou, B.-C.; Wolverton, C. Interfacial stability of θ′/Al in Al-Cu alloys. Scr. Mater. 2019, 159, 99–103. [Google Scholar] [CrossRef]
- Górny, M.; Sikora, G. Effect of Titanium Addition and Cooling Rate on Primary α(Al) Grains and Tensile Properties of Al-Cu Alloy. J. Mater. Eng. Perform. 2015, 24, 1150–1156. [Google Scholar] [CrossRef][Green Version]
- Stąpór, S.; Górny, M.; Gondek, Ł.; Gracz, B.; Kawalec, M. The Role of Molybdenum in the Formation of the Microstructure and Properties of Al-Cu Alloys. Arch. Foundry Eng. 2024, 24, 39–48. [Google Scholar] [CrossRef]
- Liang, N.; Ma, P.; Yang, J.; Liu, C. The effect of cyclic pre-deformation on the elevated temperature age hardening in Al-Cu-(Sc) alloys. Mater. Sci. Eng. A 2025, 942, 148737. [Google Scholar] [CrossRef]
- Su, M.; Zhou, M.; Yue, C.; Zhou, L.; Zheng, W.; Zheng, B.; Zuo, X.; Yuan, X. Investigation on the Influence Mechanism of Mg Contents on the Hot Cracking Behavior of Al–Cu–Mg Alloys. Met. Mater. Trans. B 2024, 56, 355–374. [Google Scholar] [CrossRef]
- Zhang, Y.; Su, M. Improvements of hot cracking in Al-Cu alloys—The positive effect of alloying on the liquid film feeding capacity. Mater. Lett. 2025, 396, 138774. [Google Scholar] [CrossRef]
- Hu, Z.; Nie, X.; Qi, Y.; Zhang, H.; Zhu, H. Cracking criterion for high strength Al–Cu alloys fabricated by selective laser melting. Addit. Manuf. 2021, 37, 101709. [Google Scholar] [CrossRef]
- Zhu, X.; Yang, X.; Huang, W.; Wang, S.; Zheng, S.; Wang, X.; Li, M. Microstructure-based modelling of steady-state fatigue crack propagation in an Al-Cu-Li alloy. Int. J. Fatigue 2025, 201, 109138. [Google Scholar] [CrossRef]
- Xu, M.; Zhang, H.; Yuan, T.; Yan, Z.; Chen, S. Microstructural characteristics and cracking mechanism of Al-Cu alloys in wire arc additive manufacturing. Mater. Charact. 2023, 197, 112677. [Google Scholar] [CrossRef]
- Yuan, L.; Liu, F.; Wu, C.; Lou, C. Improving mechanical properties and electrical conductivity of Al-Cu-Mg matrix composites by GNPs and sc additions. Sci. Rep. 2025, 15, 2418. [Google Scholar] [CrossRef] [PubMed]
- Olejnik, E.; Tokarski, T.; Sikora, G.; Sobula, S.; Maziarz, W.; Szymański, Ł.; Grabowska, B. The Effect of Fe Addition on Fragmentation Phenomena, Macrostructure, Microstructure, and Hardness of TiC-Fe Local Reinforcements Fabricated In Situ in Steel Casting. Met. Mater. Trans. A 2018, 50, 975–986. [Google Scholar] [CrossRef]
- Maziarz, W.; Wójcik, A.; Bobrowski, P.; Bigos, A.; Szymański, Ł.; Kurtyka, P.; Rylko, N.; Olejnik, E. SEM and TEM Studies on In-Situ Cast Al–TiC Composites. Mater. Trans. 2019, 60, 714–717. [Google Scholar] [CrossRef]
- Szymański, Ł.; Sobczak, J.J.; Peddeti, K.; Bigos, A.; Tokarski, T.; Maziarz, W.; Olejnik, E.; Chulist, R.; Żak, K.; Bruzda, G.; et al. Production of metal matrix composite reinforced by TiC by reactive infiltration of cast iron into Ti + C preforms. Ceram. Int. 2024, 50, 17452–17464. [Google Scholar] [CrossRef]
- Marosz, J.; Sobula, S. Analysis of Fragmentation Phenomenon of Composite Layers of Fe-TiC Type Obtained “in situ” in Steel Casting. Arch. Foundry Eng. 2024, 24, 131–136. [Google Scholar] [CrossRef]
- Marosz, J.; Kawalec, M.; Górny, M. Structure of ADI Cast Iron as a Cast “in situ” Composite Reinforced with TiC Ceramic Particles. Arch. Foundry Eng. 2024, 2024, 95–102. [Google Scholar] [CrossRef]
- Fraś, E.; Wierzbiński, S.; Janas, A.; López, H.F. Strength and Plastic Flow in “In Situ” TiC Reinforced Aluminum Composites. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2002, 33, 3831–3838. [Google Scholar] [CrossRef]
- Madhusudan, S.; Sarcar, M.M.; Bhargava, M.R.; Sunil, K.; Kumar, R. Fabrication and Deformation Studies on Al-Cu Composite Metallic Materials. Indian J. Eng. Mater. Sci. 2012, 19, 175–178. [Google Scholar] [CrossRef]
- Jain, S.; Rana, R.S.; Jain, P. Study of Microstructure and Mechanical Properties of Al-Cu Metal Matrix Reinforced with B4C Particles Composite. Int. Res. J. Eng. Technol. 2016, 3, 499–504. Available online: https://www.irjet.net/archives/V3/i1/IRJET-V3I186.pdf (accessed on 15 September 2025).
- Yu, P.; Deng, C.-J.; Ma, N.-G.; Yau, M.-Y.; Ng, D.H. Formation of nanostructured eutectic network in α-Al2O3 reinforced Al–Cu alloy matrix composite. Acta Mater. 2003, 51, 3445–3454. [Google Scholar] [CrossRef]
- Muratoğlu, M.; Aksoy, M. The effects of temperature on wear behaviours of Al–Cu alloy and Al–Cu/SiC composite. Mater. Sci. Eng. A 2000, 282, 91–99. [Google Scholar] [CrossRef]
- Duan, S.; Wu, C.; Gao, Z.; Cha, L.; Fan, T.; Chen, J. Interfacial structure evolution of the growing composite precipitates in Al-Cu-Li alloys. Acta Mater. 2017, 129, 352–360. [Google Scholar] [CrossRef]
- Gao, Y.H.; Yang, C.; Zhang, J.Y.; Cao, L.F.; Liu, G.; Sun, J.; Ma, E. Stabilizing nanoprecipitates in Al-Cu alloys for creep resistance at 300 °C. Mater. Res. Lett. 2018, 7, 18–25. [Google Scholar] [CrossRef]
- Fraś, E.; Olejnik, E.; Janas, A.; Kolbus, A. The morphology of TiC carbides produced in surface layers of carbon steel castings. Arch. Foundry Eng. 2010, 10, 39–42. Available online: https://www.journal-afe.pl/pl/339/the-morphology-of-tic-carbides-produced-in-surface-layers-of-carbon-steel-castings.pdf (accessed on 15 September 2025).
- Svendsen, L.; Jarfors, A. Al–Ti–C phase diagram. Mater. Sci. Technol. 1993, 9, 948–957. [Google Scholar] [CrossRef]
- Anand, S.; Batra, N.K. On Reactions During Melting of Aluminium-Coke Composite. In State of the Art in Cast Metal Matrix Composites in the Next Millennium; Rohatgi, P.K., Ed.; TMS–ASM International: Warrendale, PA, USA, 2000; pp. 61–68. [Google Scholar]
- Fraś, E.; Wierzbinski, S.; Janas, A.; López, H.F. Synthesis of Al–TiC in-situ Composites via SHSB Technology. In State of the Art in Cast Metal Matrix Composites in the Next Millennium; Rohatgi, P.K., Ed.; TMS–ASM International: Warrendale, PA, USA, 2000; pp. 69–79. [Google Scholar]
- Cai, X.; Zeng, C.; Yang, Q.; Jiang, Z.; Ma, B.; Li, H.; Cong, B. Wire-arc additive manufacturing of high-strength Al-Cu alloy via synergistic strengthening of Cd microalloying and TiC nanoparticle. Mater. Sci. Eng. A 2025, 940, 148572. [Google Scholar] [CrossRef]
- Zhang, W.; Yi, H.; He, H.; Cao, H. Content effects of in-situ synthesis TiC for grain refinement, porosity suppression and performance enhancement in wire arc additive manufactured Al-Cu alloy. J. Mech. Work. Technol. 2025, 340, 118875. [Google Scholar] [CrossRef]
- Zhao, D.; Xiao, Z.; Dai, Z.; Xiao, S.; Gao, X.; Chen, J.; Wan, L. Influence of TiC particles on grain boundary structure and solute atomic diffusion in TiC/Al-Cu composites. Solid State Ion. 2024, 420, 116764. [Google Scholar] [CrossRef]


| Alloy | Chemical Composition, %wt. | |||||
|---|---|---|---|---|---|---|
| Cu | Mn | Mg | Fe | Ti | Al | |
| A201 as-cast | 4.94 | 0.67 | 0.01 | 0.04 | 0.22 | Bal. |
| A201 Al3Ti | 4.98 | 0.65 | 0.01 | 0.03 | 2.40 | Bal. |
| A201 TiC | 4.88 | 0.69 | 0.01 | 0.02 | 2.30 | Bal. |
| Spot | Chemical Composition, %wt. | ||||
|---|---|---|---|---|---|
| Al | Ti | C | Cu | Mn | |
| 1 | 37.8 | 34.2 | 26.5 | 1.4 | 0.1 |
| 2 | 91.2 | 0.3 | 5.5 | 2.7 | 0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marosz, J.; Górny, M.; Morgiel, J.; Janas, A. Microstructural Evolution of Al-Cu/TiC In Situ Composites via Solid–Liquid Titanium–Carbon Reactions. Materials 2025, 18, 5374. https://doi.org/10.3390/ma18235374
Marosz J, Górny M, Morgiel J, Janas A. Microstructural Evolution of Al-Cu/TiC In Situ Composites via Solid–Liquid Titanium–Carbon Reactions. Materials. 2025; 18(23):5374. https://doi.org/10.3390/ma18235374
Chicago/Turabian StyleMarosz, Jan, Marcin Górny, Jerzy Morgiel, and Andrzej Janas. 2025. "Microstructural Evolution of Al-Cu/TiC In Situ Composites via Solid–Liquid Titanium–Carbon Reactions" Materials 18, no. 23: 5374. https://doi.org/10.3390/ma18235374
APA StyleMarosz, J., Górny, M., Morgiel, J., & Janas, A. (2025). Microstructural Evolution of Al-Cu/TiC In Situ Composites via Solid–Liquid Titanium–Carbon Reactions. Materials, 18(23), 5374. https://doi.org/10.3390/ma18235374