The Effect of Hydrogen Gas and Water Vapor in Catalytic Chemical Vapor Deposition on the Structure of Vertically Aligned Carbon Nanotubes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
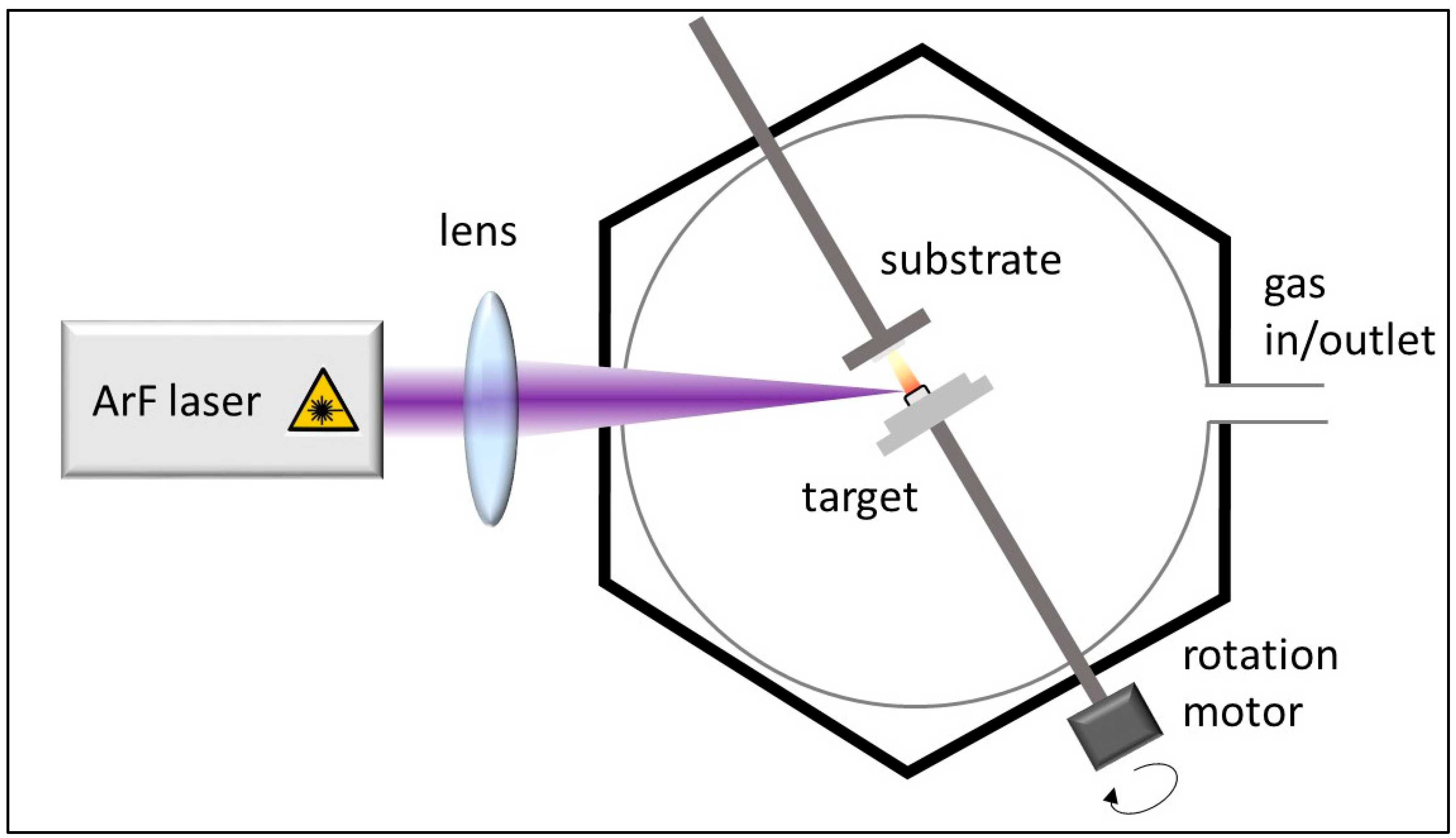
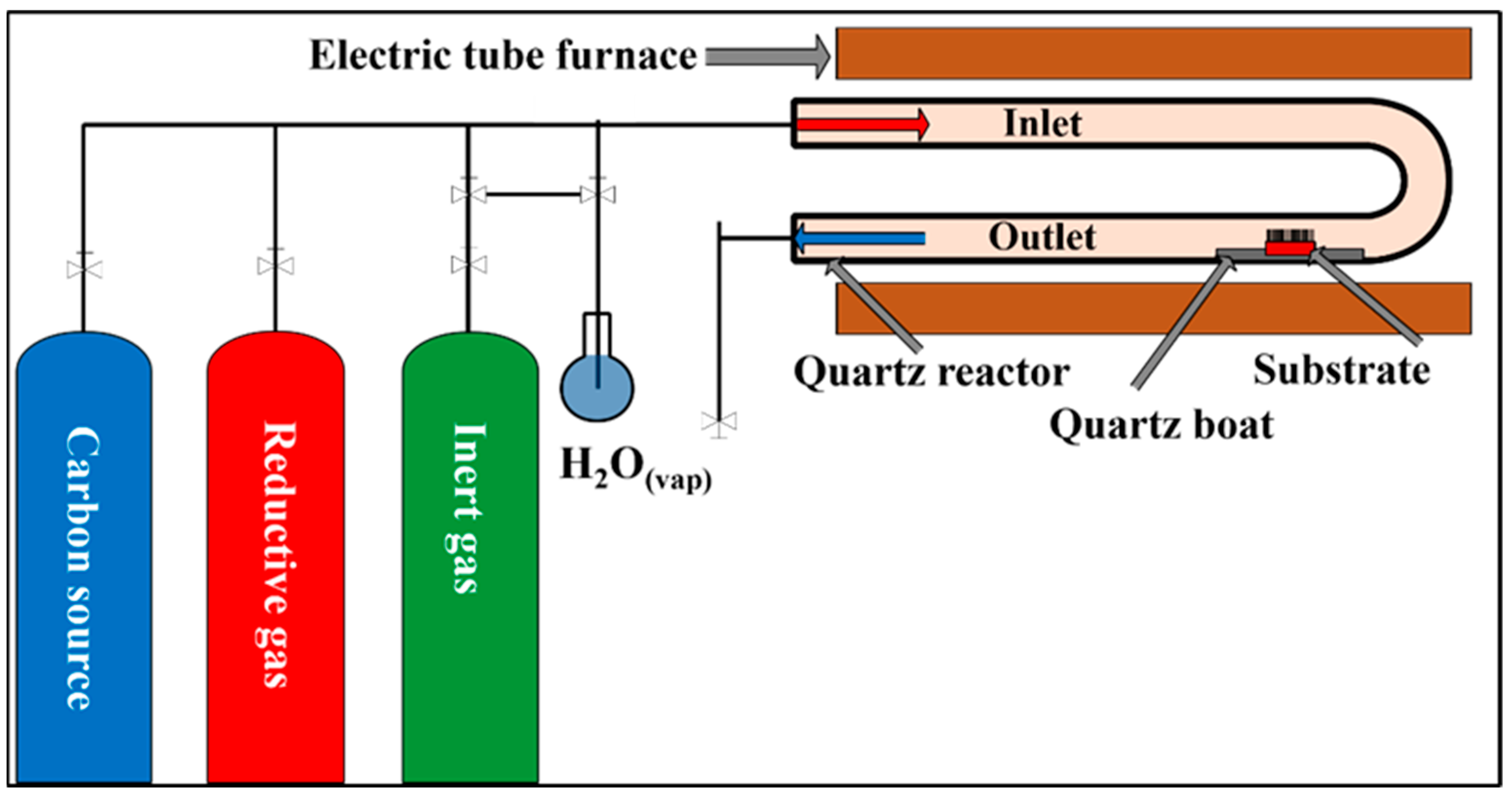
2.2. Layer Construction and CCVD Synthesis
2.3. Characterization of VACNTs
3. Results and Discussion
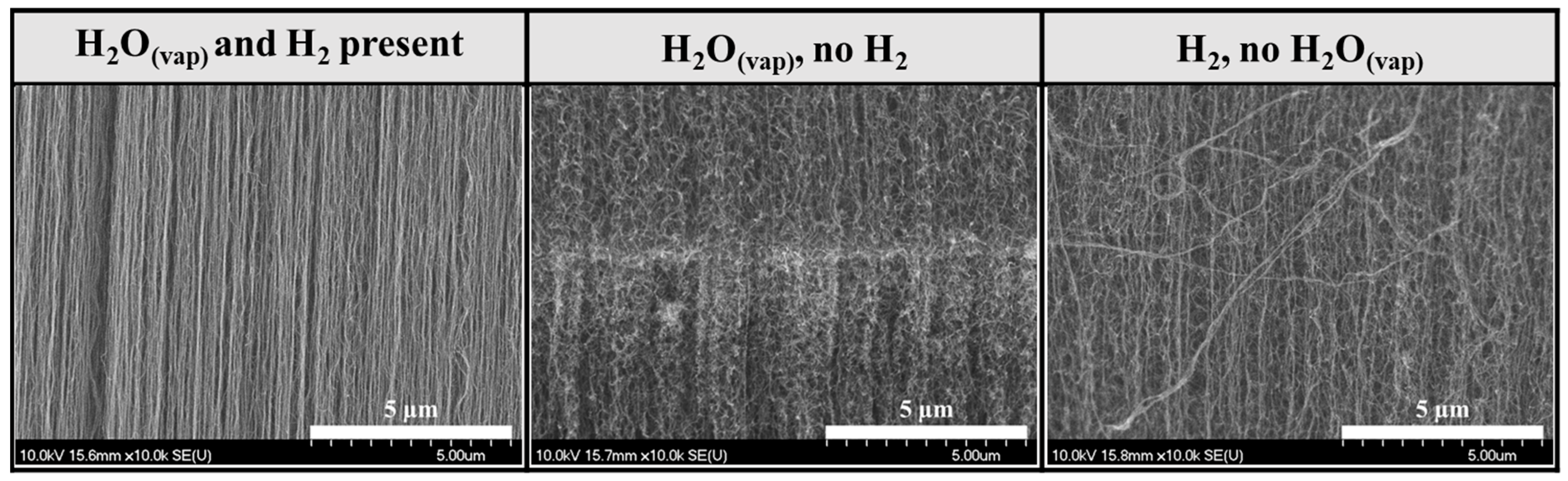
3.1. Investigation of CNT Forests Synthesized in the Presence of Water Vapor and Hydrogen
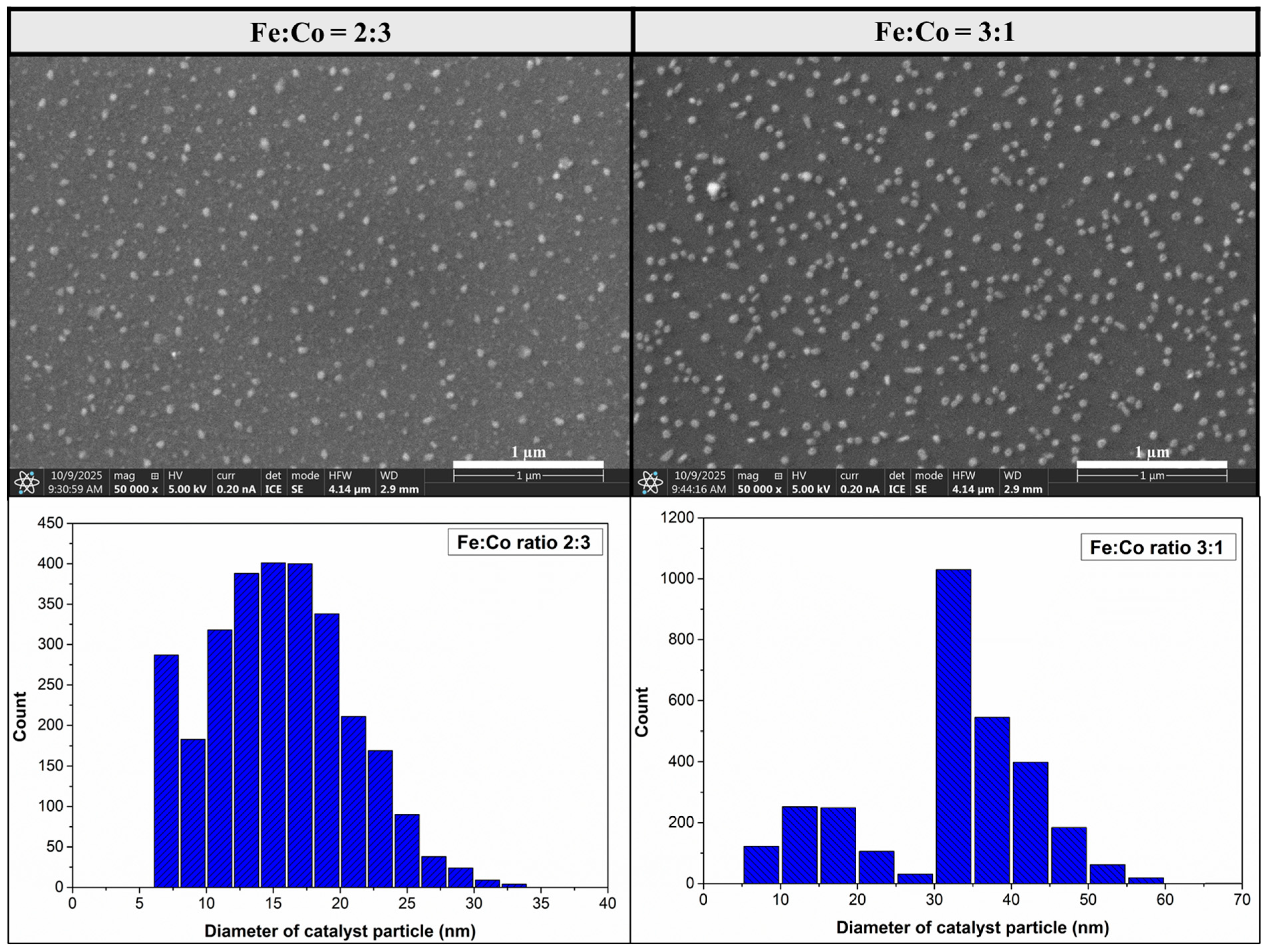
3.2. Investigation of CNT Forests Synthesized in the Presence of Hydrogen Without Water Vapor
3.3. Investigation of CNT Forests Synthesized in the Presence of Water Vapor Without Hydrogen
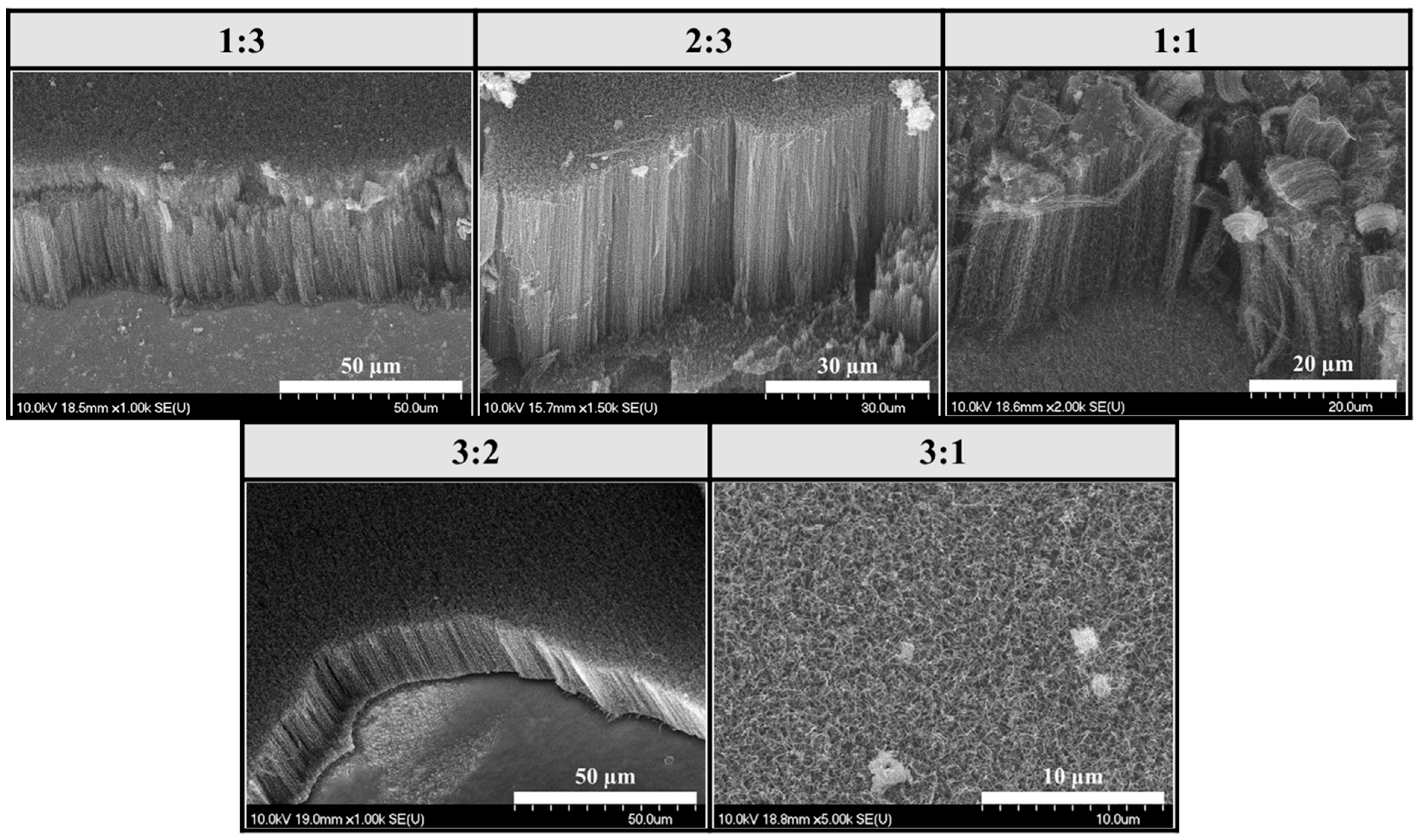
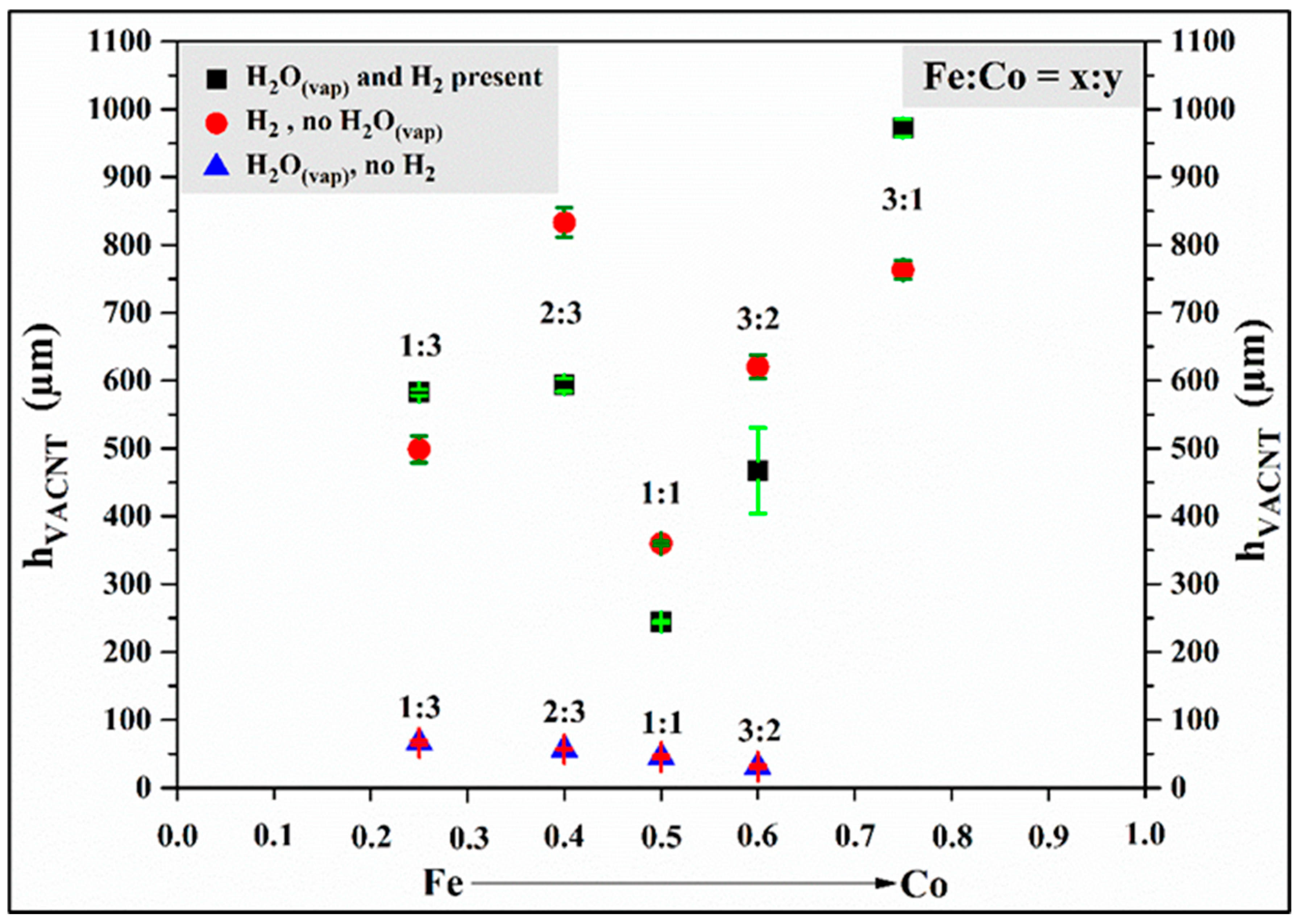
3.4. Comparison of Samples
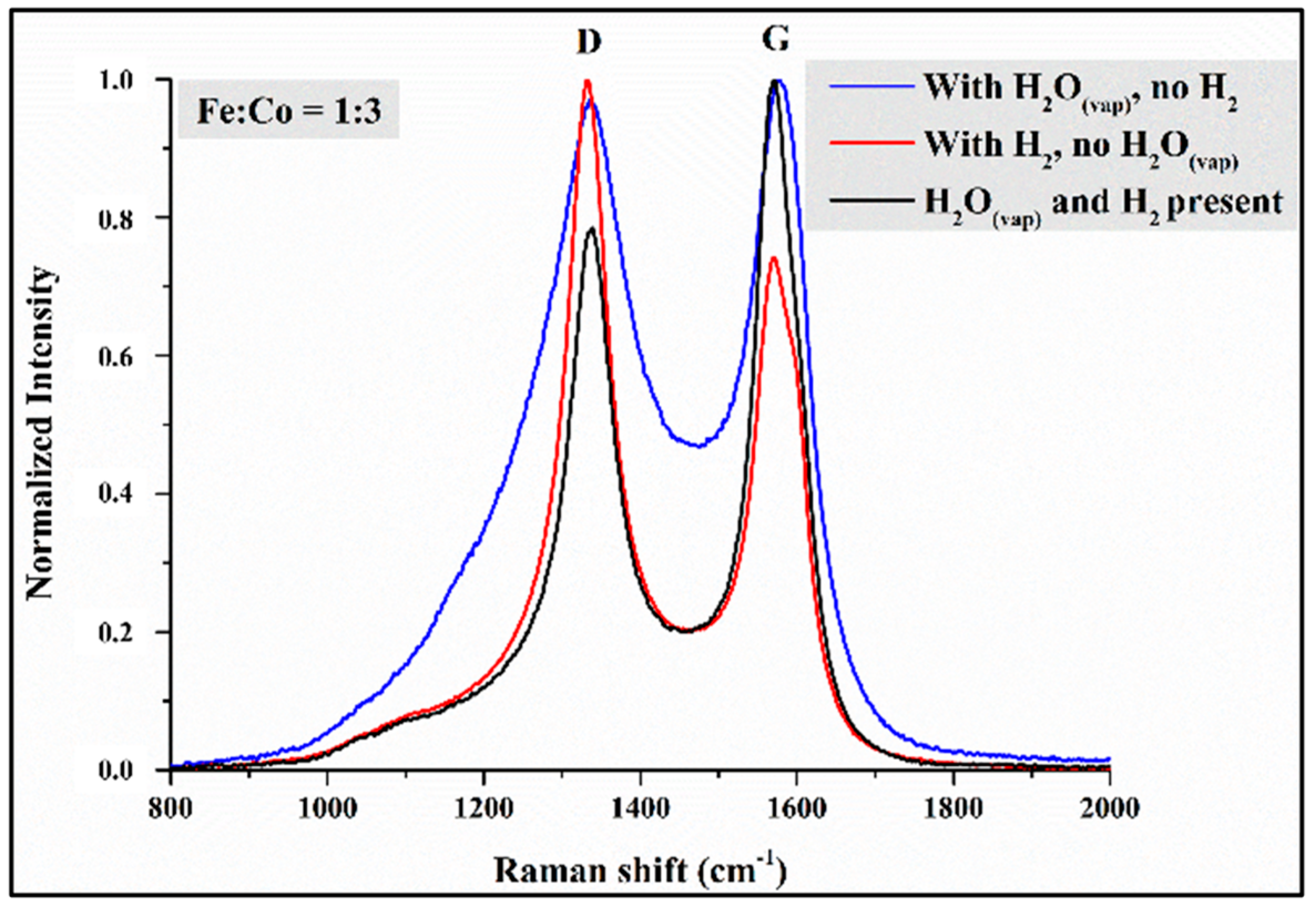
3.5. Raman Spectroscopy Observations
4. Summary
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iijima, S. Helical Microtubules of Graphitic Carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Lahiff, E.; Lynam, C.; Gilmartin, N.; O’Kennedy, R.; Diamond, D. The Increasing Importance of Carbon Nanotubes and Nanostructured Conducting Polymers in Biosensors. Anal. Bioanal. Chem. 2010, 398, 1575–1589. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, L.; de Perrot, M.; Zhao, X. Carbon Nanotubes: A Summary of Beneficial and Dangerous Aspects of an Increasingly Popular Group of Nanomaterials. Front. Oncol. 2021, 11, 693814. [Google Scholar] [CrossRef]
- Nessim, G.D. Properties, Synthesis, and Growth Mechanisms of Carbon Nanotubes with Special Focus on Thermal Chemical Vapor Deposition. Nanoscale 2010, 2, 1306. [Google Scholar] [CrossRef] [PubMed]
- Takakura, A.; Beppu, K.; Nishihara, T.; Fukui, A.; Kozeki, T.; Namazu, T.; Miyauchi, Y.; Itami, K. Strength of Carbon Nanotubes Depends on Their Chemical Structures. Nat. Commun. 2019, 10, 3040. [Google Scholar] [CrossRef]
- Purohit, R.; Purohit, K.; Rana, S.; Rana, R.S.; Patel, V. Carbon Nanotubes and Their Growth Methods. Procedia Mater. Sci. 2014, 6, 716–728. [Google Scholar] [CrossRef]
- Ando, Y.; Zhao, X.; Sugai, T.; Kumar, M. Growing Carbon Nanotubes. Mater. Today 2004, 7, 22–29. [Google Scholar] [CrossRef]
- Saraswati, T.E.; Prasiwi, O.D.I.; Masykur, A.; Handayani, N.; Anwar, M. The Modification of Carbon with Iron Oxide Synthesized in Electrolysis Using the Arc Discharge Method. IOP Conf. Ser. Mater. Sci. Eng. 2017, 176, 012046. [Google Scholar] [CrossRef]
- Meftahi, A.; Kashef Sabery, M.S.; Alibakhshi, S.; Walsh, M.; Bechelany, M.; Naseef, A.; Barhoum, A. Carbon Nanotubes and Nanofibers as Building Blocks for the Future: Structure, Synthesis, Properties, and Functionalization Perspectives. Mater. Sci. Eng. B 2025, 322, 118622. [Google Scholar] [CrossRef]
- Bulla, M.; Kumar, V.; Devi, R.; Kumar, S.; Dahiya, R.; Singh, P.; Mishra, A.K. Exploring the Frontiers of Carbon Nanotube Synthesis Techniques and Their Potential Applications in Supercapacitors, Gas Sensing, and Water Purification. J. Environ. Chem. Eng. 2024, 12, 114504. [Google Scholar] [CrossRef]
- Golshadi, M.; Maita, J.; Lanza, D.; Zeiger, M.; Presser, V.; Schrlau, M.G. Effects of Synthesis Parameters on Carbon Nanotubes Manufactured by Template-Based Chemical Vapor Deposition. Carbon 2014, 80, 28–39. [Google Scholar] [CrossRef]
- Yahyazadeh, A.; Nanda, S.; Dalai, A.K. Carbon Nanotubes: A Review of Synthesis Methods and Applications. Reactions 2024, 5, 429–451. [Google Scholar] [CrossRef]
- MacKenzie, K.J.; Dunens, O.M.; Harris, A.T. An Updated Review of Synthesis Parameters and Growth Mechanisms for Carbon Nanotubes in Fluidized Beds. Ind. Eng. Chem. Res. 2010, 49, 5323–5338. [Google Scholar] [CrossRef]
- Giannetto, M.J.; Johnson, E.P.; Watson, A.; Dimitrov, E.; Kurth, A.; Shi, W.; Fornasiero, F.; Meshot, E.R.; Plata, D.L. Modifying the Molecular Structure of Carbon Nanotubes through Gas-Phase Reactants. ACS Nanosci. Au 2023, 3, 182–191. [Google Scholar] [CrossRef]
- Eatemadi, A.; Daraee, H.; Karimkhanloo, H.; Kouhi, M.; Zarghami, N.; Akbarzadeh, A.; Abasi, M.; Hanifehpour, Y.; Joo, S.W. Carbon Nanotubes: Properties, Synthesis, Purification, and Medical Applications. Nanoscale Res. Lett. 2014, 9, 393. [Google Scholar] [CrossRef] [PubMed]
- Salah, L.S.; Ouslimani, N.; Bousba, D.; Huynen, I.; Danlée, Y.; Aksas, H. Carbon Nanotubes (CNTs) from Synthesis to Functionalized (CNTs) Using Conventional and New Chemical Approaches. J. Nanomater. 2021, 2021, 4972770. [Google Scholar] [CrossRef]
- Zhu, J.; Ba, Z.; Lou, Y.; Li, C.; Ma, L.; Liu, J.; Wen, X.; Tang, T. Green and Efficient Conversion of Polypropylene Wastes into Ni@CNTs Hybrids and Their Application for Fire Resistant Polycarbonate Composites. J. Environ. Chem. Eng. 2025, 13, 116432. [Google Scholar] [CrossRef]
- Kulkarni, R.; Lingamdinne, L.P.; Koduru, J.R.; Karri, R.R.; Kailasa, S.K.; Mubarak, N.M.; Chang, Y.-Y.; Dehghani, M.H. Exploring the Recent Cutting-Edge Applications of CNTs in Energy and Environmental Remediation: Mechanistic Insights and Remarkable Performance Advancements. J. Environ. Chem. Eng. 2024, 12, 113251. [Google Scholar] [CrossRef]
- Zhang, S.; Pang, J.; Li, Y.; Yang, F.; Gemming, T.; Wang, K.; Wang, X.; Peng, S.; Liu, X.; Chang, B.; et al. Emerging Internet of Things Driven Carbon Nanotubes-Based Devices. Nano Res. 2022, 15, 4613–4637. [Google Scholar] [CrossRef]
- Cui, K.; Chang, J.; Feo, L.; Chow, C.L.; Lau, D. Developments and Applications of Carbon Nanotube Reinforced Cement-Based Composites as Functional Building Materials. Front. Mater. 2022, 9, 861646. [Google Scholar] [CrossRef]
- Czagany, M.; Hompoth, S.; Keshri, A.K.; Pandit, N.; Galambos, I.; Gacsi, Z.; Baumli, P. Supercapacitors: An Efficient Way for Energy Storage Application. Materials 2024, 17, 702. [Google Scholar] [CrossRef]
- Jung, M.; Sivakumar, P.; Park, H.S. Carbon Nanotube Branch-Grown Nickel Nanoparticles/Graphene Composites for a High-Capacitance Electrode. J. Phys. Energy 2023, 5, 025005. [Google Scholar] [CrossRef]
- Rahat, S.M.S.M.; Hasan, K.M.Z.; Mondol, M.M.H.; Mallik, A.K. A Comprehensive Review of Carbon Nanotube-Based Metal Oxide Nanocomposites for Supercapacitors. J. Energy Storage 2023, 73, 108847. [Google Scholar] [CrossRef]
- Li, W.Z.; Xie, S.S.; Qian, L.X.; Chang, B.H.; Zou, B.S.; Zhou, W.Y.; Zhao, R.A.; Wang, G. Large-Scale Synthesis of Aligned Carbon Nanotubes. Science 1996, 274, 1701–1703. [Google Scholar] [CrossRef] [PubMed]
- Hata, K.; Futaba, D.N.; Mizuno, K.; Namai, T.; Yumura, M.; Iijima, S. Water-Assisted Highly Efficient Synthesis of Impurity-Free Single-Walled Carbon Nanotubes. Science 2004, 306, 1362–1364. [Google Scholar] [CrossRef] [PubMed]
- Nassoy, F.; Pinault, M.; Descarpentries, J.; Vignal, T.; Banet, P.; Coulon, P.-E.; Goislard de Monsabert, T.; Hauf, H.; Aubert, P.-H.; Reynaud, C.; et al. Single-Step Synthesis of Vertically Aligned Carbon Nanotube Forest on Aluminium Foils. Nanomaterials 2019, 9, 1590. [Google Scholar] [CrossRef] [PubMed]
- Szabó, A.; Kecsenovity, E.; Pápa, Z.; Gyulavári, T.; Németh, K.; Horvath, E.; Hernadi, K. Influence of Synthesis Parameters on CCVD Growth of Vertically Aligned Carbon Nanotubes over Aluminum Substrate. Sci. Rep. 2017, 7, 9557. [Google Scholar] [CrossRef]
- Goto, S.; Nakano, T.; Sugime, H.; Inoue, Y. Enhanced Growth of Ultra-High Density Carbon Nanotube Forests via Fe and Al Vapor Addition in a CVD Process. Carbon 2025, 243, 120537. [Google Scholar] [CrossRef]
- Rajab, F. Effect of the Chemical Vapor Deposition Process on the Aspect Ratio of Vertically Aligned Carbon Nanotubes (VACNTs). MRS Adv. 2022, 8, 343–348. [Google Scholar] [CrossRef]
- Ji, K.; Meng, G.; Yuan, C.; Cui, E.; Li, Y.; Sun, J.; Dai, Z. Synergistic Effect of Fe and Al2O3 Layers on the Growth of Vertically Aligned Carbon Nanotubes for Gecko-Inspired Adhesive Applications. J. Manuf. Process. 2018, 33, 238–244. [Google Scholar] [CrossRef]
- Li, H.; Yuan, G.; Shan, B.; Zhang, X.; Ma, H.; Tian, Y.; Lu, H.; Liu, J. Chemical Vapor Deposition of Vertically Aligned Carbon Nanotube Arrays: Critical Effects of Oxide Buffer Layers. Nanoscale Res. Lett. 2019, 14, 106. [Google Scholar] [CrossRef]
- Gao, L.; Peng, A.; Wang, Z.Y.; Zhang, H.; Shi, Z.; Gu, Z.; Cao, G.; Ding, B. Growth of Aligned Carbon Nanotube Arrays on Metallic Substrate and Its Application to Supercapacitors. Solid. State Commun. 2008, 146, 380–383. [Google Scholar] [CrossRef]
- Li, G.; Chakrabarti, S.; Schulz, M.; Shanov, V. Growth of Aligned Multiwalled Carbon Nanotubes on Bulk Copper Substrates by Chemical Vapor Deposition. J. Mater. Res. 2009, 24, 2813–2820. [Google Scholar] [CrossRef]
- Rao, F.-B.; Li, T.; Wang, Y.-L. Effect of Hydrogen on the Growth of Single-Walled Carbon Nanotubes by Thermal Chemical Vapor Deposition. Phys. E Low Dimens. Syst. Nanostruct. 2008, 40, 779–784. [Google Scholar] [CrossRef]
- Taoum, H.; Ezzedine, M.; Cojocaru, C.-S. Investigation of Substrate and Hydrogen Pretreatment Time to Modulate SWCNT Diameter and Growth Yield. Appl. Surf. Sci. 2025, 691, 162664. [Google Scholar] [CrossRef]
- Irfan, M.; Shukrullah, S.; Naz, M.Y.; Ahmad, I.; Shoukat, B.; Legutko, S.; Petrů, J.; Rahman, S.; Alsaiari, M.A. Si/SiO2/Al2O3 Supported Growth of CNT Forest for the Production of La/ZnO/CNT Photocatalyst for Hydrogen Production. Materials 2022, 15, 3226. [Google Scholar] [CrossRef]
- Jung, D.; Han, M.; Overzet, L.J.; Lee, G.S. Effect of Hydrogen Pretreatment on the Spin-Capability of a Multiwalled Carbon Nanotube Forest. J. Vac. Sci. Technol. B 2013, 31, 06FI02. [Google Scholar] [CrossRef]
- Pápa, Z.; Kecsenovity, E.; Fejes, D.; Budai, J.; Toth, Z.; Hernadi, K. Height and Diameter Dependence of Carbon Nanotube Forests on the Porosity and Thickness of Catalytic Layers. Appl. Surf. Sci. 2018, 428, 885–894. [Google Scholar] [CrossRef]
- Szabó, A.; Bakos, L.P.; Karajz, D.; Gyulavári, T.; Tóth, Z.-R.; Pap, Z.; Szilágyi, I.M.; Igricz, T.; Parditka, B.; Erdélyi, Z.; et al. Decoration of Vertically Aligned Carbon Nanotubes with Semiconductor Nanoparticles Using Atomic Layer Deposition. Materials 2019, 12, 1095. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Ren, H.; Shi, Z.; Zhang, L.; Wang, Z.; Zhan, K.; Yan, Y.; Yang, J.; Zhao, B. V2O5/Vertically-Aligned Carbon Nanotubes as Negative Electrode for Asymmetric Supercapacitor in Neutral Aqueous Electrolyte. J. Colloid Interface Sci. 2021, 588, 847–856. [Google Scholar] [CrossRef]
- Roguai, S.; Djelloul, A. Gold Coated Vertically Aligned Carbon Nanotubes as Electrode for Electrochemical Capacitors. Thin Solid Films 2023, 777, 139894. [Google Scholar] [CrossRef]
- Schneider, J.J. Vertically Aligned Carbon Nanotubes as Platform for Biomimetically Inspired Mechanical Sensing, Bioactive Surfaces, and Electrical Cell Interfacing. Adv. Biosyst. 2017, 1, 1700101. [Google Scholar] [CrossRef] [PubMed]
- Andričević, P.; Mettan, X.; Kollár, M.; Náfrádi, B.; Sienkiewicz, A.; Garma, T.; Rossi, L.; Forró, L.; Horváth, E. Light-Emitting Electrochemical Cells of Single Crystal Hybrid Halide Perovskite with Vertically Aligned Carbon Nanotubes Contacts. ACS Photonics 2019, 6, 967–975. [Google Scholar] [CrossRef]
- Fejes, D.; Pápa, Z.; Kecsenovity, E.; Réti, B.; Toth, Z.; Hernadi, K. Super Growth of Vertically Aligned Carbon Nanotubes on Pulsed Laser Deposited Catalytic Thin Films. Appl. Phys. A 2015, 118, 855–861. [Google Scholar] [CrossRef]
- Nánai, L.; Németh, Z.; Kaptay, G.; Hernadi, K. Experimental and Theoretical Aspects of the Growth of Vertically Aligned CNTs by CCVD on AZO Substrate. Sci. Rep. 2024, 14, 7307. [Google Scholar] [CrossRef]
- Baker, R.T.K.; Chludzinski, J.J.; Dudash, N.S.; Simoens, A.J. The Formation of Filamentous Carbon from Decomposition of Acetylene over Vanadium and Molybdenum. Carbon 1983, 21, 463–468. [Google Scholar] [CrossRef]
- Jourdain, V.; Bichara, C. Current Understanding of the Growth of Carbon Nanotubes in Catalytic Chemical Vapour Deposition. Carbon 2013, 58, 2–39. [Google Scholar] [CrossRef]
- Kaneko, A.; Yamada, K.; Kumahara, R.; Kato, H.; Homma, Y. Comparative Study of Catalytic Activity of Iron and Cobalt for Growing Carbon Nanotubes on Alumina and Silicon Oxide. J. Phys. Chem. C 2012, 116, 26060–26065. [Google Scholar] [CrossRef]
- Dörfler, S.; Felhösi, I.; Kék, I.; Marek, T.; Althues, H.; Kaskel, S.; Nyikos, L. Tailoring Structural and Electrochemical Properties of Vertical Aligned Carbon Nanotubes on Metal Foil Using Scalable Wet-Chemical Catalyst Deposition. J. Power Sources 2012, 208, 426–433. [Google Scholar] [CrossRef]



| Role of Material | Type of Material | Purity | Distribution Company |
|---|---|---|---|
| Substrate | silicon wafer | p-type (100) 0.8 mm | WRS Materials (San Jose, CA, USA) |
| Support layer | aluminum oxide | 99.99% | MERCK (Darmstadt, Germany) |
| Catalyst layer | cobalt(II) oxide | 99% | ALDRICH (Taufkirchen, Germany) |
| iron(III) oxide | 99.9% | ALDRICH (Taufkirchen, Germany) | |
| CCVD gas feed | ethylene | ≥99.9% | MESSER (Budapest, Hungary) |
| hydrogen | 99.5% | MESSER (Budapest, Hungary) | |
| nitrogen | 99.9% | MESSER (Budapest, Hungary) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nánai, L.; Gyulavári, T.; Tóth, Z.-R.; Pápa, Z.; Budai, J.; Koncz-Horvath, D.; Hernadi, K. The Effect of Hydrogen Gas and Water Vapor in Catalytic Chemical Vapor Deposition on the Structure of Vertically Aligned Carbon Nanotubes. Materials 2025, 18, 5309. https://doi.org/10.3390/ma18235309
Nánai L, Gyulavári T, Tóth Z-R, Pápa Z, Budai J, Koncz-Horvath D, Hernadi K. The Effect of Hydrogen Gas and Water Vapor in Catalytic Chemical Vapor Deposition on the Structure of Vertically Aligned Carbon Nanotubes. Materials. 2025; 18(23):5309. https://doi.org/10.3390/ma18235309
Chicago/Turabian StyleNánai, Lilla, Tamás Gyulavári, Zsejke-Réka Tóth, Zsuzsanna Pápa, Judit Budai, Daniel Koncz-Horvath, and Klara Hernadi. 2025. "The Effect of Hydrogen Gas and Water Vapor in Catalytic Chemical Vapor Deposition on the Structure of Vertically Aligned Carbon Nanotubes" Materials 18, no. 23: 5309. https://doi.org/10.3390/ma18235309
APA StyleNánai, L., Gyulavári, T., Tóth, Z.-R., Pápa, Z., Budai, J., Koncz-Horvath, D., & Hernadi, K. (2025). The Effect of Hydrogen Gas and Water Vapor in Catalytic Chemical Vapor Deposition on the Structure of Vertically Aligned Carbon Nanotubes. Materials, 18(23), 5309. https://doi.org/10.3390/ma18235309









