1. Introduction
Graphene oxide (GO), due to its unique properties such as a large specific surface area, the presence of oxygen-containing functional groups, good solubility, and colloidal stability, has found wide application in bioengineering [
1,
2]. Research presented by Lee et al. [
3] demonstrated that GO can stimulate the growth of stem and neural cells, also improving their adhesion. Scaffolds made of GO create a suitable environment for chondrocyte growth, promoting cartilage regeneration and joint repair [
4]. GO can stimulate osteogenic differentiation, supporting bone formation, which makes it a promising material for bone tissue regeneration [
3]. It is also used as an innovative component of biofilaments for 3D tissue printing, enabling the creation of advanced biomedical structures that support tissue regeneration [
5]. Due to its good solubility in water and the presence of reactive functional groups, GO can effectively absorb enzymes, which is useful for cell imaging and drug delivery applications [
6].
Ryu et al. [
7] showed that both graphene (G) and graphene oxide (GO) are biocompatible materials that provide favorable conditions for effective cell proliferation. Graphene is characterized by certain unique properties, such as high electrical conductivity and good absorption of proteins and other low-molecular-weight compounds. This has significant implications for cell development, as cells interacting with their surrounding structures secrete substances that influence the proliferation process [
8].
Wong Cheng Lee et al. [
3] conducted studies on MSCs (mesenchymal stem cells), which have the ability to differentiate into various cell types, primarily osteocytes, chondrocytes, and adipocytes. MSCs derived from human bone marrow were cultured on three substrates: graphene (G), graphene oxide (GO), and PDMS (polydimethylsiloxane)—a silicone biocompatible membrane. The conducted research [
3] showed significant differences in cell morphology. Initially, for MSCs cultured on G and GO substrates, numerous filopodia and protrusions were observed, allowing for intercellular interactions. These structures were not visible in cells cultured on PDMS matrices, where cells appeared rounder and less adherent to the surface. The number of cells grown on G and GO substrates was significantly higher compared to the control.
For hASC—human adipose-derived mesenchymal stem cells—the team of Kim et al. [
8] reported similar findings. GO films provided a favorable environment for hASC development, adhesion, proliferation, and differentiation.
Aryaei et al. [
9] evaluated the biocompatibility of graphene layers using silicon substrates. In the case of silicon plates coated with GO layers, an increased number of “attached” osteoblasts was observed (326 ± 60 cells) compared to the uncoated substrate (280 ± 70). The cells on the uncoated silicon plate exhibited undeveloped morphology with a rounded shape, while those on the GO-coated plates showed an elongated morphology and numerous filopodia. Moreover, the authors [
9] reported that no cytotoxic effects were observed in either group. The results suggest that applying graphene layers to bone implants may enhance osteoblast adhesion and growth.
Liu et al. [
10] conducted studies confirming the antibacterial activity of GO against the Escherichia coli strain. These studies showed that the activity of GO depends on incubation time and concentration (the percentage of dead bacterial cells increased proportionally with both parameters). Disruption of cell membrane structure and function (membrane stress) and excessive accumulation of reactive oxygen species (oxidative stress) were observed. The antibacterial effects of graphene oxide against various bacterial strains have also been confirmed in other scientific publications [
11,
12,
13].
The antimicrobial efficacy of GO against both Gram-positive and Gram-negative bacteria has been reported as well. In a review by Romiszewska et al. [
14], the effect of graphene oxide on bacterial growth was analyzed. Significant inhibition was observed, although the effectiveness of GO depended on physical bacterial characteristics such as shape and size.
The use of GO-enriched tissue scaffolds has been shown to improve wound healing and reduce bacterial infections [
15]. Necolau et al. [
16] presented studies on chitosan hydrogels containing graphene oxide, which were found to enhance tissue regeneration and exhibit antibacterial efficacy. Similarly, Ferrari et al. [
17] demonstrated that the use of graphene as a substrate supporting cell growth and tissue regeneration showed no signs of toxicity.
An important issue in the functional performance of implants is their corrosion resistance when exposed to body fluids. In clinical studies conducted by Bao [
18], where titanium elements were immersed in patients’ synovial fluids, the stable open circuit potential (OCP) ranged between −151 and −529 mV vs. Ag/AgCl. In typical corrosion experiments by Boraei [
19], the OCP of Ti alloys shifted toward more positive potentials. According to experimental data reported by [
20], surface coverage/passivation of TiO
2 shifts the OCP to approximately 103 mV.
Modification of the surface layer of titanium implants through micro-milling [
21], UV radiation treatment [
22], and etching [
23] can influence fibroblast viability, proliferation, adhesion, and activation. Baraka et al. [
24] presented the results of studies on the attachment of human gingival fibroblasts (HGF) of soft tissue to titanium dioxide nanotubes (TNTs) compared with commercially pure titanium (cp-Ti) and its alloys, in in vitro research. A significant increase in cell proliferation on TNT surfaces compared to cp-Ti on day 7 in three studies and on day 14 in one study was observed. A significant increase in type I collagen protein expression on TNTs compared to cp-Ti on day 6 in one study and on day 7 in two studies was also reported.
Previous studies on GO-modified titanium have focused mainly on complex hybrid systems, such as GO incorporated into anodically formed TiO
2 nanotube arrays [
25] or GO/gelatin composite coatings applied to titanium substrates [
26]. Other work has examined GO deposited on titanate nanowire scaffolds rather than on metallic titanium surfaces [
27]. These approaches differ fundamentally from the system investigated in the present study. Additionally, there are limited data that explore the GO layer deposited directly onto unalloyed, flat titanium foil, without nanotubular or nanowire oxide structures or alloying components. Moreover, earlier studies typically focused on a single biological parameter—such as osteoblast adhesion or antibacterial behavior—whereas none provided a comprehensive evaluation encompassing cytocompatibility, morphology, bacterial response, electrochemical stability, and genotoxicity. Given these limitations, there is a clear need to examine how a uniform GO coating on unalloyed titanium foil influences cellular and microbiological responses under conditions relevant to medical implants.
A review of the literature concerning the application of graphene oxide in biomedical engineering highlights its antibacterial and biocompatible properties, as well as its ability to stimulate cell growth and proliferation. This indicates the potential of GO as an innovative biomaterial supporting tissue engineering and the development of modern regenerative therapies. However, there is a lack of studies investigating the effect of graphene oxide deposited on titanium surfaces—to general implantology and medical applications—on human fibroblast cells, including healthy fibroblasts (Hs 895.Sk, CRL–7636) and cancerous fibroblasts (Hs 895.T, CRL–7637). This issue is particularly important in oncology patients requiring surgical interventions involving implant placement.
The aim of the biological part of this study was to comprehensively assess the cytocompatibility of titanium foils modified with graphene oxide (Ti + GO) in contact with human skin cells and representative bacterial strains. The research included quantitative evaluation of viability, adhesion, and proliferation of healthy skin fibroblasts (Hs 895.Sk) and melanoma-derived fibroblast-like cells (Hs 895.T) cultured on Ti and Ti + GO, verification of potential genotoxic effects using the cytokinesis-block micronucleus assay, characterization of cell morphology and cell–substrate interactions using SEM, and evaluation of antibacterial responses against Staphylococcus aureus and Pseudomonas aeruginosa via inhibition zone tests, viability assays, and SEM imaging.
It was hypothesized that titanium surface functionalization with graphene oxide would enhance cytocompatibility and cell adhesion while maintaining normal morphology and lack of genotoxicity, as well as exhibit a bacteriostatic effect compared to pure titanium, confirming the suitability of Ti + GO surfaces for implantology applications.
2. Materials and Methods
The studies on graphene oxide deposited on the titanium surface were carried out on samples with the following designations:
Model surgical implants were made from titanium foil (Ti) with a purity of 99.6% Ti according to the material data sheet (GoodFellow, Huntingdon, UK) [
28]. The samples used in the study measured 5 × 5 × 0.025 mm. All experiments presented in this work were performed in 3–5 repetitions.
The aqueous dispersion of graphene oxide was purchased from the Institute of Electronic Materials Technology (ITME, Warsaw, Poland) [
29].
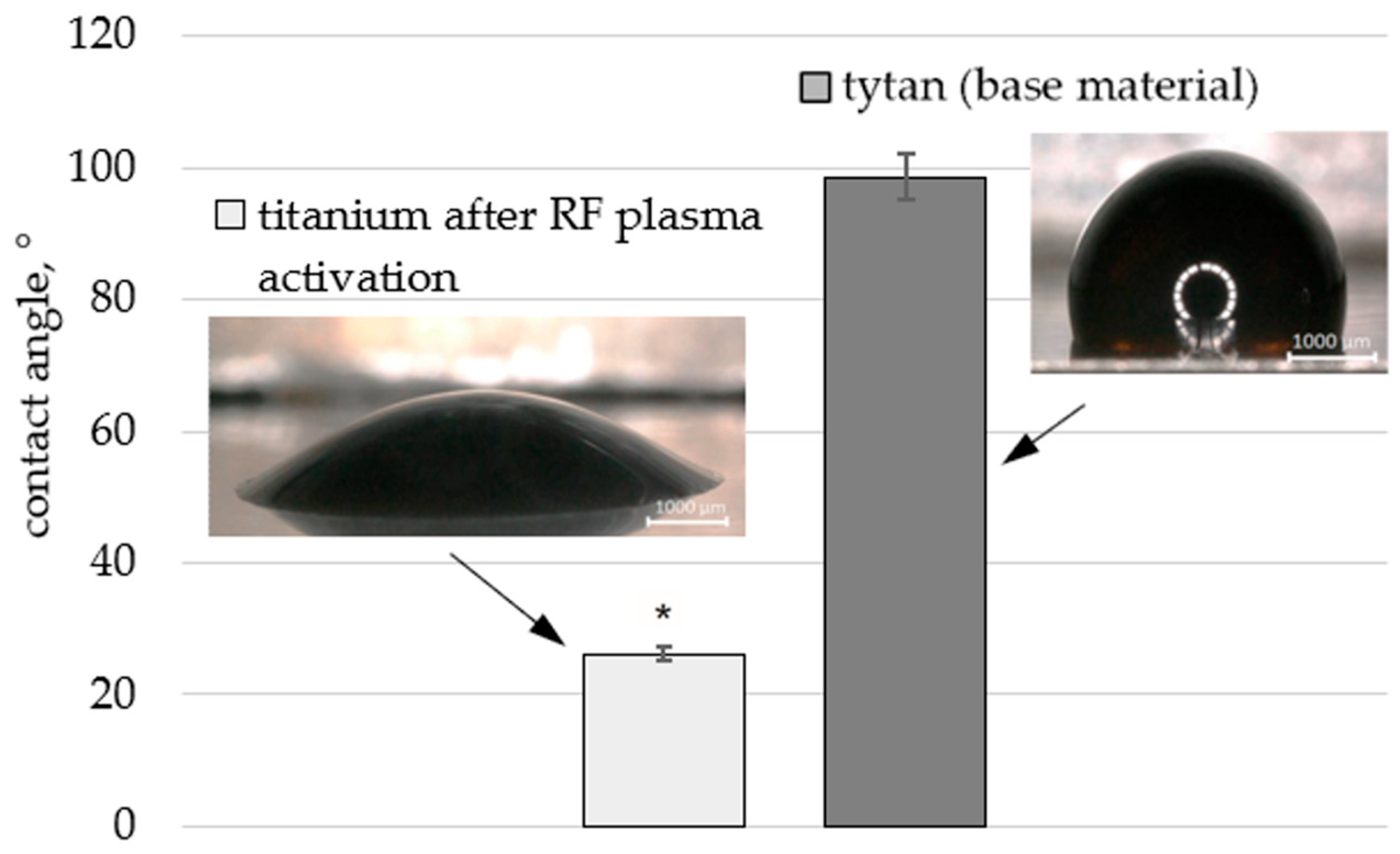
The first stage of the study involved coating the titanium foil with a layer of graphene oxide (Ti + GO). For this purpose, the samples were cleaned with ethyl alcohol, then the surface was activated and cleaned using RF plasma at a power of 100 W for approximately 15 min (Plasma Prep III, Garfield Ave, Westchester, PA, USA). This procedure was applied based on literature reports. Publications [
30,
31,
32] demonstrated that plasma treatment of steel surfaces improves their hydrophilicity, thereby enhancing the distribution of aqueous suspensions containing graphene oxide flakes.
After plasma treatment, the samples were removed from the chamber and placed on Petri dishes. A dispersed suspension of graphene oxide (4.5 g/L) (Institute of Electronic Materials Technology, ITME, Warsaw, Poland) was applied to the samples. To remove excess suspension, the spin coating technique was used (POLOS Spin150i—NPP, SPS-Europe B.V., Putten, The Netherlands) with a set rotation speed of 3000 rpm. Additionally, to evaporate excess water, the samples were placed in a Vacucell 22 vacuum oven (MMM Muenchener Medizin Mechanik Polska, Warsaw, Poland).
2.1. Structural Analysis
The contact angle was measured using an optical microscope (6000 VHX, Keyence Corporation, Osaka, Japan). In the wettability tests, individual suspensions were collected using a syringe dispenser. Then, drops of the aqueous graphene oxide suspension, with a volume of approximately 3 µL, were released onto the surface from a fixed height of 5 mm. Five measurements were performed, from which the mean value and standard deviation were calculated.
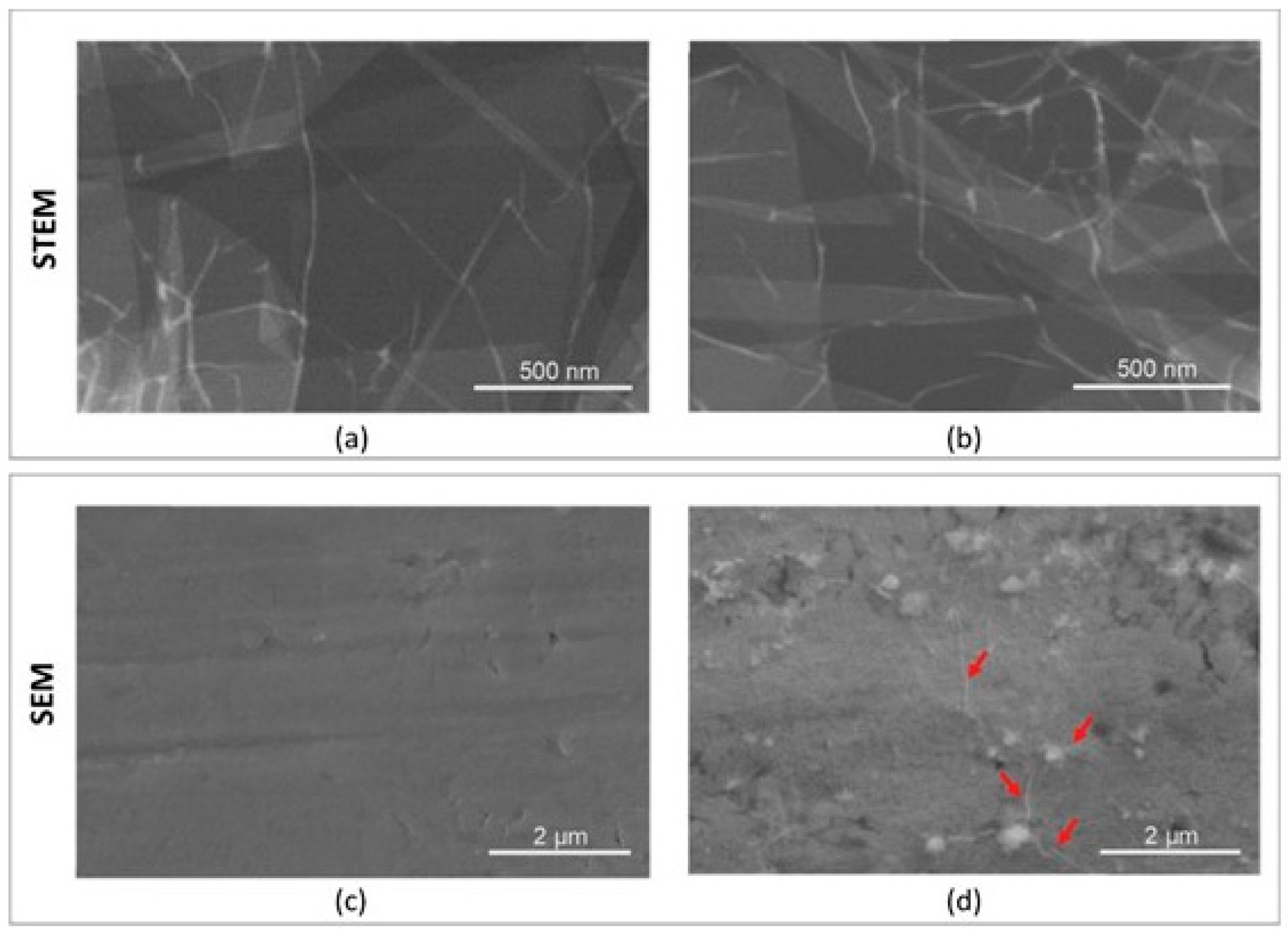
Imaging of the dispersed graphene oxide suspension was performed using a STEM detector integrated with a Quanta 250 FEG microscope (FEI, Hillsboro, OR, USA). Surface topography studies of Ti and Ti + GO samples were carried out using a CBS detector, also available in the Quanta 250 FEG scanning electron microscope. The applied accelerating voltage was 10 kV with a spot size of 3. For each sample, images were taken at magnifications of 1000× (CBS), 10,000× (CBS), and 100,000× (STEM).
For STEM analysis, copper grids were used, onto which the aqueous dispersion of graphene oxide was applied using centrifugal force (3000 rpm).
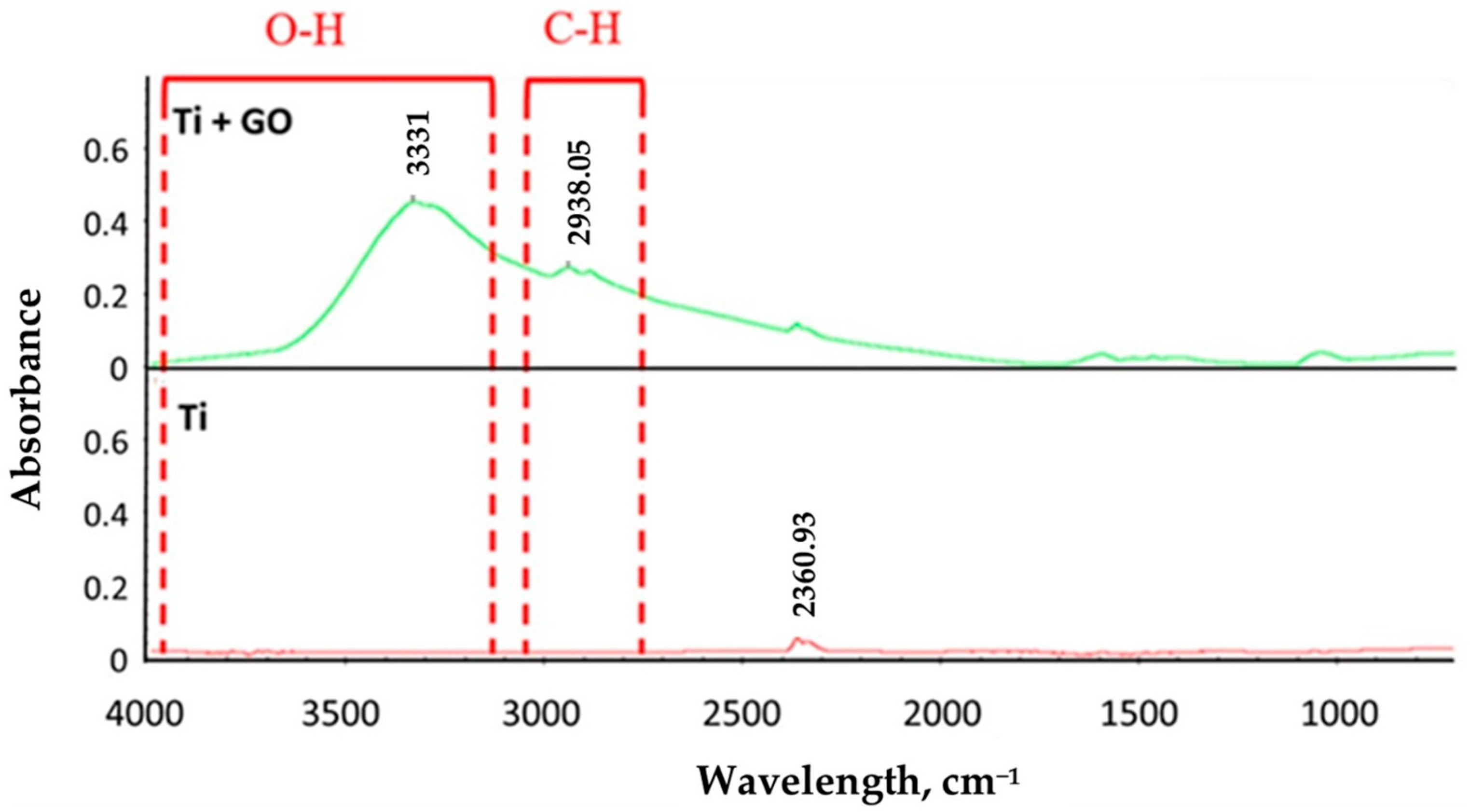
To identify carbonyl, carboxyl, and hydroxyl groups, as well as the carbon chain present on the sample surfaces after GO deposition, the samples were analyzed using a Fourier-transform infrared spectrometer (FTIR) (Nicolet IS50, Thermo Fisher Scientific, Waltham, MA, USA) equipped with an ATR (attenuated total reflectance) module. The analyses were conducted in the range of 400–4000 cm−1, with a resolution of 4 cm−1 and 64 scans applied. Three measurements were performed for each side of the sample.
The studied (Ti and Ti + GO) samples were examined by means of the X-ray diffraction using BRUKER D8 Discover diffractometer (Bruker AXS SE, Karlsruhe, Germany), equipped with CuKα radiator (λKα1 = 1.54056 Å, λKα2 = 1.54443 Å, Siemens KFL CU 2 K, 40 kV voltage, and 40 mA current in operating mode), Göbel FGM2 mirror and 1D LYNXEYE detector (no monochromator) and Bragg–Brentano geometry. Each measurement was performed with at least a 0.015° step size and an acquisition time of 2 s per step. An Anton Paar HTK-1200 N (Anton Paar GmbH, Graz, Austria) temperature chamber, was applied to stabilize the temperature at RT (around 293 K). Each measurement was reduced by the recorded background as well as the λKα2 component. Rietveld refinement and precise calculations of entire diffraction profiles, along with refined unit cell parameters, were performed using the FullProf Suite (Ver. January-2021). Phase analysis was performed using Match! Ver. 4.2 application, provided by Crystal Impact, with support of the COD database [
33].
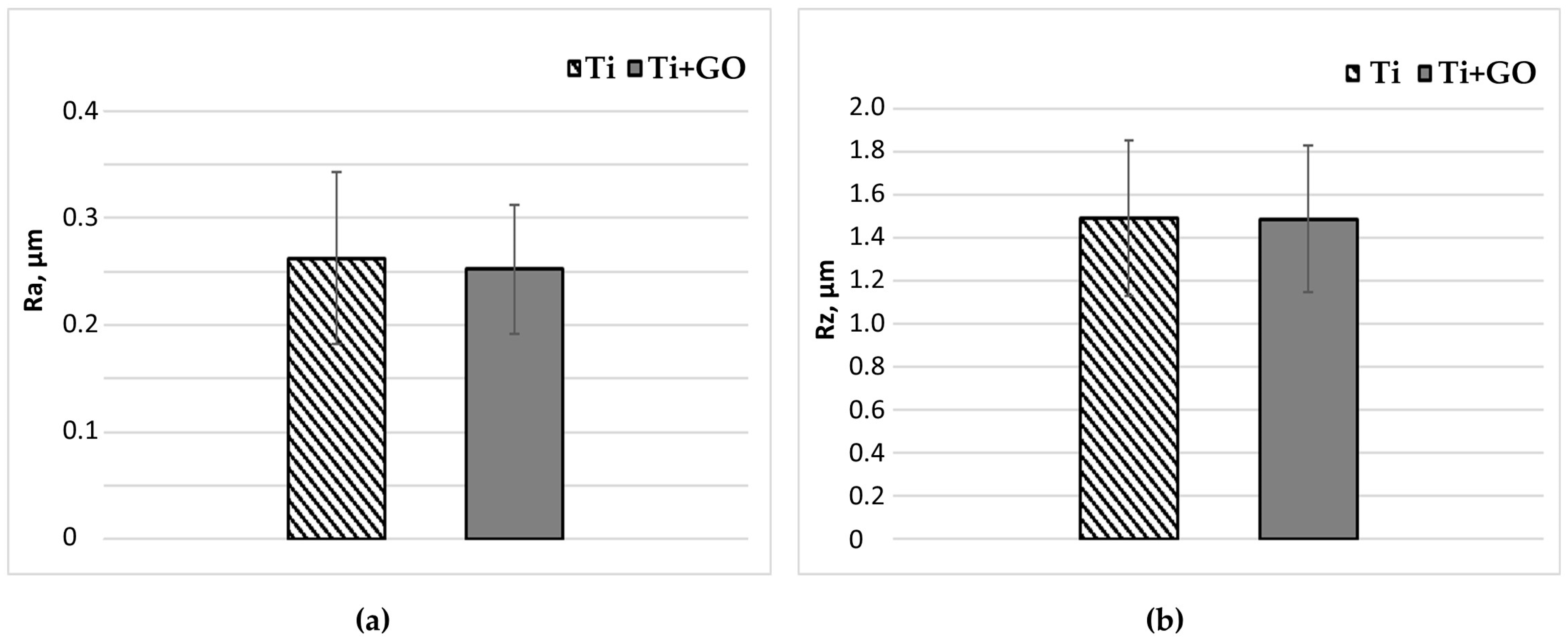
The surface roughness was measured using a Mitutoyo SJ–201 device (Mitutoyo Corporation, Takatsu, Kawasaki, Japan). The Ra and Rz parameters were determined, measurements were repeated five times, and then the arithmetic mean and standard deviation were calculated.
2.2. Corrosion Studies
Electrochemical corrosion studies of Ti and Ti + GO samples were carried out using a Metrohm Autolab PGSTAT 101 potentiostat controlled by NOVA 2.1 software. The measurement system consisted of a glass electrochemical cell equipped with a three-electrode setup, where a platinum rod (Ø = 1 mm) served as the auxiliary electrode, Ag/AgCl (3 mol/L KCl) was used as the reference electrode, and the working electrode was the tested Ti or Ti + GO sample.
During each measurement, the electrochemical cell was filled with a buffered solution formulated to simulate the composition of physiological intracellular fluids. For both titanium and graphene oxide–coated titanium samples, long-term open circuit potential (OCP) measurements were first recorded, followed by the acquisition of potentiodynamic polarization curves.
2.3. Biological Studies
2.3.1. In Vitro Culture of Human Skin Melanoma Cell Line Hs 895.T and Human Skin Fibroblast Line Hs 895.Sk
The Hs 895.Sk American Type Culture Collection (ATCC), normal skin fibroblast cell line, and Hs 895.T (ATCC©), melanoma cell line, were grown in Dulbecco’s Modified Eagle’s Medium (ATCC©) supplemented with 10% fetal bovine serum (Gibco®, Grand Island, NY, USA) and a mixture of antibiotics/antimycotic (Lonza Group AG, Basel, Switzerland): penicillin (10 U/mL) with streptomycin (10 µg/mL). Cell cultures were maintained in an incubator (HERAcell VIOS 160i, Thermo Scientific, Waltham, MA, USA) at 37 °C, 90% humidity, and in an incubator, atmosphere containing 5% CO2 (HERAcell VIOS 160i, Thermo Scientific).
The titanium foils, in their native state and with the GO layer deposited, were exposed to 70% ethyl alcohol for 15 min and then to UV-C radiation for 30 min to sterilize the surface of the samples. Then, the foil fragments were placed in a 24-well plate, and 1 mL of a cell suspension with a density of 104 cells/mL was added. The multi-well plate prepared in this manner was incubated for 24 and 48 h at 37 °C with 95% humidity and 5% CO2.
2.3.2. Cell Viability of Human Skin Melanoma Cell Line Hs 895.T and Human Skin Fibroblast Line Hs 895.Sk
The viability of human skin melanoma cells (Hs 895.T) and human skin fibroblasts (Hs 895.Sk) after 24–48 h of incubation on titanium foils coated with a graphene oxide layer was assessed using the LIVE/DEAD™ Viability/Cytotoxicity Kit (Invitrogen, Waltham, MA, USA), in accordance with the manufacturer’s instructions. After incubation, the cells were observed using a LSM 700 Axio Observer.Z1 confocal microscope (Carl Zeiss Microscopy, Jena, Germany), following prior maintenance of the samples at 37 °C and protection from light exposure.
2.3.3. Morphology of Human Skin Melanoma Cell Line Hs 895.T and Human Skin Fibroblast Line Hs 895.Sk
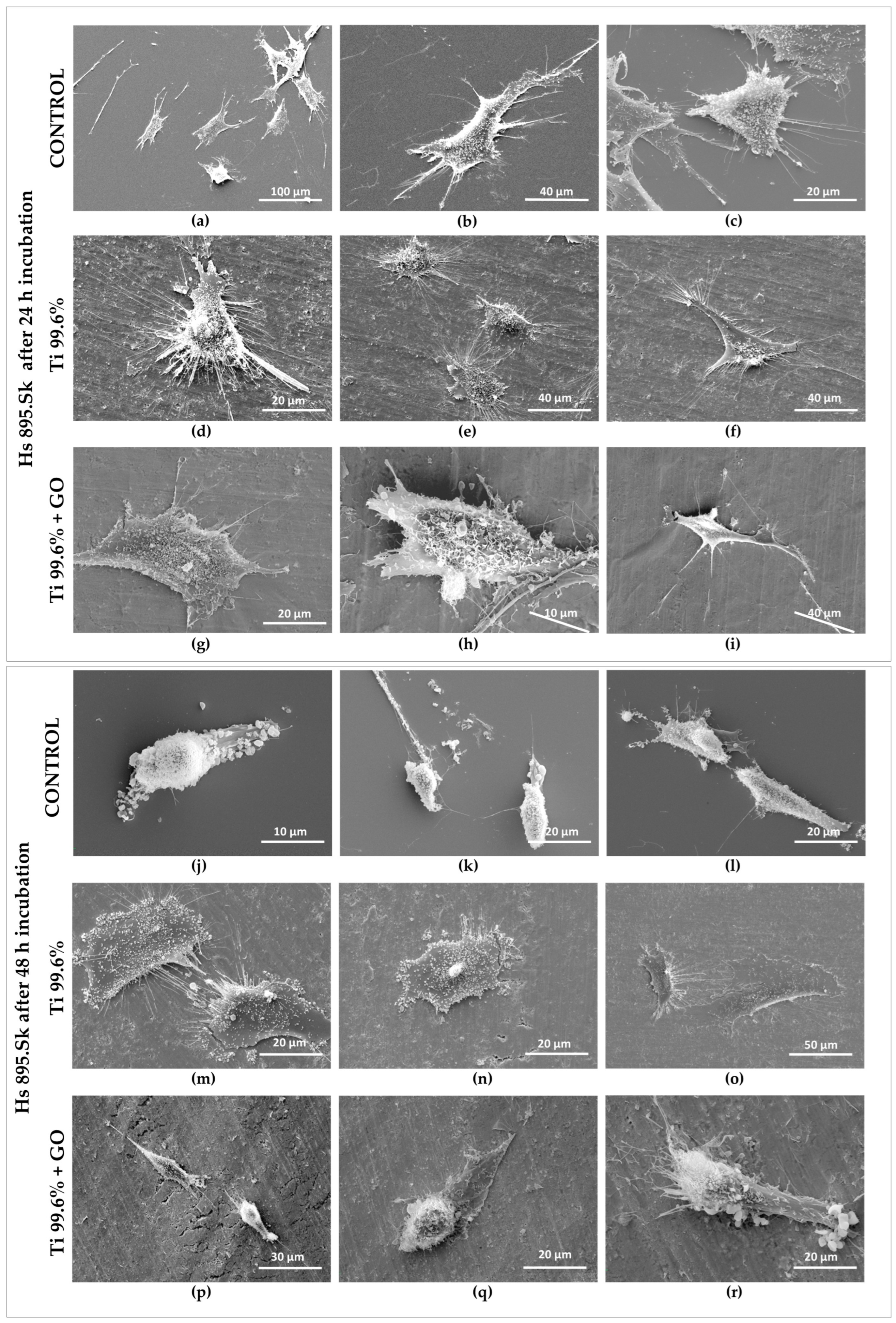
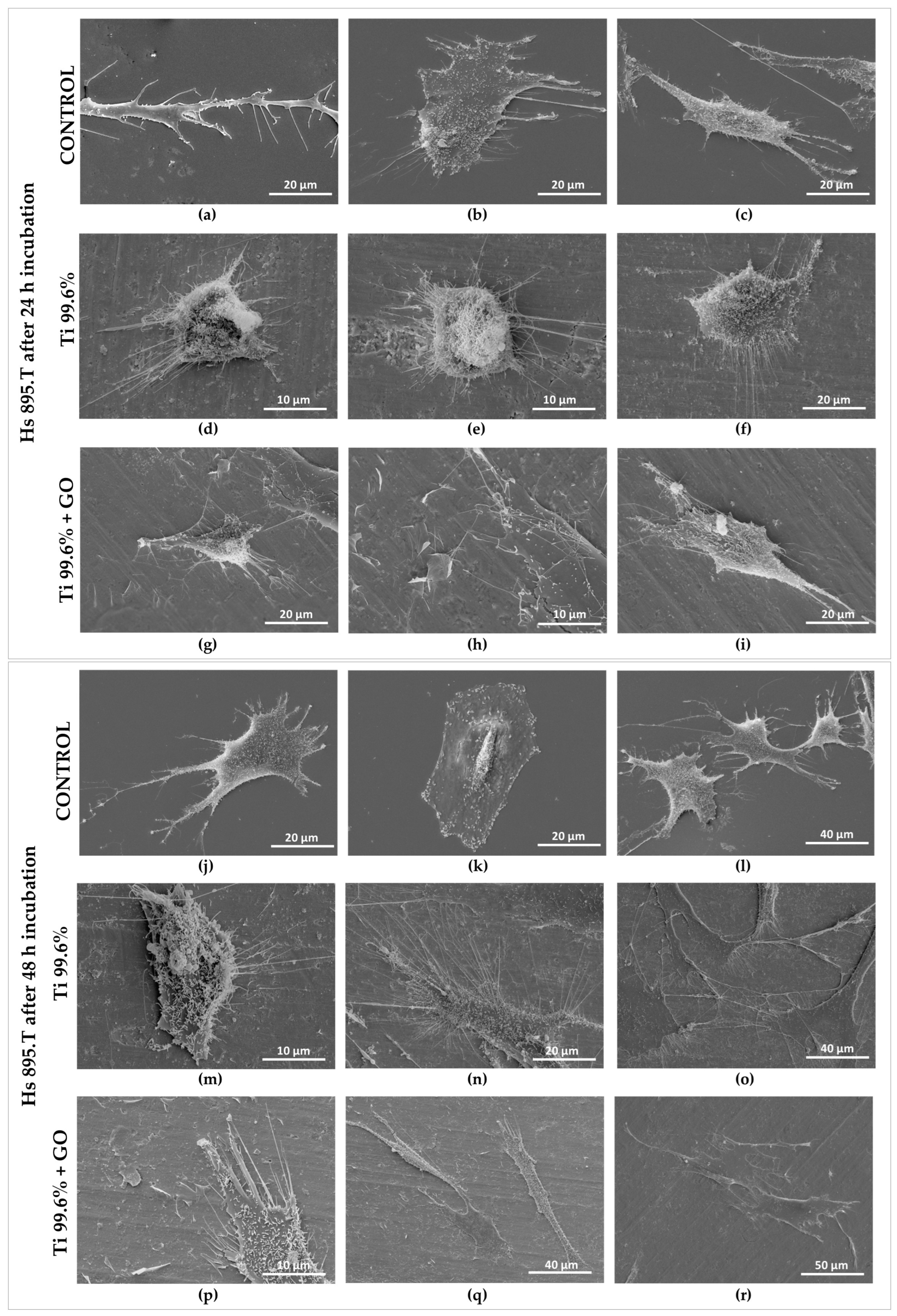
The morphology of the cells was visualized using a scanning electron microscope (SEM). For this purpose, cells proliferating on the surface of titanium foils (Ti) and titanium foils with a deposited graphene oxide layer (Ti + GO) were fixed with a mixture of 4% paraformaldehyde (Sigma-Aldrich, Burlington, MA, USA) and 0.4% glutaraldehyde (Sigma-Aldrich, Burlington, MA, USA). The cells were then contrasted in a 1% osmium tetroxide (OsO4) solution (Sigma-Aldrich, Burlington, MA, USA) at 4 °C, and dehydrated through a graded series of ethanol (POCH, Gliwice, Poland) and acetone (POCH, Gliwice, Poland) solutions ranging from 30% to 100%. After dehydration, the samples were dried at the critical point using a Leica EM CPD300 (Leica Microsystems GmbH, Wetzlar, Germany) and then coated with a thin layer of gold using a sputter coater (Leica EM ACE200, Leica Microsystems GmbH, Wetzlar, Germany).
2.3.4. Analysis of Bacterial Inhibition Zones
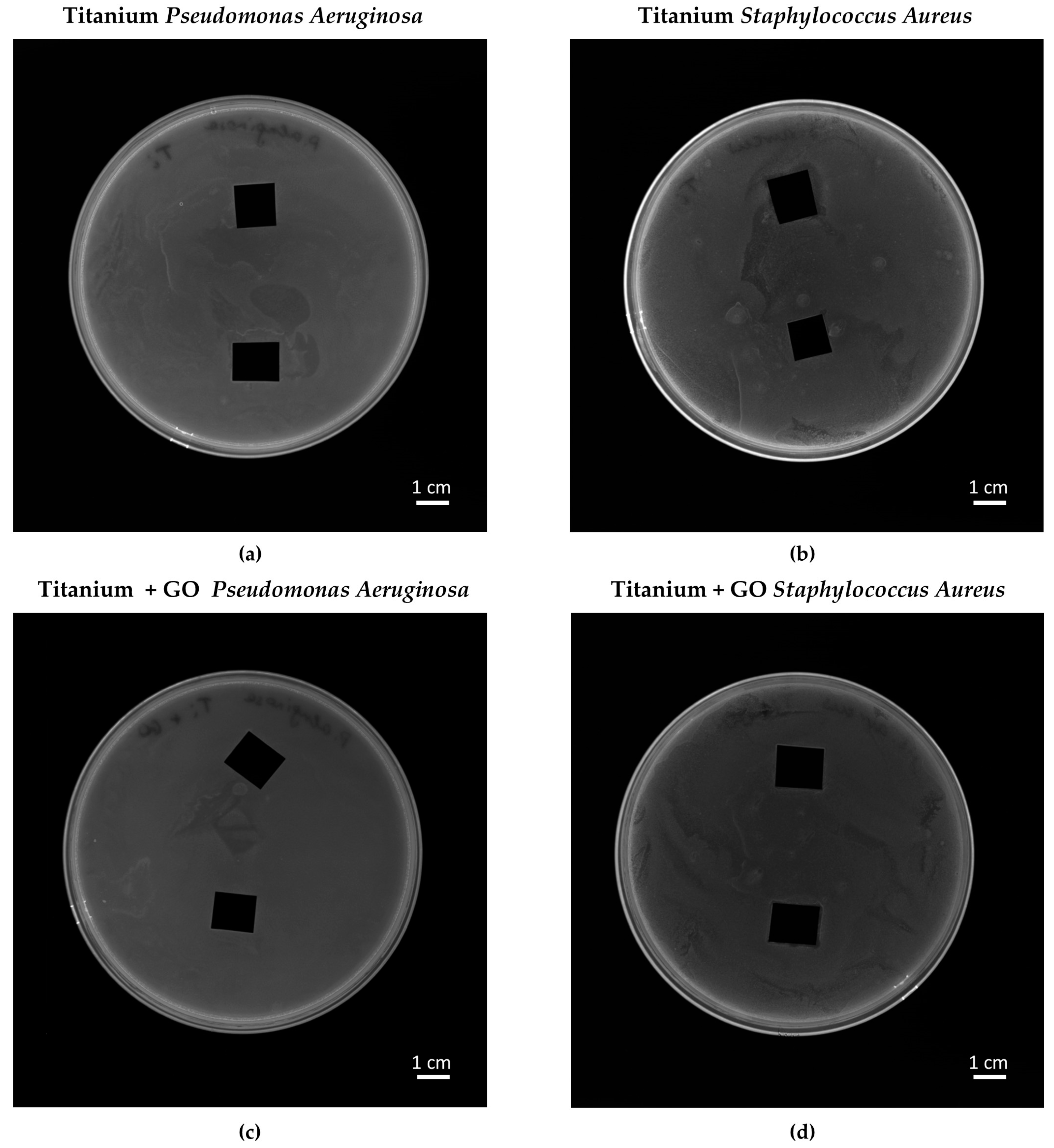
Analysis of bacterial growth and its inhibition after exposure to cement mortar was prepared with two bacterial strains:
Staphylococcus aureus (ATCC 25923) and
Pseudomonas aeruginosa (ATCC 27853), which were obtained from the American Type Culture Collection (ATCC) and they were maintained in 20% (
v/
v) glycerol at −20 °C. On the surface of solidified Mueller–Hinton agar prepared in Petri dishes (Ø90 mm), the spread plate method of bacterial suspension (0.5 on the McFarland scale) was performed, and then the samples of cement mortar (10 × 10 mm) were placed on the surface. The plates were incubated in standard conditions (24 h, 37 °C). After incubation, the inhibition zones were measured and captured with an Azure C400 (Azure Biosystem, Dublin, CA, USA). The procedure of preparing samples for this analysis was described in [
34,
35]. Thereafter, the Ti and Ti + GO samples were removed and prepared for scanning electron microscopy
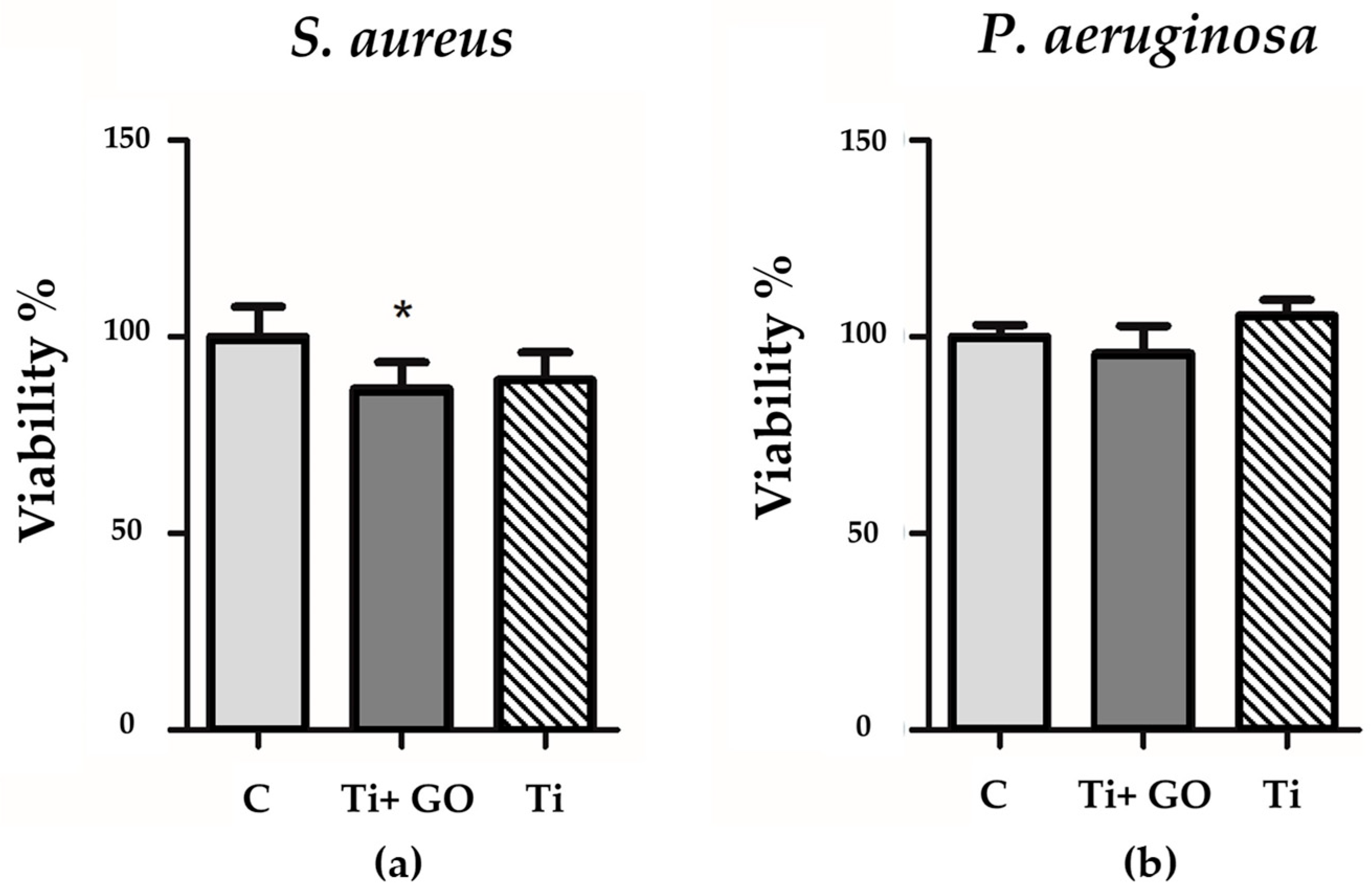
Due to the limitations that may occur in the method determining the growth inhibition zone, a viability analysis was also performed using the PrestoBlue test (ThermoFisher, Waltham, MA, USA). For this purpose, Ti and Ti + GO samples were placed in Mueller–Hinton broth, to which a 10 µL suspension of bacteria (0.5 on the McFarland scale) was added, and the whole mixture was incubated for 24 h at 37 °C. To the liquid culture, 10 µL of PrestoBlue was added to each well (the sample contained a positive control, i.e., medium with added bacteria, without the test factor, and a negative control, i.e., the medium alone). The whole was incubated for 30 min, and the absorbance was measured at a wavelength of 570 nm and a reference of 600 nm. The results were calculated as the average absorbance (test group-negative control/positive control-negative control)*100%. Each test was performed in at least 3 replicates.
2.3.5. Cell Morphology of Human Skin Melanoma Cell Line Hs 895.T and Human Skin Fibroblast Line Hs 895.Sk
The study was conducted using fibroblast cultures with an average density of 2–3 × 105 cells/mL of medium. The cells were exposed to graphene oxide flakes after 24 h of incubation. Incubation was carried out horizontally in an incubator at 37 °C with 5% CO2. After 23 h of culture, 1 µL of cytochalasin B (6 µg/mL) was added and gently mixed, and after another 24 h, the culture was collected. The cells were washed twice in PBS solution.
The next step was to terminate the culture and fix it using a methanol/acetic acid/Ringer’s solution mixture (4/1/5, 4 °C), followed by staining the cells with DAPI Fluoroshield™ with DAPI. The previously prepared fixative was kept at 4 °C for approximately 30 min before use. The culture was mixed and centrifuged for 8 min at 1000 rpm. The supernatant was discarded, leaving about 0.5 cm of medium above the cell pellet. The pellet was vortexed, and during the process, 5 mL of fixative was slowly added. The prepared solution was stored at 4 °C. The cells were then centrifuged again for 8 min at 1000 rpm. The pellet was vortexed, and 5 mL of methanol/acetic acid fixative (4/1, 4 °C) was slowly added. The pellet was vortexed again, and 40 µL of the suspension was transferred onto a microscope slide, fully covering its surface.
Cell density was assessed under a ZEISS Imager Z2 microscope (Carl Zeiss AG, Oberkochen, Germany) equipped with a UV attachment.
4. Discussion
The novelty of depositing a surface layer on titanium alloys, in contrast to the current state of knowledge, involves not only surface activation but also the possibility of embedding graphene nanostructures into the surface layer. This mechanism was confirmed in publication [
27]. Such embedding is possible not only due to surface activation but also due to improved wettability.
To determine the effect of graphene oxide (GO) deposited on titanium foil, structural, corrosion, microbiological, biological, and genotoxicity studies were carried out. Structural surface analyses performed using scanning electron microscopy (SEM) and FTIR spectroscopy confirmed the presence of GO flakes deposited on the titanium foil surface. Distinct flake structures and boundaries of individual GO sheets were observed on Ti surfaces. The spectroscopic analysis revealed strong absorption bands corresponding to the stretching vibrations of hydroxyl (O–H) groups and aliphatic C–H bonds.
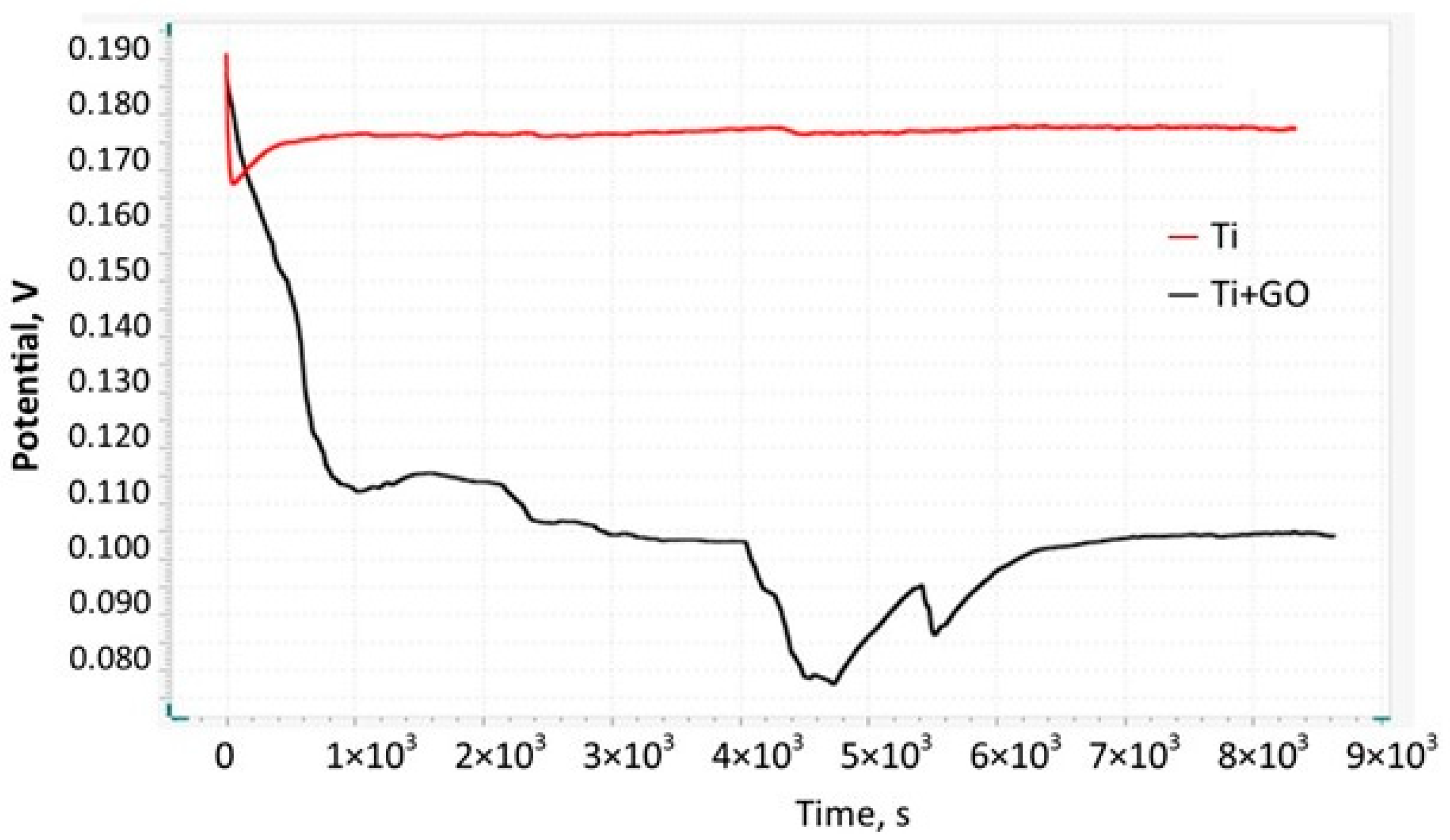
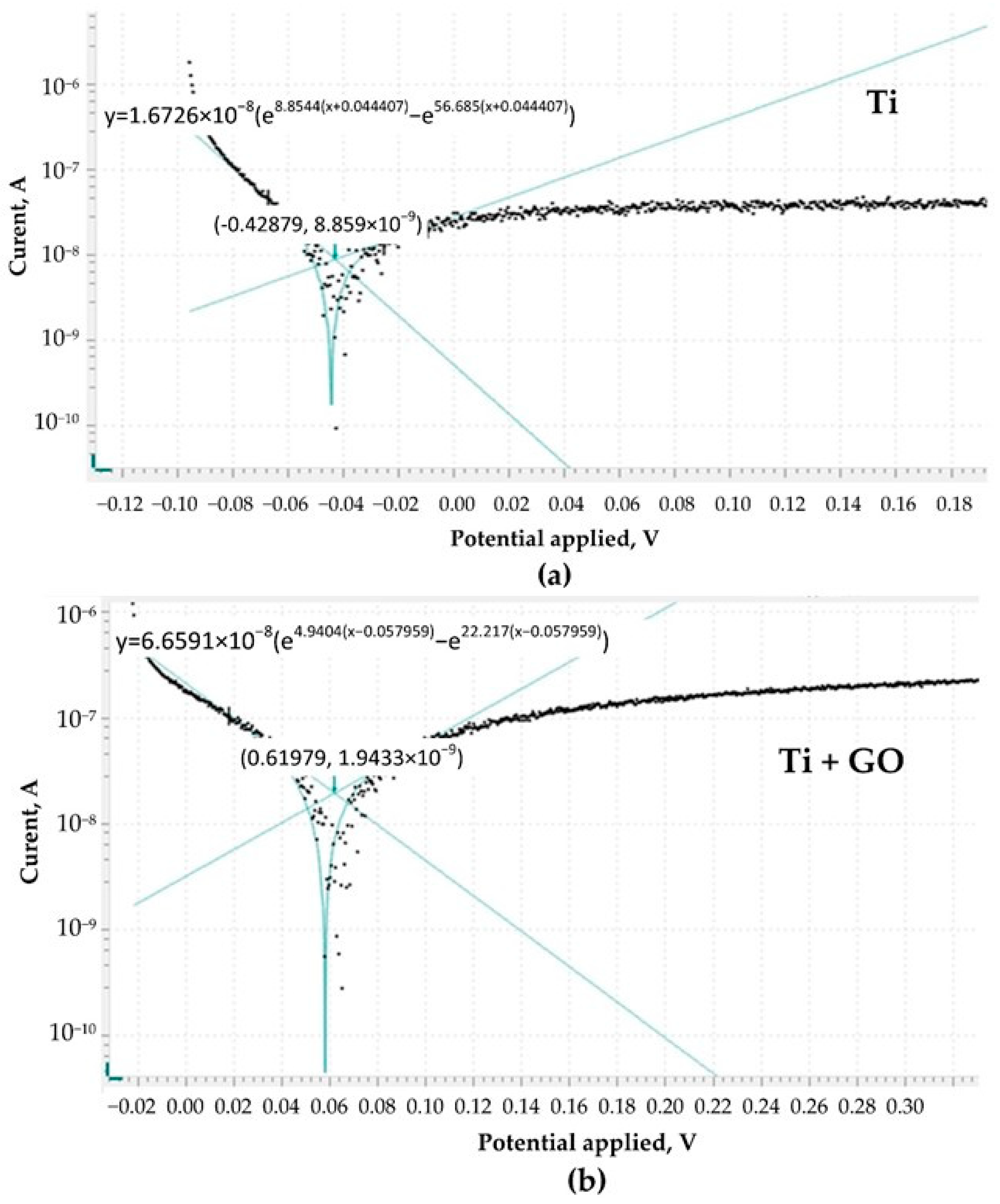
In the corrosion studies, it was observed that coating the titanium foil with graphene oxide limited the access of electrolytes and oxygen to the metal surface. The electrochemical potential of Ti + GO stabilized rapidly and remained constant over time. The higher OCP value (1.1774 V) compared to Ti indicates lower surface activity, which minimizes the risk of corrosion processes and provides better electrochemical properties. In the context of implant applications, this has a positive effect on mechanical stability and biocompatibility.
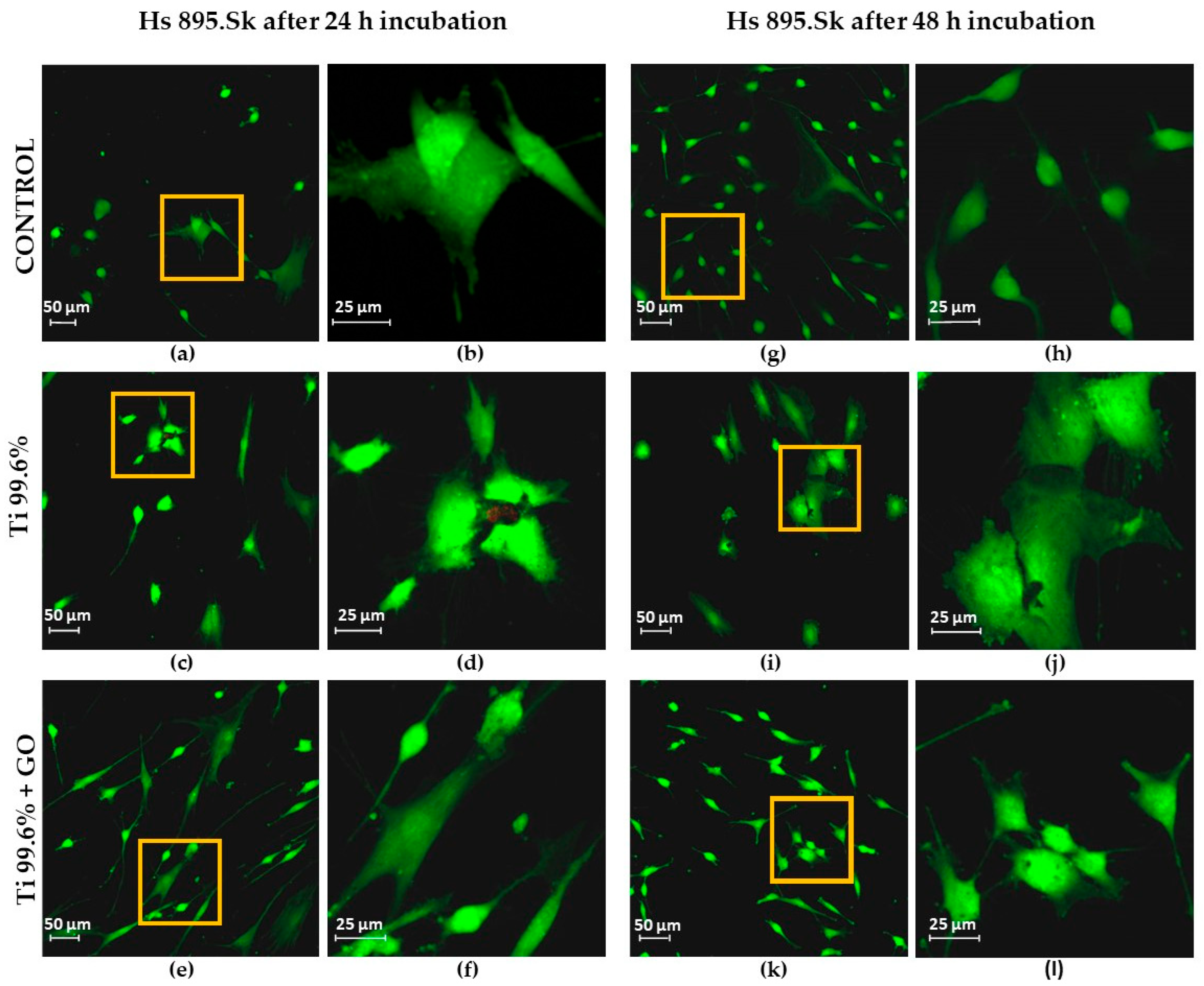
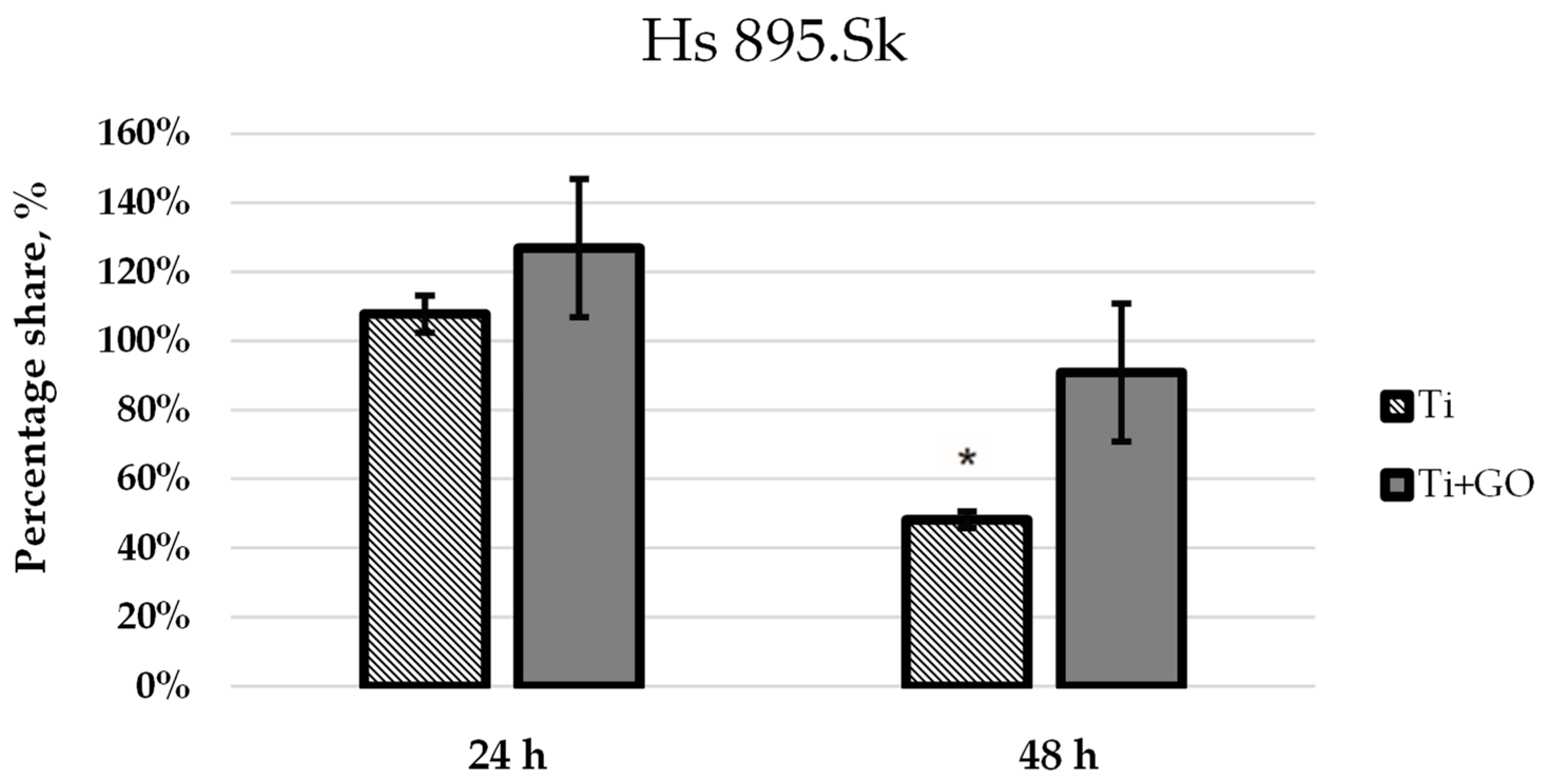
The results of biological tests clearly demonstrated that surface modification of titanium with GO promotes proper cellular behavior of human skin fibroblasts and may contribute to a reduction in microbial development. Analysis of fibroblast (Hs 895.Sk) viability and morphology showed that Ti + GO provided a more favorable microenvironment for cell growth than unmodified titanium. The cells exhibited distinct flattening and numerous cytoplasmic extensions, indicative of active adhesion and proper cytoskeletal function. These findings are consistent with literature reports suggesting that oxygen-containing functional groups present in GO enhance surface hydrophilicity and surface energy, facilitating the adsorption of adhesion proteins and promoting stable cell–substrate interactions [
3,
8,
9].
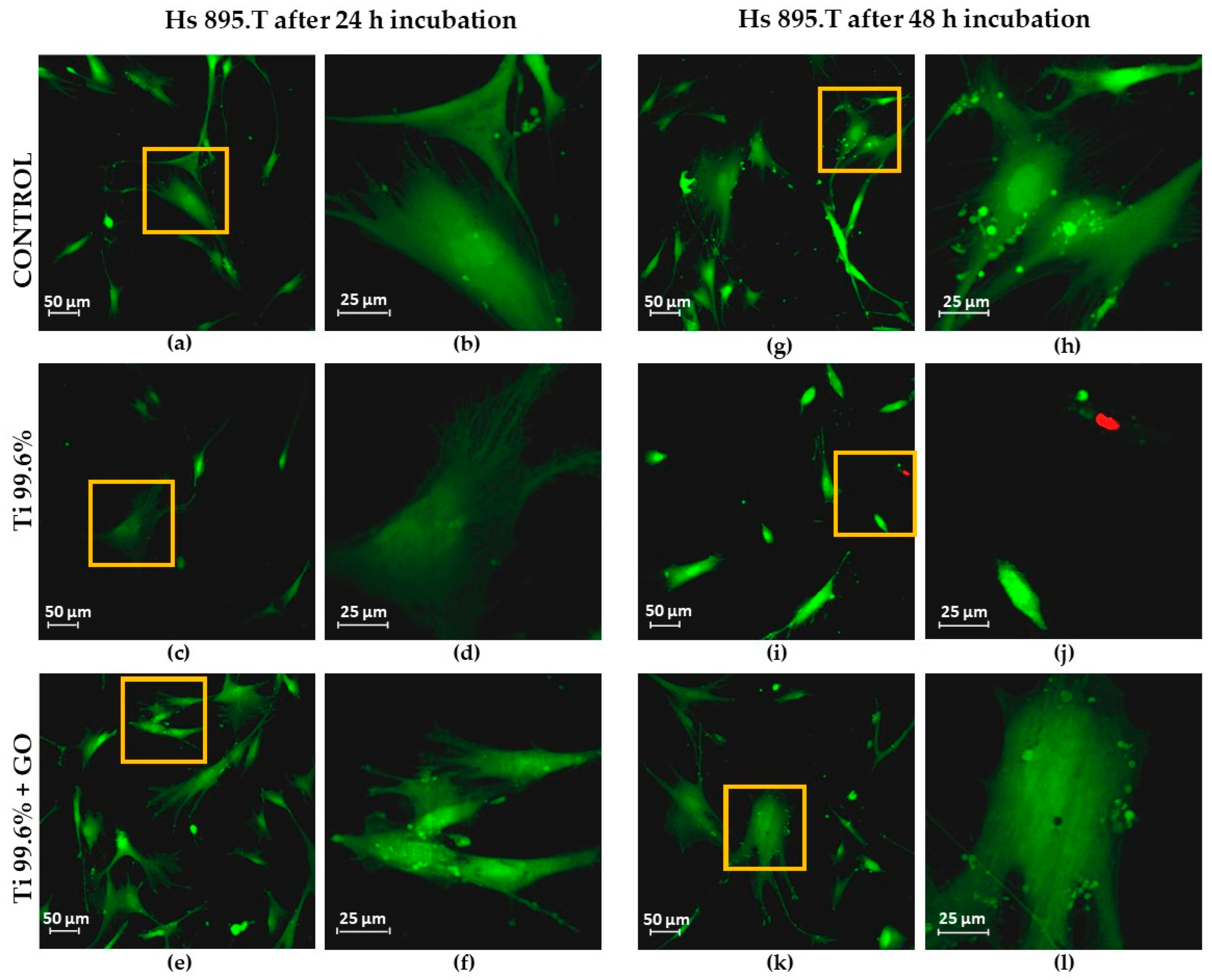
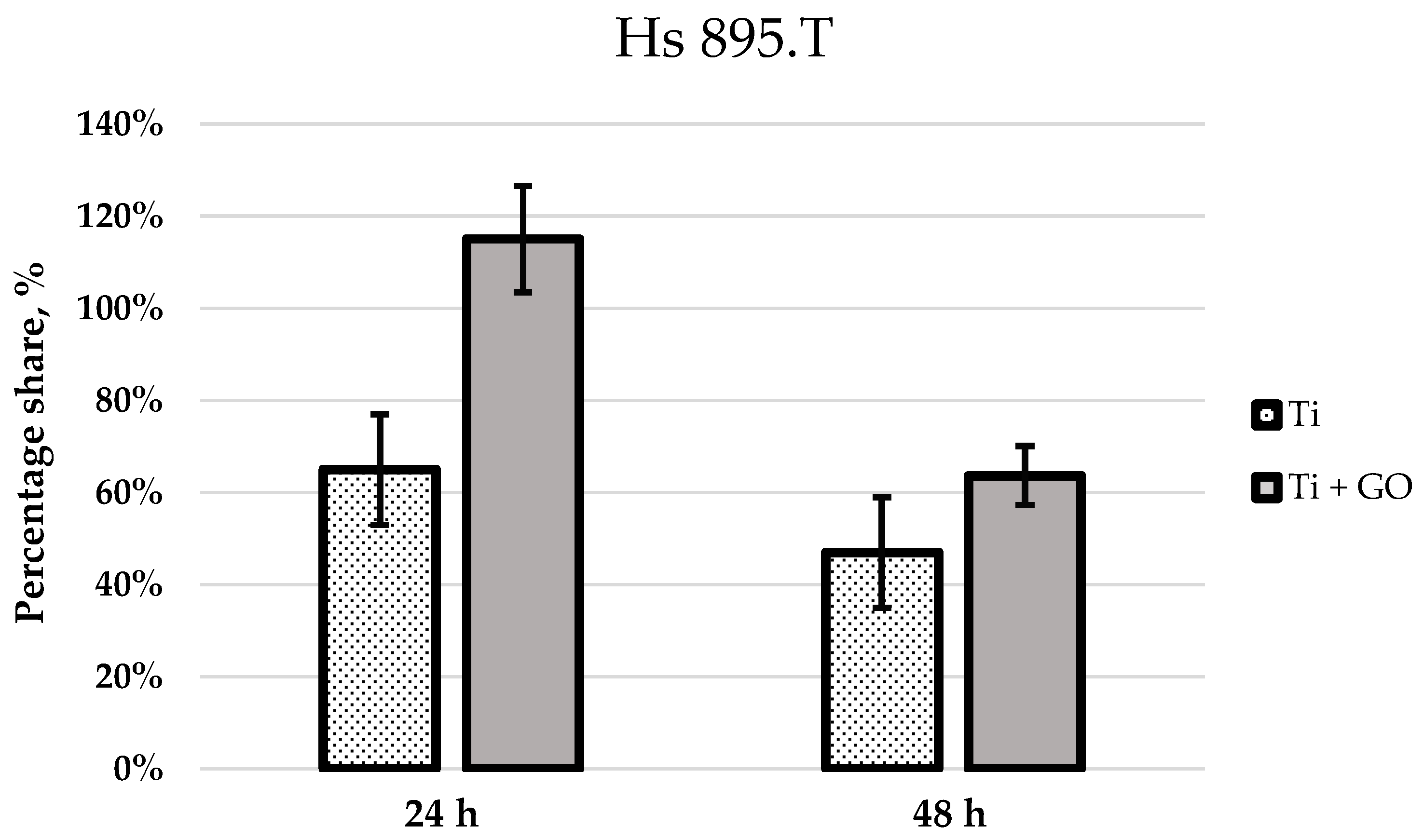
Similar trends were observed for melanoma cells (Hs 895.T); however, their proliferation and morphological response differed from fibroblasts. After 24 h, the melanoma cells adhered more strongly to Ti + GO than to Ti, while after 48 h, they exhibited more pronounced spreading and the presence of filopodia on both substrates. Hs 895.T cells cultured on Ti + GO showed a more spread and adherent morphology; however, this represents structural adaptation to the substrate rather than evidence of changes in migratory potential. Since the present work did not include migration-focused tests, no conclusions can be drawn about GO-related modulation of cancer cell motility. Nonetheless, the absence of migration-specific assays does not preclude other GO-associated cellular effects described in previous studies. Some studies have shown that GO can modulate proliferation pathways and inhibit the growth of certain cancer cells through oxidative stress generation or modification of adhesion-related signaling pathways [
10,
11,
12]. Ti + GO improves early adhesion and viability of both fibroblasts and Hs 895.T cells; this does not imply support of malignant proliferation. Additional long-term and in vivo investigations would be required to assess tumor implant interactions in oncologic patients. The results obtained here may be consistent with these findings, although further mechanistic investigation is required. The improved viability of cells on Ti–GO can be explained by the combined effect of GO’s surface chemistry and nanoscale morphology. The abundant oxygen-containing groups (–OH, –COOH, –C=O) increase hydrophilicity and surface free energy, promoting rapid adsorption of adhesion-related proteins from the culture medium, which enhances integrin-mediated attachment. Additionally, the negative surface charge of GO at physiological pH stabilizes the protein conditioning layer and facilitates the formation of a favorable cell–material interface. The wrinkled micro-/nanostructure of the GO coating provides multiple anchoring points for filopodia, supporting cell spreading and cytoskeletal organization, as confirmed by SEM. Together, these features likely contribute to the higher viability observed on Ti–GO compared with unmodified titanium. To further verify the biological safety of the coating, we next evaluated potential DNA damage and chromosomal instability using the micronucleus assay.
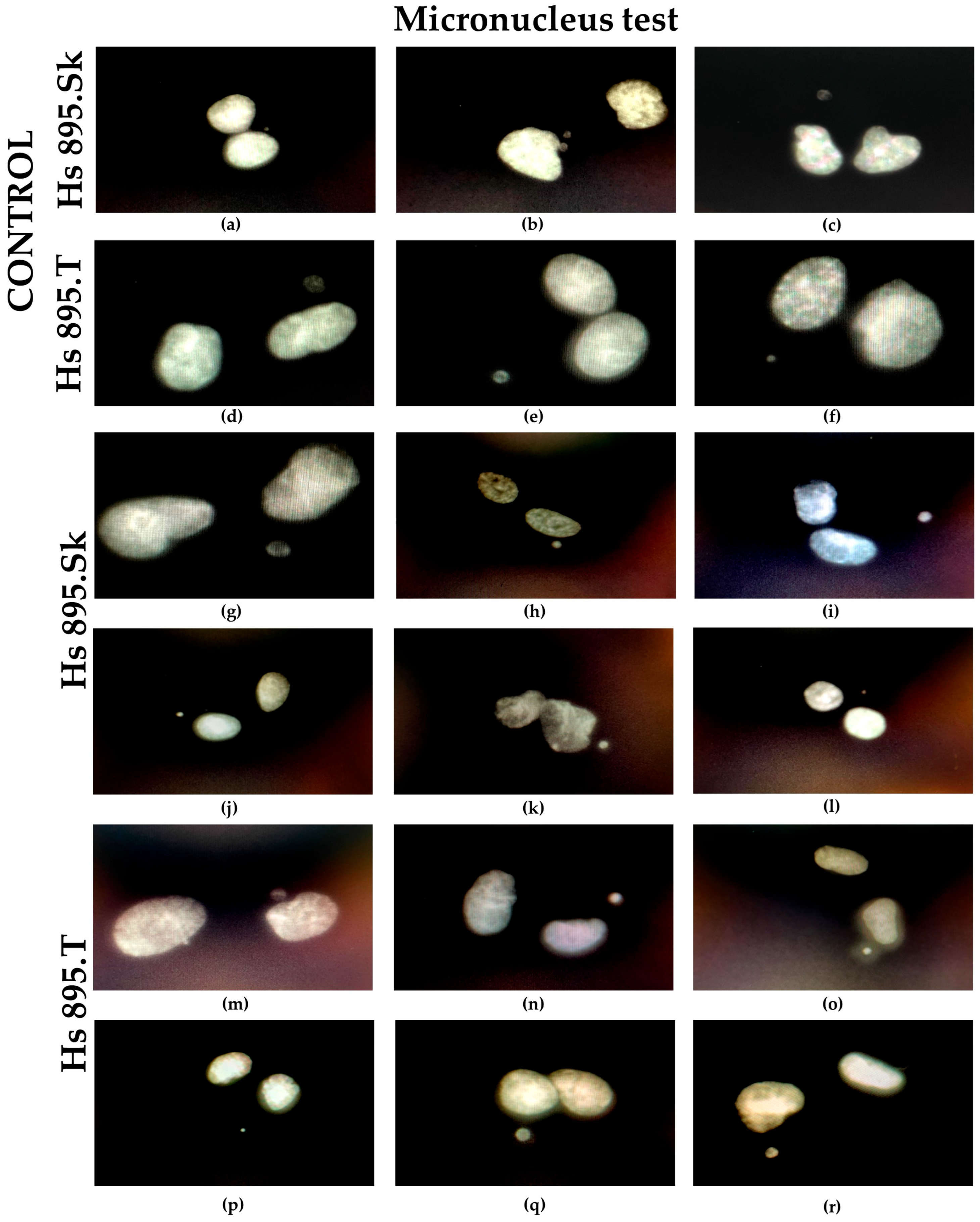
The micronucleus test results clearly confirmed the absence of genotoxic effects in both healthy fibroblasts and melanoma cells. The proportions of mono- and binucleated cells and the number of micronuclei remained similar to those of control samples, indicating preserved genoic stability and no induction of DNA damage upon exposure to Ti + GO. This is a crucial requirement for the clinical acceptance of biomaterials, especially in long-term tissue contact applications (ISO 10993 standard, International Organization for Standardization, Geneva, Switzerland) [
40].
The in vitro effects observed for Ti + GO may be relevant for implant performance in vivo. Enhanced fibroblast adhesion is widely recognized as a prerequisite for early soft-tissue integration and long-term implant stability [
41]. Studies have shown that GO-containing surfaces promote protein adsorption and fibroblast attachment in ways that support peri-implant tissue sealing [
42]. The improved electrochemical stability and passive behavior of Ti + GO observed in our study are consistent with the literature, which links stable OCP and low corrosion susceptibility to enhanced implant durability under physiological conditions [
43]. Finally, the absence of genotoxicity aligns with ISO 10993 requirements and indicates a low likelihood of initiating inflammatory or mutagenic processes in vivo [
44]. The absence of genotoxic effects is especially relevant for chronic implant applications, where prolonged biomaterial exposure must not induce DNA damage or disrupt the local tissue environment.
Bacterial response analysis showed no inhibition zones in the agar diffusion test, which may result from limited nanomaterial diffusion and the necessity of direct contact between GO and bacterial cells. However, bacterial viability assays confirmed a slight but noticeable reduction in the number of viable
Staphylococcus aureus and
Pseudomonas aeruginosa cells in contact with Ti + GO, suggesting partial antibacterial activity of the modified surface. The mechanisms of GO’s antibacterial action may involve direct membrane damage, protein and lipid adsorption, generation of reactive oxygen species (ROS), and disruption of biofilm integrity [
11,
12,
13,
14]. As no visible morphological damage of bacterial structures was observed, the likely mechanism is ROS generation—commonly attributed to graphene-family materials [
45]. Additionally, the physicochemical parameters of the applied material strongly affect its antibacterial efficacy. Given the combination of GO with titanium, another possible mechanism involves indirect isolation of bacterial cells from nutrients [
46].
Overall, the obtained results suggest that GO may serve as a beneficial component for reducing bacterial infections on implant surfaces. Nevertheless, further optimization of the nanostructure and the amount of the deposited layer is necessary to enhance its antimicrobial effectiveness.
5. Conclusions
Graphene oxide (GO) flakes were uniformly deposited onto model implant samples made of titanium foil, as confirmed by imaging using scanning transmission electron microscopy (STEM).
Infrared surface analysis (FTIR) revealed characteristic absorption bands typical of graphene oxide, corresponding to hydroxyl (O–H) and aliphatic C–H stretching vibrations.
Deposition of GO nanostructures on the titanium surface reduced the access of corrosive electrolytes and oxygen to the metallic surface of the samples. The electrochemical potential curve stabilized rapidly and remained constant over time. The higher OCP value indicated lower corrosion activity, which positively influences the practical applicability of the material for implant manufacturing.
The presence of GO nanostructures on Ti improved the adhesion and viability of the tested cell lines, Hs 895.Sk and Hs 895.T, while reducing the number of dead or damaged cells.
Neither Ti nor Ti + GO surfaces exhibited significant antibacterial properties. The materials did not alter the morphology of Staphylococcus aureus or Pseudomonas aeruginosa cells, nor did they produce inhibition zones around the samples.