Abnormal Drop Formation from Copper Films via Detachment
Abstract
1. Introduction
2. Methods
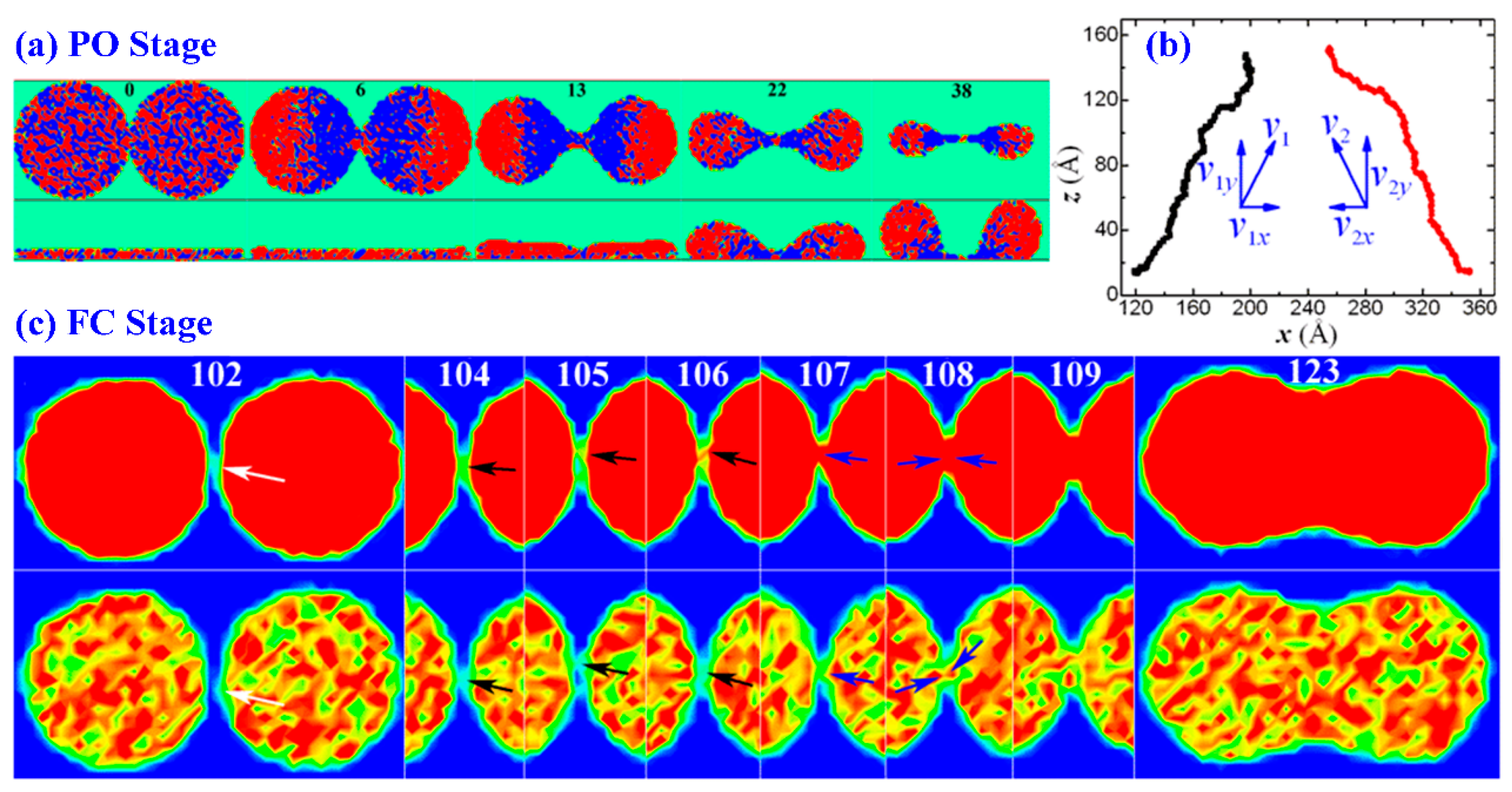
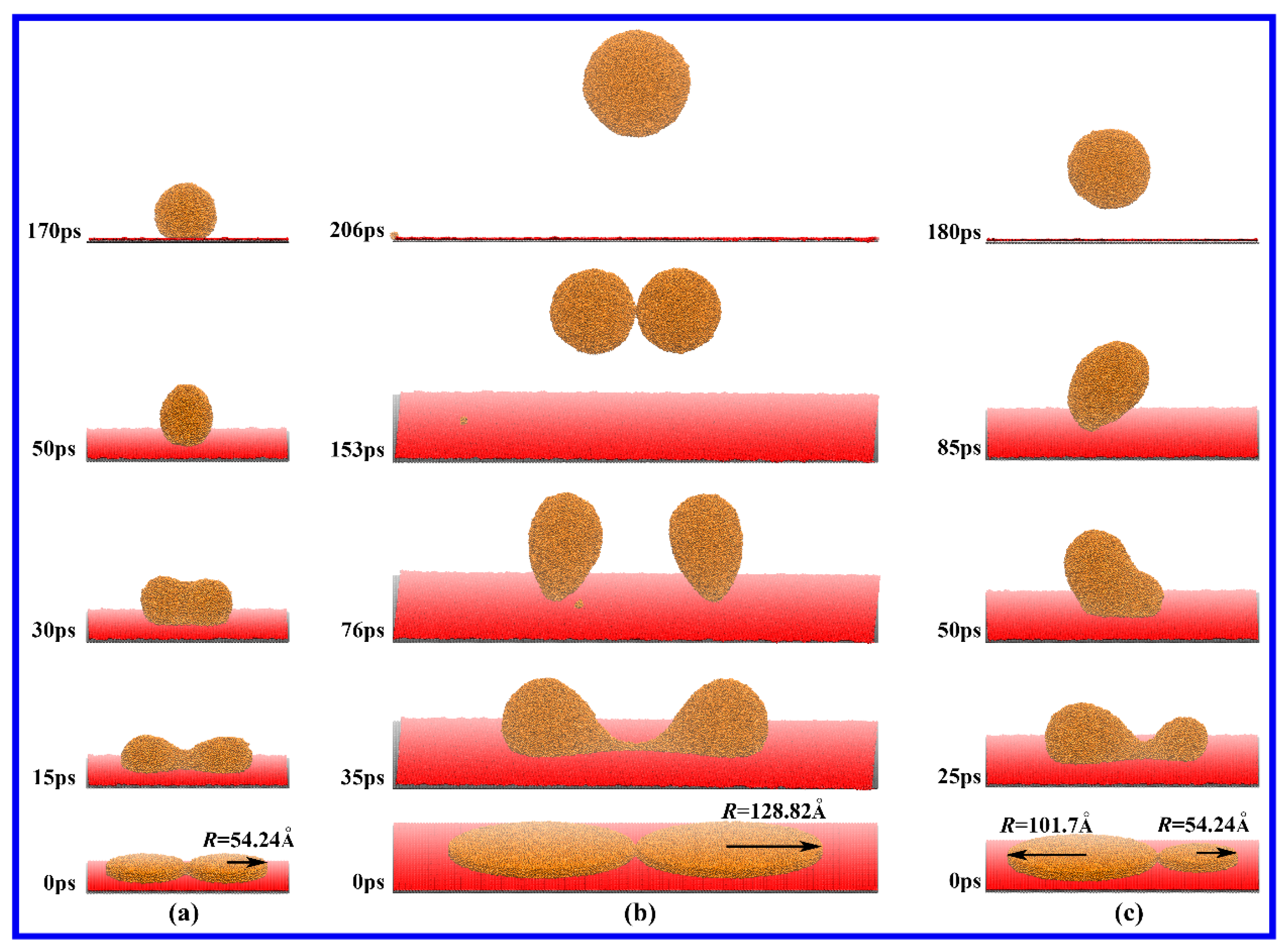
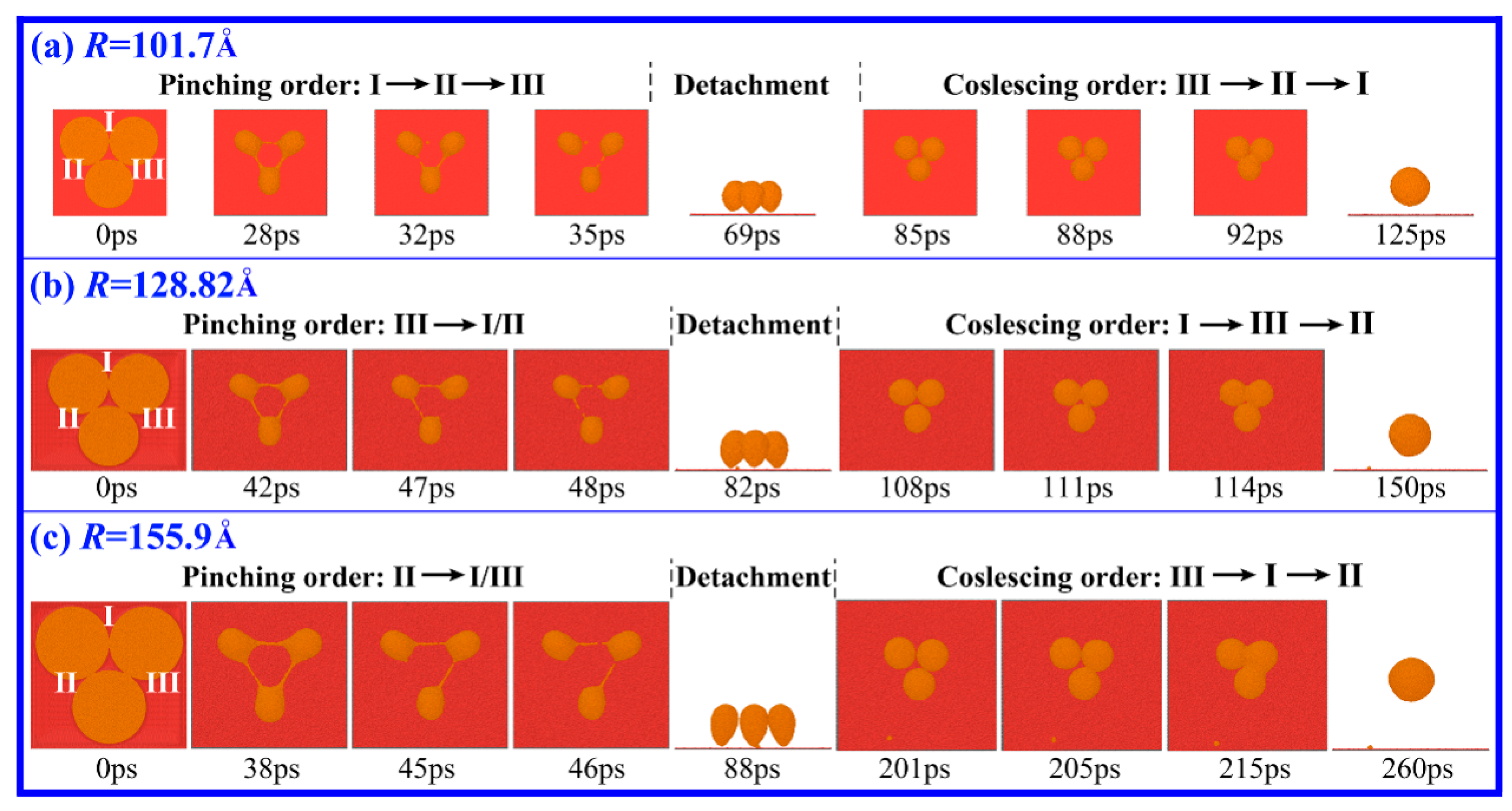
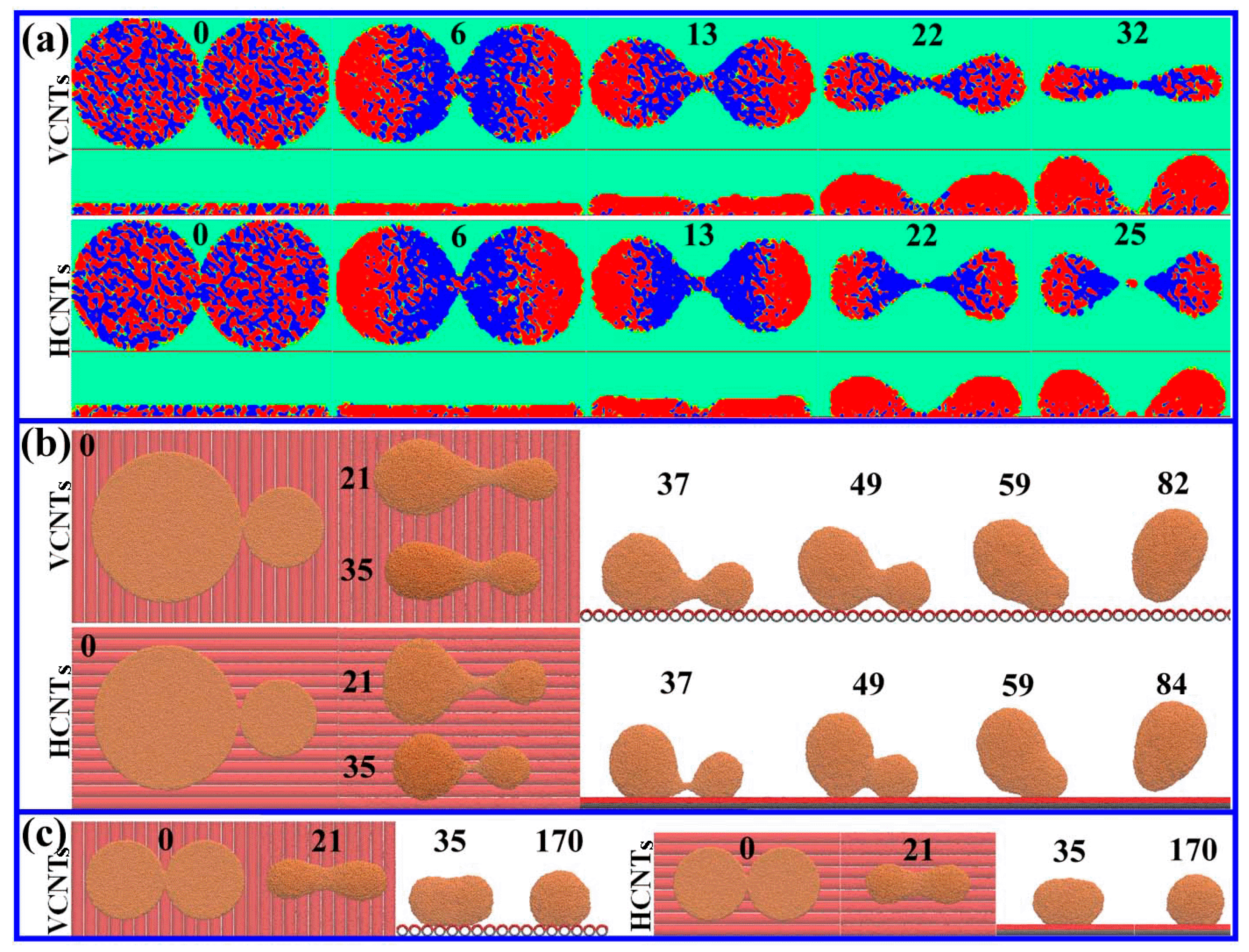
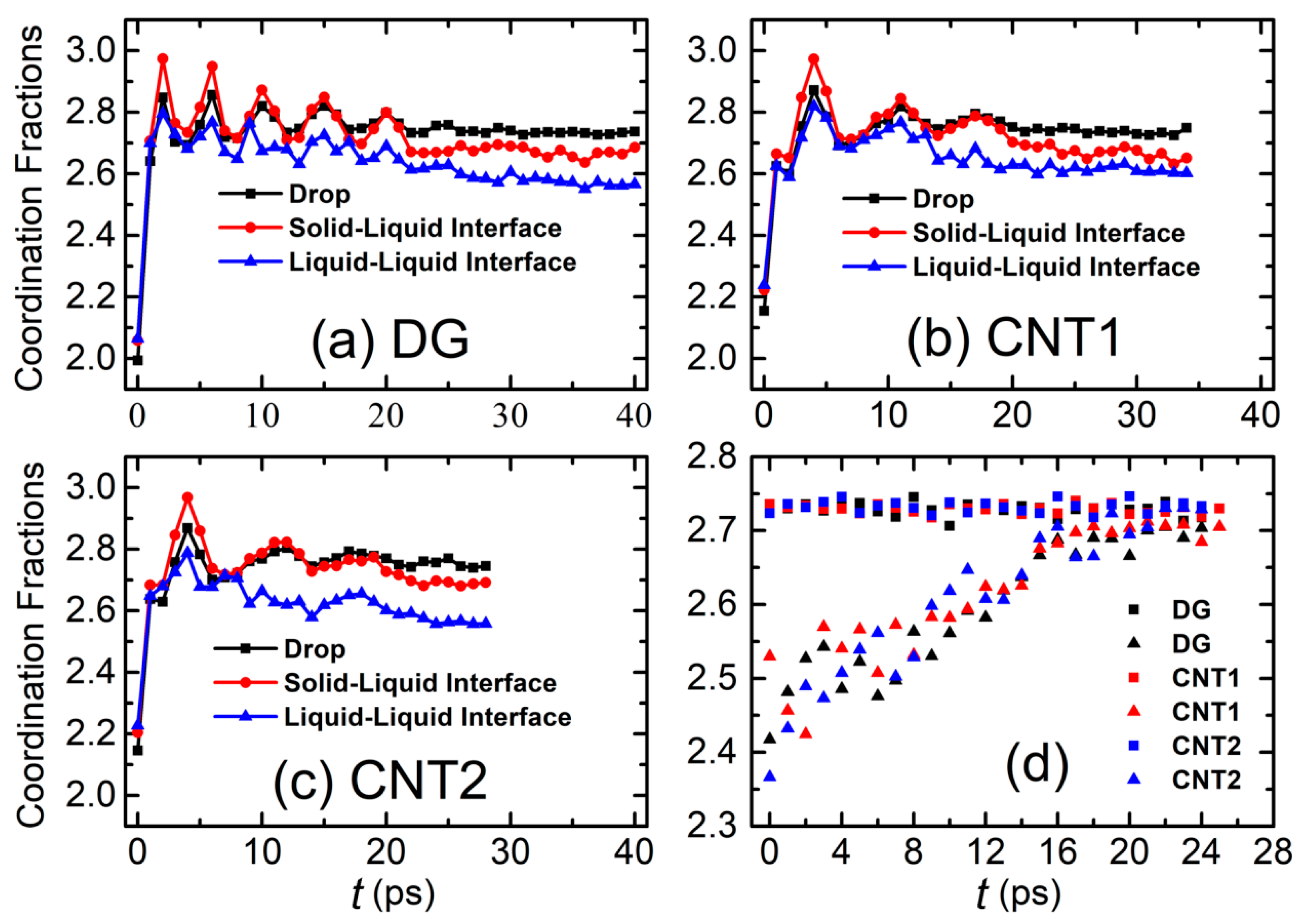
3. Results and Discussion
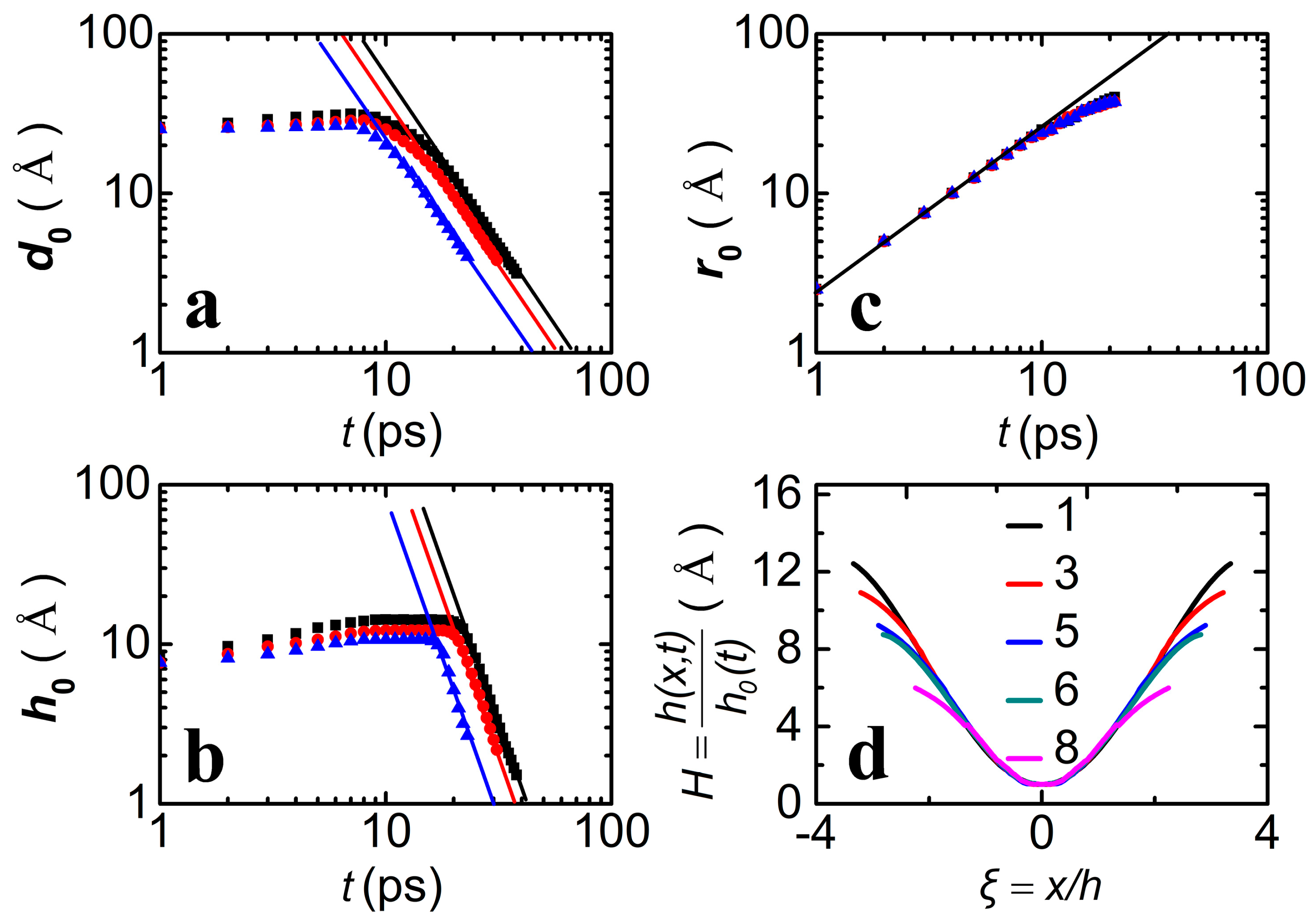
3.1. Drop Formation on DG
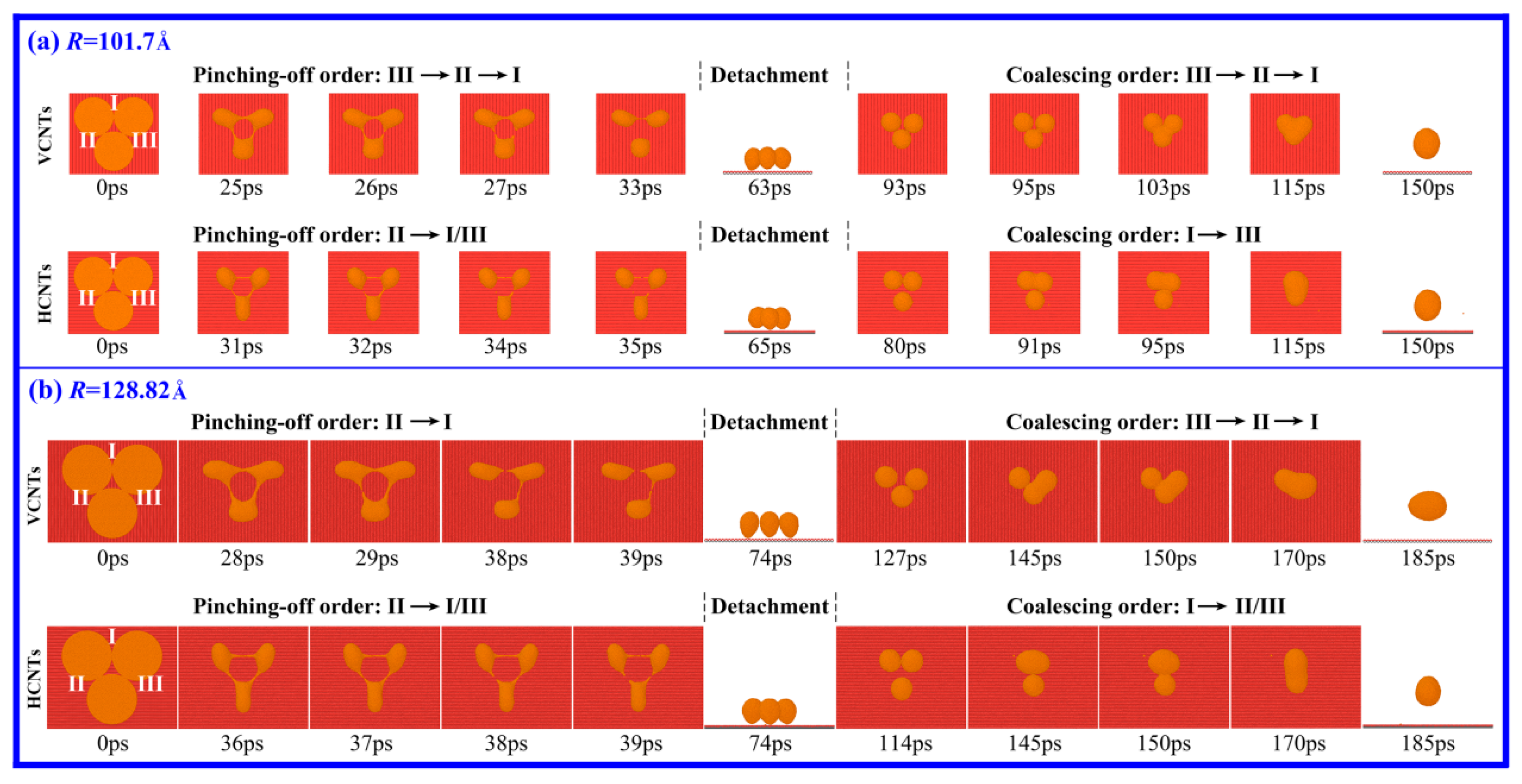
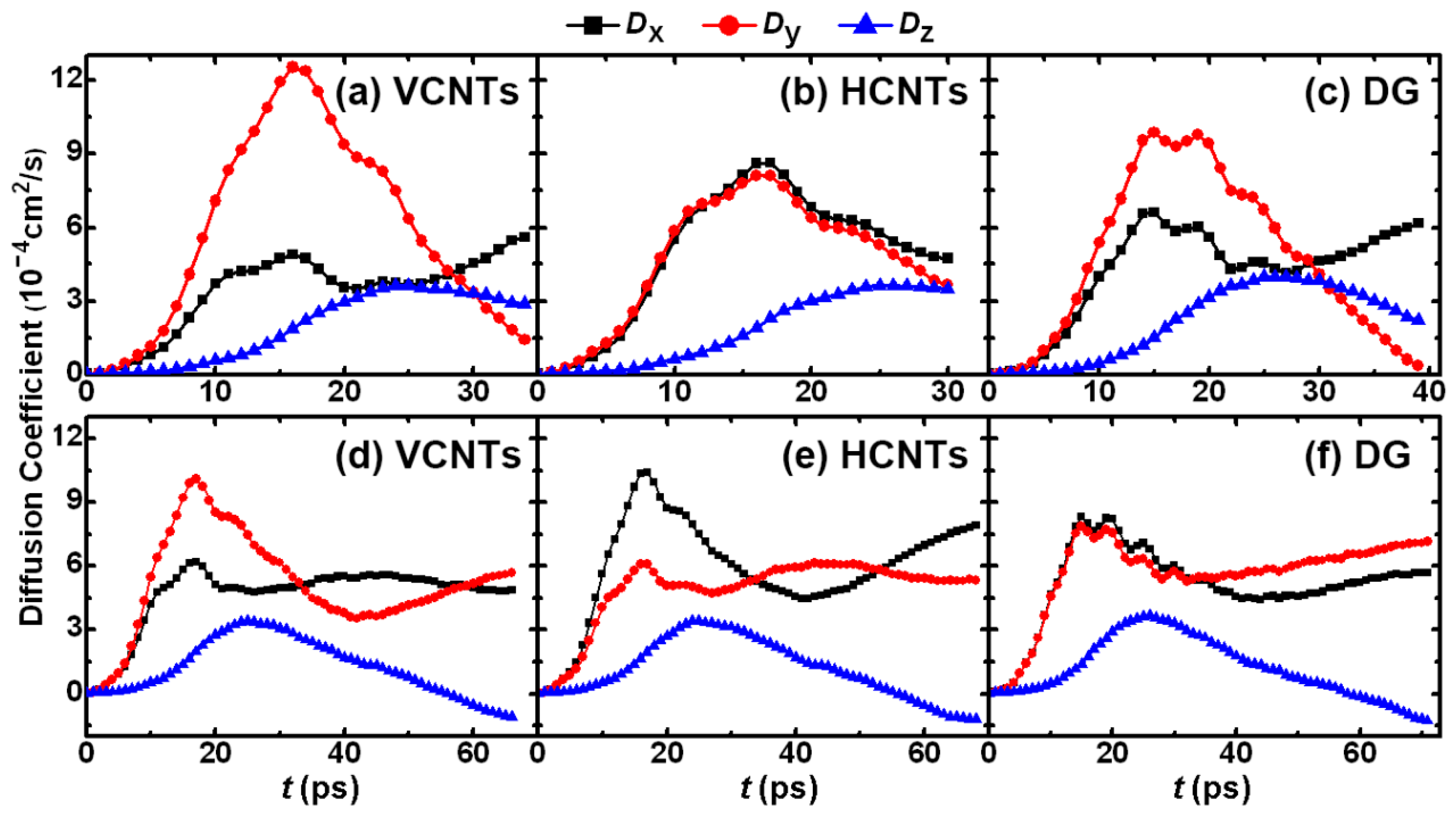
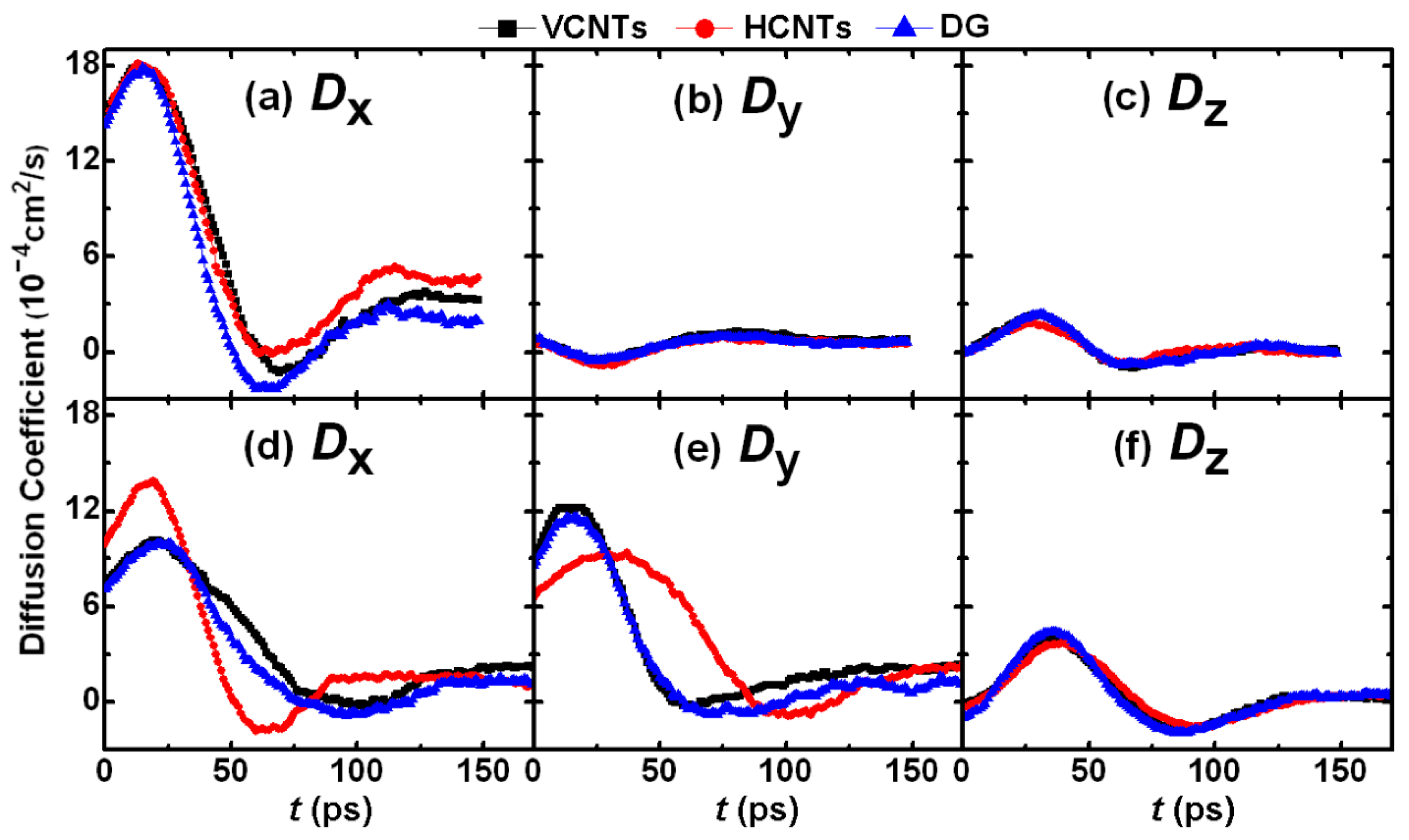
3.2. Drop Formation on CNTs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Le, H.P. Progress and trends in ink-jet printing technology. J. Imaging Sci. Technol. 1998, 42, 49–62. [Google Scholar] [CrossRef]
- You, K.; Wang, Z.; Lin, J.; Guo, X.; Lin, L.; Liu, Y.; Li, F.; Huang, W. On-Demand Picoliter-Level-Droplet Inkjet Printing for Micro Fabrication and Functional Applications. Small 2024, 20, 2402638. [Google Scholar] [CrossRef]
- Suzuki, D.; Taniguchi, H.; Yoshida, R. Autonomously oscillating viscosity in microgel dispersions. J. Am. Chem. Soc. 2009, 131, 12058–12059. [Google Scholar] [CrossRef]
- Vara Prasad, G.V.V.S.; Yadav, M.; Dhar, P.; Samanta, D. Morphed inception of dynamic Leidenfrost regime in colloidal dispersion droplets. Phys. Fluids 2023, 35, 012107. [Google Scholar] [CrossRef]
- Pumir, A.; Siggia, E.D. Incipient singularities in the Navier-Stokes equations. Phys. Rev. Lett. 1985, 55, 1749–1752. [Google Scholar] [CrossRef]
- Anthony, C.R.; Wee, H.; Garg, V.; Thete, S.S.; Kamat, P.M.; Wagoner, B.W.; Wilkes, E.D.; Notz, P.K.; Chen, A.U.; Basaran, O.A. Sharp interface methods for simulation and analysis of free surface flows with singularities: Breakup and coalescence. Annu. Rev. Fluid Mech. 2023, 55, 707–747. [Google Scholar] [CrossRef]
- Shi, X.D.; Brenner, M.P.; Nagel, S.R. A cascade of structure in a drop falling from a faucet. Science 1994, 265, 219–222. [Google Scholar] [CrossRef]
- Eggers, J.; Sprittles, J.E.; Snoeijer, J.H. Coalescence dynamics. Annu. Rev. Fluid Mech. 2024, 57, 61–87. [Google Scholar] [CrossRef]
- Eddi, A.; Winkels, K.G.; Snoeijer, J.H. Influence of droplet geometry on the coalescence of low viscosity drops. Phys. Rev. Lett. 2013, 111, 144502. [Google Scholar] [CrossRef]
- Chen, A.U.; Notz, P.K.; Basaran, O.A. Computational and experimental analysis of pinch-off and scaling. Phys. Rev. Lett. 2002, 88, 174501. [Google Scholar] [CrossRef]
- Day, R.F.; Hinch, E.J.; Lister, J.R. Self-similar capillary pinchoff of an inviscid fluid. Phys. Rev. Lett. 1998, 80, 704–707. [Google Scholar] [CrossRef]
- Papageorgiou, D.T. On the breakup of viscous liquid threads. Phys. Fluids 1995, 7, 1529–1544. [Google Scholar] [CrossRef]
- Eggers, J. Universal pinching of SD axisymmetric free-surface flow. Phys. Rev. Lett. 1993, 71, 3458–3460. [Google Scholar] [CrossRef]
- Pakdel, A.; Bando, Y.; Golberg, D. Plasma-assisted interface engineering of boron nitride nanostructure films. ACS Nano 2014, 10, 10631–10639. [Google Scholar] [CrossRef]
- Li, T.; Wang, J.; Wang, F.; Zhang, L.; Jiang, Y.; Arandiyan, H.; Li, H. The effect of surface wettability and coalescence dynamics in catalytic performance and catalyst preparation: A review. ChemCatChem 2019, 11, 1576–1586. [Google Scholar] [CrossRef]
- Li, T.; Li, J.; Yan, M.; Zhang, L.; Fu, C.; Ruan, Y.; Li, H. Investigation of triple-coalescence behaviors for comprehensively understanding the structural evolution of coalesced TiAl droplets from an atomic-level view. J. Alloys Compd. 2021, 859, 157791. [Google Scholar] [CrossRef]
- Blanchette, F.; Bigioni, T.P. Partial coalescence of drops at liquid interfaces. Nature 2006, 2, 254–257. [Google Scholar] [CrossRef]
- Boreyko, J.B. Jumping droplets. Droplet 2024, 3, e105. [Google Scholar] [CrossRef]
- Ma, C.; Wang, L.; Xu, Z.; Tong, W.; Zheng, Q. Uniform and persistent jumping detachment of condensed nanodroplets. Nano Lett. 2024, 24, 1439–1446. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, J.; He, Y.; Jiao, F. Droplet jumping stimulated by variation of surface energy: A review from mechanism to application. Adv. Colloid Interface Sci. 2025, 344, 103588. [Google Scholar] [CrossRef]
- Keller, J.B.; Miksis, M.J. Surface tension driven flows. SIAM J. Appl. Math. 1993, 43, 268–277. [Google Scholar] [CrossRef]
- Paulsena, J.D.; Burtona, J.C.; Nagela, S.R.; Appathuraib, S.; Harrisb, M.T.; Basaran, O.A. The inexorable resistance of inertia determines the initial regime of drop coalescence. Proc. Natl. Acad. Sci. USA 2012, 1091, 6857–6861. [Google Scholar] [CrossRef]
- Autumn, K.; Liang, Y.A.; Hsieh, T.; Zesch, W.; Chan, W.P.; Kenny, T.W.; Fearing, R.; Full, R.J. Adhesive force of a single gecko foot-hair. Nature 2000, 405, 681–685. [Google Scholar] [CrossRef]
- Lee, H.; Lee, B.P.; Messersmith, P.B. A reversible wet/dry adhesive inspired by mussels and geckos. Nature 2007, 448, 338–341. [Google Scholar] [CrossRef]
- Barthlott, W.; Neinhuis, C. Purity of the sacred lotus, or escape from contamination in biological surfaces. Planta 1997, 202, 1–8. [Google Scholar] [CrossRef]
- Neinhuis, C.; Barthlott, W. Characterization and distribution of water-repellent, self-cleaning plant surfaces. Ann. Bot. 1997, 79, 667–677. [Google Scholar] [CrossRef]
- Habenicht, A.; Olapinski, M.; Burmeister, F.; Leiderer, P.; Boneberg, J. Jumping nanodroplets. Science 2005, 309, 2043–2045. [Google Scholar] [CrossRef]
- Li, X.Y.; He, Y.Z.; Wang, Y.; Dong, J.C.; Li, H. Dewetting properties of metallic liquid film on nanopillared graphene. Sci. Rep. 2014, 4, 3938. [Google Scholar] [CrossRef]
- Fuentes-Cabrera, M.; Rhodes, B.H.; Baskes, M.I.; Terrones, H.; Fowlkes, J.D.; Simpson, M.L.; Rack, P.D. Controlling the velocity of jumping nanodroplets via their initial shape and temperature. ACS Nano 2011, 5, 7130–7136. [Google Scholar] [CrossRef]
- Enright, R.; Miljkovic, N.; Sprittles, J.; Nolan, K.; Mitchell, R.; Wang, E.N. How coalescing droplets jumping. ACS Nano 2014, 10, 10352–10362. [Google Scholar] [CrossRef]
- Fowlkes, J.D.; Kondic, L.; Diez, J.; Wu, Y.Y.; Rack, P.D. Self-assembly versus directed assembly of nanoparticles via pulsed laser induced dewetting of patterned metal films. Nano Lett. 2011, 11, 2478–2485. [Google Scholar] [CrossRef]
- Plimpton, S. Fast parallel algorithms for short-range molecular dynamics. J. Comput. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef]
- Zhao, H.; Min, K.; Aluru, N.R. Size and chirality dependent elastic properties of graphene nanoribbons under uniaxial tension. Nano Lett. 2009, 9, 3012–3015. [Google Scholar] [CrossRef]
- Zhou, X.W.; Wadley, H.N.G.; Johnson, R.A.; Larson, D.J.; Tabat, N.; Cerezo, A.; Petford-Long, A.K.; Smith, G.D.W.; Clifton, P.H.; Martens, R.L. Atomic scale structure of sputtered metal multilayers. Acta Mater. 2001, 49, 4005–4015. [Google Scholar] [CrossRef]
- Stuart, S.J.; Tutein, A.B.; Harrison, J.A. A reactive potential for hydrocarbons with intermolecular interactions. J. Chem. Phys. 2000, 112, 6472–6486. [Google Scholar] [CrossRef]
- He, Y.Z.; Li, H.; Li, Y.F.; Zhang, K.; Jiang, Y.Y.; Bian, X.F. Atomic insight into copper nanostructures nucleation on nending graphene. Phys. Chem. Chem. Phys. 2013, 15, 9163–9169. [Google Scholar] [CrossRef]
- Sutter, P.; Hybertsen, M.S.; Sadowski, J.T.; Sutter, E. Electronic structure of fewlayer epitaxial graphene on Ru(0001). Nano Lett. 2009, 9, 2654–2660. [Google Scholar] [CrossRef]
- Deng, B.; Xu, A.W.; Chen, G.Y.; Song, R.Q.; Chen, L.P. Synthesis of copper-core/carbon-sheath nanocables by a surfactant-assisted. Hydrothermal reduction/carbonization process. J. Phys. Chem. B 2006, 110, 11711–11716. [Google Scholar] [CrossRef]
- Fuentes-Cabrera, M.; Rhodes, B.H.; Fowlkes, J.D.; López-Benzanilla, A.; Terrones, H.; Simpson, M.L.; Rack, P.D. Molecular dynamics study of the dewetting of copper on graphite and graphene: Implications for nanoscale self-assembly. Phys. Rev. E 2011, 83, 041603. [Google Scholar] [CrossRef]
- Naidich, Y.V.; Kolesnichenko, G.A. A study of wetting of diamond and graphite by fused metals and alloys VII. The effect of vanadium, niobium, manganese, molybdenum, and tungsten on wetting of graphite by copper-based alloys. Powder Metall. Met. Ceram. 1968, 7, 563–565. [Google Scholar] [CrossRef]
- Stukowski, A. Visualization and analysis of atomistic simulation data with OVITO—The Open Visualization Tool. Model. Simul. Mater. Sci. Eng. 2009, 18, 015012. [Google Scholar] [CrossRef]
- Aarts, D.G.A.L.; Schmidt, M.; Lekkerkerker, H.N.W. Direct visual observation of thermal capillary waves. Science 2004, 304, 847–850. [Google Scholar] [CrossRef]
- Aarts, D.G.A.L.; Lekkerkerker, H.N.W.; Guo, H.; Wegdam, G.H.; Bonn, D. Hydrodynamics of droplet coalescence. Phys. Rev. Lett. 2005, 95, 164503. [Google Scholar] [CrossRef]
- You, I.; Lee, T.G.; Nam, Y.S.; Lee, H. Fabrication of a micro-mnifluidic device by omniphilic/omniphobic patterning on nanostructured surfaces. ACS Nano 2014, 9, 9016–9024. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.-Z.; Lv, X.-Q.; Li, X.-Y. Abnormal Drop Formation from Copper Films via Detachment. Materials 2025, 18, 5169. https://doi.org/10.3390/ma18225169
Liu H-Z, Lv X-Q, Li X-Y. Abnormal Drop Formation from Copper Films via Detachment. Materials. 2025; 18(22):5169. https://doi.org/10.3390/ma18225169
Chicago/Turabian StyleLiu, Heng-Zhi, Xue-Qi Lv, and Xiong-Ying Li. 2025. "Abnormal Drop Formation from Copper Films via Detachment" Materials 18, no. 22: 5169. https://doi.org/10.3390/ma18225169
APA StyleLiu, H.-Z., Lv, X.-Q., & Li, X.-Y. (2025). Abnormal Drop Formation from Copper Films via Detachment. Materials, 18(22), 5169. https://doi.org/10.3390/ma18225169







