Samarium-Doped Lead Phosphate Glass: Optical Experiments and Calculations Using the Judd–Ofelt Theory
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jayasankar, C.K.; Rukmini, E. Optical properties of Sm3+ ions in zinc and alkali zinc borosulphate glasses. Opt. Mater. 1997, 8, 193–205. [Google Scholar] [CrossRef]
- Jlassi, I.; Mnasri, S.; Elhouichet, H. Concentration dependent spectroscopic behavior of Sm3+-doped sodium fluoro-phosphates glasses for orange and reddish-orange light emitting applications. J. Lumin. 2018, 199, 516–527. [Google Scholar] [CrossRef]
- Kolavekar, S.B.; Ayachit, N.H.; Rajaramakrishna, R.; Pramod, N.G.; Kaewkhao, J. Reddish-orange emission and Judd-Ofelt investigation of Sm3+ ions doped in zinc-bismuth-phospho-tellurite glasses for solid state lighting application. J. Lumin. 2020, 226, 117498. [Google Scholar] [CrossRef]
- Manjeet; Kumar, A.; Anu; Lohan, R.; Deopa, N.; Kumar, A.; Chahal, R.P.; Dahiya, S.; Punia, R.; Rao, A.S. Impact of Sm3+ ions on structural, thermal, optical and photoluminescence properties of ZnO–Na2O–PbO–B2O3 glasses for optoelectronic device applications. Opt. Mater. 2023, 139, 113778. [Google Scholar] [CrossRef]
- Mrabet, H.; Khattech, I.; Bouzidi, S.; Kechiche, L.; Jbeli, A.; Al Harbi, N.; Bouzidi, C.; Munoz, F.; Balda, R. Influence of barium substitution on the physical, thermal, optical and luminescence properties of Sm3+ doped metaphosphate glasses for reddish orange light applications. RSC Adv. 2024, 14, 2070–2079. [Google Scholar] [CrossRef] [PubMed]
- Sharma, Y.K.; Surana, S.S.L.; Singh, R.K. Spectroscopic investigations and luminescence spectra of Sm3+ doped soda lime silicate glasses. J. Rare Earths 2009, 27, 773–780. [Google Scholar] [CrossRef]
- Herrmann, A.; Tewelde, M.; Kuhn, S.; Tiegel, M.; Rüssel, C. The effect of glass composition on the luminescence properties of Sm3+ doped alumino silicate glasses. J. Non-Cryst. Solids 2018, 502, 190–197. [Google Scholar] [CrossRef]
- Herrmann, A.; Zekri, M.; Maalej, R.; Rüssel, C. The Effect of Glass Structure on the Luminescence Spectra of Sm3+-Doped Aluminosilicate Glasses. Materials 2023, 16, 564. [Google Scholar] [CrossRef] [PubMed]
- Matos, I.R.M. Influence of Alkali Metal Ions on the Structural and Spectroscopic Properties of Sm3+-Doped Silicate Glasses. Ceramics 2023, 6, 1788–1798. [Google Scholar] [CrossRef]
- Hong, Z.; Luo, X.; Lai, F.; Wang, W.; You, W.; Huang, J. Analysis of spectroscopic characteristics of Sm3+ ions doped gallium silicate glasses for orange-red LEDs. Opt. Mater. 2024, 157, 116085. [Google Scholar] [CrossRef]
- Chen, B.J.; Shen, L.F.; Pun, E.Y.B.; Lin, H. Sm3+-doped germanate glass channel waveguide as light source for minimally invasive photodynamic therapy surgery. Opt. Exp. 2012, 20, 879–889. [Google Scholar] [CrossRef]
- Ravi Prakash, M.; Neelima, G.; Kummara, V.K.; Ravi, N.; Kiran, N.; Viswanath, C.S.D.; Rao, T.S.; Venkatramu, V. Optical and radiative properties of Sm3+ ions activated alkali-bismuth-germanate glasses. J. Lumin. 2019, 214, 116566. [Google Scholar] [CrossRef]
- Duan, Y.; Li, X.; Shen, R.; Zhang, Y.; Wang, X.; Guan, Z.; Wang, K.; Cao, Y.; Zhang, X.; Chen, B. Luminescence and optical properties of sodium germanate glasses doped with Sm3+ ions. Mater. Res. Bull. 2022, 153, 111905. [Google Scholar] [CrossRef]
- Kumar, A.; Rai, D.K.; Rai, S.B. Optical properties of Sm3+ ions doped in tellurite glass. Spectrochim. Acta A 2003, 59, 917–925. [Google Scholar] [CrossRef]
- Sasikala, T.; Moorthy, L.R.; Babu, A.M. Optical and luminescent properties of Sm3+ doped tellurite glasses. Spectrochim. Acta A 2013, 104, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Klimesz, B.; Lisiecki, R.; Ryba-Romanowski, W. Sm3+-doped oxyfluorotellurite glasses—Spectroscopic, luminescence and temperature sensor properties. J. Alloys Compd. 2019, 788, 658–665. [Google Scholar] [CrossRef]
- Sharma, Y.K.; Surana, S.S.L.; Dubedi, R.P.; Joshi, V. Spectroscopic and radiative properties of Sm3+ doped zinc fluoride borophosphate glasses. Mater. Sci. Eng. B 2005, 119, 131–135. [Google Scholar] [CrossRef]
- Maheshvaran, K.; Linganna, K.; Marimuthu, K. Composition dependent structural and optical properties of Sm3+ doped boro-tellurite glasses. J. Lumin. 2011, 131, 2746–2753. [Google Scholar] [CrossRef]
- Selvaraju, K.; Marimuthu, K. Structural and spectroscopic studies on concentration dependent Sm3+ doped boro-tellurite glasses. J. Alloys Compd. 2013, 553, 273–281. [Google Scholar] [CrossRef]
- Do, P.V.; Tuyen, V.P.; Quang, V.X.; Hung, L.X.; Thanh, L.D.; Ngoc, T.; Tam, N.V.; Huy, B.T. Investigation of spectroscopy and the dual energy transfer mechanisms of Sm3+-doped telluroborate glasses. Opt. Mater. 2016, 55, 62–67. [Google Scholar]
- Reddy, K.S.R.K.; Swapna, K.; Mahamuda, S.; Venkateswarlu, M.; Srinivas Prasad, M.V.V.K.; Rao, A.S.; Prakash, G.V. Structural, optical absorption and photoluminescence spectral studies of Sm3+ ions in Alkaline-Earth Boro Tellurite glasses. Opt. Mater. 2018, 79, 21–32. [Google Scholar] [CrossRef]
- Gokce, M.; Kocyigit, D. Optical and luminescence characteristics of Sm3+ doped B2O3-GeO2-Gd2O3 glasses. Opt. Mater. 2018, 83, 233–240. [Google Scholar] [CrossRef]
- Anantha Lakshmi, Y.; Swapna, K.; Mahamuda, S.; Venkateswarlu, M.; Rao, A.S. Photoluminescence properties of Sm3+ ions doped Bismuth Boro tellurite glasses. Solid State Sci. 2021, 116, 106609. [Google Scholar] [CrossRef]
- Kaur, S.; Katyal, V.; Plakkot, V.; Deopa, N.; Prasad, A.; Rao, A.S. Radiative emission analysis of Sm3+ ions doped borosilicate glasses for visible orange photonic devices. J. Non-Cryst. Solids 2021, 572, 121106. [Google Scholar] [CrossRef]
- Biswas, J.; Jana, S.; Ghosh, S.; Mahalingam, V. Optical and luminescence properties of Sm2O3 doped SrO–PbO–ZnO–P2O5–TeO2 glasses for visible laser applications. Solid State Sci. 2022, 129, 106910. [Google Scholar] [CrossRef]
- Zalamin, S.N.F.; Zaid, M.H.M.; Matori, K.A.; Karim, M.K.A.; Yamin, N.A.M.; Ismail, N.A.N. Comprehensive study on optical and luminescence properties of Sm3+ doped magnesium borotellurite glasses. J. Phys. Chem. Solids 2022, 163, 110563. [Google Scholar] [CrossRef]
- Sekaran, S.A.R.; Padhi, R.K.; El Sayed, Y.; Marimuthu, K. Spectroscopic studies on Sm3+ ions doped modifiers incited calcium phospho-silicate glasses for photonic and optoelectronic applications. J. Non-Cryst. Solids 2024, 623, 122692. [Google Scholar] [CrossRef]
- Gan, F.; Chen, Y. The fluorescence line narrowing of Sm3+ in ZBLAN fluoride glass. J. Non-Cryst. Solids 1993, 161, 282–285. [Google Scholar] [CrossRef]
- Němec, P.; Jedelský, J.; Frumar, M. On the effect of composition on the Judd–Ofelt parameters of Sm3+-doped chalcogenide glasses. J. Non-Cryst. Solids 2003, 326–327, 325–329. [Google Scholar] [CrossRef]
- Flórez, A.; Herrera, A.; Florez, M. Optical properties of Sm3+ ions and influence of odd third-order intensity parameters in fluoride glasses. Phys. Stat. Sol. C 2007, 4, 4156–4164. [Google Scholar] [CrossRef]
- Tang, G.; Xiong, H.; Chen, W.; Luo, L. The study of Sm3+-doped low-phonon-energy chalcohalide glasses. J. Non-Cryst. Solids 2011, 357, 2463–2467. [Google Scholar] [CrossRef]
- Mahato, K.K.; Rai, D.K.; Rasi, S.B. Optical studies of Sm3+ doped oxyfluoroborate glass. Solid State Commun. 1998, 108, 671–676. [Google Scholar] [CrossRef]
- Jayasankar, C.K.; Babu, P. Optical properties of Sm3+ ions in lithium borate and lithium fluoroborate glasses. J. Alloys Compd. 2000, 307, 82–95. [Google Scholar] [CrossRef]
- Shanmuga Sundari, S.; Marimuthu, K.; Sivraman, M.; Babu, S.S. Composition dependent structural and optical properties of Sm3+-doped sodium borate and sodium fluoroborate glasses. J. Lumin. 2010, 130, 1313–1319. [Google Scholar] [CrossRef]
- Rajesh, D.; Balakrishna, A.; Ratnakaram, Y.C. Luminescence, structural and dielectric properties of Sm3+ impurities in strontium lithium bismuth borate glasses. Opt. Mater. 2012, 35, 108–116. [Google Scholar] [CrossRef]
- Swapna, K.; Mahamuda, S.; Srinivasa Rao, A.; Sasikala, T.; Rama Moorthy, L. Visible luminescnence characteristics of Sm3+ doped zinc alumino bismuth borate glasses. J. Lumin. 2014, 146, 288–294. [Google Scholar] [CrossRef]
- Mahamuda, S.K.; Swapna, K.; Venkateswarlu, M.; Rao, A.S.; Shakya, S.; Prakash, G.V. Spectral characterisation of Sm3+ ions doped oxyfluoroborate glasses for visible orange luminescent applications. J. Lumin. 2014, 154, 410. [Google Scholar] [CrossRef]
- Deopa, N.; Rao, A.S.; Choudhary, A.; Saini, S.; Navhal, A.; Jayasimhadri, M.; Haranath, D.; Prakash, G.V. Photoluminescence investigations on Sm3+ ions doped borate glasses for tricolor w-LEDs and lasers. Mater. Res. Bull. 2018, 100, 206–212. [Google Scholar] [CrossRef]
- Amer, D.; Swapna, K.; Shanmukh Kumar, J.V.; Mahamuda, S.; Venkateswarlu, M.; Sruthi, P.; Shanmukha Sai, D.; Rao, A.S. Influence of Sm3+ ion concentration on the photoluminescence behavior of antimony lead oxy fluoro borate glasses. Mater. Res. Bull. 2022, 146, 111597. [Google Scholar] [CrossRef]
- Murty, V.R.L.; Venkateswarlu, M.; Swapna, K.; Mahamuda, S.; Rekha Rani, P.; Rao, A.S. Physical and spectroscopic studies of Sm3+ ions doped Alumino Tungsten Borate glasses for photonic applications. Radiat. Phys. Chem. 2022, 190, 109806. [Google Scholar] [CrossRef]
- Venkatramu, V.; Babu, P.; Jayasankar, C.K.; Tröster, T.; Sievers, W.; Wortmann, G. Optical spectroscopy of Sm3+ ions in phosphate and fluorophosphate glasses. Opt. Mater. 2007, 29, 1429–1439. [Google Scholar] [CrossRef]
- Zhang, L.; Peng, M.; Dong, G.; Qiu, J. Spectroscopic properties of Sm3+-doped phosphate glasses. J. Mater. Res. 2012, 27, 2111–2115. [Google Scholar] [CrossRef]
- Vijaya, R.; Venkatramu, V.; Babu, P.; Jayasankar, C.K.; Rodríguez-Mendoza, U.R.; Lavín, V. Spectroscopic properties of Sm3+ ions in phosphate and fluorophosphate glasses. J. Non-Cryst. Solids 2013, 365, 85–92. [Google Scholar] [CrossRef]
- Meza-Rocha, A.N.; Speghini, A.; Bettinelli, M.; Caldiño, U. Orange and reddish-orange light emitting phosphors: Sm3+ and Sm3+/Eu3+ doped zinc phosphate glasses. J. Lumin. 2015, 167, 305–309. [Google Scholar] [CrossRef]
- Seshadri, M.; Radha, M.; Rajesh, D.; Barbosa, L.C.; Cordeiro, C.M.B.; Ratnakaram, Y.C. Effect of ZnO on spectroscopic properties of Sm3+ doped zinc phosphate glasses. Phys. B 2015, 459, 79–87. [Google Scholar] [CrossRef]
- Shoaib, M.; Rooh, G.; Rajaramakrishna, R.; Chanthima, N.; Kiwsakunkran, N.; Kim, H.J.; Kaewkhao, J.; Tuscharoen, S. Comparative study of Sm3+ ions doped phosphate based oxide and oxy-fluoride glasses for solid state lighting applications. J. Rare Earths 2019, 37, 374–382. [Google Scholar] [CrossRef]
- Shoaib, M.; Rooh, G.; Rajaramakrishna, R.; Chanthima, N.; Kim, H.J.; Tuscharoen, S.; Kaewkhao, J. Physical and luminescence properties of samarium doped oxide and oxyfluoride phosphate glasses. Mater. Chem. Phys. 2019, 229, 514–522. [Google Scholar] [CrossRef]
- Neelima, G.; Kummar, V.K.; Ravi, N.; Suresh, K.; Rasool, S.K.N.; Tyagarajan, K.; Prasad, T.J. Investigation of spectroscopic properties of Sm3+-doped oxyfluorophosphate glasses for laser and display applications. Mater. Res. Bull. 2019, 110, 223–229. [Google Scholar] [CrossRef]
- Aboudeif, Y.M.; Moteb Alqahtani, M.; Emara, A.M.; Reben, M.; Yousef, E.-S. Luminescence of Phosphate Glasses: P2O5-ZnO-BaF2-K2TeO3-Al2O3-Nb2O5 doped with Sm3+ ions for display and laser materials. J. Electron. Mater. 2020, 49, 4144–4153. [Google Scholar] [CrossRef]
- Ratnakaram, Y.C.; Balakrishna, A.; Rajesh, D.; Seshadri, M. Influence of modifier oxides on spectroscopic properties of Sm3+ doped lithium fluoroborate glass. J. Mol. Struct. 2012, 1028, 141–147. [Google Scholar] [CrossRef]
- Indhrapriyadarshini, A.; Naseer, K.A.; Mahmoud, K.A.; Komal Poojha, M.K.; Alqahtani, M.S.; Marimuthu, K. Impact of Fluoride modifiers on Sm3+ ions doped Phospho-Borate glasses for radiation shielding applications. Radiat. Phys. Chem. 2025, 237, 113057. [Google Scholar] [CrossRef]
- Hong, Z.; Lin, Z.; Yue, H.; Luo, X.; Hou, H.; Lai, F.; Wang, W.; You, W.; Huang, J. Effect of network modifiers on spectroscopic properties of Sm3+ ions doped gallium silicate glasses. Opt. Mater. 2024, 152, 115445. [Google Scholar] [CrossRef]
- Josuva D’Silva, A.; Maheshvaran, K.; Arul Rayappan, I. Influence of alkali and alkaline-earth metal oxides on Sm3+ ions-doped borate glasses: Synthesis, structural, and optical investigations for reddish-orange solid-state-lighting applications. J. Mater. Sci. Mater. Electron. 2024, 35, 1630. [Google Scholar] [CrossRef]
- Damodaraiah, S.; Ratnakaram, Y.C. Energy transfer studies and neutral to warm white light generation in Dy3+- Sm3+ co-doped bismuth phosphate glasses for lighting applications. J. Lumin. 2019, 207, 553–560. [Google Scholar] [CrossRef]
- Jose, A.; Krishnapriya, T.; Jose, T.A.; Joseph, C.; Unnikrishnan, N.V.; Biju, P.R. Color tunable luminescence characteristics and energy transfer analysis of Dy3+/Sm3+ co-doped multicomponent borosilicate glasses. Scr. Mater. 2021, 203, 114088. [Google Scholar] [CrossRef]
- Sangwan, V.; Jayasimhadri, M.; Haranath, D. Colour-tunable and warm white light emitting thermally stable Dy3+/Sm3+ co-activated tungstate-tellurite glasses for photonic applications. J. Lumin. 2024, 266, 120276. [Google Scholar] [CrossRef]
- Biswas, J.; Jana, S.; Ghosh, S. Temperature dependent luminescence exploration in CIELab of Dy3+/Sm3+ ions co-imbued in phospho-tellurite glasses for eco-friendly light generation. J. Non-Cryst. Solids 2024, 630, 122890. [Google Scholar] [CrossRef]
- Hou, Y.; Ge, W.; Xu, J.; Wang, Z.; Cai, X.; Lu, Y.; Chen, Y. Optical and spectroscopic spectral studies: Sm3+ and Dy3+ co-doped multi-component boro-silicate glasses. Mater. Sci. Eng. B 2024, 309, 117615. [Google Scholar] [CrossRef]
- Alqarni, A.S.; Bulus, I.; Yusoff, N.M. Exploring the luminescence potentials in Dy3+/Sm3+ co-doped zinc telluro-phospho-borate glasses for photonic devices. J. Lumin. 2025, 281, 121151. [Google Scholar] [CrossRef]
- Liang, X.; Yang, Y.; Zhu, C.; Yuan, S.; Chen, G.; Pring, A.; Xia, F. Luminescence properties of Tb3+–Sm3+ codoped glasses for white light emitting diodes. Appl. Phys. Lett. 2007, 91, 091104. [Google Scholar] [CrossRef]
- Meza-Rocha, A.N.; Munoz, H.G.; Speghini, A.; Bettinelli, M.; Caldińo, U. Neutral and warm white light emission in Tb3+/Sm3+ zinc phosphate glasses. Opt. Mater. 2015, 47, 537–542. [Google Scholar] [CrossRef]
- Anil Kumar, K.; Babu, S.; Reddy Prasad, V.; Damodaraiah, S.; Ratnakaram, Y.C. Optical response and luminescence characteristics of Sm3+ and Tb3+/Sm3+ co- doped potassium-fluoro-phosphate glasses for reddish-orange lighting applications. Mater. Res. Bull. 2017, 90, 31–40. [Google Scholar] [CrossRef]
- Mohammed, A.-B.F.A.; Lakshminarayana, G.; Baki, S.O.; Bashar, K.A.; Kityk, I.V.; Mahdi, M.A. Optical and dielectric studies for Tb3+/Sm3+ co-doped borate glasses for solid-state lighting applications. Opt. Mater. 2018, 86, 387–393. [Google Scholar] [CrossRef]
- Pisarska, J.; Kos, A.; Sołtys, M.; Górny, A.; Pietrasik, E.; Pisarski, W.A. Spectroscopy and energy transfer in Tb3+/Sm3+ co-doped lead borate glasses. J. Lumin. 2018, 195, 87–95. [Google Scholar] [CrossRef]
- Soriano-Romero, O.; Huerta, E.F.; Meza-Rocha, A.N.; Caldiño, U. Orange and yellow emissions through Sm3+ and Tb3+/Sm3+ doped potassium-zinc phosphate glasses for WLED applications. Ceram. Int. 2023, 49, 36353–36359. [Google Scholar] [CrossRef]
- Dumbaugh, W.H.; Lapp, J.C. Heavy-Metal Oxide Glasses. J. Am. Ceram. Soc. 1992, 75, 2315–2326. [Google Scholar] [CrossRef]
- Lezal, D.; Pedlikova, J.; Kostka, P.; Bludska, J.; Poulain, M.; Zavadil, J. Heavy metal oxide glasses: Preparation and physical properties. J. Non-Cryst. Solids 2001, 284, 288–295. [Google Scholar] [CrossRef]
- Pisarska, J.; Żur, L.; Pisarski, W.A. Visible luminescence of dysprosium ions in oxyhalide lead borate glasses. Spectrochim. Acta A 2011, 79, 705–707. [Google Scholar] [CrossRef] [PubMed]
- Pisarski, W.A.; Pisarska, J.; Żur, L.; Goryczka, T. Structural and optical aspects for Eu3+ and Dy3+ ions in heavy metal glasses based on PbO-Ga2O3-XO2 (X = Te, Ge, Si). Opt. Mater. 2013, 35, 1051–1056. [Google Scholar] [CrossRef]
- Ersundu, A.E.; Çelikbilek, M.; Baazouzi, M.; Soltani, M.T.; Troles, J.; Aydin, S. Characterization of new Sb2O3-based multicomponent heavy metal oxide glasses. J. Alloys Compd. 2014, 615, 712–718. [Google Scholar] [CrossRef]
- Marzouk, M.A.; Fayad, A.M. Heavy metal oxide glass responses for white light emission. J. Mater. Sci. Mater. Electron. 2020, 31, 14502–14511. [Google Scholar] [CrossRef]
- Tekin, H.O.; Susoy, G.; Issa, S.A.M.; Ene, A.; Almisned, G.; Rammah, Y.S.; Ali, F.T.; Algethami, M.; Zakaly, H.M.H. Heavy metal oxide (HMO) glasses as an effective member of glass shield family: A comprehensive characterization on gamma ray shielding properties of various structures. J. Mater. Res. Technol. 2022, 18, 231–244. [Google Scholar] [CrossRef]
- Pisarski, W.A.; Żur, L.; Pisarska, J. Optical transitions of Eu3+ and Dy3+ ions in lead phosphate glasses. Opt. Lett. 2011, 36, 990–992. [Google Scholar] [CrossRef]
- Srinivasa Rao, C.; Upendra Kumar, K.; Babu, P.; Jayasankar, C.K. Optical properties of Ho3+ ions in lead phosphate glasses. Opt. Mater. 2012, 35, 102–107. [Google Scholar] [CrossRef]
- Basavapoornima, C.; Kesavulu, C.R.; Maheswari, T.; Pecharapa, W.; Depuru, S.R.; Jayasankar, C.K. Spectral characteristics of Pr3+-doped lead based phosphate glasses for optical display device applications. J. Lumin. 2020, 228, 117585. [Google Scholar] [CrossRef]
- Sołtys, M.; Pisarska, J.; Leśniak, M.; Sitarz, M.; Pisarski, W.A. Structural and spectroscopic properties of lead phosphate glasses doubly doped with Tb3+ and Eu3+ ions. J. Mol. Struct. 2018, 1163, 418–427. [Google Scholar] [CrossRef]
- Venkata Krishnaiah, K.; Rajeswari, R.; Upendra Kumar, K.; Surendra Babu, S.; Martín, I.R.; Jayasankar, C.K. Spectroscopy and radiation trapping of Yb3+ ions in lead phosphate glasses. J. Quant. Spectrosc. Radiat. Transf. 2014, 140, 37–47. [Google Scholar] [CrossRef]
- Pisarska, J.; Pisarski, W.A.; Goryczka, T.; Lisiecki, R.; Ryba-Romanowski, W. Thermal analysis and near-infrared luminescence of Er3+-doped lead phosphate glasses modified by PbF2. J. Lumin. 2015, 160, 57–63. [Google Scholar] [CrossRef]
- Jana, S.; Mitra, S. Compositional dependence of the luminescence properties of Nd3+ ions in lead phosphate glasses: The efficient laser active materials. Opt. Laser Technol. 2021, 141, 107123. [Google Scholar] [CrossRef]
- Yan, Y.; Faber, A.J.; de Waal, H. Luminescence quenching by OH groups in highly Er-doped phosphate glasses. J. Non-Cryst. Solids 1995, 181, 283–290. [Google Scholar] [CrossRef]
- Pisarski, W.A.; Żur, L.; Goryczka, T.; Sołtys, M.; Pisarska, J. Structure and spectroscopy of rare earth—Doped lead phosphate glasses. J. Alloys Compd. 2014, 587, 90–98. [Google Scholar] [CrossRef]
- Zhou, L.; Zhou, Y.-X.; Dai, S.-X.; Xu, T.-F.; Nie, Q.-H.; Shen, X. Effect of Ga2O3 on the spectroscopic properties of erbium-doped boro-bismuth glasses. Spectrochim. Acta A 2007, 68, 548–553. [Google Scholar]
- Schwarz, J.; Vosejková, K. Thermal properties of Ga2O3-PbO-P2O5 glass system. J. Therm. Anal. Calorim. 2011, 104, 1051–1054. [Google Scholar] [CrossRef]
- Manoj Kumar, G.; Shivakiran Bhaktha, B.N.; Narayana Rao, D. Self-quenching of spontaneous emission in Sm3+ doped lead-borate glass. Opt. Mater. 2006, 28, 1266–1270. [Google Scholar] [CrossRef]
- Żmojda, J.; Kochanowicz, M.; Miluski, P.; Leśniak, M.; Sitarz, M.; Pisarski, W.; Pisarska, J.; Dorosz, D. Effect of GeO2 content on structural and spectroscopic properties of antimony glasses doped with Sm3+ ions. J. Mol. Struct. 2016, 1126, 207–212. [Google Scholar] [CrossRef]
- Khan, I.; Rooh, G.; Rajaramakrishna, R.; Srisittipokakun, N.; Kim, H.J.; Kothan, S.; Kaewkhao, J.; Kirdsiri, K. Comparative study of optical and luminescence properties of Sm3+-ions doped Li2O–Gd2O3–PbO–SiO2 and Li2O-GdF3-PbO–SiO2 glasses for orange emission solid state device application. J. Lumin. 2020, 222, 117136. [Google Scholar] [CrossRef]
- Rajesh, M.; Kavaz, E.; Raju, B.D.P. Photoluminescence, radiative shielding properties of Sm3+ ions doped fluoroborosilicate glasses for visible (reddish-orange) display and radiation shielding applications. Mater. Res. Bull. 2021, 142, 111383. [Google Scholar] [CrossRef]
- Roopa; Eraiah, B. Impact of samarium ion concentration on the physical, structural and optical properties of multi-component borate glasses. J. Non-Cryst. Solids 2022, 596, 121866. [Google Scholar] [CrossRef]
- Ayari, O.; Bouzidi, C.; Khlissa, F.; Garbout, A.; Hraiech, S. Investigation of spectroscopic properties of Sm3+ doped phosphate glasses for reddish orange light applications. Displays 2022, 74, 102266. [Google Scholar] [CrossRef]
- Arunkumar, S.; Marimuthu, K. Concentration effect of Sm3+ ions in B2O3–PbO–PbF2–Bi2O3–ZnO glasses—Structural and luminescence investigations. J. Alloys Compd. 2013, 565, 104–114. [Google Scholar] [CrossRef]
- Vijayakumar, M.; Marimuthu, K.; Sudarsan, V. Concentration dependent spectroscopic behavior of Sm3+ doped lead fluoro-borophosphate glasses for laser and LED applications. J. Alloys Compd. 2015, 647, 209–220. [Google Scholar] [CrossRef]
- Selvi, S.; Marimuthu, K.; Muralidharan, G. Structural and luminescence behavior of Sm3+ ions doped lead boro-telluro-phosphate glasses. J. Lumin. 2015, 159, 207–218. [Google Scholar] [CrossRef]
- Judd, B.R. Optical absorption intensities of rare-earth ions. Phys. Rev. 1962, 127, 750–761. [Google Scholar] [CrossRef]
- Ofelt, G.S. Intensities of crystal spectra of rare-earth ions. J. Chem. Phys. 1962, 37, 511–520. [Google Scholar] [CrossRef]
- Luo, W.; Liao, J.; Li, R.; Chen, X. Determination of Judd–Ofelt intensity parameters from the excitation spectra for rare-earth doped luminescent materials. Phys. Chem. Chem. Phys. 2010, 12, 3276–3282. [Google Scholar] [CrossRef]
- Luo, M.; Chen, B.; Li, X.; Zhang, J.; Xu, S.; Zhang, X.; Cao, Y.; Sun, J.; Zhang, Y.; Wang, X.; et al. Fluorescence decay route of optical transition calculation for trivalent rare earth ions and its application for Er3+-doped NaYF4 phosphor. Phys. Chem. Chem. Phys. 2020, 22, 25177–25183. [Google Scholar] [CrossRef] [PubMed]
- Pisarski, W.A. Judd–Ofelt Analysis and Emission Properties of Dy3+ Ions in Borogermanate Glasses. Materials 2022, 15, 9042. [Google Scholar] [CrossRef]
- Kesavulu, C.R.; Jayasankar, C.K. White light emission in Dy3+-doped lead fluorophosphate glasses. Mater. Chem. Phys. 2011, 130, 1078–1085. [Google Scholar] [CrossRef]
- Pisarska, J.; Sołtys, M.; Żur, L.; Pisarski, W.A.; Jayasankar, C.K. Excitation and luminescence of rare earth-doped lead phosphate glasses. Appl. Phys. B 2014, 116, 837–845. [Google Scholar] [CrossRef]
- Ahrens, H.; Wollenhaupt, M.; Fröbel, P.; Lin, J.; Bärner, K.; Sun, G.S.; Braunstein, R. Determination of the Judd-Ofelt parameters of the optical transitions of Sm3+ in lithiumborate tungstate glasses. J. Lumin. 1999, 82, 177–186. [Google Scholar] [CrossRef]
- Jayasimhadri, M.; Cho, E.-J.; Jang, K.-W.; Lee, H.S.; Kim, S.I. Spectroscopic properties and Judd–Ofelt analysis of Sm3+ doped lead–germanate–tellurite glasses. J. Appl. D Appl. Phys. 2008, 41, 175101. [Google Scholar] [CrossRef]
- Bhardwaj, S.; Shukla, R.; Sanghi, S.; Agarwal, A.; Pal, I. Spectroscopic properties of Sm3+ doped lead bismosilicate glasses using Judd–Ofelt theory. Spectrochim. Acta A 2014, 117, 191–197. [Google Scholar] [CrossRef]
- Kuhn, S.; Herrmann, A.; Rüssel, C. Judd–Ofelt analysis of Sm3+-doped lanthanum-aluminosilicate glasses. J. Lumin. 2015, 157, 390–397. [Google Scholar] [CrossRef]
- Mawlud, S.Q.; Ameen, M.M.; Sahar, M.R.; Mahraz, Z.A.S.; Ahmed, K.F. Spectroscopic properties of Sm3+ doped sodium-tellurite glasses: Judd-Ofelt analysis. Opt. Mater. 2017, 69, 318–327. [Google Scholar] [CrossRef]
- Largot, H.; Aiadi, K.E.; Ferid, M.; Hraiech, S.; Bouzidi, C.; Charnay, C.; Horchani-Naifer, K. Spectroscopic investigations of Sm3+ doped phosphate glasses: Judd-Ofelt analysis. Phys. B 2019, 552, 184–189. [Google Scholar] [CrossRef]
- Ravina; Naveen; Sheetal; Kumar, V.; Dahiya, S.; Deopa, N.; Punia, R.; Rao, A.S. Judd-Ofelt itemization and influence of energy transfer on Sm3+ ions activated B2O3–ZnF2–SrO–SiO2 glasses for orange-red emitting devices. J. Lumin. 2021, 229, 117651. [Google Scholar] [CrossRef]
- Bansal, K.; Mishra, N.K.; Abdullahi, I.; Singh, P.J.; Tyagi, M.; Singh, S. Studies of luminescence traits and Judd-Ofelt analysis of Sm3+ activated oxyfluoride glasses. Opt. Mater. 2024, 147, 114579. [Google Scholar] [CrossRef]
- Carnall, W.T.; Fields, P.R.; Rajnak, K. Electronic energy levels in the trivalent lanthanide aquo ions. I. Pr3+, Nd3+, Pm3+, Sm3+, Dy3+, Ho3+, Er3+, and Tm3+. J. Chem. Phys. 1968, 49, 4424–4442. [Google Scholar] [CrossRef]
- Srivastava, P.; Rai, S.B.; Rai, D.K. Optical properties of Sm3+ doped calibo glass with addition of lead oxide. Spectrochim. Acta A 2004, 60, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Ratnakaram, Y.C.; Thirupathi Naidu, D.; Chakradhar, R.P.S. Spectral studies of Sm3+ and Dy3+ doped lithium cesium mixed alkali borate glasses. J. Non-Cryst. Solids 2006, 352, 3914–3922. [Google Scholar] [CrossRef]
- Ratnakaram, Y.C.; Thirupathi Naidu, D.; Vijaya Kumar, A.; Gopal, N.O. Influence of mixed alkalies on absorption and emission properties of Sm3+ ions in borate glasses. Phys. B 2005, 358, 296–307. [Google Scholar] [CrossRef]
- Jamalaiah, B.C.; Vijaya Kumar, M.V.; Rama Gopal, K. Fluorescence properties and energy transfer mechanism of Sm3+ ion in lead telluroborate glasses. Opt. Mater. 2011, 33, 1643–1647. [Google Scholar] [CrossRef]
- Deopa, N.; Rao, A.S. Spectroscopic studies of Sm3+ ions activated lithium lead alumino borate glasses for visible luminescent device applications. Opt. Mater. 2017, 72, 31–39. [Google Scholar] [CrossRef]
- Mohan Babu, A.; Jamalaiah, B.C.; Sasikala, T.; Saleem, S.A.; Moorthy, L.R. Absorption and emission spectral studies of Sm3+-doped lead tungstate tellurite glasses. J. Alloys Compd. 2011, 509, 4743–4747. [Google Scholar] [CrossRef]
- Herrera, A.; Fernandes, R.G.; de Camargo, A.S.S.; Hernandes, A.C.; Buchner, S.; Jacinto, C.; Balzaretti, N.M. Visible–NIR emission and structural properties of Sm3+ doped heavy-metal oxide glass with composition B2O3–PbO–Bi2O3–GeO2. J. Lumin. 2016, 171, 106–111. [Google Scholar] [CrossRef]
- Praveena, R.; Venkatramu, V.; Babu, P.; Jayasankar, C.K. Fluorescence spectroscopy of Sm3+ ions in P2O5–PbO–Nb2O5 glasses. Phys. B 2008, 403, 3527–3534. [Google Scholar] [CrossRef]
- Suhasini, T.; Suresh Kumar, J.; Sasikala, T.; Jang, K.; Lee, H.S.; Jayasimhadri, M.; Jeong, J.H.; Yi, S.S.; Moorthy, L.R. Absorption and fluorescence properties of Sm3+ ions in fluoride containing phosphate glasses. Opt. Mater. 2009, 31, 1167–1172. [Google Scholar] [CrossRef]
- Rao, C.S.; Jayasankar, C.K. Spectroscopic and radiative properties of Sm3+-doped K–Mg–Al phosphate glasses. Opt. Commum. 2013, 286, 204–210. [Google Scholar]
- Kesavulu, C.R.; Jayasankar, C.K. Spectroscopic properties of Sm3+ ions in lead fluorophosphate glasses. J. Lumin. 2012, 132, 2802–2809. [Google Scholar] [CrossRef]
- Lim, K.-S.; Vijaya, N.; Kesavulu, C.R.; Jayasankar, C.K. Structural and luminescence properties of Sm3+ ions in zinc fluorophosphate glasses. Opt. Mater. 2013, 35, 1557–1563. [Google Scholar]
- Basavapoornima, C.; Jayasankar, C.K. Spectroscopic and photoluminescence properties of Sm3+ ions in Pb–K–Al–Na phosphate glasses for efficient visible lasers. J. Lumin. 2014, 153, 233–241. [Google Scholar] [CrossRef]
- Srihari, T.; Jayasankar, C.K. Spectral investigations of Sm3+-doped niobium phosphate glasses. Opt. Mater. 2017, 66, 35–42. [Google Scholar] [CrossRef]
- Bayoudhi, D.; Bouzidi, C.; Matei, E.; Secu, M.; Galca, A.C. Optical characterization of Sm3+ doped phosphate glasses for potential orange laser applications. J. Lumin. 2024, 265, 120204. [Google Scholar] [CrossRef]
- Goyal, P.; Sharma, Y.K.; Pal, S.; Bind, U.C.; Huang, S.-C.; Chung, S.-L. Structural, optical and physical analysis of B2O3–SiO2–Na2O–PbO–ZnO glass with Sm3+ ions for reddish–orange laser emission. J. Lumin. 2017, 192, 1227–1234. [Google Scholar] [CrossRef]
- Seshadri, M.; Rao, K.V.; Rao, J.L.; Ratnakaram, Y.C. Spectroscopic and laser properties of Sm3+ doped different phosphate glasses. J. Alloys Compd. 2009, 476, 263–270. [Google Scholar] [CrossRef]
- Damodaraiah, S.; Reddy Prasad, V.; Ratnakaram, Y.C. Structural and luminescence properties of Sm3+-doped bismuth phosphate glass for orange-red photonic applications. Luminescence 2018, 33, 594–603. [Google Scholar] [CrossRef]
- Chandana, G.; Nageswara Rao, C.; Vasudeva Rao, P.; Al-Musawi, M.J.S.; Samatha, K.; Dhar, G.G. Luminescent properties of Sm3+ doped metal fluoro phosphate glasses. Optik 2020, 208, 163909. [Google Scholar] [CrossRef]
- Sobczyk, M.; Starynowicz, P.; Lisiecki, R.; Ryba-Romanowski, W. Synthesis, optical spectra and radiative properties of Sm2O3:PbO:P2O5 glass materials. Opt. Mater. 2008, 30, 1571–1575. [Google Scholar] [CrossRef]
- Indhrapriyadarshini, A.; Naseer, K.A.; Komal Poojha, M.K.; Kebaili, I.; Marimuthu, K. Spectroscopic assessments on Sm3+ ions doped phospho-borate glasses mixed with fluoride modifiers for the applications of reddish-orange visible light. Spectrochim. Acta A 2025, 324, 124963. [Google Scholar] [CrossRef]
- Poolprasroed, S.; Pengpat, K.; Srichumpong, T.; Deechob, S.; Eitssayeam, S.; Intatha, U.; Kamnoy, M.; Chattrapiban, N.; Kantarak, E. Low-cost samarium-doped mixed alkali borosilicate glasses: Structural, thermal, and optical characterization for luminescent applications in art and decoration. J. Alloys Compd. 2025, 1043, 184167. [Google Scholar] [CrossRef]
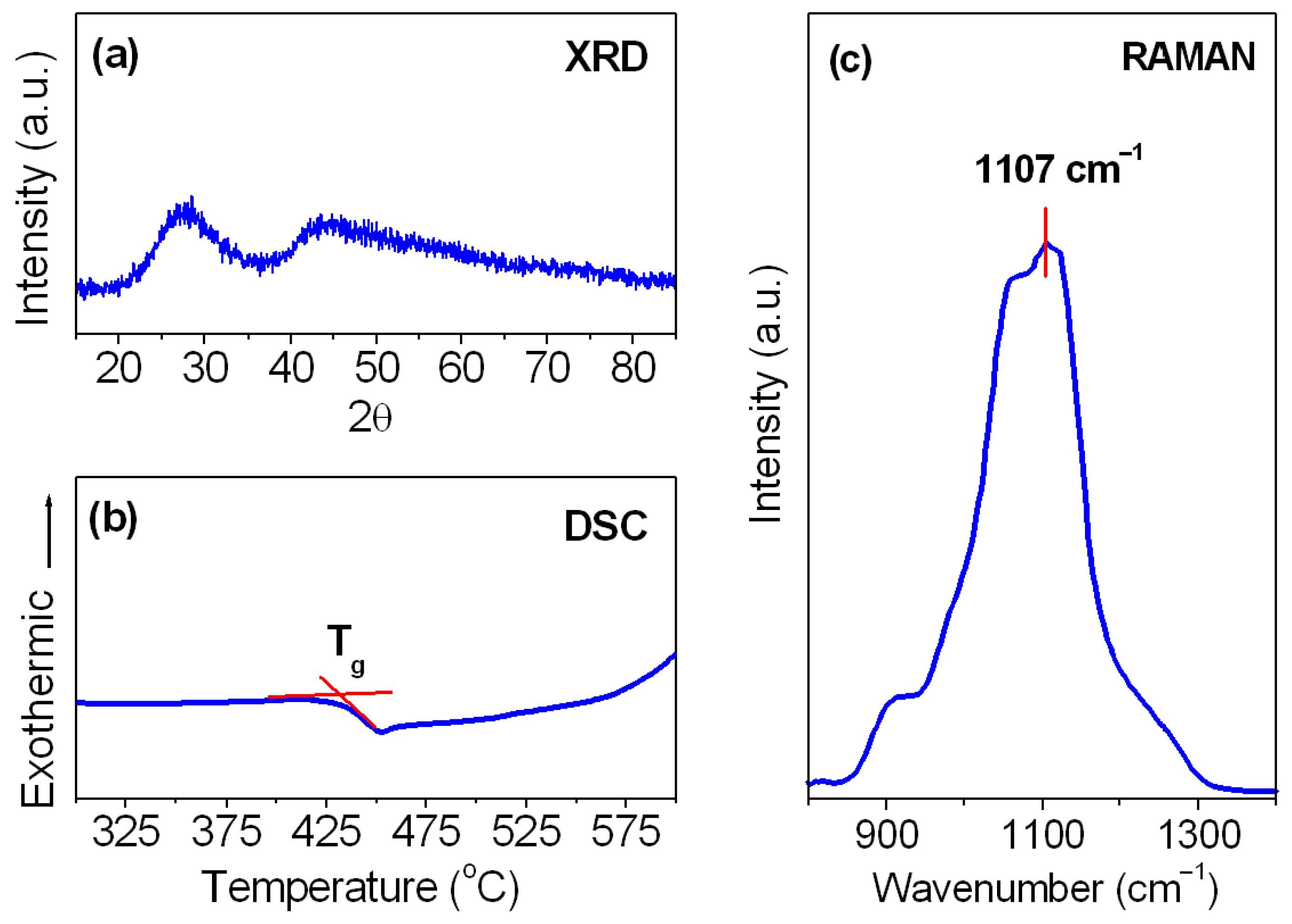
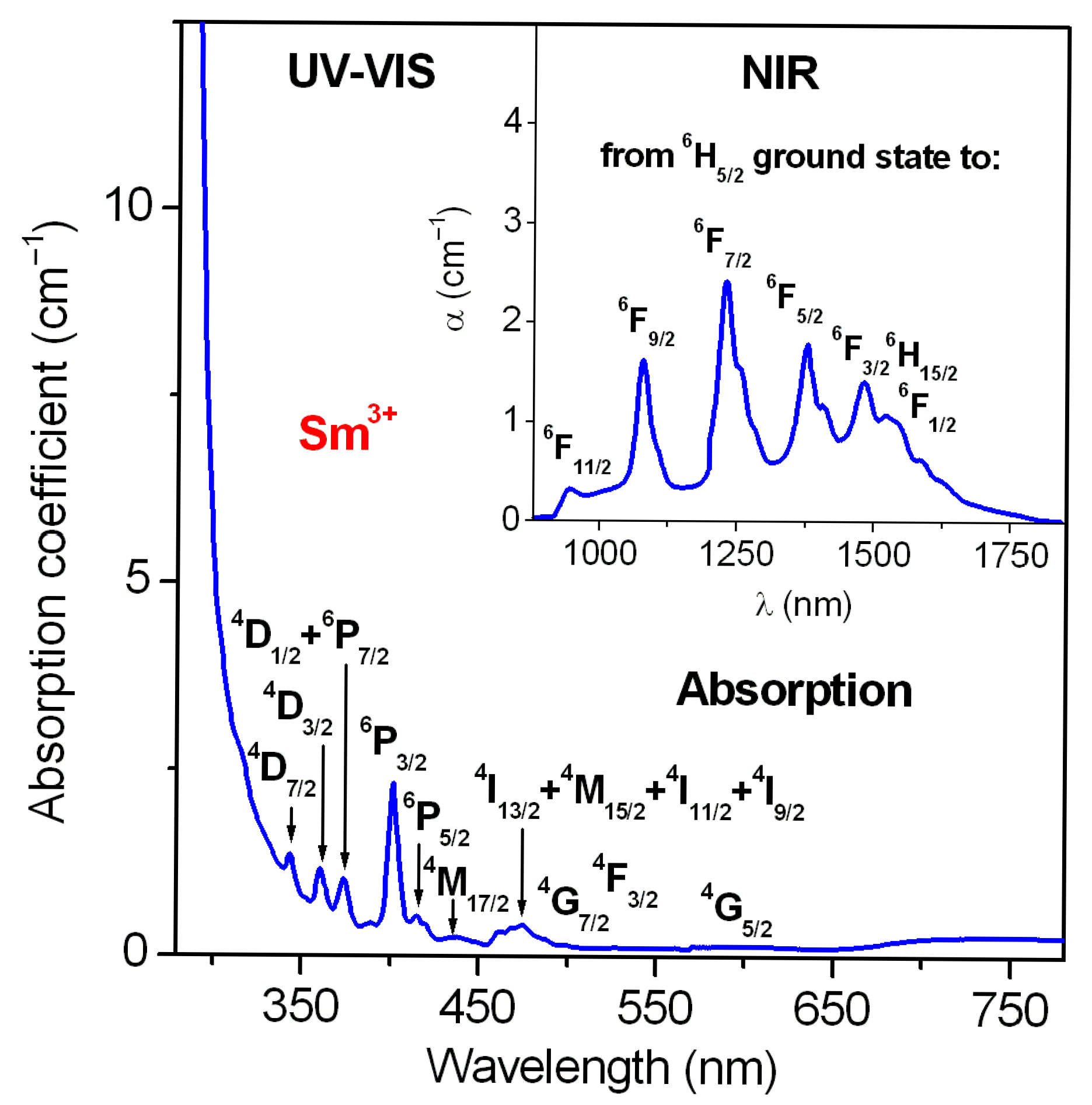
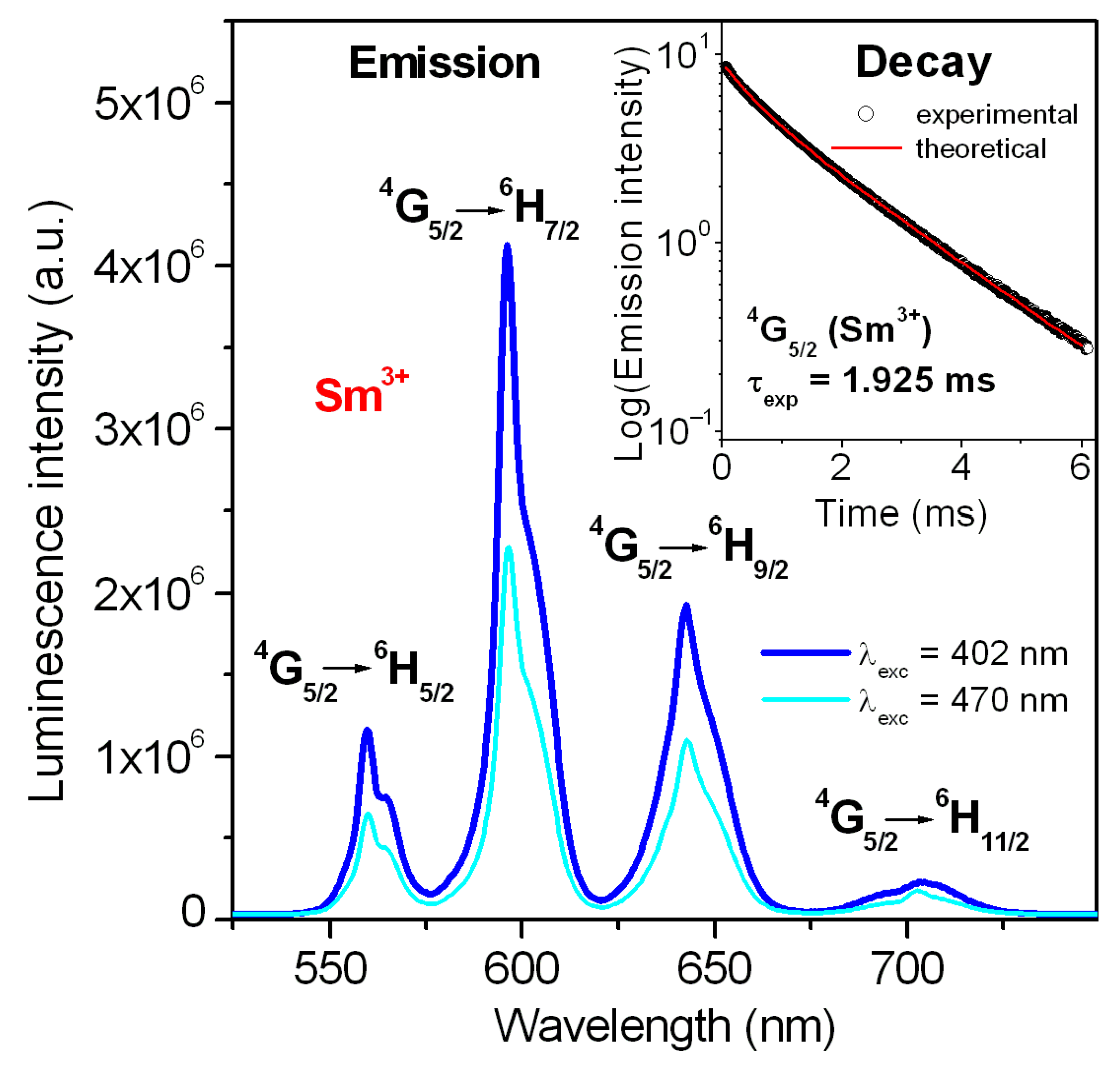

| Parameters | Lead Phosphate Glass |
|---|---|
| Chemical composition (molar%) | 45PbO-45P2O5-9.5Ga2O3-0.5Sm2O3 |
| Average molecular weight (M g mol−1) | 183.9 |
| Density (d g cm−3) | 5.12 |
| Activator (Sm3+) content (molar %) | 0.5 |
| Sm3+ ion concentration (N × 1020 ions cm−3) | 1.68 |
| Refractive index (n) | 1.75 |
| Phonon energy (hω cm−1) | 1107 |
| Glass transition temperature (Tg °C) | 435 |
| Level | Energy (cm−1) | Pmeas | Pcalc | Pmeas − Pcalc * |
|---|---|---|---|---|
| 6F1/2 | 6300 | 0.257 | 0.262 | 0.005 |
| 6F3/2 | 6750 | 0.880 | 0.877 | 0.003 |
| 6F5/2 | 7257 | 1.510 | 1.528 | 0.018 |
| 6F7/2 | 8130 | 3.950 | 3.905 | 0.045 |
| 6F9/2 | 9276 | 2.850 | 2.916 | 0.066 |
| 6F11/2 | 10,600 | 0.495 | 0.484 | 0.011 |
| Glass Host | Judd–Ofelt Parameters Ωt (in 10−20 cm2 Units) | Trend | Ref. | ||
|---|---|---|---|---|---|
| Ω2 | Ω4 | Ω6 | |||
| 45PbO-45P2O5-9.5Ga2O3-0.5Sm2O3 | 0.76 ± 0.19 | 2.43 ± 0.09 | 2.98 ± 0.05 | Ω2 < Ω4 < Ω6 | this work |
| 68H3BO3-30PbO-2Sm2O3 | 0.75 | 2.67 | 3.01 | Ω2 < Ω4 < Ω6 | [109] |
| 67H3BO3-12Li2CO3-20Cs2CO3-1Sm2O3 | 0.81 | 3.22 | 4.28 | Ω2 < Ω4 < Ω6 | [110] |
| 67B2O3-12Li2O-20K2O-1Sm2O3 | 0.88 | 3.93 | 4.66 | Ω2 < Ω4 < Ω6 | [111] |
| 67B2O3-12Li2O-20Na2O-1Sm2O3 | 0.77 | 6.39 | 7.09 | Ω2 < Ω4 < Ω6 | [111] |
| 67B2O3-12Na2O-20K2O-1Sm2O3 | 1.47 | 2.00 | 3.09 | Ω2 < Ω4 < Ω6 | [111] |
| 30PbF2-30TeO2-39H3BO3-1Sm2O3 | 0.21 | 1.42 | 1.8 | Ω2 < Ω4 < Ω6 | [112] |
| Transition | λ (nm) | AJ (s−1) * | β |
|---|---|---|---|
| 4G5/2 ⟶ 6F11/2 | 1460 | 0.34 | 0.11 |
| 4G5/2 ⟶ 6F19/2 | 1198 | 0.96 | 0.35 |
| 4G5/2 ⟶ 6F7/2 | 1038 | 2.60 | 0.94 |
| 4G5/2 ⟶ 6F5/2 | 953 | 6.82 | 2.48 |
| 4G5/2 ⟶ 6F3/2 | 908 | 0.82 | 0.30 |
| 4G5/2 ⟶ 6H15/2 | 902 | 0.50 | 0.18 |
| 4G5/2 ⟶ 6F1/2 | 893 | 0.66 | 0.24 |
| 4G5/2 ⟶ 6H13/2 | 795 | 7.16 | 2.60 |
| 4G5/2 ⟶ 6H11/2 | 710 | 32.87 | 11.93 |
| 4G5/2 ⟶ 6H9/2 | 648 | 70.47 | 25.58 |
| 4G5/2 ⟶ 6H7/2 | 596 | 145.84 | 52.94 |
| 4G5/2 ⟶ 6H5/2 | 560 | 6.46 | 2.35 |
| Glass Composition (mol%) | λp (nm) | FWHM (nm) | τexp (ms) | τrad (ms) | η (%) | σem (cm2 × 10−22) | Ref. |
|---|---|---|---|---|---|---|---|
| 45PbO-45P2O5-9.5Ga2O3-0.5Sm2O3 | 596 | 10.50 | 1.925 | 3.630 | 53 | 7.60 | this work |
| 10Li2O-10PbO-9Al2O3-70B2O3-1Sm2O3 | 597 | 10.59 | 0.966 | 1.600 | 60.4 | 7.23 | [113] |
| 15PbF2-59TeO2-25WO3-1Sm2O3 | 600 | - | 0.620 | 1.360 | 45.6 | 6.26 | [114] |
| 26.66B2O3-52.33PbO-16GeO2-4Bi2O3-1Sm2O3 | 601 | 15.03 | 1.392 | 2.580 | 54 | 6.08 | [115] |
| 55P2O5-39PbO-5Nb2O5-1Sm2O3 | 598 | 10.40 | 1.896 | 3.268 | 58 | 6.76 | [116] |
| 55P2O5-14K2O-6KF-15BaO-9Al2O3-1Sm2O3 | 597 | 12 | 2.400 | 4.290 | 56 | 5.92 | [117] |
| 58.5P2O5-15K2O-16.5MgO-9Al2O3-1Sm2O3 | 598 | 11.20 | 1.800 | 3.140 | 57 | 5.80 | [118] |
| 44P2O5-17K2O-9Al2O3-23PbF2-6Na2O-1Sm2O3 | 601 | 13.96 | 1.580 | 2.04 | 77 | 8.98 | [119] |
| 41P2O5-17K2O-8Al2O3-23ZnF2-10LiF-1Sm2O3 | 601 | 14.63 | 1.690 | 2.124 | 80 | 9.53 | [120] |
| 44P2O5-17K2O-9Al2O3-23PbO-6Na2O-1Sm2O3 | 598 | 11.20 | 1.570 | 1.880 | 83.5 | 11.50 | [121] |
| 44P2O5-17K2O-9Al2O3-29Nb2O5-1Sm2O3 | 602 | 15.07 | 1.175 | - | 98 | 11.52 | [122] |
| 45P2O5-45Na2O-2Al2O3-8BaO-0.5Sm2O3 | 600 | - | 2.400 | 2.710 | 88 | 12.36 | [123] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pisarska, J.; Pisarski, W.A. Samarium-Doped Lead Phosphate Glass: Optical Experiments and Calculations Using the Judd–Ofelt Theory. Materials 2025, 18, 5254. https://doi.org/10.3390/ma18225254
Pisarska J, Pisarski WA. Samarium-Doped Lead Phosphate Glass: Optical Experiments and Calculations Using the Judd–Ofelt Theory. Materials. 2025; 18(22):5254. https://doi.org/10.3390/ma18225254
Chicago/Turabian StylePisarska, Joanna, and Wojciech A. Pisarski. 2025. "Samarium-Doped Lead Phosphate Glass: Optical Experiments and Calculations Using the Judd–Ofelt Theory" Materials 18, no. 22: 5254. https://doi.org/10.3390/ma18225254
APA StylePisarska, J., & Pisarski, W. A. (2025). Samarium-Doped Lead Phosphate Glass: Optical Experiments and Calculations Using the Judd–Ofelt Theory. Materials, 18(22), 5254. https://doi.org/10.3390/ma18225254





