Wood Biomass Ash (WBA) Used in Conjunction with Post-Fermentation Mass (PFM) as a Way to Stabilize Soil Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Assumptions
2.2. Characteristics of the Initial Soil and Materials (WBA, ULF, SSF and SLF)
2.3. Description of Analytical Methods
2.4. Description of Statistical Methods
3. Results
3.1. Reaction of Soil
3.2. Content of TC, TN of Soil and Ratio TC/TN
3.3. Soil Sorption Complex
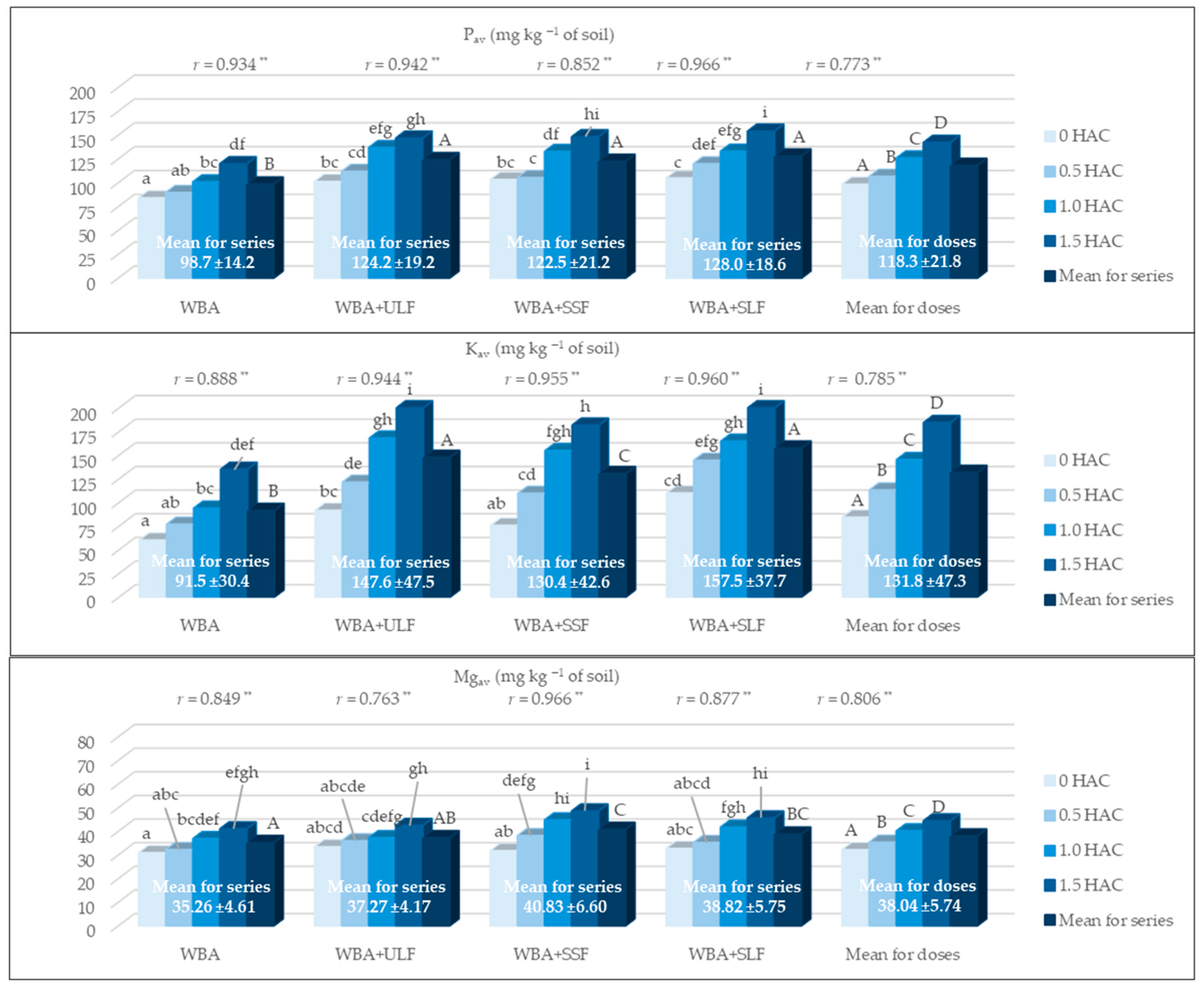
3.4. Content of Available Forms of Macronutrients (Pav, Kav i Mgav)
3.5. Soil Salinity
3.6. Interrelationships Between the Described Features
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| WBA | woody biomass ash |
| PFM | post-fermentation mass |
| ULF | unseparated liquid post-fermentation mass |
| SSF | separated solid post-fermentation mass |
| SLF | separated liquid post-fermentation mass |
References
- Ministry of Climate and Environment. Regulation of the Minister of Climate of 18 January 2020 on the waste catalogue. J. Laws 2020, 10, 1–48. [Google Scholar]
- Demeyer, A.; Voundi Nkana, J.C.; Verloo, M.G. Characteristics of wood ash and influence on soil properties and nutrient uptake: An overview. Bioresour. Technol. 2001, 77, 287–295. [Google Scholar] [CrossRef]
- Odzijewicz, J.I.; Wołejko, E.; Wydro, U.; Wasil, M.; Jabłońska-Trypuć, A. Utilization of ashes from biomass combustion. Energies 2022, 15, 9653. [Google Scholar] [CrossRef]
- Ike, M.; Kawagoe, H.; Oshita, K.; Takaoka, M. Detailed estimation of generated woody biomass ash for use as fertilizer material. Waste Manag. 2025, 195, 275–283. [Google Scholar] [CrossRef]
- Ministry of Agriculture and Rural Development. Act on Fertilisers and Fertilisation of 10 July 2007. J. Laws 2007, 147, 1033. [Google Scholar]
- Ministry of Agriculture and Rural Development. Regulation of the Minister of Agriculture and Rural Development of 18 June 2008 on the implementation of certain provisions of the Act on fertilisers and fertilisation. J. Laws 2008, 119, 765. [Google Scholar]
- Ministry of Agriculture and Rural Development. Regulation of the Minister of Agriculture and Rural Development of October 12, 2023 on a detailed list of substrates that can be used in agricultural biogas plants. J. Laws 2023, 2230. Available online: https://eli.gov.pl/eli/DU/2023/2230/ogl (accessed on 11 November 2025).
- Witorożec-Piechnik, A.; Kopiński, J.; Markowska-Strzemska, E.; Woźniak, M. Environmental safety aspects of using the digestate from an agricultural biogas plant. Pol. J. Agron. 2023, 52, 54–61. [Google Scholar] [CrossRef]
- Ragályi, P.; Szécsy, O.; Uzinger, N.; Magyar, M.; Szabó, A.; Rékási, M. Factors influencing the impact of anaerobic digestates on soil properties. Soil Syst. 2025, 9, 78. [Google Scholar] [CrossRef]
- Rolka, E.; Żołnowski, A.C.; Wyszkowski, M.; Zych, W.; Skorwider–Namiotko, A. Wood biomass ash (WBA) from the heat production process as a mineral amendment for improving selected soil properties. Energies 2023, 16, 5110. [Google Scholar] [CrossRef]
- Rolka, E.; Wyszkowski, M.; Żołnowski, A.C.; Skorwider-Namiotko, A.; Szostek, R.; Wyżlic, K.; Borowski, M. Digestate from an agricultural biogas plant as a factor shaping soil properties. Agronomy 2024, 14, 1528. [Google Scholar] [CrossRef]
- Abdulraheem, M.I. Effects of ash on soil properties and yield of crops. Agric. Obser. 2020, 1, 61–66. [Google Scholar]
- Symanowicz, B.; Becher, M.; Jaremko, D.; Skwarek, K. Possibilities for the use of wood ashes in agriculture. J. Ecol. Eng. 2018, 19, 191–196. [Google Scholar] [CrossRef]
- Romanowska-Duda, Z.; Janas, R.; Grzesik, M. Increasing fertilization efficiency of biomass ash by the synergistically acting digestate and extract from water plants sequestering CO2 in sorghum crops. Molecules 2024, 29, 4397. [Google Scholar] [CrossRef]
- Smołka-Danielowska, D.; Jabłońska, M. Chemical and mineral composition of ashes from wood biomass combustion in domestic wood-fired furnaces. Int. J. Environ. Sci. Technol. 2022, 19, 5359–5372. [Google Scholar] [CrossRef]
- Zając, G.; Szyszlak-Bargłowicz, J.; Gołębiowski, W.; Szczepanik, M. Chemical characteristics of biomass ashes. Energies 2018, 11, 2885. [Google Scholar] [CrossRef]
- Füzesi, I.; Heil, B.; Kovács, G. Effects of wood ash on the chemical properties of soil and crop vitality in small plot experiments. Acta Silv. Lignaria Hung. 2015, 11, 55–64. [Google Scholar] [CrossRef]
- Rolka, E.; Wyszkowski, M.; Żołnowski, A.C.; Skorwider-Namiotko, A. Role of woody biomass ash material in immobilization of cadmium, lead and zinc in soil. Materials 2024, 17, 2206. [Google Scholar] [CrossRef] [PubMed]
- Gill, K.S.; Malhi, S.S.; Lupwayi, N.Z. Wood ash improved soil properties and crop yield for nine years and saved fertilizer. J. Agric. Sci. 2015, 7, 72–83. [Google Scholar] [CrossRef]
- Johansen, J.L.; Nielsen, M.L.; Vestergård, M.; Mortensen, L.H.; Cruz-Paredes, C.; Rønn, R.; Kjøller, R.; Hovmand, M.; Christensen, S.; Ekelund, F. The complexity of wood ash fertilization disentangled: Effects on soil pH, nutrient status, plant growth and cadmium accumulation. Environ. Exp. Bot. 2021, 185, 104424. [Google Scholar] [CrossRef]
- Weimers, K.; Bergstrand, K.-J.; Hultberg, M.; Asp, H. Liquid anaerobic digestate as sole nutrient source in soilless horticulture–or spiked with mineral nutrients for improved plant growth. Front. Plant Sci. 2022, 13, 770179. [Google Scholar] [CrossRef]
- Van Midden, C.; Harris, J.; Shaw, L.; Sizmur, T.; Pawlet, M. The impact of anaerobic digestate on soil life: A review. Appl. Soil Ecol. 2023, 191, 105066–105078. [Google Scholar] [CrossRef]
- Verdi, L.; Kuikman, P.J.; Orlandini, S.; Mancini, M.; Napoli, M.; Dalla Martaa, A. Does the use of digestate to replace mineral fertilizers have less emissions of N2O and NH3? Agric. For. Meteorol. 2019, 269, 112–118. [Google Scholar] [CrossRef]
- Tymchuk, I.; Malovanyy, M.; Zhuk, V.; Kochubei, V.; Yatsukh, K.; Luchyt, L. Towards increasing the utilization of anaerobic digestate from biogas production in agrotechnologies. Ecol. Quest. 2023, 34. [Google Scholar] [CrossRef]
- Akhiar, A.; Battimelli, A.; Torrijos, M.; Carrere, H. Comprehensive characterization of the liquid fraction of digestates from full-scale anaerobic co-digestion. Waste Manag. 2017, 59, 118–128. [Google Scholar] [CrossRef]
- Mazur-Pączka, A.; Butt, K.R.; Jaromin, M.; Hajduk, E.; Garczyńska, M.; Kostecka, J.; Pączka, G. Fertilization effects of solid digestate treatments on earthworm community parameters and selected soil attributes. Agriculture 2025, 15, 1511. [Google Scholar] [CrossRef]
- Jian, C.; Hamamoto, T.; Inoue, C.; Chien, M.-F.; Naganuma, H.; Mori, T.; Sawada, A.; Hidaka, M.; Setoyama, H.; Makino, T. Effects of wood ash fertilizer on element dynamics in soil solution and crop uptake. Agronomy 2025, 15, 1097. [Google Scholar] [CrossRef]
- Park, B.B.; Yanai, R.D.; Sahm, J.M.; Lee, D.K.; Abrahamson, L.P. Wood ash effects on plant and soil in a willow bioenergy plantation. Biomass Bioenergy 2005, 28, 355–365. [Google Scholar] [CrossRef]
- An, J.Y.; Park, B.B. Effects of wood ash and N fertilization on soil chemical properties and growth of Zelkova serrata across soil types. Sci. Rep. 2021, 11, 14489. [Google Scholar] [CrossRef]
- Couch, R.L.; Luckai, N.; Morris, D.; Diochon, A. Short-term effects of wood ash application on soil properties, growth, and foliar nutrition of Picea mariana and Picea glauca seedlings in a plantation trial. Can. J. Soil Sci. 2021, 101, 203–215. [Google Scholar] [CrossRef]
- Kujawska, J.; Cel, W.; Kwiatkowski, C.A.; Harasim, E.; Zamorska, J. The effect of digestate from biogas plants alone and with the addition of biochar and zeolite on soil properties and sorghum yield. Bioresources 2025, 20, 5765–5789. [Google Scholar] [CrossRef]
- Siebielec, S.; Siebielec, G.; Woźniak, M. Potential of using digestate to regenerate soil and stimulate its biological life. Pol. J. Agron. 2023, 52, 157–170. [Google Scholar] [CrossRef]
- Kataki, S.; Hazarika, S.; Baruah, D.C. Recycling of bioenergy by-products as crop nutrient: Application in different phases for improvement of soil and crop. Environ. Prog. Sustain. Energy. 2019, 38, 4. [Google Scholar] [CrossRef]
- Szwed, M.; Koszel, M.; Przywara, A.; Kachel-Górecka, M. Impact of digestate fertilization on crop production–a comprehensive review. Adv. Sci. Technol. Res. J. 2024, 18, 347–353. [Google Scholar] [CrossRef]
- Doyeni, M.O.; Stulpinaite, U.; Baksinskaite, A.; Suproniene, S.; Tilvikiene, V. The effectiveness of digestate use for fertilization in an agricultural cropping system. Plants 2021, 10, 1734. [Google Scholar] [CrossRef] [PubMed]
- Przygocka-Cyna, K.; Grzebisz, W. The multifactorial effect of digestate on the availability of soil elements and grain yield and its mineral profile—The case of maize. Agronomy 2020, 10, 275. [Google Scholar] [CrossRef]
- Rolka, E.; Wyszkowski, M.; Skorwider-Namiotko, A.; Szostek, P. Fertilisation Potential of combined use of wood biomass ash and digestate in maize cultivation. Agronomy 2025, 15, 1968. [Google Scholar] [CrossRef]
- Rolka, E.; Wyszkowski, M. Availability of trace elements in soil with simulated cadmium, lead and zinc pollution. Minerals 2021, 11, 879. [Google Scholar] [CrossRef]
- Żołnowski, A.C.; Bakuła, T.; Rolka, E.; Klasa, A. Effect of mineral–microbial deodorizing preparation on the value of poultry manure as soil amendment. Int. J. Environ. Res. Public Health 2022, 19, 16639. [Google Scholar] [CrossRef] [PubMed]
- Panalytical Malvern. Mastersizer 3000. Brochure. Available online: https://www.malvernpanalytical.com/en/assets/mastersizer%203000%20brochure%20(en)_tcm50-58994.pdf (accessed on 14 April 2023).
- Karczewska, A.; Kabała, C. Methodology of Laboratory Analyzes of Soils and Plants; University of Life Sciences: Wrocław, Poland, 2008. [Google Scholar]
- Bremner, J.M. Nitrogen–Total. In Methods of Soil Analysis: Part 3, Chemical Methods; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Sumner, M.E., Eds.; Soil Science Society of America: Madison, WI, USA, 1996; pp. 1087–1123. [Google Scholar]
- Ostrowska, A.; Gawliński, S.; Szczubiałka, Z. Methods of Analysis and Assessment of Soil and Plants Properties, 1st ed.; Institute of Environmental Protection: Warsaw, Poland, 1991. [Google Scholar]
- Egner, H.; Riehm, H.; Domingo, W.R. Untersuchungen Uber Die Chemische Bodenanalyse Als Grundlage Fur Die Beurteilung Des Nahrstoffzustandes Der Boden. II. Chemische Extraktionsmethoden Zur Phosphorund Kaliumbestimmung. K. Lantbrukshogskolans Ann. 1960, 26, 199–215. [Google Scholar]
- Panak, H. Methodological Guide for Agricultural Chemistry Exercises; Agricultural and Technical University in Olsztyn: Olsztyn, Poland, 1995. [Google Scholar]
- Tibco. Statistica Data Analysis Software System; Tibco Software Inc.: Palo Alto, CA, USA, 2021. [Google Scholar]
- Burdzy, J. Statistical Tables; Lublin University of Technology Publishing House: Lublin, Poland, 1999. [Google Scholar]
- Microsoft. MS Excel® for Microsoft 365 MSO; Microsoft Corporation: Albuquerque, NM, USA, 2021. [Google Scholar]
- Adeyemo, A.J.; Daramola, A.R.; Adejoro, S.A.; Ojeniyi, S.O. Application of urea and wood ssh on soil nutrient composition, growth and yield of okra under degraded humid tropical alfisol of South Western Nigeria. J. Horti. Sci. Crop. Res. 2022, 2, 101. [Google Scholar]
- Adamovics, A.; Sivicka, I. Influence of biogas digestate, wood ash and their mixtures on the yield and quality of cucumbers. AGROFOR Int. J. 2023, 8, 68–75. Available online: https://agrofor.ues.rs.ba/data/20230410-09_Adamovics_and_Sivicka.pdf (accessed on 11 November 2025).
- Zuševica, A.; Adamovics, A.; Duminš, K.; Vendina, V.; Žigure, S.; Lazdina, D. Soil fertility improvement with mixtures of wood ash and biogas digestates enhances leaf photosynthesis and extends the growth period for deciduous trees. Plants 2023, 12, 1152. [Google Scholar] [CrossRef]
- Barłóg, P.; Hlisnikovský, L.; Kunzová, E. Effect of digestate on soil organic carbon and plant-available nutrient content compared to cattle slurry and mineral fertilization. Agronomy 2020, 10, 379. [Google Scholar] [CrossRef]
- Brtnicky, M.; Kintl, A.; Holatko, A.; Hammerschmiedt, T.; Mustafa, A.; Kucerik, J.; Vitez, T.; Prichystalova, J.; Baltazar, T.; Elbl, J. Effect of digestates derived from the fermentation of maize-legume intercropped culture and maize monoculture application on soil properties and plant biomass production. Chem. Biol. Technol. Agric. 2022, 9, 43. [Google Scholar] [CrossRef]
- Mórtola, N.; Romaniuk, R.; Cosentino, V.; Eiza, M.; Carfagno, P.; Rizzo, P.; Bres, P.; Riera, N.; Roba, M.; Butti, M.; et al. Potential use of a poultry manure digestate as a biofertiliser: Evaluation of soil properties and Lactuca sativa growth. Pedosphere 2019, 29, 60–69. [Google Scholar] [CrossRef]
- Abelenda, A.M.; Semple, K.T.; Lag-Brotons, A.J.; Herbert, B.M.J.; Aggidis, G.; Aiouache, F. Effects of wood ash-based alkaline treatment on nitrogen, carbon, and phosphorus availability in food waste and agro-industrial waste digestates. Waste Biomass Valori. 2021, 12, 3355–3370. [Google Scholar] [CrossRef]
- Abelenda, A.M.; Aiouache, F. Wood ash based treatment of anaerobic digestate: State-of-the-art and possibilities. Processes 2022, 10, 147. [Google Scholar] [CrossRef]
- Adamovičs, A.; Poiša, L. The effects of digestate and wood ash mixtures on the productivity and yield quality of winter wheat. In Environment. Technology. Resources. Proceedings of the 16th International Scientific and Practical Conference, Rezekne, Latvia, 19–20 June 2025; Riga Technical University: Rezekne, Latvia, 2025; Volume 1, pp. 17–21. [Google Scholar]
- Wójcik, M.; Stachowicz, F.; Masłoń, A. The use of wood biomass ash in sewage sludge treatment in terms of its agricultural utilization. Waste Biomass Valori. 2020, 11, 753–768. [Google Scholar] [CrossRef]




| Series of Experiment | |||
|---|---|---|---|
| WBA (Doses of HAC) | WBA + ULF | WBA + SSF | WBA + SLF |
| 0 WBA + NPK | 0 WBA + ULF + NPK | 0 WBA + SSF + NPK | 0 WBA + SLF + NPK |
| 0.5 WBA + NPK | 0.5 WBA + ULF + NPK | 0.5 WBA + SSF + NPK | 0.5 WBA + SLF + NPK |
| 1.0 WBA + NPK | 1.0 WBA + ULF + NPK | 1.0 WBA + SSF + NPK | 1.0 WBA + SLF + NPK |
| 1.5 WBA + NPK | 1.5 WBA + ULF + NPK | 1.5 WBA + SSF + NPK | 1.5 WBA + SLF + NPK |
| Parameter | Unit | Starting Soil | WBA | ULF | SSF | SLF |
|---|---|---|---|---|---|---|
| Dry mas | % | - | 60.28 ± 0.65 | 5.51 ± 0.24 | 24.56 ± 0.62 | 5.24 ± 0.04 |
| Soil reaction (pHKCl) | −log10(H+) | 3.96 ± 0.02 | 11.97 ± 0.09 | 7.48 ± 0.03 | 9.53 ± 0.03 | 7.49 ± 0.03 |
| Electrical conductivity (EC) | mS cm−1 | 0.05 ± 0.00 | 10.49 ± 0.23 | 29.13 ± 1.04 | 25.15 ± 1.12 | 28.87 ± 0.94 |
| Hydrolytic acidity (HAC) | mmol kg−1 | 28.25 ± 1.02 | 600.0 ± 4.08 | 285.0 ± 8.50 | 116.3 ± 3.75 | 300.0 ± 5.00 |
| Sum of base cations (SBC) | mmol kg−1 | 45.33 ± 0.61 | - | - | - | - |
| Cation exchange capacity (CEC) | mmol kg−1 | 73.58 ± 1.63 | - | - | - | - |
| Base saturation (BS) | % | 61.60 ± 0.53 | - | - | - | - |
| Total carbon (TC) | g kg−1 | 4.09 ± 0.09 | 231.7 ± 8.33 | 20.95 ± 0.12 | 39.05 ± 1.34 | 19.51 ± 0.58 |
| Total nitrogen (TN) | g kg−1 | 0.54 ± 0.01 | 3.55 ± 0.33 | 2.80 ± 0.11 | 5.83 ± 0.18 | 2.47 ± 0.18 |
| Total macronutrients: | ||||||
| Phosphorus (Ptot) | g kg−1 | - | 10.51 ± 0.33 | 2.11 ± 0.16 | 13.36 ± 0.49 | 2.05 ± 0.07 |
| Potassium (Ktot) | g kg−1 | - | 33.41 ± 0.13 | 8.56 ± 0.06 | 7.72 ± 0.21 | 8.36 ± 0.13 |
| Magnesium (Mgtot) | g kg−1 | - | 10.31 ± 0.05 | 0.38 ± 0.00 | 0.48 ± 0.00 | 0.37 ± 0.01 |
| Calcium (Catot) | g kg−1 | - | 168.9 ± 0.51 | 2.37 ± 0,10 | 4.77 ± 0.02 | 2.14 ± 0.23 |
| Sodium (Natot) | g kg−1 | - | 2.18 ± 0.02 | 3.41 ± 0.07 | 3.25 ± 0.10 | 3.51 ± 0.12 |
| Available macronutrients: | ||||||
| Phosphorus (Pav) | mg kg−1 | 68.63 ± 4.38 | - | - | - | - |
| Potassium (Kav) | mg kg−1 | 97.69 ± 1.70 | - | - | - | - |
| Magnesium (Mgav) | mg kg−1 | 31.00 ± 1.13 | - | - | - | - |
| Alkalinity | % CaO | - | 26.93 ± 0.88 | - | - | - |
| WBA Doses of HAC | Series of Experiment | ||||
|---|---|---|---|---|---|
| WBA | WBA + ULF | WBA + SSF | WBA + SLF | Mean for Doses | |
| TC (g kg−1 of soil) | |||||
| 0 | 4.169 ± 0.096 a | 4.296 ± 0.088 ab | 4.393 ± 0.087 abcd | 4.308 ± 0.067 abc | 4.292 ± 0.117 A |
| 1 | 4.198 ± 0.090 a | 4.328 ± 0.147 abc | 4.483 ± 0.103 bcde | 4.457 ± 0.047 bcde | 4.367 ± 0.154 AB |
| 2 | 4.503 ± 0.127 bcde | 4.475 ± 0.118 bcde | 4.693 ± 0.070 e | 4.608 ± 0.187 de | 4.570 ± 0.158 C |
| 3 | 4.378 ± 0.070 abcd | 4.545 ± 0.142 bcde | 4.563 ± 0.125 cde | 4.409 ± 0.074 abcd | 4.474 ± 0.135 BC |
| Mean for series | 4.312 ± 0.168 A | 4.411 ± 0.162 AB | 4.533 ± 0.148 C | 4.445 ± 0.153 BC | 4.425 ± 0.177 |
| r | 0.621 n.s. | 0.616 n.s. | 0.546 n.s. | 0.330 n.s. | 0.474 ** |
| TN (g kg−1 of soil) | |||||
| 0 | 0.541 ± 0.017 abc | 0.527 ± 0.026 abc | 0.532 ± 0.023 abc | 0.579 ± 0.013 c | 0.545 ± 0.029 A |
| 1 | 0.551 ± 0.024 abc | 0.546 ± 0.000 abc | 0.518 ± 0.030 ab | 0.523 ± 0.026 abc | 0.534 ± 0.027 A |
| 2 | 0.551 ± 0.013 abc | 0.513 ± 0.037 ab | 0.551 ± 0.013 abc | 0.551 ± 0.053 abc | 0.541 ± 0.037 A |
| 3 | 0.499 ± 0.024 a | 0.523 ± 0.017 abc | 0.546 ± 0.023 abc | 0.569 ± 0.013 bc | 0.534 ± 0.033 A |
| Mean for series | 0.536 ± 0.029 AB | 0.527 ± 0.027 A | 0.537 ± 0.026 AB | 0.555 ± 0.038 B | 0.539 ± 0.032 |
| r | −0.482 n.s. | −0.193 n.s. | 0.316 n.s. | −0.017 n.s. | −0.085 n.s. |
| TC/TN (ratio) | |||||
| 0 | 7.715 ± 0.425 abc | 8.162 ± 0.336 abcd | 8.273 ± 0.418 abcd | 7.448 ± 0.160 a | 7.900 ± 0.485 B |
| 1 | 7.631 ± 0.189 ab | 7.926 ± 0.270 abcd | 8.683 ± 0.524 d | 8.548 ± 0.433 cd | 8.197 ± 0.575 AB |
| 2 | 8.178 ± 0.187 abcd | 8.752 ± 0.501 d | 8.526 ± 0.166 bcd | 8.416 ± 0.505 bcd | 8.468 ± 0.430 A |
| 3 | 8.792 ± 0.523 d | 8.704 ± 0.384 d | 8.377 ± 0.492 bcd | 7.746 ± 0.126 abc | 8.405 ± 0.582 A |
| Mean for series | 8.079 ± 0.586 AB | 8.386 ± 0.520 AB | 8.465 ± 0.451 B | 8.039 ± 0.575 A | 8.242 ± 0.567 |
| r | 0.720 ** | 0.527 n.s. | 0.038 n.s. | 0.148 n.s. | 0.352 * |
| Elements | pH | EC | TC | TN | Pav | Kav | Mgav | HAC | SBC | CEC |
|---|---|---|---|---|---|---|---|---|---|---|
| EC | 0.922 ** | |||||||||
| TC | 0.523 ** | 0.493 ** | ||||||||
| TN | −0.030 | −0.010 | 0.134 | |||||||
| Pav | 0.899 ** | 0.893 ** | 0.493 ** | 0.053 | ||||||
| Kav | 0.921 ** | 0.954 ** | 0.460 ** | 0.011 | 0.942 ** | |||||
| Mgav | 0.820 ** | 0.731 ** | 0.452 ** | −0.026 | 0.803 ** | 0.764 ** | ||||
| HAC | −0.960 ** | −0.885 ** | −0.514 ** | 0.069 | −0.841 ** | −0.856 ** | −0.787 ** | |||
| SBC | 0.935 ** | 0.897 ** | 0.558 ** | −0.051 | 0.864 ** | 0.889 ** | 0.846 ** | −0.928 ** | ||
| CEC | 0.863 ** | 0.848 ** | 0.547 ** | −0.038 | 0.823 ** | 0.853 ** | 0.826 ** | −0.830 ** | 0.978 ** | |
| BS | 0.954 ** | 0.908 ** | 0.559 ** | −0.060 | 0.858 ** | 0.883 ** | 0.826 ** | −0.974 ** | 0.982 ** | 0.926 ** |
 —positive correlations;
—positive correlations;  —negative correlations.
—negative correlations.Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rolka, E.; Wyszkowski, M.; Żołnowski, A.C.; Skorwider-Namiotko, A.; Szostek, R. Wood Biomass Ash (WBA) Used in Conjunction with Post-Fermentation Mass (PFM) as a Way to Stabilize Soil Properties. Materials 2025, 18, 5176. https://doi.org/10.3390/ma18225176
Rolka E, Wyszkowski M, Żołnowski AC, Skorwider-Namiotko A, Szostek R. Wood Biomass Ash (WBA) Used in Conjunction with Post-Fermentation Mass (PFM) as a Way to Stabilize Soil Properties. Materials. 2025; 18(22):5176. https://doi.org/10.3390/ma18225176
Chicago/Turabian StyleRolka, Elżbieta, Mirosław Wyszkowski, Andrzej Cezary Żołnowski, Anna Skorwider-Namiotko, and Radosław Szostek. 2025. "Wood Biomass Ash (WBA) Used in Conjunction with Post-Fermentation Mass (PFM) as a Way to Stabilize Soil Properties" Materials 18, no. 22: 5176. https://doi.org/10.3390/ma18225176
APA StyleRolka, E., Wyszkowski, M., Żołnowski, A. C., Skorwider-Namiotko, A., & Szostek, R. (2025). Wood Biomass Ash (WBA) Used in Conjunction with Post-Fermentation Mass (PFM) as a Way to Stabilize Soil Properties. Materials, 18(22), 5176. https://doi.org/10.3390/ma18225176







