Non-Wettable Galvanic Coatings for Metal Protection: Insights from Nature-Inspired Solutions
Abstract
1. Introduction
2. Materials and Methods
3. Non-Wettable Surfaces
4. Superhydrophobic Coatings
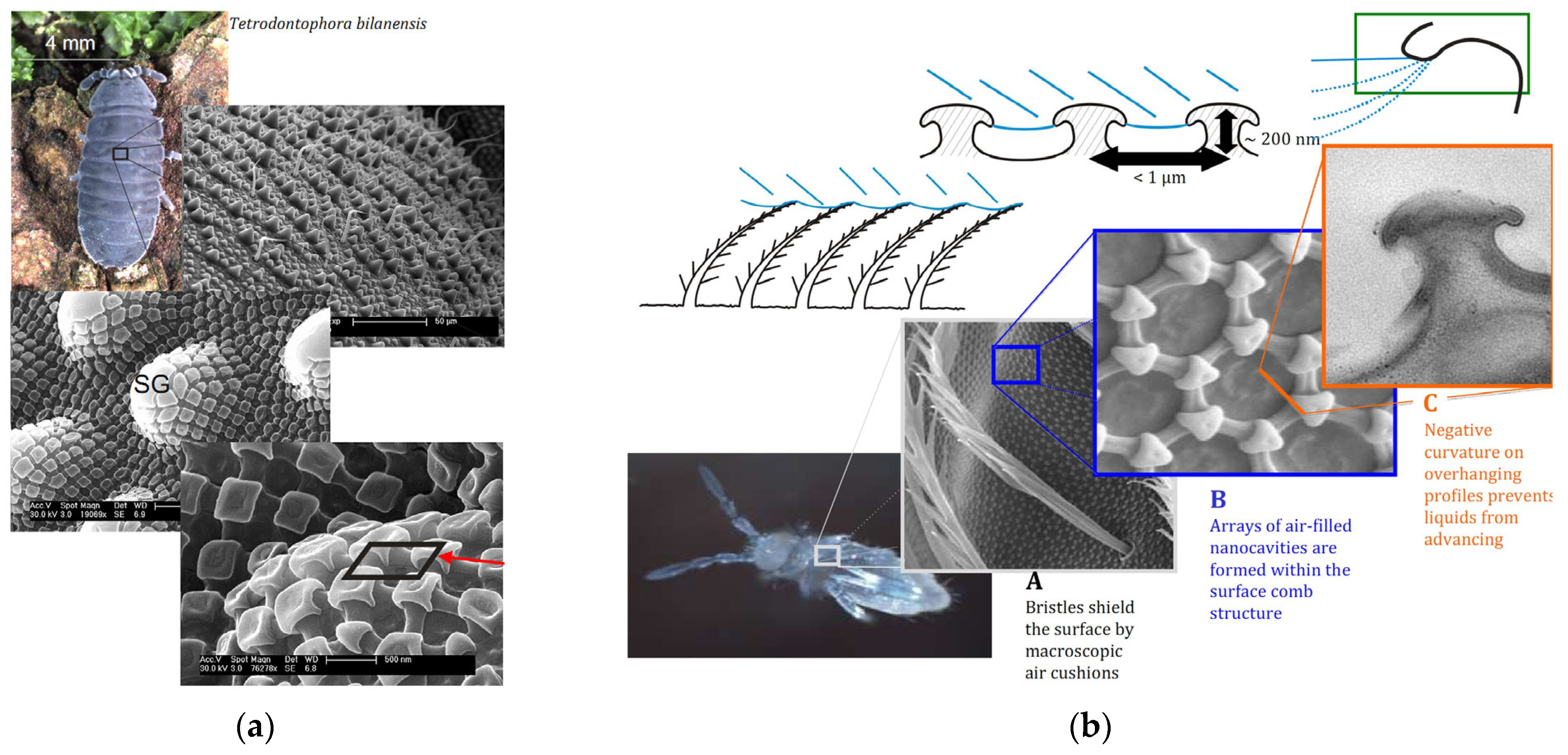
4.1. Nature Inspirations
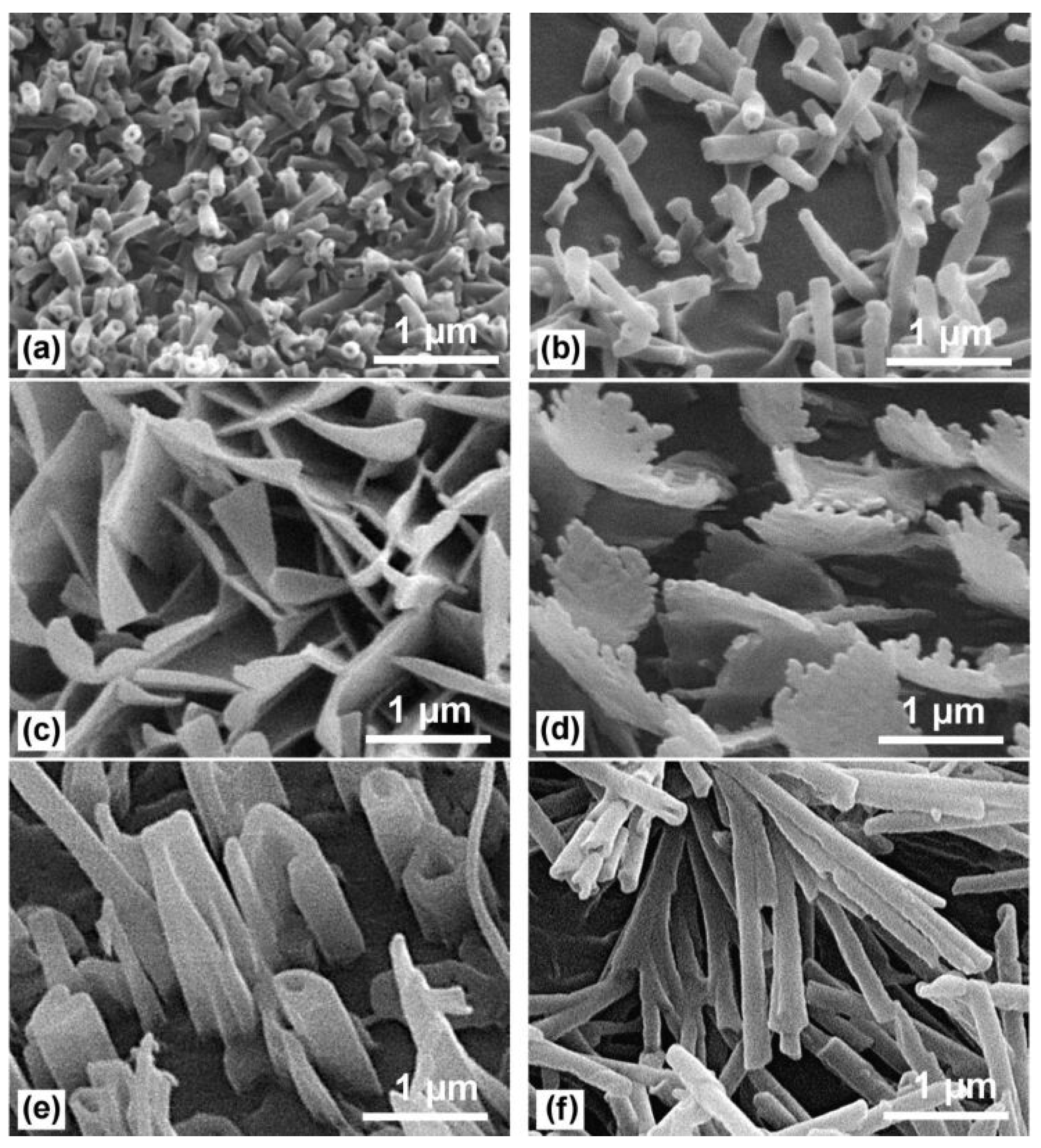
4.2. Electrodeposited Coatings
4.2.1. One-Step Electrolysis
| Hydrophobicity Drivers | Coating | Fabrication Technique | Surface Topography | WCA | Corrosion Resistance * | Ref. |
|---|---|---|---|---|---|---|
| One-step Electrolysis | ||||||
| surface roughness | Ni | chloride bath + C2H10Cl2N2 modifier galvanostatic deposition | hierarchical | 150–155° | −220 mV 10−9–10−8 A/cm2 | [54] |
| Ni | sulfate–ethanol bath + C12H24O2 modifier, galvanostatic deposition | hierarchical micro-flower | 168° | −550 mV 10−6 A/cm2 | [57] | |
| Cu-Mn | chloride bath + C14H28O2 modifier, galvanostatic deposition | hierarchical masonry-like | 161° | −450 mV 10−5 A/cm2 | [58] | |
| Zn-Ni | chloride–sulfate–gluconate bath, galvanostatic deposition, hydrogen-bubble template | porous cauliflower-like clusters | 152° | −1360 mV 10−7 A/cm2 | [59] | |
| molecules of low surface energy | Zn | acetate bath, potentiostatic deposition; modification with stearic acid | multiscale needle and branch-shaped fractal | 156–166° | −960 mV 10−5 A/cm2 | [60] |
| Ni | chloride bath, galvanostatic deposition; electrochemical modification with polysiloxane | microcones | 157° | −270 mV * 10−8 A/cm2 | [64] | |
| Ni | chloride bath, galvanostatic deposition; modification with myristic acid | hierarchical starfishes | 152–157° | −482 mV 10−8 A/cm2 | [65] | |
| Ni-Fe | chloride–sulfate–glycerol bath, galvanostatic deposition; modification with myristic acid | hierarchical Echinopsis multiplex (cactus)-like | 166° | −838 mV 10−6 A/cm2 | [68] | |
| oxide layer | Sn | chloride bath, potentiostatic deposition; post-annealing in air | porous tremella-like | 170° | one-year stability in air | [72] |
| Zn | acetate bath, potentiostatic deposition; post-annealing in air | willow-leaf-like | 170° | −240 mV 10−10 A/cm2 | [73] | |
| Two-step Electrolysis | ||||||
| surface roughness | Ni | chloride bath + C2H10Cl2N2 modifier; galvanostatic depositions | micro- and nanocones | 156° | −140 mV 10−6 A/cm2 | [77] |
| Ni-Zn | chloride bath + NH4Cl modifier; galvanostatic depositions | micro- and nanocones | 155° | −245 mV 10−6 A/cm2 | [78] | |
| molecules of low surface energy | Cu | sulfate bath, potentiostatic depositions; modification with stearic acid | hierarchical cauliflower-like | 160–164° | −220 mV 10−9–10−6 A/cm2 | [63] |
| Electrochemical Additive Manufacturing | ||||||
| surface roughness | Ni | chloride bath + NH4Cl modifier; galvanostatic scanning deposition | porous cauliflower-like clusters | 155° | stable properties in water (after 6 months) or hot air | [81] |
| Ni | chloride–sulfate bath + Ni nanoparticles; galvanostatic scanning deposition + magnetic field | porous cauliflower-like clusters | 155° | −240 mV 10−8 A/cm2 | [82] | |
| molecules of low surface energy | Cu | sulfate bath; galvanostatic jet deposition; modification with stearic acid | hierarchical cauliflower-like | 151° | −225 mV 10−5 A/cm2 | [83] |
4.2.2. Two-Step Electrolysis
4.2.3. Electrochemical Additive Manufacturing
4.2.4. Non-Aqueous Electrolytes
| Hydrophobicity Drivers | Coating | Fabrication Technique | Surface Topography | WCA | Corrosion Resistance * | Ref. |
|---|---|---|---|---|---|---|
| Conventional Solvent-based Baths | ||||||
| surface roughness of low surface energy | Ni | chloride–ethanol bath + myristic acid modifier; one-step galvanostatic deposition | hierarchical protrusions | 172° | −166 mV 10−9 A/cm2 | [80] |
| Mn | chloride-DMSO bath + stearic acid modifier; one-step galvanostatic deposition | porous cauliflower-like clusters | 154–159° | 106 Ω∙cm2 | [90] | |
| Deep Eutectic Solvent-based Baths | ||||||
| surface roughness of low surface energy | Zn | chloride–choline chloride–ethylene glycol bath + stearic acid modifier; one-step galvanostatic deposition | hierarchical ordered micro-slices and nanoconcaves | 165° | −920 mV 10−6 A/cm2 | [91] |
| Ni | chloride–choline chloride–ethylene glycol bath + stearic acid modifier; constant voltage deposition | hierarchical flowers, nanostrips or nanosheets | 162–166° | −710 mV 10−6 A/cm2 | [92] | |
| Cu | chloride–choline chloride–ethylene glycol bath + stearic acid modifier; one-step galvanostatic deposition | porous layered clusters | 158° | −180 mV 10−7 A/cm2 | [93] | |
| molecules of low surface energy | Cu | chloride–choline chloride– ethylene glycol bath; one-step galvanostatic deposition; modification with stearic acid | porous broccoli-like clusters | 152° | −230 mV 10−7 A/cm2 | [94] |
| Cu | chloride–choline chloride– ethylene glycol bath; one-step galvanostatic deposition; modification with stearic acid | flower-like clusters | 158° | −220 mV 10−7 A/cm2 | [95] | |
| Zn | chloride–choline chloride–ethylene glycol bath + thiourea modifier; one-step galvanostatic deposition; modification with polypropylene | spongy microscale network structure with tiny sheets | 170° | −934 mV 10−5 A/cm2 | [96] | |
5. Superhydrophobic-(Super)oleophobic Coatings
5.1. Nature Inspirations
5.2. Electrodeposited Coatings
6. Slippery Coatings
6.1. Nature Inspirations
6.2. Electrodeposited Coatings
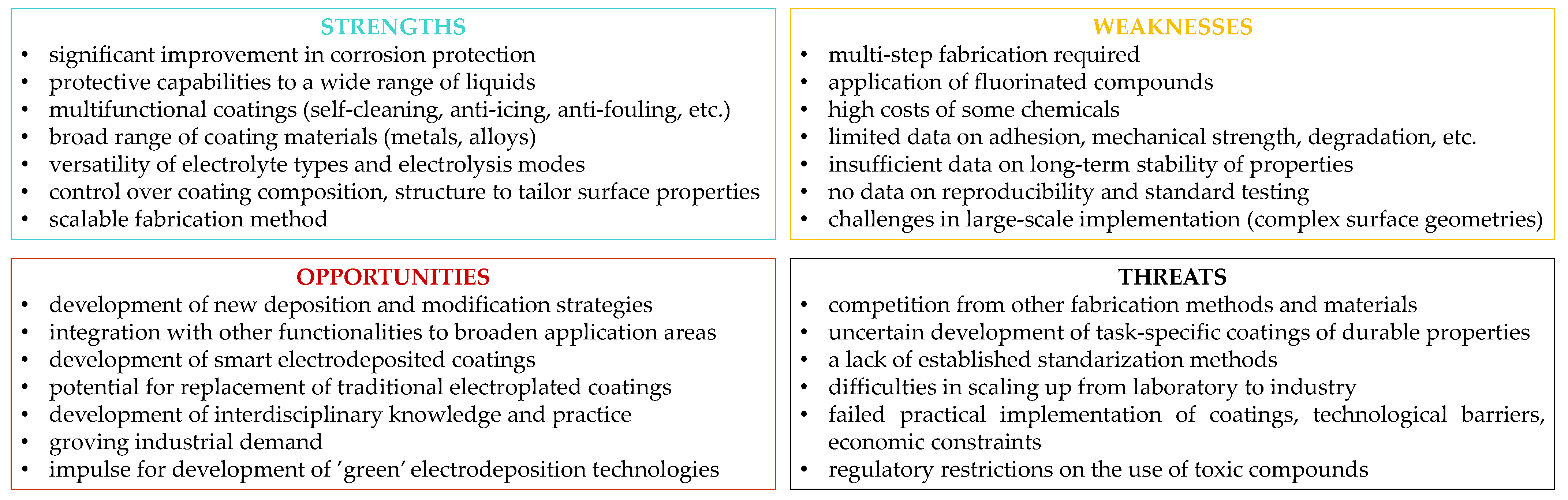
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Revie, W.R. (Ed.) Uhlig’s Corrosion Handbook, 3rd ed.; Revie, W.R. (Ed.) Wiley: Hoboken, NJ, USA, 2011. [Google Scholar]
- Koch, G.; Varney, J.; Thompson, N.; Moghissi, O.; Gould, M.; Payer, J. International Measures of Prevention, Application, and Economics of Corrosion Technologies Study; NACE International: Houston, TX, USA, 2016. [Google Scholar]
- Thompson, N.G.; Yunowich, M.; Dunmire, D. Cost of corrosion and corrosion maintenance strategies. Corr. Rev. 2007, 25, 247–261. [Google Scholar] [CrossRef]
- Hou, B.; Li, X.; Ma, X.; Du, C.; Zhang, D.; Zheng, D.; Xu, W.; Lu, D.; Ma, F. The cost of corrosion in China. Mater. Degrad. 2017, 1, 4. [Google Scholar] [CrossRef]
- Mazumder, M.A.J. Global impact of corrosion: Occurrence, cost and mitigation. Glob. J. Eng. Sci. 2020, 5, 1–5. [Google Scholar] [CrossRef]
- Petrović, Z.C. Catastrophes caused by corrosion. Milit. Techn. Courier 2016, 64, 1048–1064. [Google Scholar] [CrossRef]
- Valipour, N.; Birjandi, F.C.; Sargolzaei, J. Super-non-wettable surfaces: A review. Coll. Surf. A Physicochem. Eng. Aspects 2014, 448, 93–106. [Google Scholar] [CrossRef]
- Tam, J.; Palumbo, G.; Erb, U. Recent advances in superhydrophobic electrodeposits. Materials 2016, 9, 151. [Google Scholar] [CrossRef]
- Fihri, A.; Bovero, E.; Al-Shahrani, A.; Al-Ghamdi, A.; Alabedi, G. Recent progress in superhydrophobic coatings used for steel protection: A review. Coll. Surf. A Physicochem. Eng. Aspects 2017, 520, 378–390. [Google Scholar] [CrossRef]
- Vazirinasab, E.; Jafari, R.; Momen, G. Application of superhydrophobic coatings as a corrosion barrier: A review. Surf. Coat. Technol. 2018, 341, 40–56. [Google Scholar] [CrossRef]
- Mamagin, H.P.; Pati, P.R.; Samanta, K.K.; Brajpuriya, R.; Gupta, R.; Pandey, J.K.; Giri, J.; Sathish, T.; Kanan, M. A review on bio-ispired corrosion resistant superhydrophobic coating on copper substrate: Recent advances, mechanisms, constraints, and future prospects. Res. Eng. 2025, 25, 103863. [Google Scholar]
- Lian, Z.; Xu, J.; Wang, Z.; Yu, H. Biomimetic superlyophobic metallic surfaces: Focusing on their fabrication and applications. J. Bionic Eng. 2020, 17, 1–33. [Google Scholar] [CrossRef]
- Senez, V.; Thomy, V.; Dufor, R. Nanotechnologies for Synthetic Super Non-Wetting Surfaces; Wiley: Hoboken, NJ, USA, 2014. [Google Scholar]
- Mortazavi, V.; Khonsari, M.M. On the degradation of superhydrophobic surfaces: A review. Wear 2017, 372–373, 145–157. [Google Scholar] [CrossRef]
- Parvate, S.; Dixit, P.; Chattopadhyay, S. Superhydrophobic surfaces: Insights from theory and experiment. J. Phys. Chem. B 2020, 124, 1323–1360. [Google Scholar] [CrossRef] [PubMed]
- Yong, J.; Chen, F.; Yang, Q.; Huo, J.; Hou, X. Superoleophobic surfaces. Chem. Soc. Rev. 2017, 46, 4168–4217. [Google Scholar] [CrossRef] [PubMed]
- Chu, Z.; Seeger, S. Superamphiphobic surfaces. Chem. Soc. Rev. 2014, 43, 2784–2798. [Google Scholar] [CrossRef]
- Barthlott, W.; Mail, M.; Neinhuis, C. Superhydrophobic hierarchically structured surfaces in biology: Evolution, structural principles and biomimetic applications. Phil. Trans. R. Soc. A 2016, 374, 20160191. [Google Scholar] [CrossRef]
- Hensel, R.; Neinhuis, C.; Werner, C. The springtail cuticule as a blueprint for omniphobic surfaces. Chem. Soc. Rev. 2016, 45, 323–341. [Google Scholar] [CrossRef]
- Darmanin, T.; Guittard, F. Superhydrophobic and superoleophobic properties in nature. Mater. Today 2015, 18, 233–285. [Google Scholar] [CrossRef]
- Young, T. An essay on the cohesion of fluids. Philos. Trans. R. Soc. Lond. 1805, 95, 65–87. [Google Scholar]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Marmur, A. Wetting on hydrophobic rough surfaces: To be heterogeneous or not to be? Langmuir 2003, 19, 8343–8348. [Google Scholar] [CrossRef]
- Verho, T.; Korhonen, J.T.; Sainiemi, L.; Jokinen, V.; Bower, C.; Franze, K.; Franssila, S.; Andrew, P.; Ikkala, O.; Ras, R.H.A. Reversible switching between superhydrophobic states on a hierarchically structured surface. Proc. Nat. Acad. Sci. USA 2012, 109, 10210–10213. [Google Scholar] [CrossRef]
- Wong, T.S.; Kang, S.H.; Tang, S.K.Y.; Smythe, E.J.; Hatton, B.D.; Grinthal, A.; Aizenberg, J. Bioinspired self-repairing slippery surfaces with pressure-stable omniphobicity. Nature 2011, 477, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Bormashenko, E. Progress in understanding wetting transitions on rough surfaces. Adv. Coll. Interf. Sci. 2015, 222, 92–103. [Google Scholar] [CrossRef]
- Marmur, A. From hygrophilic to superhygrophobic: Theoretical conditions for making high-contact-angle surfaces from low-contact-angle materials. Langmuir 2008, 24, 7573–7579. [Google Scholar] [CrossRef]
- Tuteja, A.; Choi, W.; McKinley, G.H.; Cohen, R.E.; Rubner, M.F. Design parameters for superhydrophobicity and superoleophobicity. MRS Bull. 2008, 33, 752–758. [Google Scholar] [CrossRef]
- Kota, A.K.; Kwon, G.; Tuteja, A. The design and applications of superomniphobic surfaces. NPG Asia Mater. 2014, 6, e109. [Google Scholar] [CrossRef]
- Neinhuis, C.; Barthlott, W. Purity of the sacred lotus, or escape from contamination in biological surfaces. Planta 1997, 202, 1–8. [Google Scholar]
- Neinhuis, C.; Barthlott, W. Characterization and distribution of water-repellent, self-cleaning plant surfaces. Ann. Bot. 1997, 79, 667–677. [Google Scholar] [CrossRef]
- Ensikat, H.J.; Ditsche-Kuru, P.; Neinhuis, C.; Barthlott, W. Superhydrophobicity in perfection: The outstanding properties of the lotus leaf. Beilstein J. Nanotechnol. 2011, 2, 152–161. [Google Scholar] [CrossRef]
- Guo, Z.; Liu, W. Biomimic from the superhydrophobic plant leaves in nature: Binary structure and unitary structure. Plant Sci. 2007, 172, 1103–1112. [Google Scholar] [CrossRef]
- Kumar, M.; Bhardwaj, R. Wetting characteristics of Colocasia esculenta (Taro) leaf and a bioinspired surface tereof. Sci. Rep. 2020, 10, 935. [Google Scholar]
- Zhu, H.; Guo, Z. Wetting characterizations of oilseed rapes. J. Bionic Eng. 2016, 13, 213–219. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, Y.; Xi, J.; Zhu, Y.; Wang, N.; Xia, F.; Jiang, L. Petal effect: A superhydrophobic state with high adhesive force. Langmuir 2008, 24, 4114–4119. [Google Scholar] [CrossRef]
- Chakraborty, M.; Weibel, J.A.; Schaber, J.A.; Garimella, S.V. The wetting state of water on a rose petal. Adv. Mater. Interf. 2019, 6, 1900652. [Google Scholar] [CrossRef]
- Han, Z.; Fu, J.; Wang, Z.; Wang, Y.; Li, B.; Mu, z.; Zhang, J. Long-term durability of superhydrophobic properties of butterfly wing scales after continuous contact with water. Coll. Surf. A Physicochem. Eng. Aspects 2017, 518, 139–144. [Google Scholar] [CrossRef]
- Zheng, Y.; Gao, X.; Jiang, L. Directional adhesion of superhydrophobic butterfly wings. Soft Matter 2007, 3, 178–182. [Google Scholar] [CrossRef]
- Oh, J.; Dana, C.E.; Hong, S.; Roman, J.K.; Jo, K.D.; Hong, J.W.; Nguyen, J.; Cropel, D.M.; Alleyne, M.; Milijkovic, N. Exploring the role of habitat on the wettability of cicada wings. ACS Appl. Mater. Interf. 2017, 9, 27173–27184. [Google Scholar] [CrossRef]
- Sun, M.; Watson, G.S.; Zheng, Y.; Watson, J.A.; Liang, A. wetting properties on nanostructured surfaces of cicada wings. J. Experim. Biol. 2009, 212, 3148–3155. [Google Scholar] [CrossRef]
- Wei, P.J.; Chen, C.C.; Lin, J.F. Adhesion forces and contact angles of water strider legs. Langmuir 2009, 25, 1526–1528. [Google Scholar] [CrossRef]
- Watson, G.S.; Cribb, B.W.; Watson, J.A. Experimental determination of the efficiency of nanostructuring on non-wetting legs of the water strider. Acta Biomater. 2010, 6, 4060–4064. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Guo, Z.; Liu, W. Interfacial effects of superhydrophobic plant surfaces: A review. J. Bionic Eng. 2014, 11, 325–345. [Google Scholar] [CrossRef]
- Gou, X.; Guo, Z. Superhydrophobic leaves with micro-line structures: An optimal biomimetic objective in bionic engineering. J. Bionic Eng. 2018, 15, 851–858. [Google Scholar] [CrossRef]
- Koch, K.; Barthlott, W. Superhydrophobic and superhydrophilic plant surfaces: An inspiration for biomimetic materials. Phil. Trans. R. Soc. A 2009, 367, 1487–1509. [Google Scholar] [CrossRef]
- Barthlott, W.; Büdel, B.; Mail, M.; Neumann, K.M.; Bartels, D.; Fischer, E. Superhydrophobic terrestrial cyanobacteria and land plant transition. Front. Plant Sci. 2022, 13, 880439. [Google Scholar] [CrossRef]
- Holloway, J.P. Surface factors affecting the wetting of leaves. Pestic. Sci. 1970, 1, 156–163. [Google Scholar] [CrossRef]
- Barthlott, W.; Schimmel, T.; Wiersch, S.; Koch, K.; Brede, M.; Barczewski, M.; Walheim, S.; Weis, A.; Kaltenmaier, A.; Leder, A.; et al. The Salvinia paradox: Superhydrophobic surfaces with hydrophilic pins for air retention under water. Adv. Mater. 2010, 22, 2325–2328. [Google Scholar] [CrossRef]
- Bhushan, B.; Nosonovsky, M. The rose petal effect and the modes of superhydrophobicity. Phil. Trans. R. Soc. A 2010, 368, 4713–4728. [Google Scholar] [CrossRef]
- Gou, X.; Guo, Z. Superhydrophobic plant leaves: The variation in surface morphologies and wettability during the vegetation period. Langmuir 2019, 35, 1047–1053. [Google Scholar] [CrossRef]
- Rudnik, E.; Chat, K.; Włoch, G.; Osuch, P. Influence of chloride and sulfate ions on electrodeposition, wettability and corrosion resistance of zinc coatings produced from gluconate solutions. J. Electrochem. Soc. 2019, 166, D323–D332. [Google Scholar] [CrossRef]
- Farzaneh, A.; Asl, S.K.; Hosseini, M.G. Evaluation effect of electrodeposition parameters on superhydrophobicity and corrosion performance of nickel coatings. Prot. Met. Phys. Chem. Surf. 2017, 53, 88–93. [Google Scholar] [CrossRef]
- Chat-Wilk, K.; Rudnik, E.; Włoch, G. Effect of chloride and sulfate ions on electrodeposition and surface properties of alloys produced from zinc-nickel-manganese gluconate baths. J. Electrochem. Soc. 2022, 16, 092515. [Google Scholar] [CrossRef]
- Liu, P.; Cao, L.; Zhao, W.; Xia, Y.; Huang, W.; Li, Z. Insights into the superhydrophobicity of metallic surfaces prepared by electrodeposition involving spontaneous adsorption of airborne hydrocarbons. Appl. Surf. Sci. 2015, 324, 576–583. [Google Scholar] [CrossRef]
- Zhou, Z.; Ma, B.; Zhang, X.; Tang, L.; Lin, X.; Hu, C.; Ren, K. Enhanced corrosion resistance and self-cleaning properties of superhydrophobic nickel coating fabricated by one-step electrodeposition. Ceram. Int. 2023, 49, 13109–13118. [Google Scholar] [CrossRef]
- Tang, L.; Liu, X.; Zhang, X.; Yang, C.; Li, T.; Hu, C. Preparation of copper-manganese superhydrophobic coating with self-cleaning and anticorrosion resistance by one-step electrodeposition. Coll. Surf. A. Physicochem. Eng. Aspects 2024, 692, 134032. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.; Zhang, G. Hydrogen bubble-templated electrodeposition of superhydrophobic Zn-Ni films. New. J. Chem. 2023, 47, 844–851. [Google Scholar] [CrossRef]
- Jain, R.; Pitchumani, R. Fabrication and characterization of zinc-based superhydrophobic coatings. Surf. Coat. Technol. 2018, 337, 223–231. [Google Scholar] [CrossRef]
- Polyakov, N.A.; Botyrakova, I.G.; Glukhov, V.G.; Redkina, G.V.; Kuznetsov, Y.I. Formation and anticorrosion properties of superhydrophobic zinc coatings. Chem. Eng. J. 2021, 421, 127775. [Google Scholar] [CrossRef]
- Mousavi, S.M.A.; Pitchumani, R. A study of corrosion on electrodeposited superhydrophobic copper surfaces. Corr. Sci. 2021, 186, 109420. [Google Scholar] [CrossRef]
- Liang, J.; Li, D.; Wang, D.; Liu, K.; Chen, L. Preparation of stable superhydrophobic film on stainless steel substrate by combined approach using electrodeposition and fluorinated modification. Appl. Surf. Sci. 2014, 293, 265–270. [Google Scholar] [CrossRef]
- Song, Z.; Ding, L.; Zhang, S.; Zhao, S.; Zuo, L. The development of a porous nickel/polysiloxane superhydrophobic coating with long-time corrosion resistance properties. Surf. Coat. Technol. 2024, 493, 131296. [Google Scholar] [CrossRef]
- Xiang, T.; Ding, S.; Li, C.; Zheng, S.; Hu, W.; Wang, J.; Liu, P. Effect of current density on wettability and corrosion resistance of superhydrophobic nickel coating deposited on low carbon steel. Mat. Design 2017, 114, 65–72. [Google Scholar] [CrossRef]
- Brassard, J.D.; Sarkar, D.K.; Perron, J.; Audibert-Hayet, A.; Melot, D. Nano-micro structured zinc coating on steel for prevention of corrosion and ice adhesion. J. Coll. Interf. Sci. 2015, 447, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhang, D.; Qiu, R.; Hou, B. Superhydrophobic film prepared on zinc as corrosion barrier. Corr. Sci. 2011, 53, 2080–2086. [Google Scholar] [CrossRef]
- Tian, G.; Zhang, M.; Zhao, Y.; Li, J.; Wang, H.; Zhang, X.; Yan, H. High corrosion protection performance of a novel non-fluorinated biomimetic superhydrophobic Zn-Fe coating with Echinopsis multiplex-like structure. ACS Appl. Mater. Interf. 2019, 11, 38205–38217. [Google Scholar] [CrossRef]
- Li, H.; Yu, S.; Han, X.; Zhang, S.; Zhao, Y. A simple method for fabrication of bionic superhydrophobic zinc coating with crater-like structures on steel substrate. J. Bionic Eng. 2016, 13, 622–630. [Google Scholar] [CrossRef]
- Wang, L.; Guo, S.; Dong, S. Facile electrochemical route to directly fabricate hierarchical spherical cupreous microstructures: Toward superhydrophobic surface. Electrochem. Comm. 2008, 10, 655–658. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, H.; Chen, Y.; Chen, S.; Wang, D. Self-assembled super-hydrophobic multilayer films with corrosion resistance on copper substrate. RSC Adv. 2016, 6, 2379–2386. [Google Scholar] [CrossRef]
- He, G.; Lu, S.; Xu, W.; Yu, J.; Wu, B.; Cui, S. Fabrication of durable superhydrophobic electrodeposited tin surfaces with tremella-like structure on copper substrate. Surf. Coat. Technol. 2017, 309, 590–599. [Google Scholar] [CrossRef]
- He, G.; Lu, S.; Xu, W.; Szunerits, S.; Boukherroub, R.; Zhang, H. Controllable growth of durable superhydrophobic coatings on a copper substrate via electrodeposition. Phys. Chem. Chem. Phys. 2015, 17, 10871–10880. [Google Scholar] [CrossRef]
- Li, H.; Yu, S.; Hu, J.; Liu, E. A robust superhydrophobic coating with ZnO nanosheets on steel substrate and its self-cleaning property. Thin Solid Films 2018, 666, 100–107. [Google Scholar] [CrossRef]
- He, S.; Zheng, M.; Yao, L.; Yuan, X.; Li, M.; Ma, L.; Shen, W. Preparation and properties of ZnO nanostructures by electrochemical anodization method. Appl. Surf. Sci. 2010, 256, 255–2562. [Google Scholar] [CrossRef]
- He, G.; Lu, S.; Xu, W.; Ye, P.; Liu, G.; Wang, H.; Dai, T. Stable superhydrophobic Zn/ZnO surfaces fabricated via electrodeposition on tin substrate for self-cleaning behavior and switchable wettability. J. All. Compd. 2018, 747, 772–782. [Google Scholar] [CrossRef]
- Khorsand, S.; Raeissi, K.; Ashrafizadeh, F. Corrosion resistance and long-term durability of super-hydrophobic nickel film prepared by electrodeposition process. Appl. Surf. Sci. 2014, 305, 498–505. [Google Scholar] [CrossRef]
- Soleimangoli, F.; Hosseini, S.A.; Davoodi, A.; Mokhtari, A.; Alishahi, M. Effect of NH4Cl on the microstructure, wettability and corrosion behavior of electrodeposited Ni-Zn coatings with hierarchical nano/microstructure. Surf. Coat. Technol. 2020, 394, 125825. [Google Scholar] [CrossRef]
- Hang, T.; Hu, A.; Ling, H.; Li, M.; Mao, D. Super-hydrophobic nickel films with micro-nano hierarchical structure prepared by electrodeposition. Appl. Surf. Sci. 2010, 256, 2400–2404. [Google Scholar] [CrossRef]
- Daneshnia, A.; Raeissi, K.; Salehikahrizsangi, P.; Khorsand, S. A comparative study on the corrosion resistance and long-term stability of hierarchical superhydrophobic nickel coatings obtained by one-step and two-step electrodeposition processes. J. Mater. Eng. Perform. 2024. [Google Scholar] [CrossRef]
- Shen, L.; Fan, M.; Qiu, M.; Jiang, W.; Wang, Z. Superhydrophobic nickel coating fabricated by scanning electrodeposition. Appl. Surf. Sci. 2019, 483, 706–712. [Google Scholar] [CrossRef]
- Shen, L.; Xu, M.; Jiang, W.; Qiu, M.; Fan, M.; Ji, G.; Tian, Z. A novel superhydrophobic Ni/Nip coating fabricated by magnetic field induced selective scanning electrodeposition. Appl. Surf. Sci. 2019, 489, 25–33. [Google Scholar] [CrossRef]
- Jinsong, C.; Jian, G.; Mingbo, Q.; Jianming, Y.; Dazhi, H.; Xiaoli, W.; Yunfei, D. Preparation of copper-based superhydrophobic surfaces by jet-electrodeposition. Mater. Trans. 2018, 59, 793–798. [Google Scholar] [CrossRef]
- Wang, S.; Hou, C.; Wu, M.; Li, X.; Wang, W.; Mitsuzaki, N.; Chen, Z. Effect of choline chloride on electrodeposited superhydrophobic nickel film and the corrosion protection application. Coll. Surf. A Physicochem. Eng. Aspects 2021, 614, 126185. [Google Scholar] [CrossRef]
- Gu, W.; Jiang, Y. Electrochemical additive manufacturing of micro/nano functional materials. Mater. Today Sustain. 2024, 27, 100793. [Google Scholar]
- Simka, W.; Puszczyk, D.; Nawrat, G. Electrodeposition of metals from non-aqueous solutions. Electrochim. Acta 2009, 54, 5307–5319. [Google Scholar] [CrossRef]
- Wang, Q.; Yuan, R.; Yang, M.; Gao, W.; Jiao, S.; Tian, D.; Jiao, H.; Yu, H.; Sun, D. Exploring coating electrodeposition protocols from a cross-electrolyte and cross-metal perspective. J. Mater. Sci. Technol. 2025, 226, 122–134. [Google Scholar] [CrossRef]
- Chiorcea-Paquim, A.M.; Brett, C.M.A. Electrodeposition in deep eutectic solvents: Perspectives towards advanced corrosion protection. Appl. Mater. Today 2025, 44, 102746. [Google Scholar] [CrossRef]
- Chen, Z.; Hao, L.; Song, Q.; Chen, C. A rapid one-step process for fabrication of superhydrophobic surface by electrodeposition method. Electrochim. Acta 2012, 59, 168–171. [Google Scholar] [CrossRef]
- Zaffora, A.; Di Franco, F.; Megna, B.; Santamaria, M. One-step electrodeposition of superhydrophobic coating on 316L stainless steel. Metals 2021, 11, 1867. [Google Scholar] [CrossRef]
- Li, R.; Gao, Q.; Dong, Q.; Luo, C.; Sheng, L.; Liang, J. Template-free electrodeposition of ultra-high adhesive superhydrophobic Zn/Zn stearate coating with ordered hierarchical structure from deep eutectic solvent. Surf. Coat. Technol. 2020, 403, 126267. [Google Scholar] [CrossRef]
- Gu, C.; Tu, J. One-step fabrication of nanostructured Ni film with lotus effect from deep eutectic solvent. Langmuir 2011, 27, 10132–10140. [Google Scholar] [CrossRef]
- Kuang, Y.; Jiang, F.; Zhu, T.; Wu, H.; Yang, X.; Li, S.; Hu, C. One-step electrodeposition of superhydrophobic copper coating from ionic liquid. Mater. Lett. 2021, 303, 130579. [Google Scholar] [CrossRef]
- Jiang, F.; Zhu, T.; Kuang, Y.; Wu, H.; Li, S. Superhydrophobic copper coating with ultrahigh corrosion resistance by electrodeposition process in a deep eutectic solvent. Chem. Phys. Lett. 2023, 811, 140197. [Google Scholar] [CrossRef]
- Jiang, F.; Song, T.; Kuang, Y.; Wu, H. Facile fabrication of a flower-like superhydrophobic surface with superior corrosion resistance. Int. J. Mater. Res. 2024, 115, 540–545. [Google Scholar] [CrossRef]
- Zhang, X.; Liang, J.; Liu, B.; Peng, Z. Preparation of superhydrophobic zinc coating for corrosion protection. Coll. Surf. A Physicochem. Eng. Aspects 2014, 454, 113–118. [Google Scholar] [CrossRef]
- Helbig, R.; Nickerl, J.; Neinhuis, C.; Werner, C. Smart skin patterns protect springtails. PLoS ONE 2011, 6, e25105. [Google Scholar] [CrossRef]
- Gundersen, H.; Leinaas, H.P.; Thaulow, C. Surface structure and wetting characteristics of Collembola cuticules. PLoS ONE 2014, 9, e86783. [Google Scholar] [CrossRef]
- Schmüser, L.; Zhang, W.; Marx, M.T.; Encinas, N.; Vollmer, D.; Gorb, S.; Baio, J.E.; Räder, H.J.; Weidner, T. Role of surface chemistry in the superhydrophobicity of the springtail Orchesella cinta (Insecta: Collembola). ACS Appl. Mater. Interf. 2020, 12, 12294–12304. [Google Scholar] [CrossRef]
- Nickerl, J.; Tsurkan, M.; Hensel, R.; Nieinhuis, C.; Werner, C. The multi-layered protective cuticule of Collembola: A chemical analysis. J. R. Soc. Interf. 2014, 11, 20140619. [Google Scholar] [CrossRef]
- Bello, E.; Alleyne, M. Brochosome size variation and its influence on leafhopper (Hemitera: Cicadellidae) wing wettability. J. Insect Sci. 2024, 24, 6. [Google Scholar] [CrossRef]
- Rakitov, R.; Gorb, S.N. Brochosomal coats turn leafhopper (Insecta, Hemiptera, Cicadellidae) ingument to superhydrophobic state. Proc. R. Soc. B 2013, 280, 20122391. [Google Scholar] [CrossRef]
- Epstein, A.K.; Pokroy, B.; Seminara, A.; Aizenberg, J. Bacterial biofilm shows persistent resistance to liquid wetting and gas penetration. Proc. Natl. Acad. Sci. USA 2011, 108, 995–1000. [Google Scholar] [CrossRef]
- Werb, M.; Garcia, C.F.; Bach, N.C.; Grumbein, S.; Sieber, S.A.; Optiz, M.; Lieleg, O. Surface topology affects wetting behavior of Bactillus subtilis biofilms. NPJ Biofilms Microbiomes 2017, 3, 11. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wang, S.; Wei, Z.; Song, Y.; Jiang, L. Bioinspired design of a superoleophobic and low adhesive water/solid interface. Adv. Mater. 2009, 21, 665–669. [Google Scholar] [CrossRef]
- Waghmare, P.R.; Gunda, N.S.K.; Mitra, S.K. Under-water superoleophobicity of fish scales. Sci. Rep. 2014, 4, 7454. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Lin, L.; Xue, Z.; Liu, M.; Wang, S.; Jiang, L. Filefish-inspired surface design for anisotropic underwater oleophobicity. Adv. Funct. Mater. 2014, 24, 809–816. [Google Scholar] [CrossRef]
- Nosonovsky, M.; Bhushan, B. Why re-entrant surface topography is needed for robust oleophobicity. Phil Trans. R Soc A 2016, 374, 20160185. [Google Scholar] [CrossRef]
- Liu, Y.; Li, S.; Wang, Y.; Gao, K.; Han, Z.; Ren, L. Superhydrophobic and superoleophobic surface by electrodeposition on magnesium alloy substrate: Wettability and corrosion inhibition. J. Coll. Interf. Sci. 2016, 478, 164–171. [Google Scholar] [CrossRef]
- Qing, Y.; Yang, C.; Zhao, Q.; Hu, C.; Liu, C. Simple fabrication of superhydrophobic/superoleophobic surfaces on copper substrate by two-step method. J. Alloys Compd. 2017, 695, 1878–1883. [Google Scholar] [CrossRef]
- Alinezhadfar, M.; Abad, S.N.K.; Mozammel, M. Multifunctional cobalt coating with exeptional amphiphobic properties: Self-cleaning and corrosion inhibition. Surf. Interf. 2020, 21, 100744. [Google Scholar] [CrossRef]
- Bai, C.; Hu, C.; Zhang, X.; Zhang, W.; Ma, B.; Li, T. A rapid two-step method for fabrication of superhydrophobic-superoleophobic alloy coating with self-cleaning and anticorrosion properties. Coll. Surf. A Psicochem. Eng. Aspects 2022, 651, 129635. [Google Scholar] [CrossRef]
- Daneshnia, A.; Reaeissi, K.; Salehikahrizsangi, P. Rapid one-step electrodeposition of robust superhydrophobic and oleophobic Ni coating with anti-corrosion and selfcleaning properties. Surf. Coat. Technol. 2022, 450, 129007. [Google Scholar] [CrossRef]
- Daneshnia, A.; Reaeissi, K.; Salehikahrizsangi, P. Corrosion protection of superhydrophobic/amphiphobic cobalt coating with anti-icing and self-cleaning properties fabricated by a one-step electrodeposition method. J. Alloys Compd. 2023, 948, 169767. [Google Scholar] [CrossRef]
- Yu, S.; Li, H. Fabrication of superhydrophobic and oleophobic zinc coatings on steel surface. Mater. Sci. Technol. 2017, 33, 1290–1297. [Google Scholar] [CrossRef]
- Li, H.; Yu, S. A stable superamphiphobic Zn coating with self-cleaning property on steel surface fabricated via a deposition method. J. Taiwan Inst. Chem. Eng. 2016, 63, 411–420. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, J.; He, Y.; Wu, Y. Effect of temperature, current density, and deposition time on superhydrophobic and superoleophobic properties of Ni coating designed by laser-electrodeposition process. J. Mater. Eng. Perform. 2025. [Google Scholar] [CrossRef]
- Bauer, U.; Di Gusto, B.; Skeeper, J.; Grafe, T.U.; Federle, W. With a flick of the lid: A novel trapping mechanism in Nepenthes gracilis piter plants. PLoS ONE 2012, 7, e38951. [Google Scholar] [CrossRef]
- Ouyang, Y.; Zhao, J.; Qiu, R.; Hu, S.; Chen, M.; Wang, P. Liquid-infused superhydrophobic dendritic silver matrix: A bioinspired strategy to prohibit biofouling on titanium. Surf. Coat. Technol. 2019, 367, 148–155. [Google Scholar] [CrossRef]
- Ouyang, Y.; Zhao, J.; Qiu, R.; Hu, S.; Niu, H.; Chen, M.; Wang, P. Biomimetics leading to liquid-infused surface based on vertical dendritic Co matrix: A barrier to inhibit bioadhesion and microbiologically induced corrosion. Coll. Surf. A 2019, 583, 124006. [Google Scholar] [CrossRef]
- Mousavi, S.M.A.; Pitchumani, R. Bioinspired nonwetting surfaces for corrosion inhibition over a range of temperature and corrosivity. J. Coll. Interf. Sci. 2022, 607, 323–333. [Google Scholar] [CrossRef]
- Stoddard, R.; Nithyanandam, K.; Pitchumani, R. Fabrication and durability characterization of superhydrophobic and lubricant-infused surfaces. J. Coll. Interf. Sci. 2022, 608, 662–672. [Google Scholar] [CrossRef]
- Xiang, T.; Liu, J.; Liu, Q.; Wei, F.; Lv, Z.; Yang, Y.; Shi, L.P.; Li, C.; Cheng, D.; Xu, G. Self-healing solid slippery surface with porous structure and enhanced corrosion resistance. Chem. Eng. J. 2021, 417, 128083. [Google Scholar] [CrossRef]
- Qiu, Z.; Qiu, R.; Xiao, Y.; Zheng, J.; Lin, C. Slippery liquid-infused porous surface fabricated on CuZn: A barrier to abiotic seawater corrosion and microbiologically induced corrosion. Appl. Surf. Sci. 2018, 457, 468–476. [Google Scholar] [CrossRef]
- Quyang, Y.; Zhao, J.; Qiu, R.; Shi, Z.; Hu, Z.; Hu, S.; Zhang, Y.; Chen, M. Liquid infused surface based on hierarchical dendritic iron wire array: An exceptional barrier to prohibit biofouling and biocorrosion. Prog. Organ. Coat. 2019, 136, 105216. [Google Scholar]
- Qiu, R.; Zhang, Q.; Wang, P.; Jiang, L.; Hou, J.; Guo, W.; Zhang, H. Fabrication of slippery liquid-infused porous surface based on carbon fiber with enhanced corrosion inhibition property. Coll. Surf. A Physicochem. Eng. Aspects 2014, 453, 132–141. [Google Scholar] [CrossRef]
- Hussain, M.M.; Kunwar, A.; Majeed, M.K.; Wang, Y.; Saleem, A.; Ma, H. Superhydrophobic surface and lubricant-infused surface: Implementing two extremes on electrodeposited Ni-TiO2 surface to drive optimal wettability regimes for droplets’ multifunctional behaviors. Adv. Eng. Mater. 2021, 23, 2100266. [Google Scholar] [CrossRef]
- Nezhad, A.H.; Davoodi, A.; Zahrani, E.M.; Arefinia, R. The effects of an inorganic corrosion inhibitor on the electrochemical behavior of superhydrophobic micro-nano structured Ni films in 3.5% NaCl solution. Surf. Coat. Technol. 2020, 395, 125946. [Google Scholar] [CrossRef]
- Tripathi, D.; Ray, P.; Singh, A.V.; Kishore, V.; Singh, S.L. Durability of slippery liquid-infused surfaces: Challenges and advances. Coatings 2023, 13, 1093. [Google Scholar] [CrossRef]
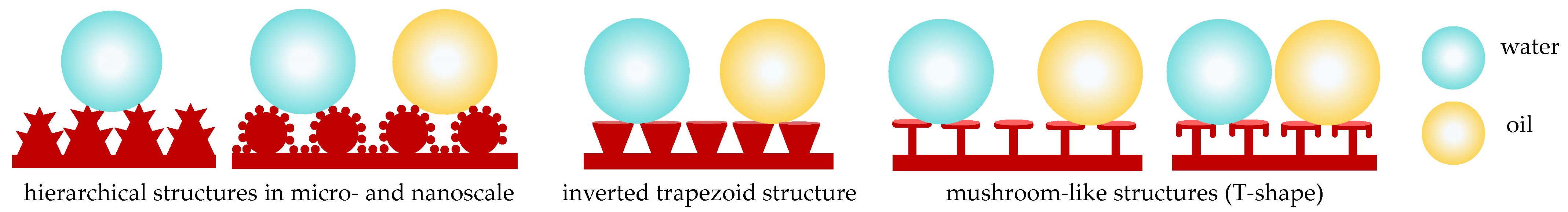


| Model | Liquid Droplet | Contact Angle | Remarks | Ref. |
|---|---|---|---|---|
| Young | 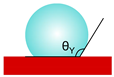 | cosθY = (γSG − γSL)/γLG θY—contact angle γ—surface free energy (or surface tension) | a liquid droplet on a flat surface; an equilibrium between superficial energies at the solid–liquid–gas interface | [21] |
| Wenzel | 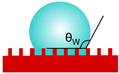 | cosθW = r·cosθY r—roughness factor | a liquid droplet remains in contact with peaks and valleys of the rough surface (homogenous wetting) | [22] |
| Cassie–Baxter | 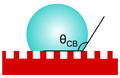 | cosθCB = fs·cosθY + fs − 1 fs—area fraction of solid phase of the rough surface | a liquid droplet is suspended by the surface peaks and does not penetrate the valleys occupied with air (heterogeneous wetting) | [23] |
| Marmur | 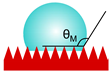 | cosθM = rf·fs·cosθY + fs − 1 rf—roughness of the solid that touches the liquid fs—area fraction of solid phase of the rough surface | a liquid droplet partially wets the surface and partially sits on the air pockets (heterogeneous wetting) | [24] |
| Cassie-type states |  micro-Cassie state | micro-Cassie state: the plastron of microscale dimensions occupies the space between microposts | reversible, localized, and instantaneous transition between two Cassie wetting states facilitated by two-level topography of a superhydrophobic surface | [25] |
 nano-Cassie state | nano-Cassie state: the space between the posts is mostly filled with water, but air remains in the nanofilament layer (hundreds of nanometers thick) | |||
| SLIPS | 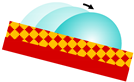 | r·(γB·cosθB − γA·cosθA) − γAB > 0 r·(γB·cosθB − γA·cosθA) + γA − γB > 0 A—repelled liquid B—lubricating fluid AB—liquid–liquid interface | nano/microstructured porous surface that retains a lubricating fluid, forming stable, smooth, and omniphobic slippery interface | [26] |
| Species | WCA | Surface Structure | Ref. |
|---|---|---|---|
| Plant Leaves | |||
| Lotus Nelumbo nucifiera | 160–162° | papillae covered with dense wax tubules | [31,32,33,34] |
| Taro Colocasia esculenta | 150–165° | hierarchical honeycomb-like microstructures | [32,35] |
| Spurge Euphorbia myrsinites | 162° | microsized papillae covered with nanosized platelet wax | [32,33] |
| Rice Oryza sativa * | 157–162° | papillae (arranged parallely) covered with dense nanopins | [32,34] |
| Tulip Tulipa praestans | 160° | convex epidermis covered with dense wax tubules | [32] |
| Canna Canna glauca | 159–165° | papillae covered with platelet wax | [32,34] |
| Nasturtium Tropaeolum majus | 160° | convex epidermis covered with tiny clumps of waxy tubes | [32] |
| Ramee Boehmeria nivea | 164° | unitary structure of micrometer fibers (rear leaf side) | [34] |
| Flower Petals | |||
| Oilseed rape Brassica campestris L. | 154–155° | interlocking intestine-like microstructures on both petal sides with carotenoid pigment in upper epidermis cells | [36] |
| Rose Rosa rubiginosa ** | 151–152° | tight-packed periodic arrays of microscale bumps with nanoscale striae (wrinkled folds) on the top of each micropapilla | [37,38] |
| Insects | |||
| Butterfly wing * | 151–152° | hierarchical structure composed of overlapping scales with longitudinal ridges and nanoscale grooves | [39,40] |
| Cicada wing | 140–164° | a nanopillar array structure, with regularly spaced, cone-shaped protrusions | [41,42] |
| Water strider leg * | 168–170° | hierarchical needle-like microsetae decorated with nanoscale grooves | [43,44] |
| Amphiphobicity Drivers | Coating | Fabrication Technique | Surface Topography | WCA OCA | Corrosion Resistance * | Ref. |
|---|---|---|---|---|---|---|
| One-step Electrolysis | ||||||
| surface roughness + molecules of low surface energy | Ni | chloride bath; galvanostatic deposition; modification with perfluorooctanoic acid | hierarchical cauliflower-like clusters | 160° 152° | −800 mV 10−6 A/cm2 | [109] |
| Zn | sulfate bath; galvanostatic deposition; modification with stearic acid; modification with TiO2/FAS | hierarchical protrusions | 162° 152° | no data | [110] | |
| Co | sulfate bath; galvanostatic deposition; activation in ethanol–hexane mixture; modification with trichloro perfluorooctyl silane | micro-scaled pyramids with nano-scaled fur-like structures | 161° 141° | 10−7 A/cm2 stable properties in air for 6 months | [111] | |
| Ni-Cu | sulfate bath; galvanostatic deposition; modification with perfluorodecyltrimethoxysilane | pagoda-like micro/nano structures | 163° 155° | −250 mV 10−6 A/cm2 | [112] | |
| surface roughness of low surface energy | Ni | chloride–absolute ethanol bath + myristic acid modifier | mushroom-like structures | 172° 160° | stable properties after 5 months in air | [113] |
| Co | chloride–absolute ethanol bath + myristic acid modifier | sedum-like structures | 172° 160° | −200 mV 10−7 A/cm2 | [114] | |
| Two-step Electrolysis | ||||||
| surface roughness + molecules of low surface energy | Zn | sulfate bath; galvanostatic depositions; modification with pentadecafluorooctanoic acid | concave structures on hexagonal crystals | 153° 149° | stable properties in air for 120 days | [115] |
| Zn | sulfate bath; galvanostatic depositions; modification with perfluorooctanoic acid | hierarchical structure with grooves | 155° 154° | stable properties in air for 6 months, in NaCl solution | [116] | |
| Ni | laser ablation; sulfate bath + ethylenediamine modifier; galvanostatic depositions; modification with perfluorooctanoic acid | hierarchical micro- and nanocones | 161° 153° | no data | [117] | |
| Coating | Fabrication Technique | Surface Topography | WCA TA | Corrosion Resistance * | Ref. |
|---|---|---|---|---|---|
| Ag | nitrate–ammonia bath; galvanostatic deposition; modification with dodecanethiol; dimethyl silicone oil lubricant | hierarchical dendrites | no data 8° | no data | [119] |
| Co | chloride-sulfate bath; potentiostatic deposition; chemical oxidation; modification with dopamine; modification with dodecanethiol; dimethyl silicone oil lubricant | hierarchical dendrites | 96° 6° | −220 mV 10−6 A/cm2 | [120] |
| Cu | sulfate bath; two-step potentiostatic deposition; chemical oxidation; modification with stearic acid; silicone oil lubricant | hierarchical cauliflower-like clusters | 93° 3° | −220 mV 10−7–10−6 A/cm2 | [121] |
| Cu | sulfate bath; two-step deposition: potentiostatic–galvanostatic; modification with n-hexa methyl mercaptan; perfluoroether oil lubricant | boulder-like, needle-like or cauliflower-like | 120° 2° ** | stabile properties for 1.5-years | [122] |
| Ni | chloride bath; two-step galvanostatic deposition, hydrogen-bubble template; modification with myristic acid; paraffin lubricant | hierarchical cauliflower-like clusters | 108/40° *** 33/7° | 105 Ω∙cm2 | [123] |
| Cu | chloride bath; potentiostatic Cu deposition; anodic oxidation; modification with dodecanethiol; perfluorinated lubricant | nanoscale bundle clusters | no data 11° | −220 mV 10−4 A/cm2 | [124] |
| Fe | sulfate bath; potentiostatic deposition; chemical oxidation; modification with dopamine; modification with dodecanethiol; deep eutectic solvent lubricant | dendritic wire clusters with ravine-like gaps | 82° 8° | −260 mV 10−3 A/cm2 | [125] |
| Cu | sulfate bath; potentiostatic deposition; modification with carbon fibers; perfluorinated lubricant | sponge-like | no data 5° | 10−7A/cm2 | [126] |
| Ni/TiO2 | Watts bath; constant voltage deposition; modification with myristic acid; perfluorinated lubricant | hierarchical flower-like | 118° 4° | 200 mV 10−8 A/cm2 | [127] |
| Microorganism | Contact Time | Bare Surface | Slippery Surface | Ref. |
|---|---|---|---|---|
| Diatom Navicula minima | 3 days | Ti: 1.4 × 1010 Ti/SHC: 4.1 × 108 | Ti/SC: 6.6 × 106 | [119] |
| 14 days | Ti: 1.6 × 1011 Ti/SHC: 2.5 × 109 | Ti/SC: 6.8 × 107 | ||
| Green algae Chlorella vulgaris | 7 days | Ti: 7.7 × 1010 Ti/SHC: 5.5 × 108 | Ti/SC: 9.9 × 106 | |
| 14 days | Ti: 1.6 × 1011 Ti/SHC: 5.6 × 109 | Ti/SC: 5.0 × 107 | ||
| Sulfate-reducing bacteria culture | 3 days | Cu: 1.3 × 1010 | Cu/SC: 1.3 × 109 | [120] |
| 24 h | CuZn: 3.4 × 106 | CuZn/SC: 2.9 × 105 | [124] | |
| Diatom Navicula minima | 14 days | Cu: 2.0 × 1011 | Cu/SC: 2.0 × 107 | [125] |
| Green algae Chlorella vulgaris | 14 days | no data | Cu/SC: 3.1 × 107 | |
| Sulfate-reducing bacteria culture | 7 days | Cu: 3.0 × 1010 | Cu/SC: 4.0 × 106 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rudnik, E. Non-Wettable Galvanic Coatings for Metal Protection: Insights from Nature-Inspired Solutions. Materials 2025, 18, 2890. https://doi.org/10.3390/ma18122890
Rudnik E. Non-Wettable Galvanic Coatings for Metal Protection: Insights from Nature-Inspired Solutions. Materials. 2025; 18(12):2890. https://doi.org/10.3390/ma18122890
Chicago/Turabian StyleRudnik, Ewa. 2025. "Non-Wettable Galvanic Coatings for Metal Protection: Insights from Nature-Inspired Solutions" Materials 18, no. 12: 2890. https://doi.org/10.3390/ma18122890
APA StyleRudnik, E. (2025). Non-Wettable Galvanic Coatings for Metal Protection: Insights from Nature-Inspired Solutions. Materials, 18(12), 2890. https://doi.org/10.3390/ma18122890






