1. Introduction
With the rapid development of industry, the disposal of waste rubber presents serious challenges. Given the current global trend toward promoting a green circular economy, processing waste rubber into rubber powder for resource recycling shows great development potential [
1]. More than 30 years of design and construction experience have been accumulated in China’s expressway construction, and a relatively complete highway transportation network has been established. However, due to the need for maintenance during use, the demand for high-performance asphalt has increased dramatically [
2]. Rubber powder/SBS modified asphalt is primarily composed of base asphalt, rubber powder, SBS modifier, and stabilizers. Due to the advantages of both rubber powder and SBS as modifiers, the road performance of asphalt has been greatly improved [
3,
4,
5]. Jatoi et al. [
6] also pointed out that the incorporation of polymers like SBS and rubber into asphalt can significantly enhance the mechanical properties of asphalt mixtures. It was also noted that this approach improves pavement durability and reduces lifecycle maintenance costs, which is beneficial for sustainable development. Therefore, rubber powder/SBS-composite-modified asphalt has been widely used in road construction.
However, many environmental issues regarding the use of rubber powder/SBS-modified asphalt under high-temperature conditions have not been resolved. Li et al. [
7] found that significant amounts of VOCs are released by asphalt samples when temperatures exceed 150 °C; Gong et al. [
8] found that VOCs were released by asphalt pavements during use, posing environmental and health risks. A thorough understanding of asphalt VOCs emissions is considered essential for the development of targeted reduction measures. It has been shown in studies that VOCs emitted from asphalt can affect human growth and development [
9], impact the nervous system [
10], cause organ irritation, breathing difficulties, and even certain cancers [
11]. From an environmental perspective, land and water sources can be contaminated by particulate matter generated by VOCs [
12] and the atmospheric environment can be affected [
13]. Compared with traditional asphalt, more complex and higher concentrations of VOCs emissions are exhibited by rubber powder/SBS asphalt. The rubber powder in unsulfurized rubber powder/SBS-modified asphalt (UDRA) is mostly derived from waste rubber tires, and the main component of tire rubber is vulcanized polymer. Under high-temperature conditions, sulfur-containing fumes and volatile organic compounds are easily released during the modification process [
14] and large amounts of VOCs and sulfur compounds were generated [
15,
16,
17]. These substances are not only the main components of rubber exhaust gases but also the primary sources of malodorous gases, and this is why rubber exhaust gases generally have a strong and pungent odor. The pollutants contained in it pose serious hazards to human health and the ecological environment, and they are likely to cause respiratory, blood, nervous system, and central nervous system diseases in humans [
10]. Li et al. [
11] found that VOCs emissions from rubber asphalt in the early stages originate from the rubber, transitioning to petroleum asphalt in the later stages; Wang et al. [
18] demonstrated that increasing the rubber powder content and heating temperature in rubber asphalt significantly increased VOCs emissions. A large number of VOCs, such as benzene, toluene, ethylbenzene, xylene, and sulfur compounds, were identified in rubber asphalt emissions by Gągol et al. [
19].
GC-MS is widely used for the detection of VOCs in asphalt materials due to its high detection sensitivity. Compared to Fourier Transform Infrared Spectroscopy (FTIR), specific gas products can be identified by GC-MS rather than a group of compounds with the same functional group characteristics [
20]. The advantage of the GC-MS analysis method is that compounds in complex mixtures can be separated through chromatography, and then analyzed by mass spectrometry ionization, and standard spectral libraries are used to accurately identify unknown compounds. Yang et al. [
21] used pyrolysis GC-MS to study the emissions of carbon dioxide and VOCs in asphalt fumes. Qin et al. [
22] used GC-MS to track the evolution of light components in recycled asphalt. Tang et al. [
23] dissolved samples collected under different conditions in carbon disulfide and used GC-MS to quantify the emissions.
Currently, the primary methods for reducing emissions from rubber powder/SBS-modified asphalt are divided into two categories: the addition of emission reduction agents and mechanical desulfurization. Wang et al. [
24] and E et al. [
25] reduced VOCs emissions from road asphalt at high temperatures by preparing asphalt emission reduction agents. Lin et al. [
26] reduced VOCs emissions from rubber asphalt by using inhibitors. Jia et al. [
27] absorbed pollutants from asphalt fumes using molecular sieves to lower the preparation temperature. However, the performance of asphalt in road applications may be affected by the addition of emission reduction agents. Mechanical desulfurization has the advantages of simple equipment, high production efficiency, and low cost [
28]. The C-S bonds in the gum powder can be effectively broken, reducing the generation of volatile organic compounds under high-temperature conditions.
To reduce VOCs emissions from rubber powder/SBS-modified asphalt under high temperatures, we investigated the key road performance characteristics of asphalt modified with different proportions of rubber powder/SBS and determined the optimal rubber powder proportion. The VOCs emission behavior of rubber powder/SBS-modified asphalt before and after desulfurization was compared and analyzed using GC-MS, with infrared spectroscopy analysis further validating the GC-MS results. Finally, the VOCs emission mechanisms were revealed through microstructural testing.
2. Methodology
Figure 1 lists the raw materials used in this study, from left to right: devulcanized rubber powder, SBS modifier, and undevulcanized rubber powder.
2.1. Raw Material
Double Dragon 70# base asphalt from Suzhou Sanchuang Road Engineering Company was employed. The basic indicators of the base asphalt were determined according to the test requirements specified in ASTM International, including ASTM D6114 [
29], ASTM D5603 [
30], and ASTM D5755 [
31], as shown in
Table 1.
The unsulfurized rubber powder was obtained directly by grinding used tires. The desulfurized rubber powder (80 mesh) was produced using desulfurized rubber powder processed by the screw extrusion method as the raw material for research. The rubber powder was mixed with the desulfurizing agent under high shear force and extruded, allowing the desulfurizing agent to penetrate the interior of the rubber powder. Under the action of heat and oxygen, a chemical reaction occurred, breaking the molecular chains of the rubber powder and generating new active groups, which activated and regenerated the rubber powder [
32]. The technical specifications were listed in
Table 2. The addition ratio of the SBS modifier was 4%. The technical specifications are shown in
Table 3.
Desulfurized rubber powder is abbreviated as DR, unsulfurized rubber powder is abbreviated as UDR, desulfurized rubber powder/SBS-modified asphalt is abbreviated as DR/SBSA, and unsulfurized rubber powder/SBS-modified asphalt is abbreviated as UDR/SBSA. SBS-modified asphalt is abbreviated as SBSA.
Preparation of SBSA: The base asphalt was heated to 145 °C. An amount of 4% SBS was added, and the mixture was sheared for approximately 40 min by a high-speed emulsifying shear mixer (Shanghai Aido BME100LT, Shanghai, China). Then, 0.3% stabilizer was added, stirred for 1 min, and cured at 175 °C for 1 h.
Preparation of DR/SBSA: The base asphalt was heated to 145 °C. Devulcanized rubber powder at 10%, 15%, and 20% by weight was added, and the mixture was thoroughly mixed for approximately 5 min to ensure uniform dispersion of the rubber powder in the base asphalt. Under 4000 rpm and 170–175 °C, 4% SBS was added, and the mixture was sheared for approximately 40 min by a high-speed emulsifying shear mixer (Shanghai Aido BME100LT). Then, 0.3% stabilizer was added, stirred for 1 min, and cured at 175 °C for 1 h.
Preparation of UDR/SBSA: The base asphalt was heated to 145 °C. Undevulcanized rubber powder at 10%, 15%, and 20% by weight was added, and the mixture was thoroughly mixed for approximately 5 min to ensure uniform dispersion of the rubber powder in the base asphalt. Under 4000 rpm and 170–175 °C, 4% SBS was added, and the mixture was sheared for approximately 40 min by a high-speed emulsifying shear mixer (Shanghai Aido BME100LT). Then, 0.3% stabilizer was added, stirred for 1 min, and cured at 175 °C for 1 h.
2.2. Test Methods
Table 4 presents the tests conducted on all asphalt samples in this study.
2.2.1. Basic Performance Test
The test methods were conducted following the testing standards specified by ASTM International. All tests in this study were conducted in triplicate, and the reported values are the average of three independent measurements. The experimental errors comply with the requirements of the relevant standards, according to ASTM D6114 [
29], ASTM D5603 [
30], and ASTM D5755 [
31].
Needle penetration: The Wuxi South SYD-2801F fully automatic needle penetration tester produced by Wuxi Huanan Experimental Instrument Co., Ltd. (Wuxi, China) was used. Penetration was determined by the depth to which a standard needle was vertically inserted into the asphalt sample under specified load, temperature, and time conditions. The unit of measurement was 1/10 mm.
Extensibility: The Wuxi Petroleum LYY-10A-1 Extensibility Tester (Wuxi, China) produced by Wuxi Petroleum Instrument Equipment Co., Ltd. was used. The test temperature was 5 °C, and the stretching speed was 5 cm/min. First, the asphalt sample was poured into an octagonal mold. The sample was cooled at room temperature for 1.5 h, then shaved flat along the mold surface using a knife heated by an alcohol lamp. The asphalt and mold were placed in water at 5 °C and cooled for 1 h. Finally, the mold was removed, and the asphalt sample was taken out. It was then placed on the elongation tester to conduct a tensile test and determine the elongation value of the sample.
Softening point: The instrument used was the SYD-2806F produced by Wuxi Huanan Experimental Instrument Co., Ltd. (Wuxi, China). During the test, the water bath temperature was controlled to increase at a rate of 5 °C per minute. First, the asphalt sample was poured into the sample ring until it slightly exceeded the ring surface. After cooling at room temperature for 0.5 h, the sample was scraped along the mold surface to ensure it was flush with the ring surface. The sample, mold, steel ball, and metal support were placed in a water bath maintained at 5 °C for at least 15 min. Then, according to the specified method, the sample was placed in the test container for heating. The temperature was recorded when the steel ball sunk to the specified distance from the asphalt.
Rolling thin-film oven test (RTFOT): The 81-PV1632 rolling thin-film oven was used. First, 35 ± 0.5 g of asphalt sample was poured into a clean, dry glass bottle, and the initial mass was recorded. The oven was then preheated to 163 ± 0.5 °C. The air flow was adjusted to 4000 mL/min. The sample was placed in the oven, and the rotation was started. The aging process was timed for 75 min.
2.2.2. Rheological Property Test
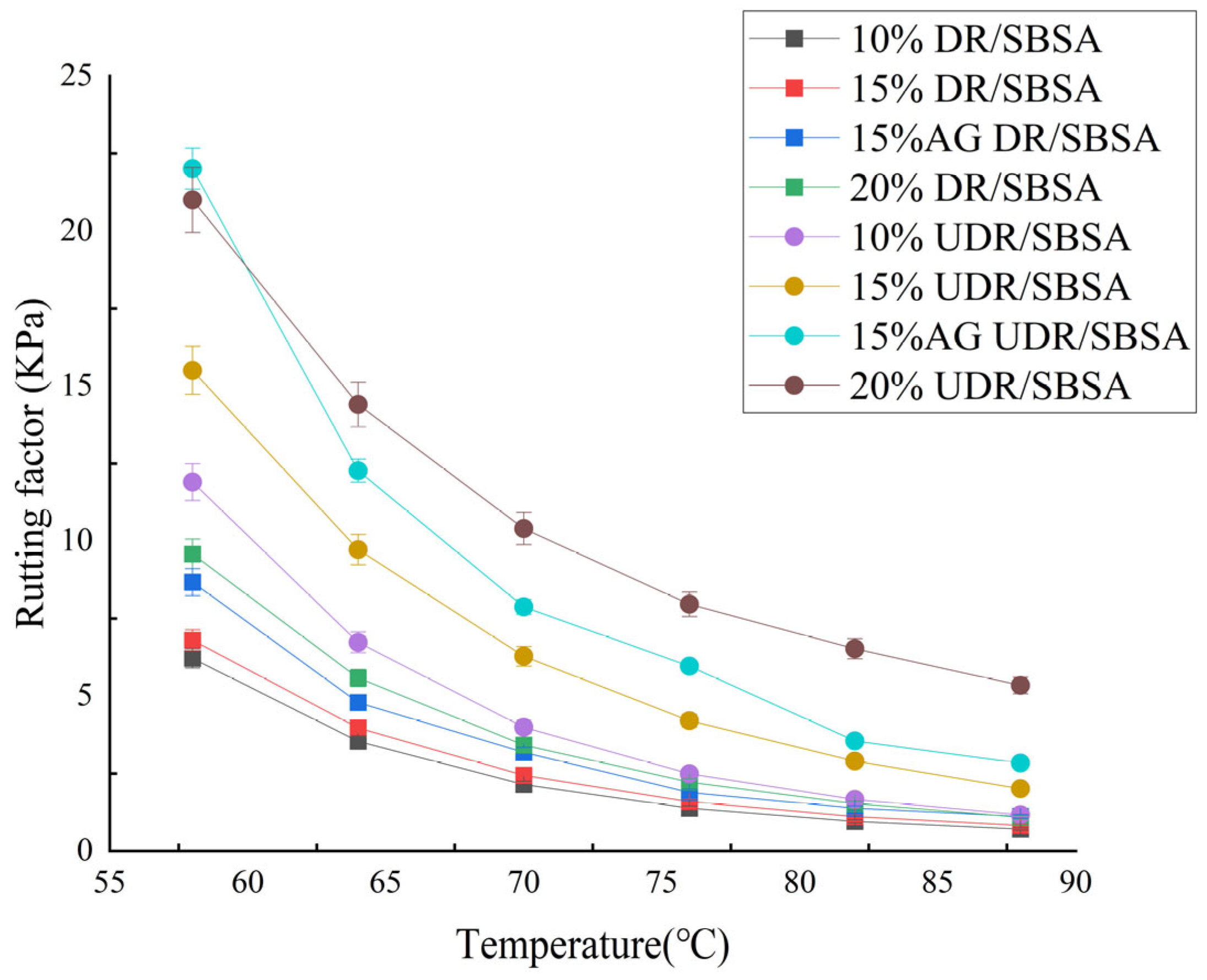
The instrument used was the American TA HR10 produced by TA Instruments (Newcastle, DE, USA). The asphalt sample was poured into a silicone sheet to form a chess piece-shaped sample. A 25 mm parallel plate was used for the test. After the instrument was calibrated, the scraper was heated with an alcohol lamp to remove excess asphalt. The temperature was scanned from 58 °C to 88 °C with a temperature gradient of 6 °C during the test.
2.2.3. Volatile Organic Compound Test
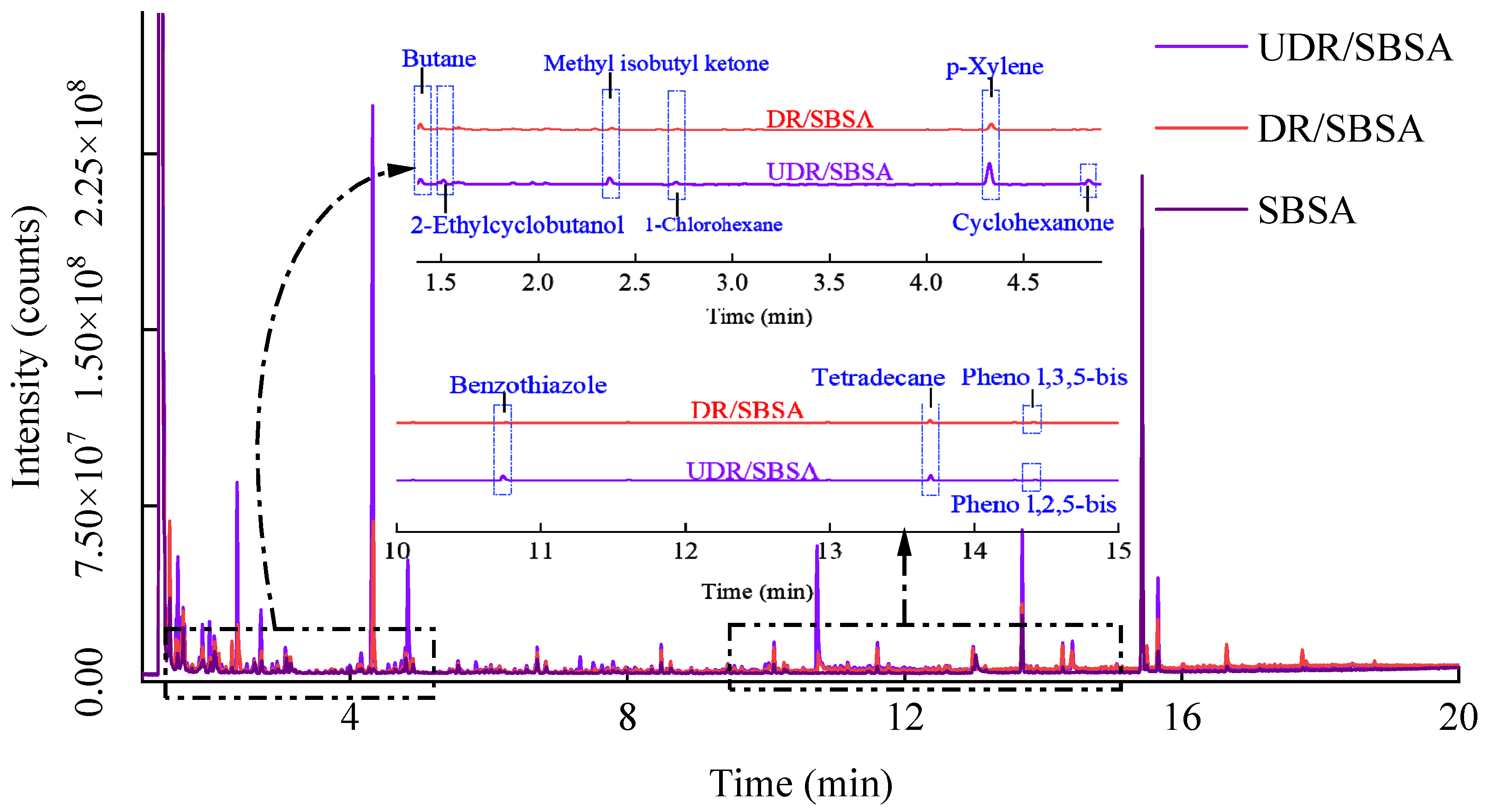
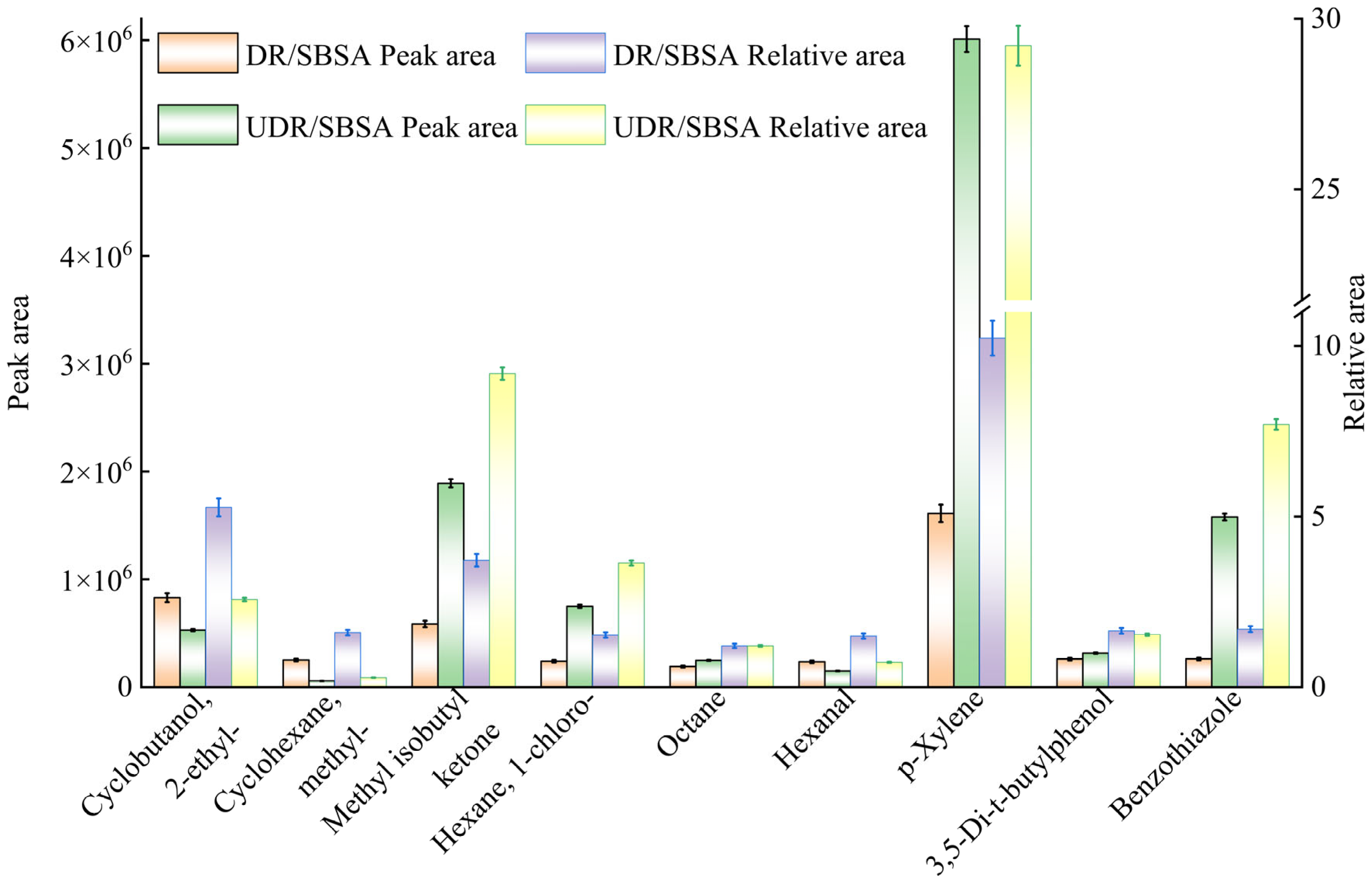
The test was conducted using the Thermo Fisher Scientific ISQ 7000 single quadrupole gas chromatography–mass spectrometry (GC-MS) instrument produced by Guoyue Trading (Shanghai) Co., Ltd. (Shanghai, China). First, the samples were processed and injected into headspace vials, then heated to promote the evaporation of volatile substances. The headspace sampler automatically extracted gases from the samples at a set temperature and time, which were subsequently separated by gas chromatography and detected by mass spectrometry. Unknown substances were identified by comparing their mass-to-charge ratio (m/z) distributions and relative abundances with reference spectra in the database, where match factors (MF) exceeding 90% served as the reliable identification threshold.
2.2.4. Fourier Transform Infrared Spectroscopy (FTIR) Test
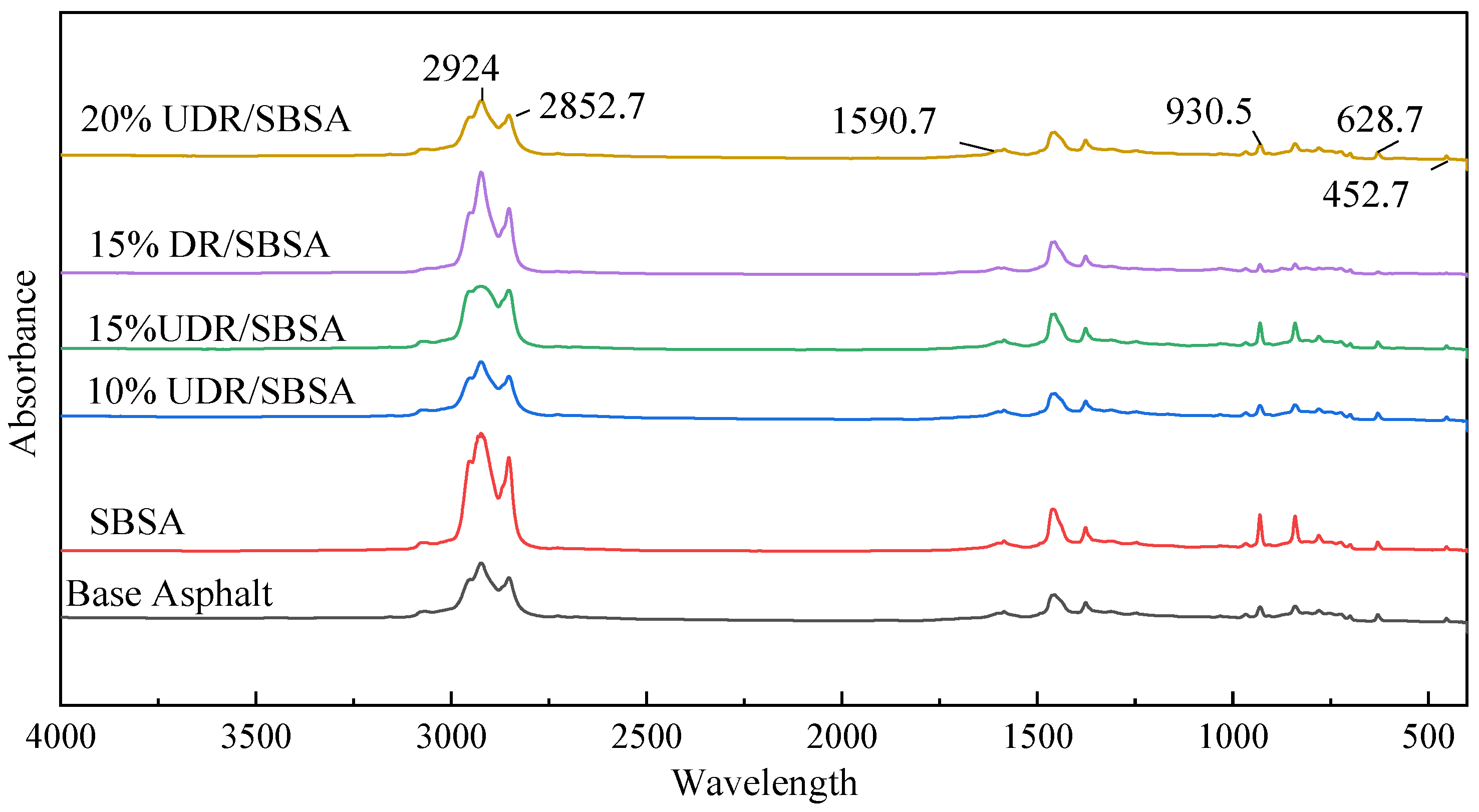
A Thermo Fisher IS5 infrared spectrometer produced by Shanghai Kerui Instrument Technology Co., Ltd. (Shanghai, China) was used. Asphalt samples and trichloroethylene were mixed in a 1:10 ratio to form a solution. One drop of the solution was placed on a potassium bromide (KBr) substrate using a dropper, and the solvent was allowed to evaporate naturally to form a uniform asphalt film. The film should be bubble-free and of consistent thickness. The KBr plate with the asphalt film was then placed in the sample chamber of the infrared spectrometer, and the spectrum was recorded.
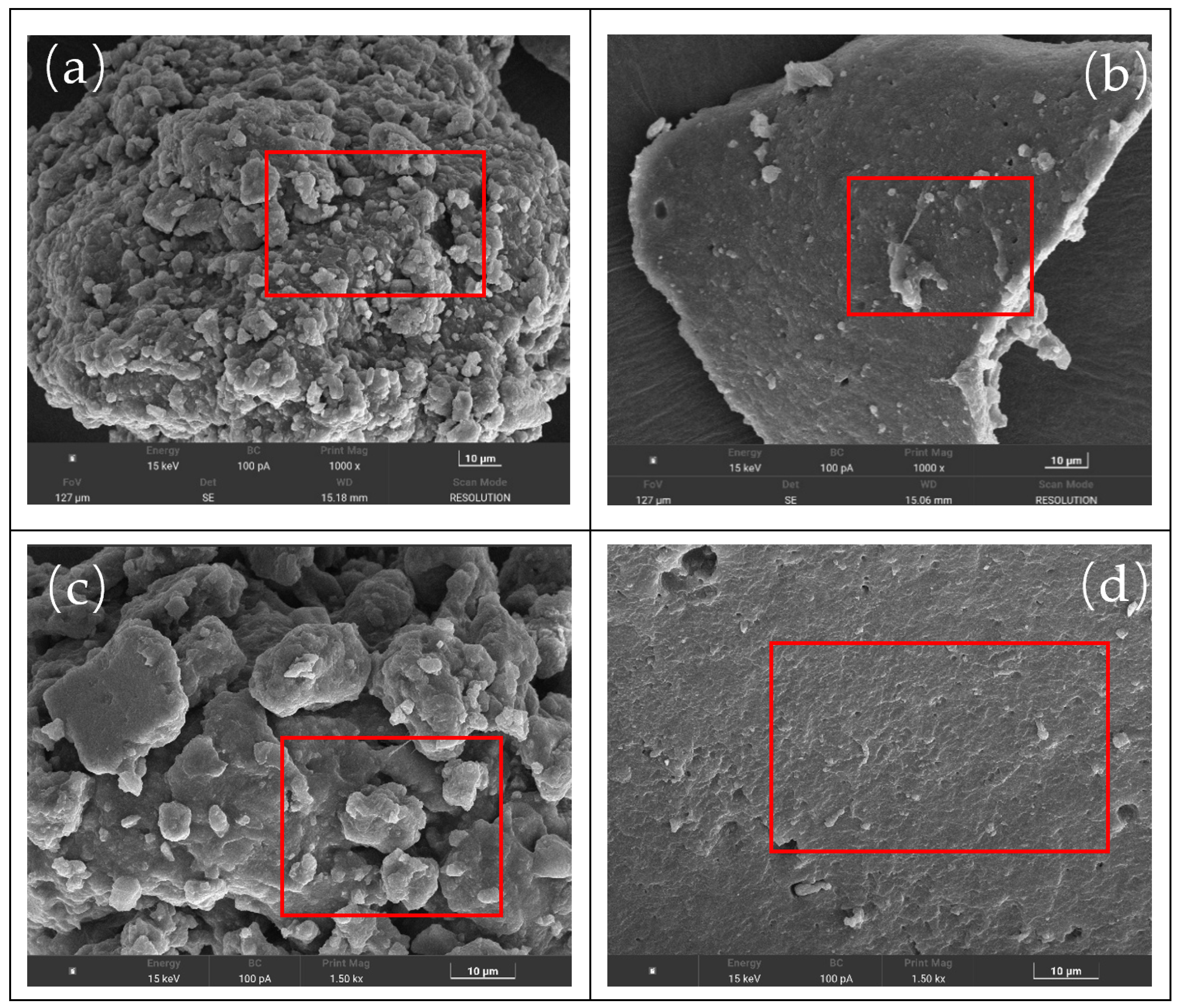
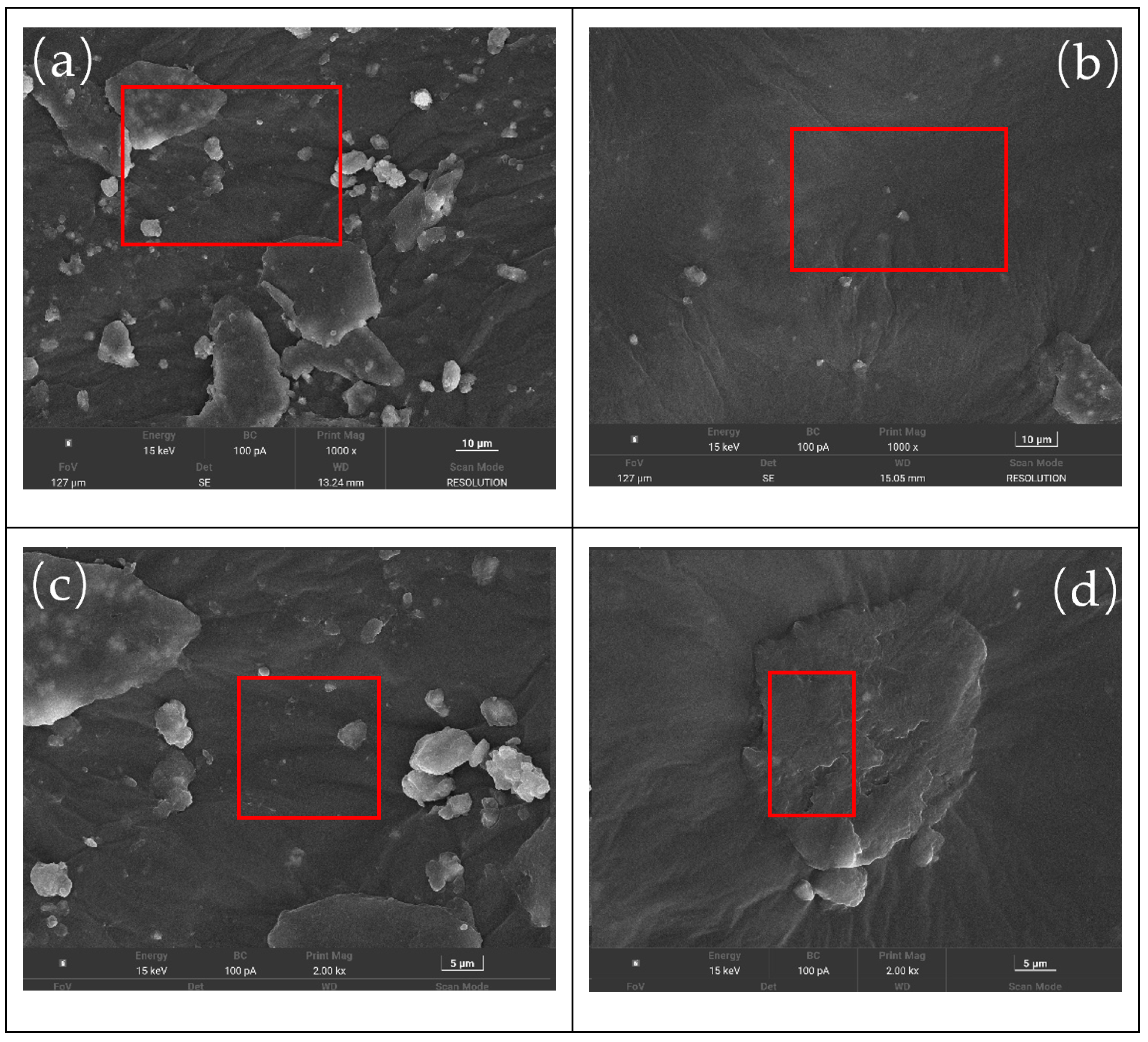
2.2.5. Scanning Electron Microscopy (SEM) Test
Czech TESCAN MIRA LMS produced by TESCAN (Shanghai) company (Shanghai, China): The asphalt sample was rapidly frozen in liquid nitrogen and then transferred to the SEM cryo-stage to prevent shrinkage or structural deformation caused by drying. A small sample (diameter ≤ 1 cm, height ≤ 5 mm) was taken and directly adhered to the conductive adhesive. The Quorum SC7620 sputtering coating instrument produced by Mecano Technology Co., Ltd. (Hong Kong, China) was used to deposit platinum for 45 s at a current of 10 mA. This step ensures uniform coating deposition across the sample surface, effectively mitigating charge accumulation effects. Subsequently, the sample morphology was captured. During morphology imaging, the acceleration voltage was set to 3 kV.