Efficient Removal of Cr(VI) from Water by Carbon-Based Composites Derived from Oil-Based Drill Cuttings
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation Method
2.2. Cr(VI) Adsorption Experiment
2.3. Characterization and Analysis Methods
3. Results and Discussion
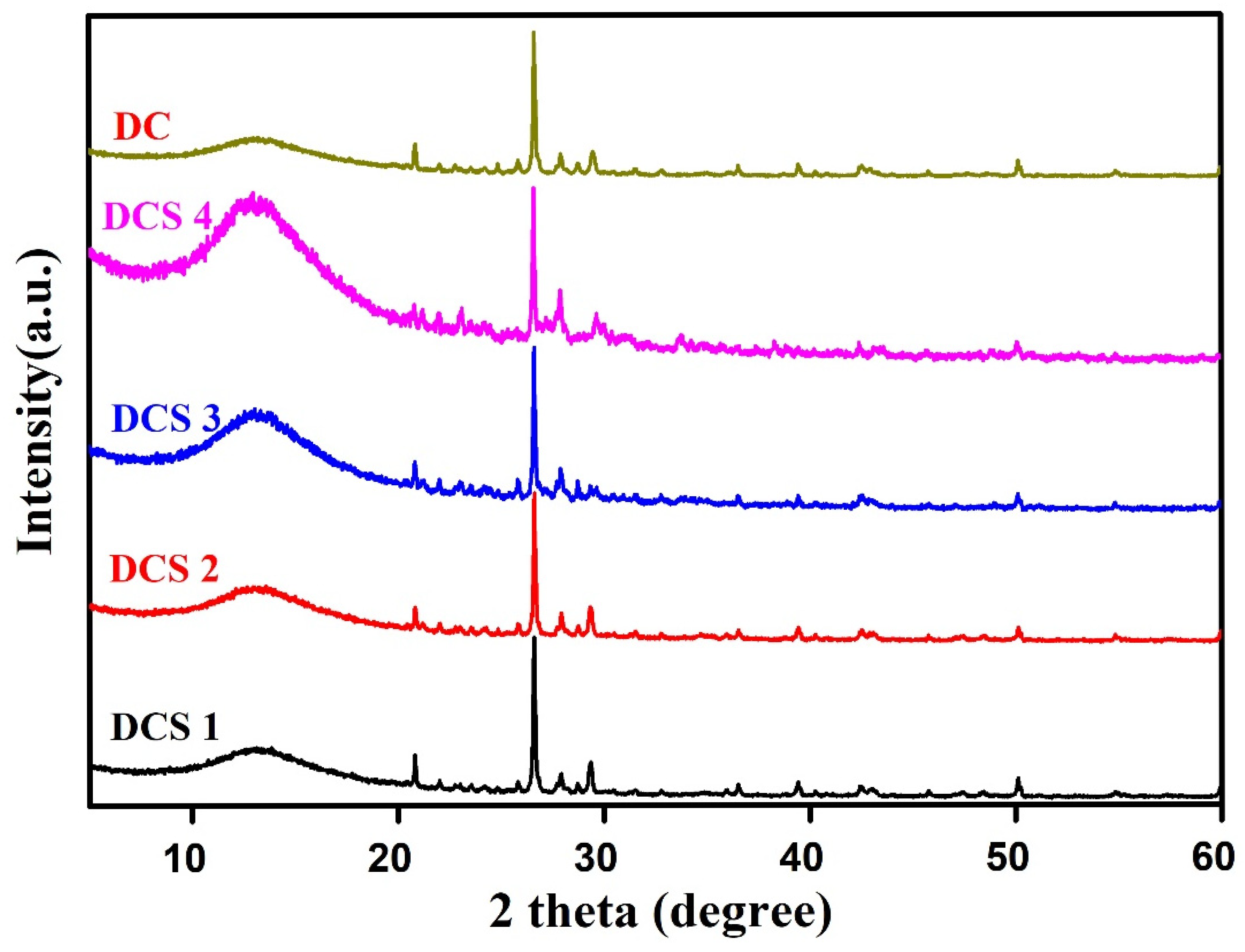
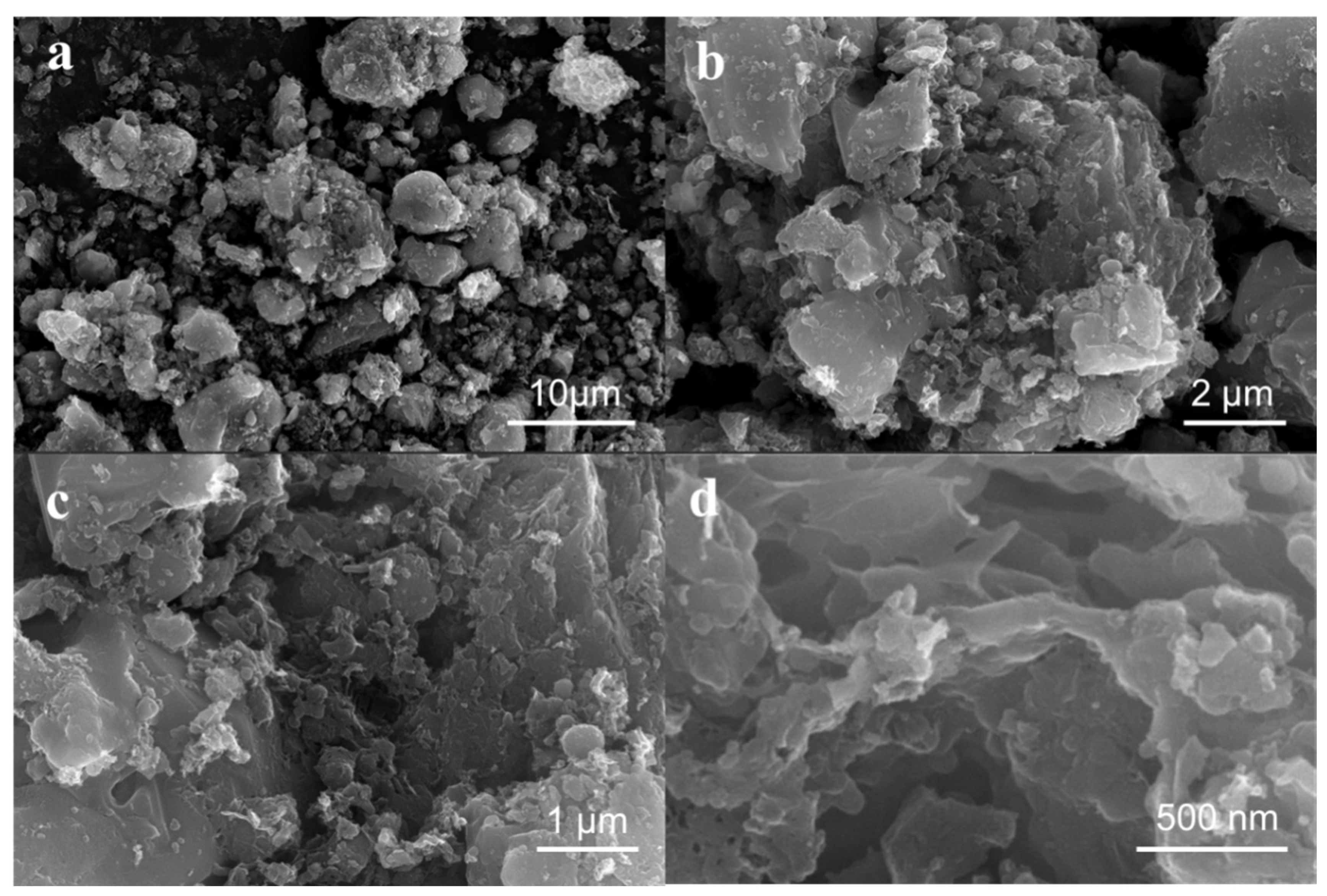
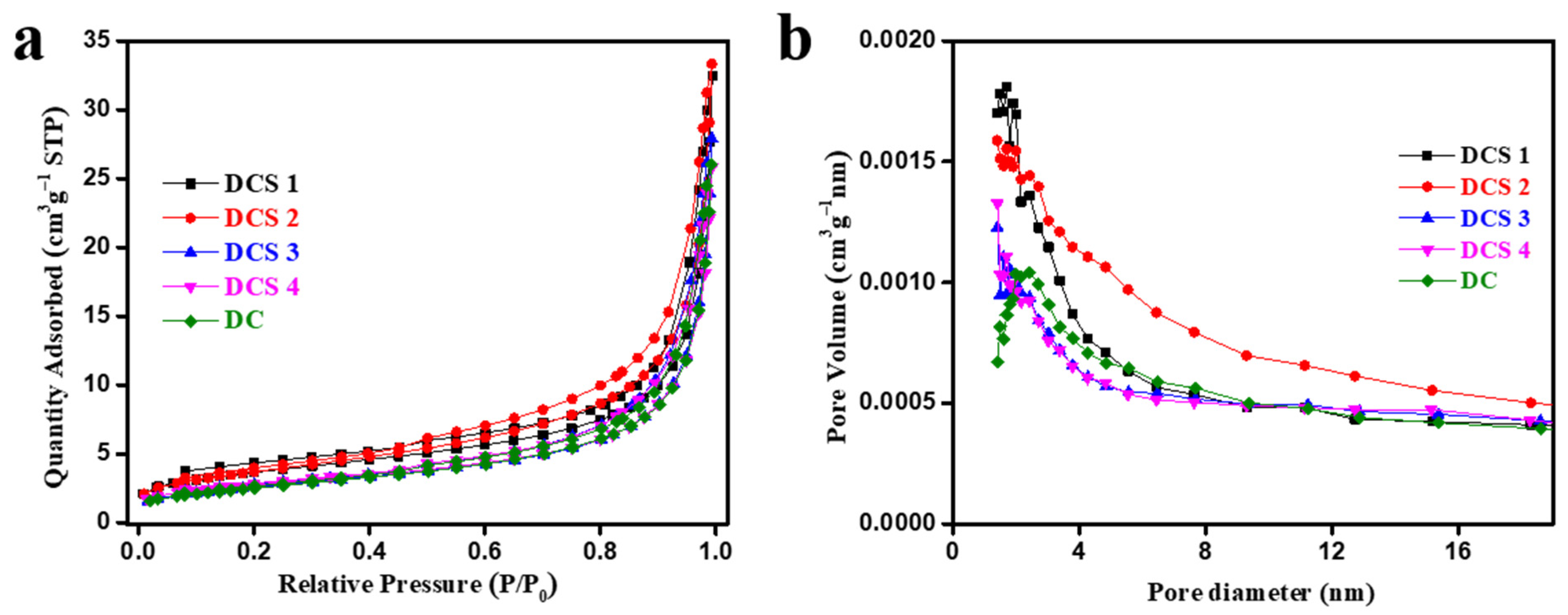
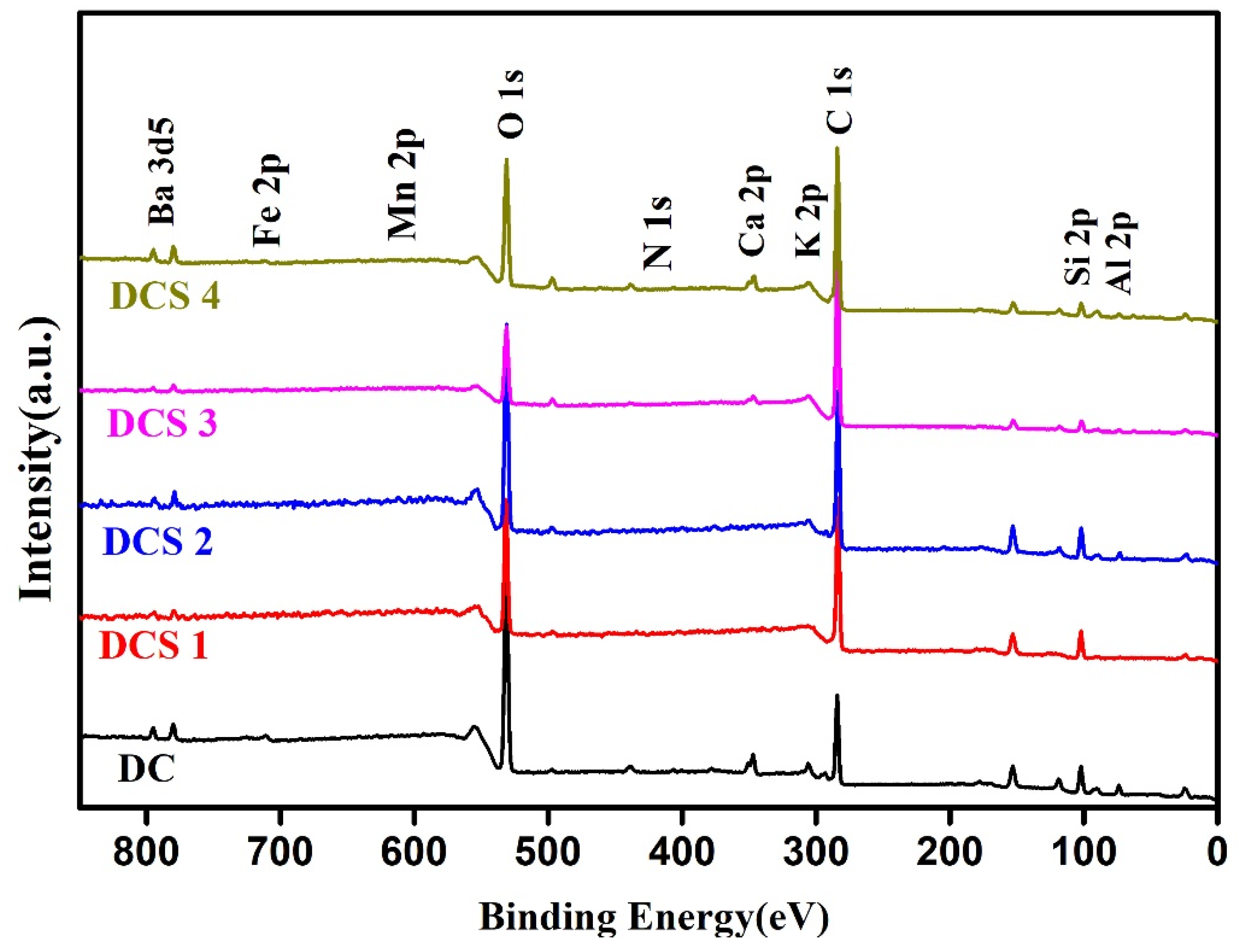
3.1. Structural Characterization of Carbon-Based Composites
3.2. Adsorption Performance
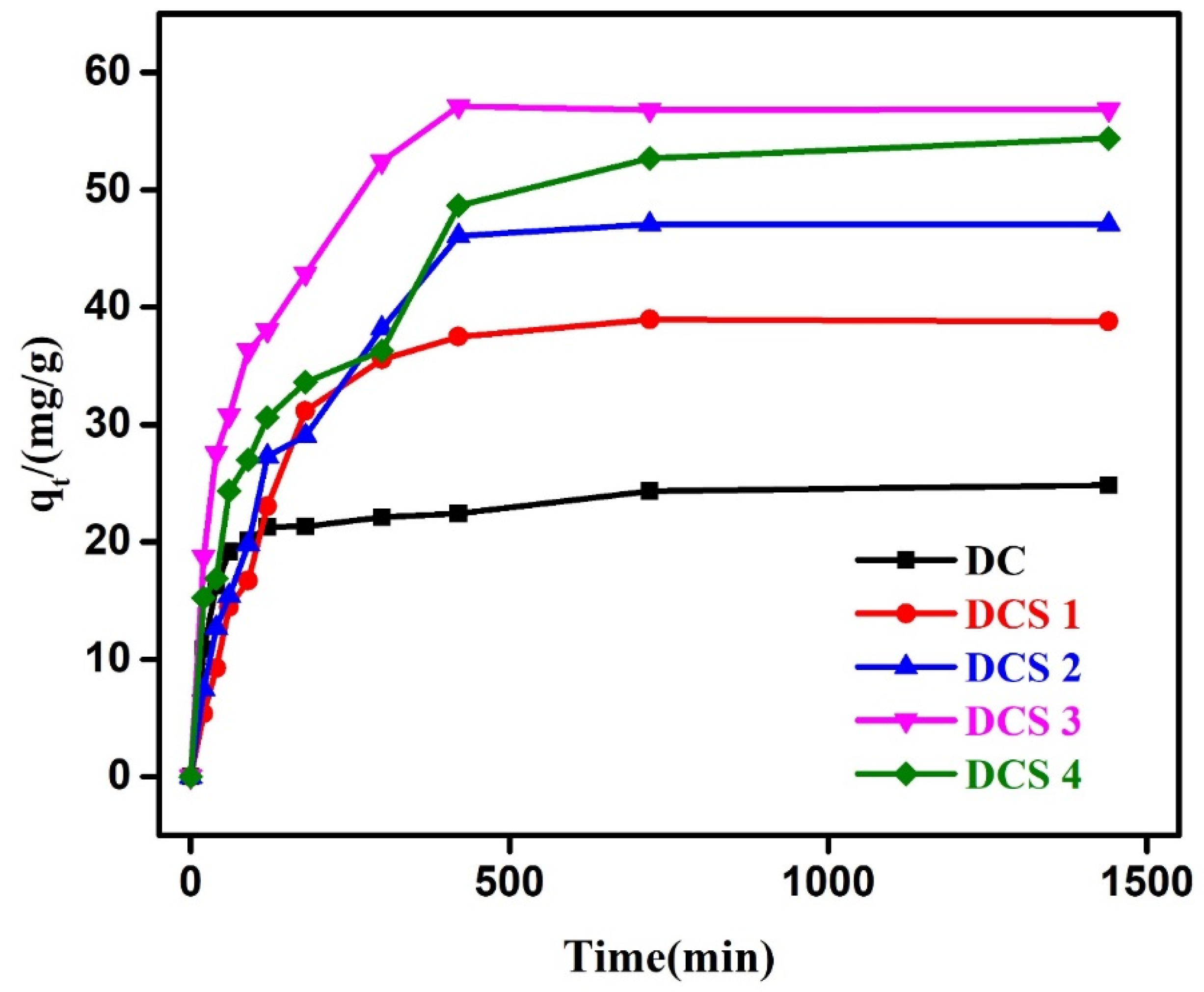
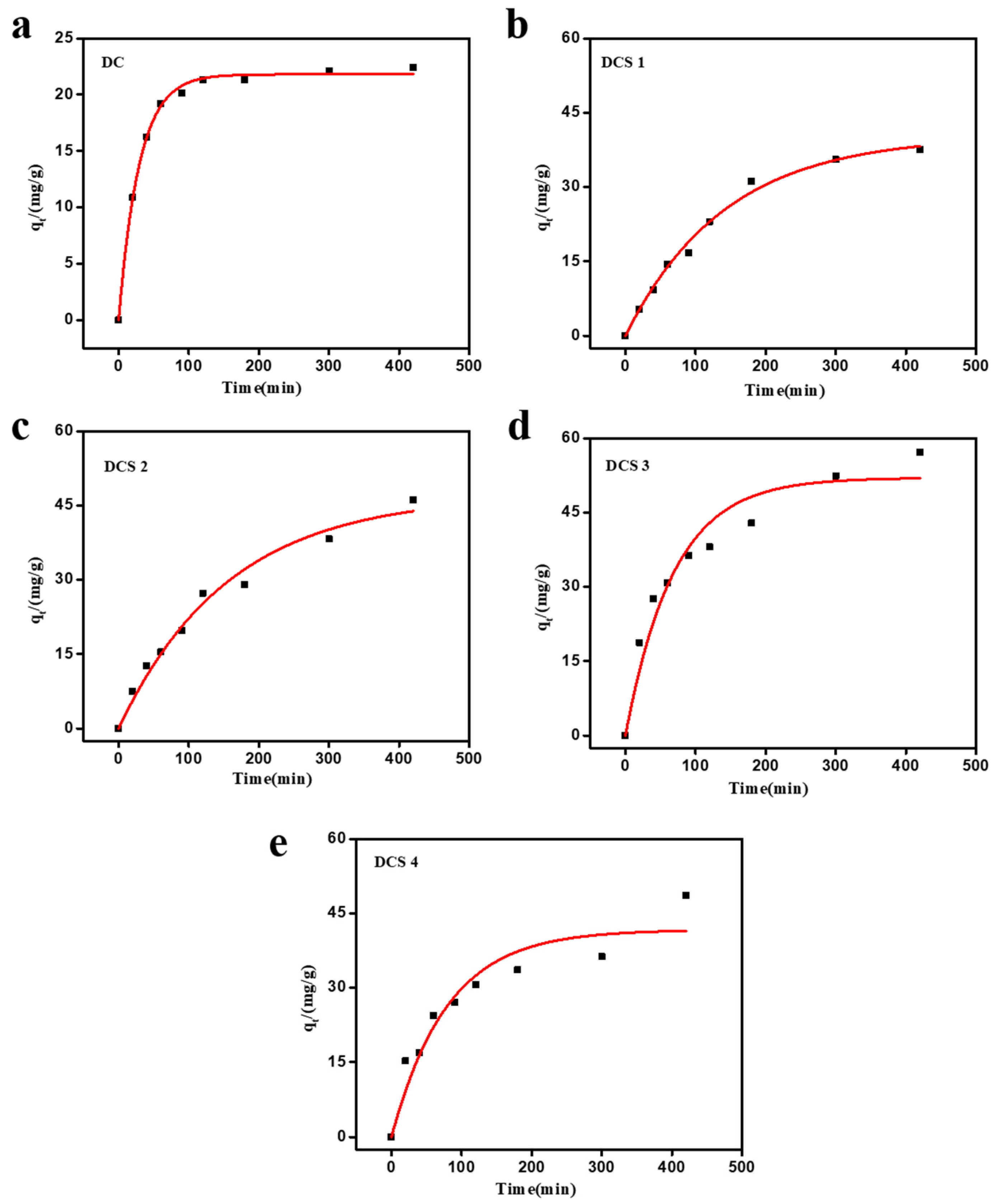
3.3. Adsorption Kinetics
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jew, A.D.; Druhan, J.L.; Ihme, M.; Kovscek, A.R.; Battiato, I.; Kaszuba, J.P.; Bargar, J.R.; Brown, G.E. Chemical and Reactive Transport Processes Associated with Hydraulic Fracturing of Unconventional Oil/Gas Shales. Chem. Rev. 2022, 122, 9198–9263. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Chang, Z.; Xiao, Y.; Huang, Y.; El-Din, M.G.; Liu, C.; Xu, L. Self-driven microbial fuel cells-electrolytic cell coupling system for the shale gas flowback wastewater and oil-based drill sludge degradation. Energy Convers. Manag. 2025, 325, 119445. [Google Scholar] [CrossRef]
- Zhang, S.; Feng, Y.; Li, B.; Deng, J.; Geng, T.; Zhang, J. Fracture development during disposal of hazardous drilling cuttings by deep underground injection: A review. J. Rock Mech. Geotech. Eng. 2022, 14, 1652–1670. [Google Scholar] [CrossRef]
- Yang, H.; Diao, H.; Zhang, Y.; Xia, S. Treatment and novel resource-utilization methods for shale gas oil based drill cuttings—A review. J. Environ. Manag. 2022, 317, 115462. [Google Scholar] [CrossRef]
- Adhami, S.; Jamshidi-Zanjani, A.; Darban, A.K. Remediation of oil-based drilling waste using the electrokinetic-Fenton method. Process Saf. Environ. Prot. 2021, 149, 432–441. [Google Scholar] [CrossRef]
- Khodadadi, M.; Moradi, L.; Dabir, B.; Moghadas Nejad, F.; Khodaii, A. Reuse of drill cuttings in hot mix asphalt mixture: A study on the environmental and structure performance. Constr. Build. Mater. 2020, 256, 119453. [Google Scholar] [CrossRef]
- Ravindiran, G.; Rajamanickam, S.; Janardhan, G.; Hayder, G.; Alagumalai, A.; Mahian, O.; Lam, S.S.; Sonne, C. Production and modifications of biochar to engineered materials and its application for environmental sustainability: A review. Biochar 2024, 6, 62. [Google Scholar] [CrossRef]
- Alves, G.M.; Petri, I. Microwave remediation of oil-contaminated drill cuttings—A review. J. Pet. Sci. Eng. 2021, 207, 109137. [Google Scholar] [CrossRef]
- Araka, P.P.; Okparanma, R.N.; Ayotamuno, J.M. Diagnostic screening of organic contaminant level in solidified/stabilized pre-treated oil-based drill cuttings. Heliyon 2019, 5, e02644. [Google Scholar] [CrossRef]
- Xia, Z.; Yang, H.; Sun, J.; Zhou, Z.; Wang, J.; Zhang, Y. Co-pyrolysis of waste polyvinyl chloride and oil-based drilling cuttings: Pyrolysis process and product characteristics analysis. J. Clean. Prod. 2021, 318, 128521. [Google Scholar] [CrossRef]
- Zhang, G.; Zhao, F.; Cheng, X.; Huang, S.; Zhang, C.; Zhou, M.; Mei, K.; Zhang, L. Resource utilization from solid waste originated from oil-based shale drilling cutting during shale gas development. Chemosphere 2022, 298, 134318. [Google Scholar] [CrossRef] [PubMed]
- Ostakh, O.; Uzyakova, E.; Grechishcheva, N.; Kusheeva, V. Ecotoxicological assessment of soil-like mixtures made of drill cuttings. J. Eng. Des. Technol. 2021, 19, 1433–1450. [Google Scholar] [CrossRef]
- Alavi, N.; Daneshpajou, M.; Shirmardi, M.; Goudarzi, G.; Neisi, A.; Babaei, A.A. Investigating the efficiency of co-composting and vermicomposting of vinasse with the mixture of cow manure wastes, bagasse, and natural zeolite. Waste Manag. 2017, 69, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Liu, H.; Chen, C.; Hou, H.; Li, J.; Hewage, K.; Sadiq, R. Low-temperature thermal desorption and secure landfill for oil-based drill cuttings management: Pollution control, human health risk, and probabilistic cost assessment. J. Hazard. Mater. 2021, 410, 124570. [Google Scholar] [CrossRef]
- Kogbara, R.B. A review of the mechanical and leaching performance of stabilized/solidified contaminated soils. Environ. Rev. 2014, 22, 66–86. [Google Scholar] [CrossRef]
- Liuyang, X.; Yang, H.; Huang, S.; Zhang, Y.; Xia, S. Resource utilization of secondary pyrolysis oil-based drilling cuttings ash for removing Cr (VI) contaminants: Adsorption properties, kinetics and mechanism. J. Environ. Chem. Eng. 2020, 8, 104474. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, Z.; Yin, F.; Wang, G.; Chen, H.; He, C.; Xu, Y. Supercritical water oxidation of oil-based drill cuttings. J. Hazard. Mater. 2017, 332, 205–213. [Google Scholar] [CrossRef]
- Chen, Z.; Zheng, Z.; Li, D.; Chen, H.; Xu, Y. Continuous supercritical water oxidation treatment of oil-based drill cuttings using municipal sewage sludge as diluent. J. Hazard. Mater. 2020, 384, 121225. [Google Scholar] [CrossRef]
- Lv, Q.; Wang, L.a.; Jiang, J.; Ma, S.; Liu, L.; Zhou, Z.; Liu, L.; Wang, X.; Bai, J. Catalytic pyrolysis of oil-based drill cuttings over metal oxides: The product properties and environmental risk assessment of heavy metals in char. Process Saf. Environ. Prot. 2022, 159, 354–361. [Google Scholar] [CrossRef]
- Santos, J.M.; Petri, I.J.; Mota, A.C.S.; Morais, A.d.S.; Ataíde, C.H. Optimization of the batch decontamination process of drill cuttings by microwave heating. J. Pet. Sci. Eng. 2018, 163, 349–358. [Google Scholar] [CrossRef]
- Ye, Y.; Li, J.; Zhang, Q.; Feng, J.; Zhu, J.; Yin, D. Nanoemulsion for oil-contaminated oil-based drill cuttings removel in lab. Int. J. Hydrogen Energy 2017, 42, 18734–18740. [Google Scholar] [CrossRef]
- Hu, L.; Yu, H.; Yan, X.; Sun, Y.; Zhu, B. Analysis of molten slag from high-temperature plasma treatment of oil-based drill cuttings. Process Saf. Environ. Prot. 2023, 172, 97–104. [Google Scholar] [CrossRef]
- Onwosi, C.O.; Odibo, F.J.C.; Enebechi, C.K.; Nwankwegu, A.S.; Ikele, A.I.; Okeh, O.C. Bioremediation of Diesel-contaminated Soil by Composting with Locally Generated Bulking Agents. Soil Sediment Contam. Int. J. 2017, 26, 438–456. [Google Scholar] [CrossRef]
- Liu, P.; Zhan, Y.; Quan, K.; Li, K.; Jia, Y. The Damage Characteristics of Oil-Based Drilling Cuttings Coke Based on Ultrasonic Vibration. Rock Mech. Rock Eng. 2024, 57, 4925–4934. [Google Scholar] [CrossRef]
- Khankhaje, E.; Rafieizonooz, M.; Salim, M.R.; Khan, R.; Mirza, J.; Siong, H.C.; Salmiati. Sustainable clean pervious concrete pavement production incorporating palm oil fuel ash as cement replacement. J. Clean. Prod. 2018, 172, 1476–1485. [Google Scholar] [CrossRef]
- Wiemes, L.; Pawlowsky, U.; Mymrin, V. Incorporation of industrial wastes as raw materials in brick’s formulation. J. Clean. Prod. 2017, 142, 69–77. [Google Scholar] [CrossRef]
- Kovaleva, E.I.; Guchok, M.V.; Terekhova, V.A.; Demin, V.V.; Trofimov, S.Y. Drill cuttings in the environment: Possible ways to improve their properties. J. Soils Sediments 2021, 21, 1974–1988. [Google Scholar] [CrossRef]
- Wienchol, P.; Szlęk, A.; Ditaranto, M. Waste-to-energy technology integrated with carbon capture—Challenges and opportunities. Energy 2020, 198, 117352. [Google Scholar] [CrossRef]
- Xie, B.; Qin, J.; Sun, H.; Wang, S.; Li, X. Release characteristics of polycyclic aromatic hydrocarbons (PAHs) leaching from oil-based drilling cuttings. Chemosphere 2022, 291, 132711. [Google Scholar] [CrossRef]
- Yang, H.; Huang, S.; Zhang, Y.; Zhou, B.; Manzoor Ahmed, S.; Liu, H.; Liu, Y.; He, Y.; Xia, S. Remediation effect of Cr (VI)-contaminated soil by secondary pyrolysis oil-based drilling cuttings ash. Chem. Eng. J. 2020, 398, 125473. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, H.; Sun, J.; Zhang, Y.; Xia, S. Enhanced Adsorption of Rhodamine B on Modified Oil-Based Drill Cutting Ash: Characterization, Adsorption Kinetics, and Adsorption Isotherm. ACS Omega 2021, 6, 17086–17094. [Google Scholar] [CrossRef]
- Yang, H.; Liu, Y.; Bai, G.; Liao, R.; Zhang, Y.; Xia, S. Enhanced and selective adsorption of tamoxifen using sodium dodecyl sulfate modified oil-based drill cutting ash. Sep. Purif. Technol. 2021, 278, 119660. [Google Scholar] [CrossRef]
- Costa, L.C.; Carvalho, C.F.; Soares, A.S.F.; Souza, A.C.P.; Bastos, E.F.T.; Guimaraes, E.; Santos, J.C.; Carvalho, T.; Calderari, V.H.; Marinho, L.S.; et al. Physical and chemical characterization of drill cuttings: A review. Mar. Pollut. Bull. 2023, 194, 115342. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Wei, C.; Yu, Z.; Ji, L.; Liu, M.; Cao, Q.; Wu, L.; Li, F. Fabrication of Iron-Containing Biochar by One-Step Ball Milling for Cr(VI) and Tetracycline Removal from Wastewater. Langmuir 2023, 39, 18958–18970. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, W.; Yuan, F.; Yang, Z.; Lin, J.; Huang, Y.; Ding, M. Effect of Bi5O7I/calcined ZnAlBi-LDHs composites on Cr(VI) removal via adsorption and photocatalytic reduction. Appl. Surf. Sci. 2021, 562, 150129. [Google Scholar] [CrossRef]
- Wu, S.; Yin, X.; Liu, Z.; Zhang, W.; Huang, Y.; Dong, F.; Zhang, D. Electrolyte Flow-Driven Coupling of Oxygen Evolution Reaction and CO2 Electroreduction for Promoting Selective HCOOH Production under Acidic Conditions. ACS Sustain. Chem. Eng. 2025, 13, 7062–7073. [Google Scholar] [CrossRef]
- Huang, Y.; Yin, X.; He, P.; Kou, S.; Zhang, X.; Wang, L.; Lu, P. Peroxymonosulfate Activation by Bi2WO6/BiOCl Heterojunction Nanocomposites under Visible Light for Bisphenol A Degradation. Nanomaterials 2021, 11, 3130. [Google Scholar] [CrossRef]
- Brzhezinskaya, M.; Zhivulin, V.E. Controlled modification of polyvinylidene fluoride as a way for carbyne synthesis. Polym. Degrad. Stab. 2022, 203, 110054. [Google Scholar] [CrossRef]
- Kou, J.; Tao, D.; Xu, G. A study of adsorption of dodecylamine on quartz surface using quartz crystal microbalance with dissipation. Colloids Surf. A Physicochem. Eng. Asp. 2010, 368, 75–83. [Google Scholar] [CrossRef]
- Brzhezinskaya, M.; Irzhak, A.; Irzhak, D.; Kang, T.W.; Kononenko, O.; Matveev, V.; Panin, G.; Roshchupkin, D. Direct growth of graphene film on piezoelectric La3Ga5.5Ta0.5O14 crystal. Phys. Status Solidi Rapid Res. Lett. 2016, 10, 639–644. [Google Scholar] [CrossRef]
- Zhu, N.; Yan, T.; Qiao, J.; Cao, H. Adsorption of arsenic, phosphorus and chromium by bismuth impregnated biochar: Adsorption mechanism and depleted adsorbent utilization. Chemosphere 2016, 164, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Jena, H.M. Adsorption of Cr(VI) from aqueous phase by high surface area activated carbon prepared by chemical activation with ZnCl2. Process Saf. Environ. Prot. 2017, 109, 63–71. [Google Scholar] [CrossRef]
- Li, F.; Zimmerman, A.R.; Hu, X.; Yu, Z.; Huang, J.; Gao, B. One-pot synthesis and characterization of engineered hydrochar by hydrothermal carbonization of biomass with ZnCl2. Chemosphere 2020, 254, 126866. [Google Scholar] [CrossRef] [PubMed]
- Niam, A.C.; Fenelon, E.; Ningsih, E.; Mirzayanti, Y.W.; Kristanti, E. High-Efficiency Adsorption of Hexavalent Chromium from Aqueous Solution by Samanea saman Activated Carbon. Adsorpt. Sci. Technol. 2022, 2022, 8960379. [Google Scholar] [CrossRef]
- Abewaa, M.; Arka, A.; Haddis, T.; Mengistu, A.; Takele, T.; Adino, E.; Abay, Y.; Bekele, N.; Andualem, G.; Girmay, H. Hexavalent chromium adsorption from aqueous solution utilizing activated carbon developed from Rumex abyssinicus. Results Eng. 2024, 22, 102274. [Google Scholar] [CrossRef]
- Barad, J.M.; Kohli, H.P.; Chakraborty, M. Adsorption of hexavalent chromium from aqueous stream by maghemite nanoparticles synthesized by the microemulsion method. Energy Nexus 2022, 5, 100035. [Google Scholar] [CrossRef]
- Park, J.E.; Shin, J.H.; Oh, W.; Choi, S.J.; Kim, J.; Kim, C.; Jeon, J. Removal of Hexavalent Chromium(VI) from Wastewater Using Chitosan-Coated Iron Oxide Nanocomposite Membranes. Toxics 2022, 10, 98. [Google Scholar] [CrossRef]




| Element | Unit | Sample Number | |||||
|---|---|---|---|---|---|---|---|
| DC | DCS 1 | DCS 2 | DCS 3 | DCS 4 | |||
| Al2O3 | Al | % | 10.05 | 9.09 | 9.24 | 10.77 | 10.82 |
| CaO | Ca | % | 7.62 | 8.06 | 7.69 | 7.13 | 7.64 |
| Fe2O3 | Fe | % | 2.60 | 2.59 | 2.51 | 2.67 | 2.43 |
| K2O | K | % | 1.47 | 1.44 | 1.32 | 1.55 | 1.47 |
| MgO | Mg | % | 0.85 | 0.78 | 0.72 | 0.85 | 0.79 |
| Na2O | Na | % | 2.45 | 3.24 | 5.66 | 8.14 | 8.07 |
| SiO2 | Si | % | 55.30 | 50.88 | 49.03 | 49.41 | 47.76 |
| Ba | Ba | % | 3.53 | 3.17 | 3.24 | 3.53 | 3.56 |
| Mn | Mn | % | 0.22 | 0.22 | 0.21 | 0.23 | 0.20 |
| Ti | Ti | % | 0.98 | 0.89 | 0.90 | 0.97 | 0.98 |
| Sample Number | C [%] | H [%] | C/H |
|---|---|---|---|
| DCS 1 | 5.62 | 0.445 | 12.65 |
| DCS 2 | 4.97 | 0.447 | 11.12 |
| DCS 3 | 3.18 | 0.282 | 11.28 |
| DCS 4 | 2.23 | 0.212 | 10.49 |
| DC | 1.94 | 0.16 | 12.13 |
| Sample Name | Specific Surface Area (m2/g) | Pore Volume (cm3/g) | Average Pore Size (nm) |
|---|---|---|---|
| DC | 9.06 | 0.040 | 17.08 |
| DCS 1 | 12.82 | 0.049 | 16.44 |
| DCS 2 | 13.26 | 0.051 | 15.21 |
| DCS 3 | 9.27 | 0.043 | 18.32 |
| DCS 4 | 9.64 | 0.040 | 17.31 |
| Raw Material | Synthesis Temperature | qm/(mg/g) | Reference |
|---|---|---|---|
| Bi5O7I/ZnAlBi-CLDHs-1/10 | - | 18.73 | [35] |
| Bismuth impregnated biochar | 600 | 12.20 | [41] |
| Activated carbon | 30 | 43.45 | [42] |
| Hydrochar by biomass with ZnCl2 | 200 | 14.00 | [43] |
| Fe@MBC | 600 | 25.46 | [34] |
| Carbon-Modified Oil-Based Cuttings | 700 | 56.84 | This Study |
| Adsorbent Material | Pseudo-First-Order Kinetics | Pseudo-Second-Order Kinetics | ||
|---|---|---|---|---|
| k1 (min−1) | R2 | k2 (g·mg−1·min−1) | R2 | |
| DC | 0.03399 | 1.00 | 0.002592 | 1.00 |
| DCS 1 | 0.00703 | 0.99 | 0.0001 | 0.97 |
| DCS 2 | 0.00638 | 0.98 | 5.46 × 10−5 | 0.85 |
| DCS 3 | 0.01452 | 0.94 | 0.000355 | 0.99 |
| DCS 4 | 0.01255 | 0.92 | 0.000443 | 0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Li, L.; Qiao, J.; Wu, X.; Zhang, Y.; Bai, B.; Yang, L.; Pan, S.; Feng, Q.; Liu, L. Efficient Removal of Cr(VI) from Water by Carbon-Based Composites Derived from Oil-Based Drill Cuttings. Materials 2025, 18, 5295. https://doi.org/10.3390/ma18235295
Huang Y, Li L, Qiao J, Wu X, Zhang Y, Bai B, Yang L, Pan S, Feng Q, Liu L. Efficient Removal of Cr(VI) from Water by Carbon-Based Composites Derived from Oil-Based Drill Cuttings. Materials. 2025; 18(23):5295. https://doi.org/10.3390/ma18235295
Chicago/Turabian StyleHuang, Yongkui, Linzhi Li, Jiangrong Qiao, Xiaochuan Wu, Ye Zhang, Bo Bai, Liu Yang, Shubiao Pan, Qi Feng, and Li Liu. 2025. "Efficient Removal of Cr(VI) from Water by Carbon-Based Composites Derived from Oil-Based Drill Cuttings" Materials 18, no. 23: 5295. https://doi.org/10.3390/ma18235295
APA StyleHuang, Y., Li, L., Qiao, J., Wu, X., Zhang, Y., Bai, B., Yang, L., Pan, S., Feng, Q., & Liu, L. (2025). Efficient Removal of Cr(VI) from Water by Carbon-Based Composites Derived from Oil-Based Drill Cuttings. Materials, 18(23), 5295. https://doi.org/10.3390/ma18235295









