Construction of Graphite Shells on Ferromanganese Oxide for Electromagnetic Wave Absorption
Abstract
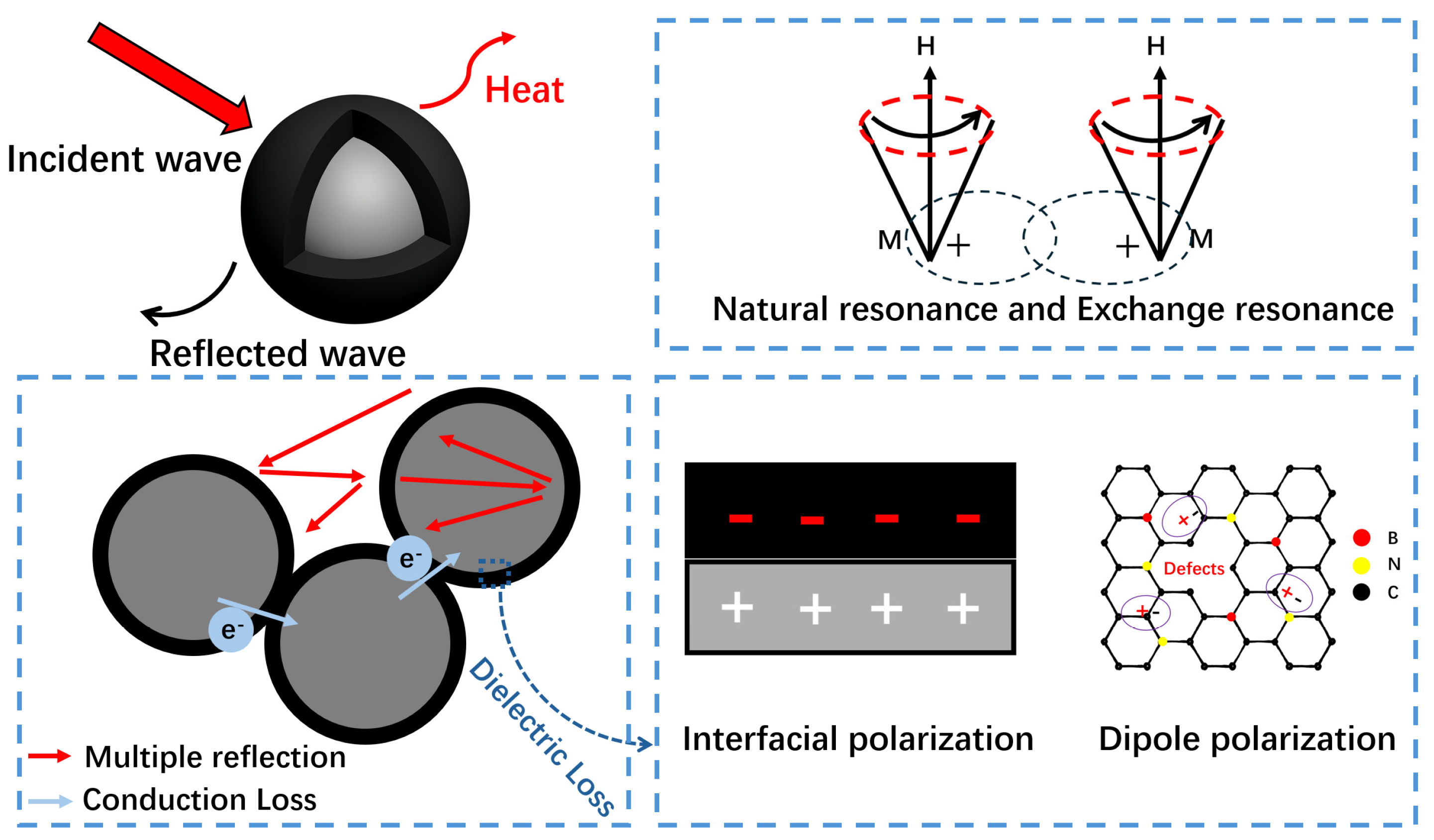
1. Introduction
2. Materials and Methods
2.1. Fabrication of the FMO@C Powder
2.2. Characterization
3. Results and Discussion
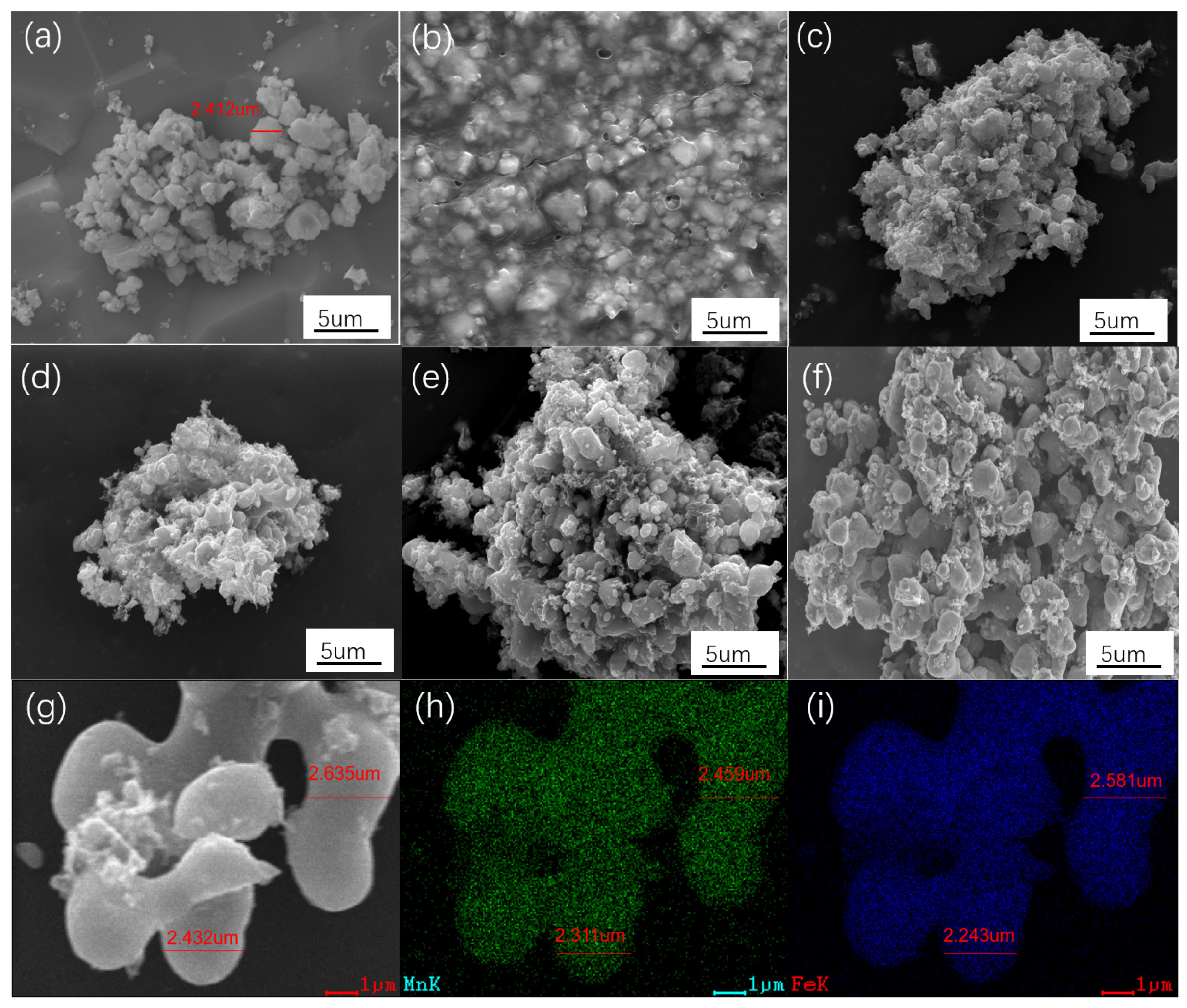
3.1. The Phase Evolution of FMO@C
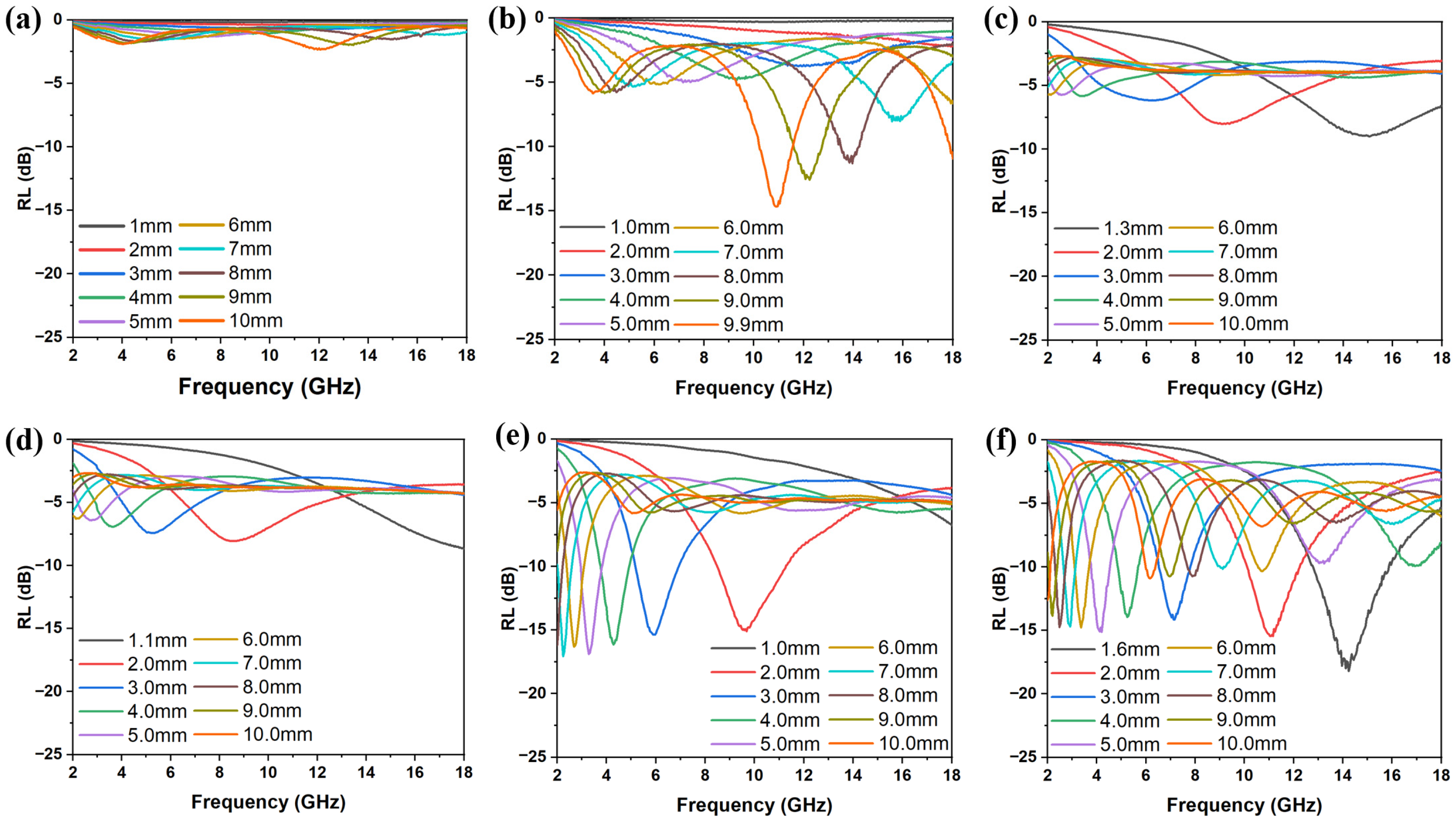
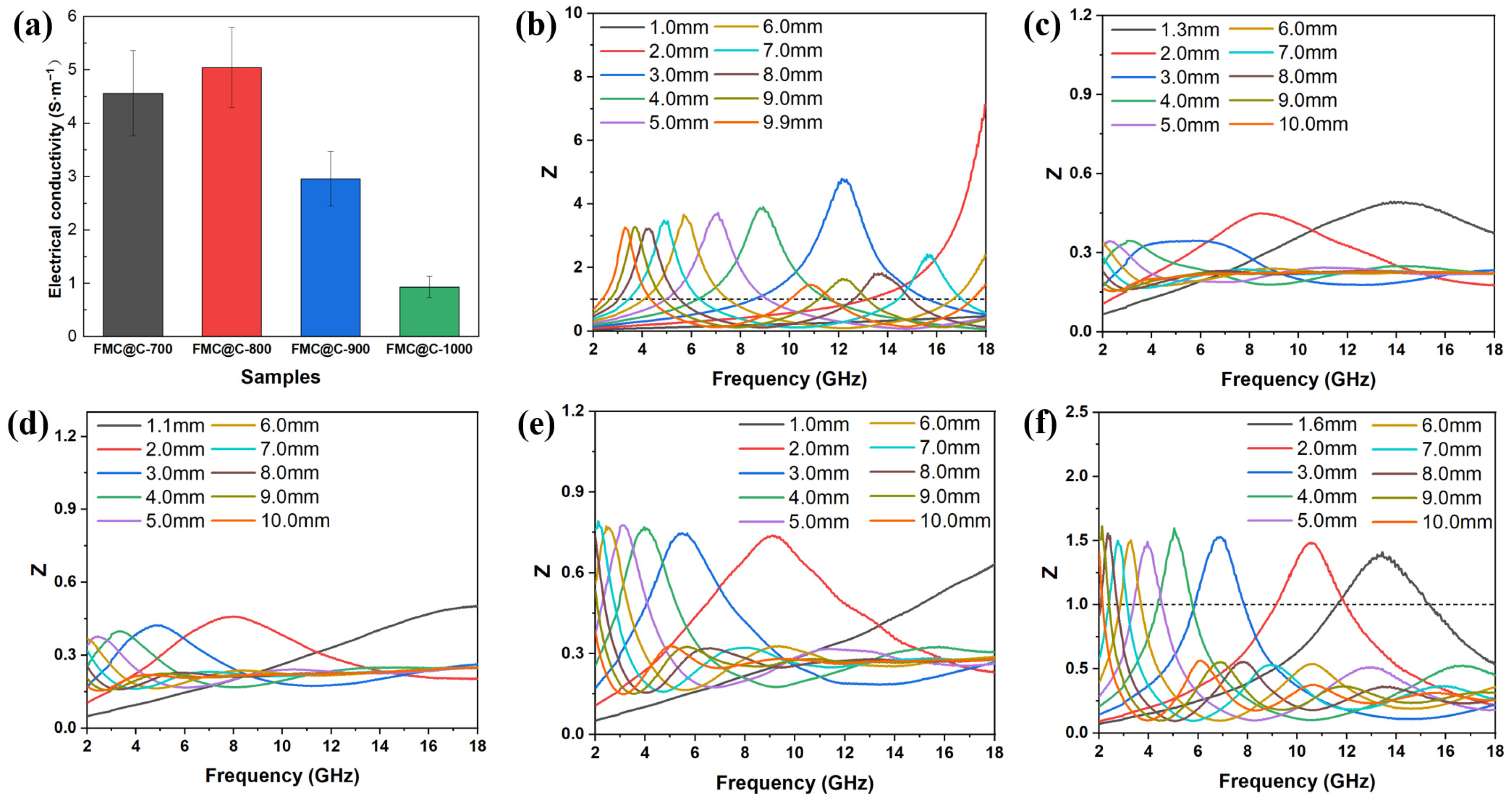
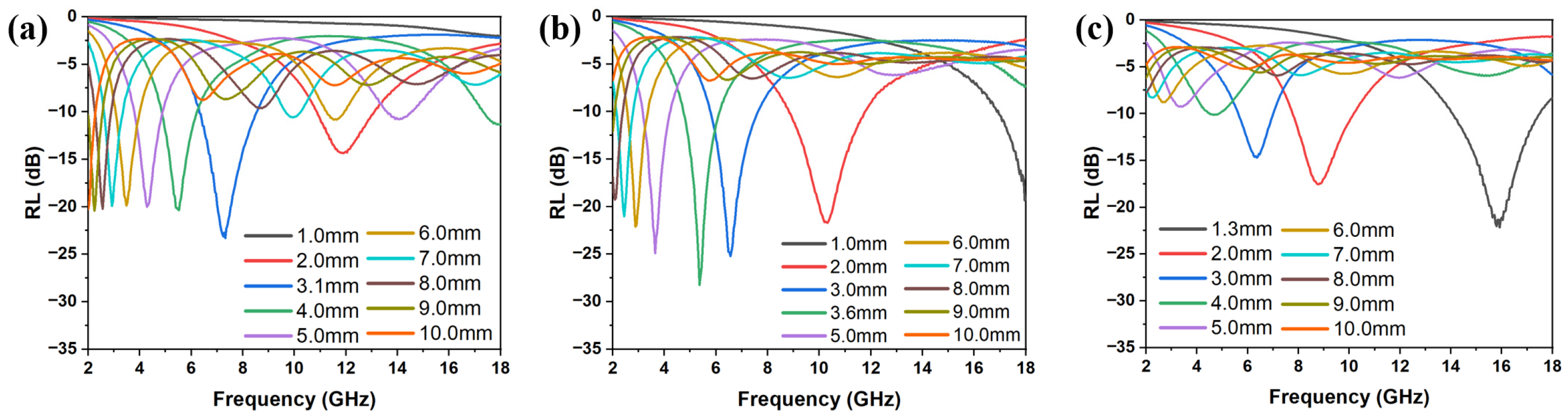
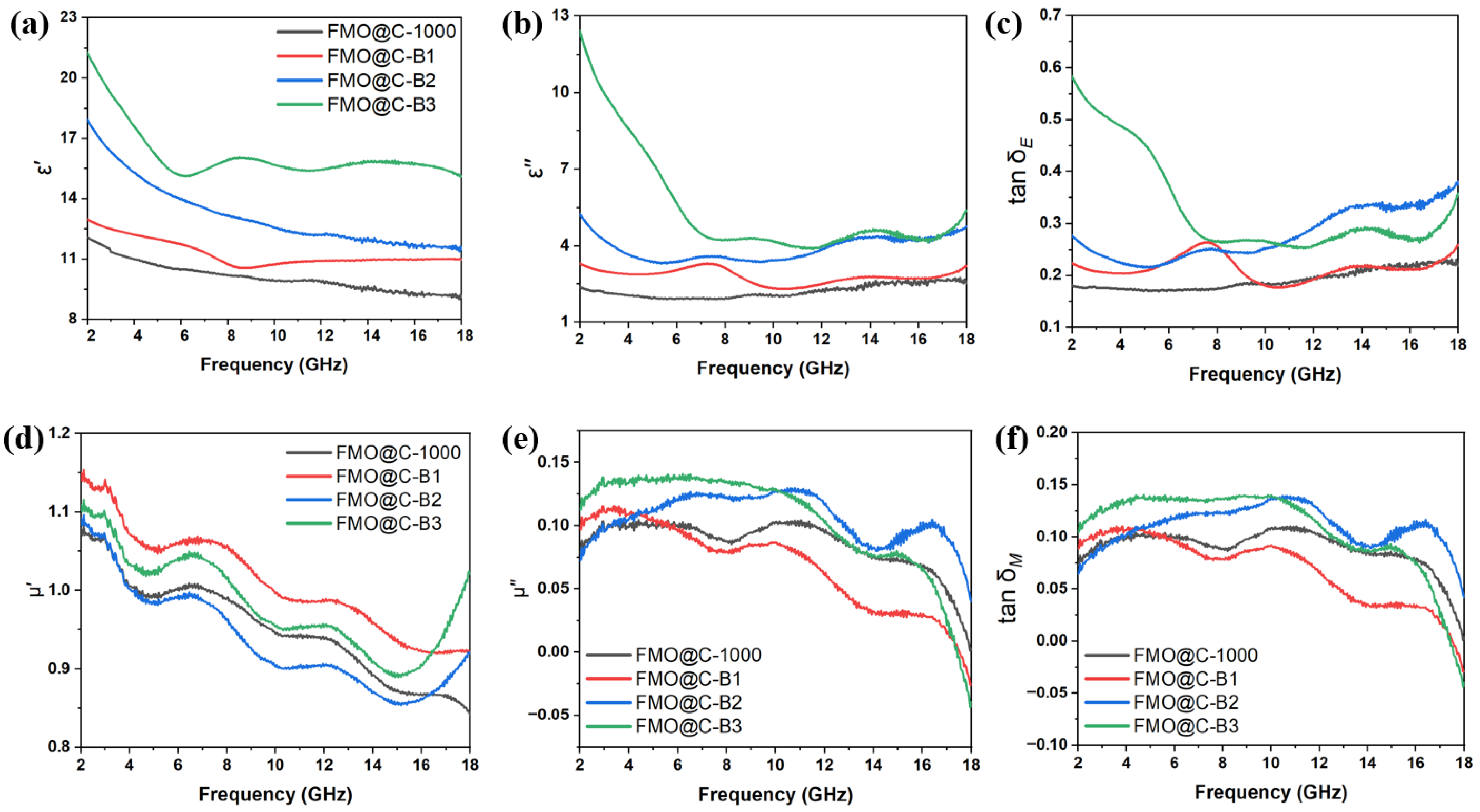
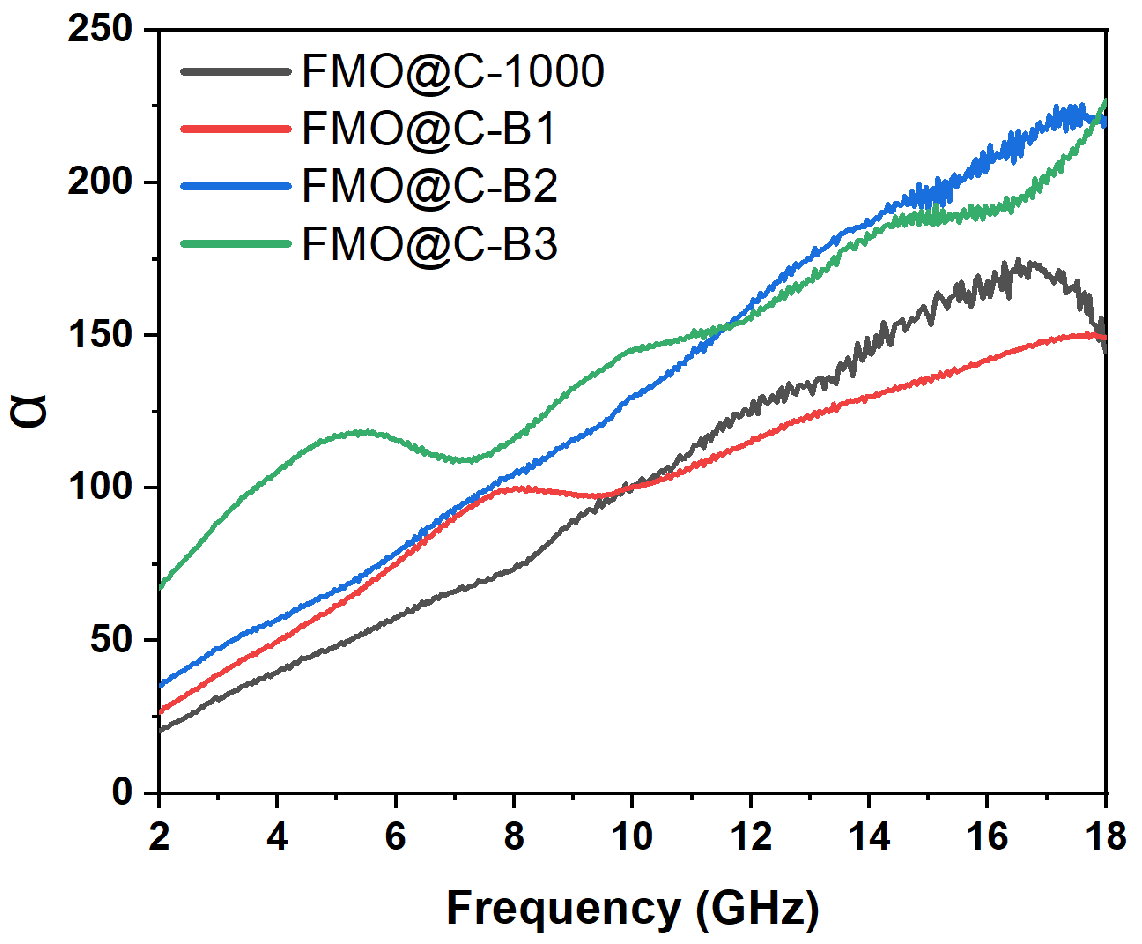
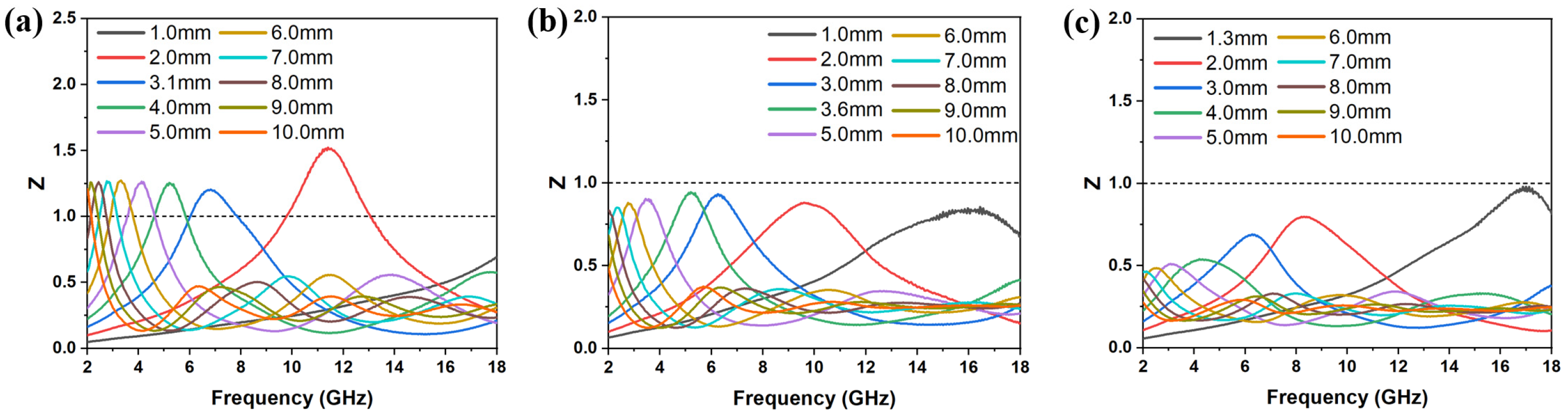
3.2. EMW Absorption Properties of FMO@C
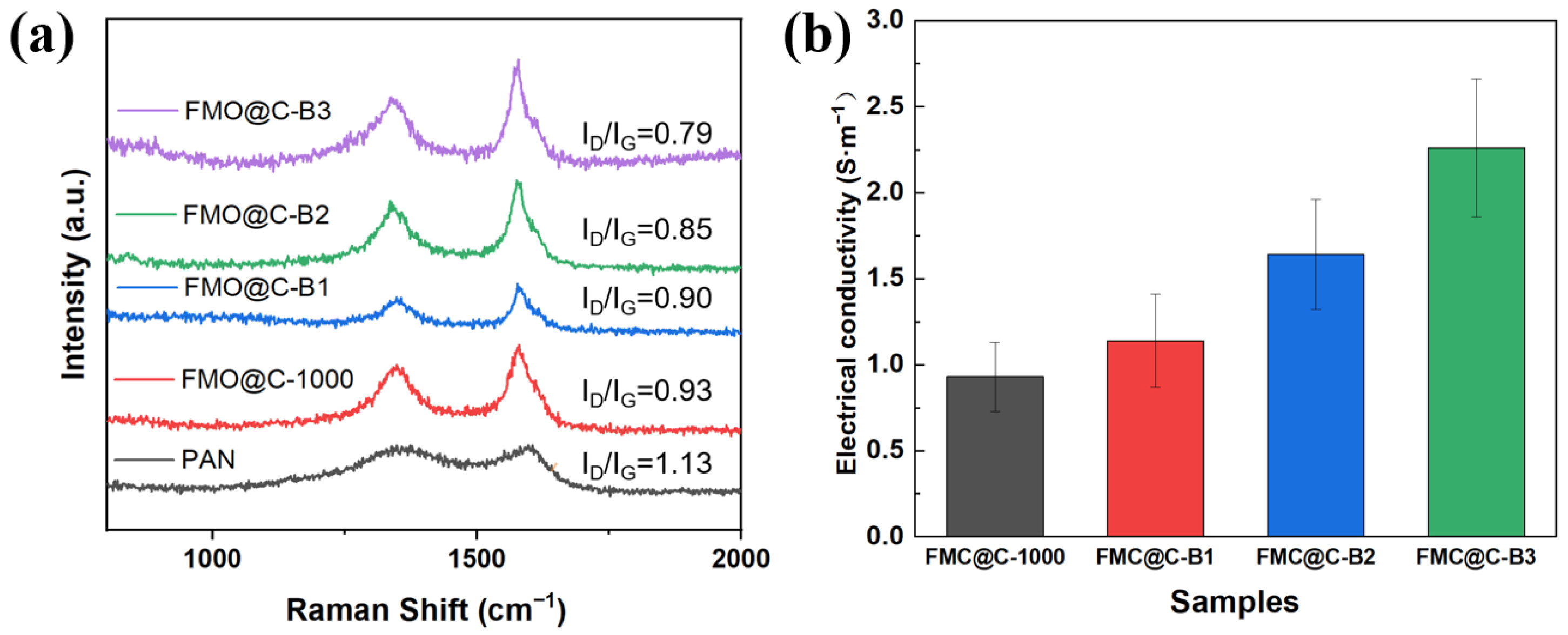
3.3. Effects of Boric Acid Catalyzation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, D.; Peng, J.; Liu, S.; Huang, T.; Xiong, Z.; Huang, S.; Zhao, F.; Peng, Y.; Cao, M.; Liu, C. Prospects of porous-carbon-based electromagnetic wave absorbing materials. Carbon 2025, 244, 120650. [Google Scholar] [CrossRef]
- Chen, X.; Lan, D.; Zhou, L.; Liu, H.; Song, X.; Wang, S.; Zou, Z.; Wu, G. Review of recent advances in ferrite-based materials: From synthesis techniques to electromagnetic wave absorption performance. Int. J. Miner. Metall. Mater. 2025, 32, 591–608. [Google Scholar] [CrossRef]
- Zhao, R.; Liang, B.; Shi, Y.; Dong, Q.; Li, T.; Gu, J.; Ma, Y.; Zhang, J.; Melhi, S.; Alshammari, A.S.; et al. Recent progress of carbon-based magnetic fibers for electromagnetic wave absorption. Carbon 2024, 229, 119513. [Google Scholar] [CrossRef]
- Ozturk, M.; Depci, T.; Bahceci, E.; Karaaslan, M.; Akgol, O.; Sevim, U.K. Production of new electromagnetic wave shielder mortar using waste mill scales. Constr. Build. Mater. 2020, 242, 118028. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, J.; Liu, Y.; Li, J. Use of steel slag as carbonation material: A review of carbonation methods and evaluation, environmental factors and carbon conversion process. J. CO2 Util. 2024, 88, 102947. [Google Scholar] [CrossRef]
- Dai, Y.; Wu, J.; Wang, D.; Li, R.; Lu, C.; Xu, Z. Electromagnetic Wave-Absorbing Properties of Steel Slag. J. Mater. Eng. Perform. 2018, 28, 535–542. [Google Scholar] [CrossRef]
- Ma, C.; Wu, Z.; Xie, S.; Wang, Y.; Ji, Z.; Wang, J. Synergistic effects of carbon black and waste iron powder on electromagnetic wave absorbing and mechanical properties of cement mortar. J. Ind. Eng. Chem. 2025. [Google Scholar] [CrossRef]
- Jakubas, A.; Łada-Tondyra, E.; Makówka, M.; Suchecki, Ł. A Study on the Possibility of Using Iron Scale in the Construction of Electromagnetic Field Shields. Energies 2022, 15, 1332. [Google Scholar] [CrossRef]
- Alwaeli, M. The implementation of scale and steel chips waste as a replacement for raw sand in concrete manufacturing. J. Clean. Prod. 2016, 137, 1038–1044. [Google Scholar] [CrossRef]
- Lin, D.; Peng, J.; Guo, J.; Jiang, X. Unique frequency reversible conversion and bandwidth regulation of electromagnetic wave absorption performance for core-shell structured Fe3O4 and manganese oxide composites. Mater. Today Nano 2025, 29, 100568. [Google Scholar] [CrossRef]
- Meng, X.; Qiao, J.; Yang, Y.; Zhang, X.; Yang, Z.; Zheng, S.; Liu, J.; Wu, L.; Wang, Z.; Wang, F. Three-dimensional porous manganese oxide/nickel/carbon microspheres as high-performance electromagnetic wave absorbers with superb photothermal property. J. Colloid. Interface Sci. 2023, 629, 884–894. [Google Scholar] [CrossRef]
- Peng, H.; Zhang, D.; Xie, Z.; Lu, S.; Liu, Y.; Liang, F. Recent Advances in Structural Design of Carbon/Magnetic Composites and their Electromagnetic Wave Absorption Applications. Small 2025, 21, e2408570. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Guo, X.; Lan, D.; Wang, Y.; Huang, H.; Zhang, C.; Wu, G.; Zhang, S.; Jia, Z. Multifunctional electromagnetic wave absorbing materials: Research progress from component structural design to intelligent integration. Carbon 2025, 245, 120818. [Google Scholar] [CrossRef]
- Bhattacharjee, Y.; Bose, S. Core–Shell Nanomaterials for Microwave Absorption and Electromagnetic Interference Shielding: A Review. ACS Appl. Nano Mater. 2021, 4, 949–972. [Google Scholar] [CrossRef]
- Gai, L.; Zhao, H.; Wang, F.; Wang, P.; Liu, Y.; Han, X.; Du, Y. Advances in core—Shell engineering of carbon-based composites for electromagnetic wave absorption. Nano Res. 2022, 15, 9410–9439. [Google Scholar] [CrossRef]
- Wu, Z.; Cheng, H.W.; Jin, C.; Yang, B.; Xu, C.; Pei, K.; Zhang, H.; Yang, Z.; Che, R. Dimensional Design and Core-Shell Engineering of Nanomaterials for Electromagnetic Wave Absorption. Adv. Mater. 2022, 34, e2107538. [Google Scholar] [CrossRef]
- Ma, G.; Yin, P.; Zhang, L.; Huang, H.; Sun, X.; Zhang, Y.; Wang, J.; Feng, X. Biomass-derived porous carbon combined with CoFe2O4/CoFe@C for available low-frequency microwave dissipation. Powder Technol. 2023, 415, 118196. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, Y.; Yang, H. Facile fabrication for core-shell BaFe12O19@C composites with excellent microwave absorption properties. J. Alloys Compd. 2019, 805, 130–137. [Google Scholar] [CrossRef]
- Qiao, M.; Lei, X.; Ma, Y.; Tian, L.; Su, K.; Zhang, Q. Dependency of tunable microwave absorption performance on morphology-controlled hierarchical shells for core-shell Fe3O4@MnO2 composite microspheres. Chem. Eng. J. 2016, 304, 552–562. [Google Scholar] [CrossRef]
- Wang, B.; Fu, Y.; Li, J.; Wu, Q.; Wang, X.; Liu, T. Construction of Co@C nanocapsules by one-step carbon reduction of single-crystal Co3O4 nanoparticles: Ultra-wideband microwave absorber verified via coaxial and arch methods. Chem. Eng. J. 2022, 445, 136863. [Google Scholar] [CrossRef]
- Lee, S.; Cho, S.Y.; Chung, Y.S.; Choi, Y.C.; Lee, S. High electrical and thermal conductivities of a PAN-based carbon fiber via boron-assisted catalytic graphitization. Carbon 2022, 199, 70–79. [Google Scholar] [CrossRef]
- Cui, A.; Wang, C.; Miao, Y.; Wang, X.; Wang, Y.; Lan, D.; Wu, S.; Song, G.; Wang, T.; Tian, Z.; et al. B─C Bonding Configuration Manipulation Strategy Toward Synergistic Optimization of Polarization Loss and Conductive Loss for Highly Efficient Electromagnetic Wave Absorption. Adv. Funct. Mater. 2024, 35, 2420292. [Google Scholar] [CrossRef]
- Huckstaedt, T.; Erdmann, J.; Lehmann, A.; Protz, R.; Ganster, J. Boric Acid as A Low-Temperature Graphitization Aid and Its Impact on Structure and Properties of Cellulose-Based Carbon Fibers. Polymers 2023, 15, 4310. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Sun, M.; Yin, J.; Abou-Hamad, E.; Schwingenschlogl, U.; Costa, P.; Alshareef, H.N. A Cyclized Polyacrylonitrile Anode for Alkali Metal Ion Batteries. Angew. Chem. Int. Ed. Engl. 2021, 60, 1355–1363. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Hu, G.; Peng, Z.; Cao, Y.; Zhu, F.; Zhang, Y.; Gao, H.; Du, K. Achieving a bifunctional conformal coating on nickel-rich cathode LiNi0.8Co0.1Mn0.1O2 with half-cyclized polyacrylonitrile. Electrochim. Acta 2021, 386, 138440. [Google Scholar] [CrossRef]
- Sayyar, S.; Moskowitz, J.; Fox, B.; Wiggins, J.; Wallace, G. Wet-spinning and carbonization of graphene/PAN-based fibers: Toward improving the properties of carbon fibers. J. Appl. Polym. Sci. 2019, 136, 47932. [Google Scholar] [CrossRef]
- Wang, T.; Wang, H.; Chi, X.; Li, R.; Wang, J. Synthesis and microwave absorption properties of Fe–C nanofibers by electrospinning with disperse Fe nanoparticles parceled by carbon. Carbon 2014, 74, 312–318. [Google Scholar] [CrossRef]
- Gai, L.; Zhao, H.; Li, X.; Wang, P.; Yu, S.; Chen, Y.; Wang, C.; Lan, D.; Han, F.; Du, Y. Shell engineering afforded dielectric polarization prevails and impedance amelioration toward electromagnetic wave absorption enhancement in nested-network carbon architecture. Chem. Eng. J. 2024, 501, 157556. [Google Scholar] [CrossRef]
- Xing, Y.; Fan, Y.; Yan, Z.; Zhao, B.; Huang, Y.; Pan, W. Core-shell LaOCl/LaFeO3 nanofibers with matched impedance for high-efficiency electromagnetic wave absorption. Sci. China Mater. 2022, 66, 1587–1596. [Google Scholar] [CrossRef]
- Liu, Q.; Cao, Q.; Bi, H.; Liang, C.; Yuan, K.; She, W.; Yang, Y.; Che, R. CoNi@SiO2 @TiO2 and CoNi@Air@TiO2 Microspheres with Strong Wideband Microwave Absorption. Adv. Mater. 2016, 28, 486–490. [Google Scholar] [CrossRef]
- Xu, X.; Wang, Y.; Yue, Y.; Wang, C.; Xu, Z.; Liu, D. Core-shell MXene/nitrogen-doped C heterostructure for wide-band electromagnetic wave absorption at thin thickness. Ceram. Int. 2022, 48, 30317–30324. [Google Scholar] [CrossRef]
- Yuan, S.; Wang, T.; Feng, T.; Kong, J. Electromagnetic wave absorption of fabricated Fe/Fe3O4/C hollow fibers derived from ceiba fiber templates. Mater. Sci. Eng. B 2024, 299, 117057. [Google Scholar] [CrossRef]
- Han, C.; Zhang, M.; Cao, W.-Q.; Cao, M.-S. Electrospinning and in-situ hierarchical thermal treatment to tailor C–NiCo2O4 nanofibers for tunable microwave absorption. Carbon 2021, 171, 953–962. [Google Scholar] [CrossRef]
- Wu, X.; Liu, K.; Ding, J.; Zheng, B.; Gao, F.; Qian, K.; Ma, Y.; Feng, Y.; Chen, L.; Zhang, P.; et al. Construction of Ni-based alloys decorated sucrose-derived carbon hybrid towards: Effective microwave absorption application. Adv. Compos. Hybrid Mater. 2022, 5, 2260–2270. [Google Scholar] [CrossRef]
- Mohammed, M.I.; Abud-Alnur, S.; Aliwi, S.M. Study of the mechanism of graphitization of phenolic resin carbon catalyzed by boron oxide. J. Polym. Res. 2021, 28, 174. [Google Scholar] [CrossRef]
- Du, Y.; Liu, W.; Qiang, R.; Wang, Y.; Han, X.; Ma, J.; Xu, P. Shell thickness-dependent microwave absorption of core-shell Fe3O4@C composites. ACS Appl. Mater. Interfaces 2014, 6, 12997–13006. [Google Scholar] [CrossRef]
- Krishnan, S.G.; White, C.E.; Zeng, K.; Kalarikkal, N.; Ok, Y.S.; Arnold, C.B.; Thomas, S.; Nzihou, A. Recent developments on multi- versus single-metallic catalytic graphitisation of biocarbon: A review. Fuel 2025, 396, 135330. [Google Scholar] [CrossRef]
- Liang, X.; Liu, S.; Zhong, S.; Zhang, S.; Meng, X.; Zhang, Y.; Yu, M.; Wang, C. A novel synthesis of Porous Fe(4)N/carbon hollow microspheres for thin and efficient electromagnetic wave absorbers. J. Colloid Interface Sci. 2023, 637, 123–133. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Shen, S.; Li, J. Construction of Graphite Shells on Ferromanganese Oxide for Electromagnetic Wave Absorption. Materials 2025, 18, 5336. https://doi.org/10.3390/ma18235336
Zhang Y, Shen S, Li J. Construction of Graphite Shells on Ferromanganese Oxide for Electromagnetic Wave Absorption. Materials. 2025; 18(23):5336. https://doi.org/10.3390/ma18235336
Chicago/Turabian StyleZhang, Yuxiang, Shuling Shen, and Jing Li. 2025. "Construction of Graphite Shells on Ferromanganese Oxide for Electromagnetic Wave Absorption" Materials 18, no. 23: 5336. https://doi.org/10.3390/ma18235336
APA StyleZhang, Y., Shen, S., & Li, J. (2025). Construction of Graphite Shells on Ferromanganese Oxide for Electromagnetic Wave Absorption. Materials, 18(23), 5336. https://doi.org/10.3390/ma18235336





