Exploring the Biological and Chemical Properties of Emerging 3D-Printed Dental Resin Composites Compared to Conventional Light-Cured Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimen Preparation
2.2. Aging Protocol
2.3. Cell Viability
2.3.1. Cell Culture
2.3.2. Preparation of the Eluates
2.3.3. MTT Assay
2.4. Live/Dead Cell Fluorescent Microscopy
2.5. Raman Spectroscopy
2.6. Statistical Analysis
3. Results
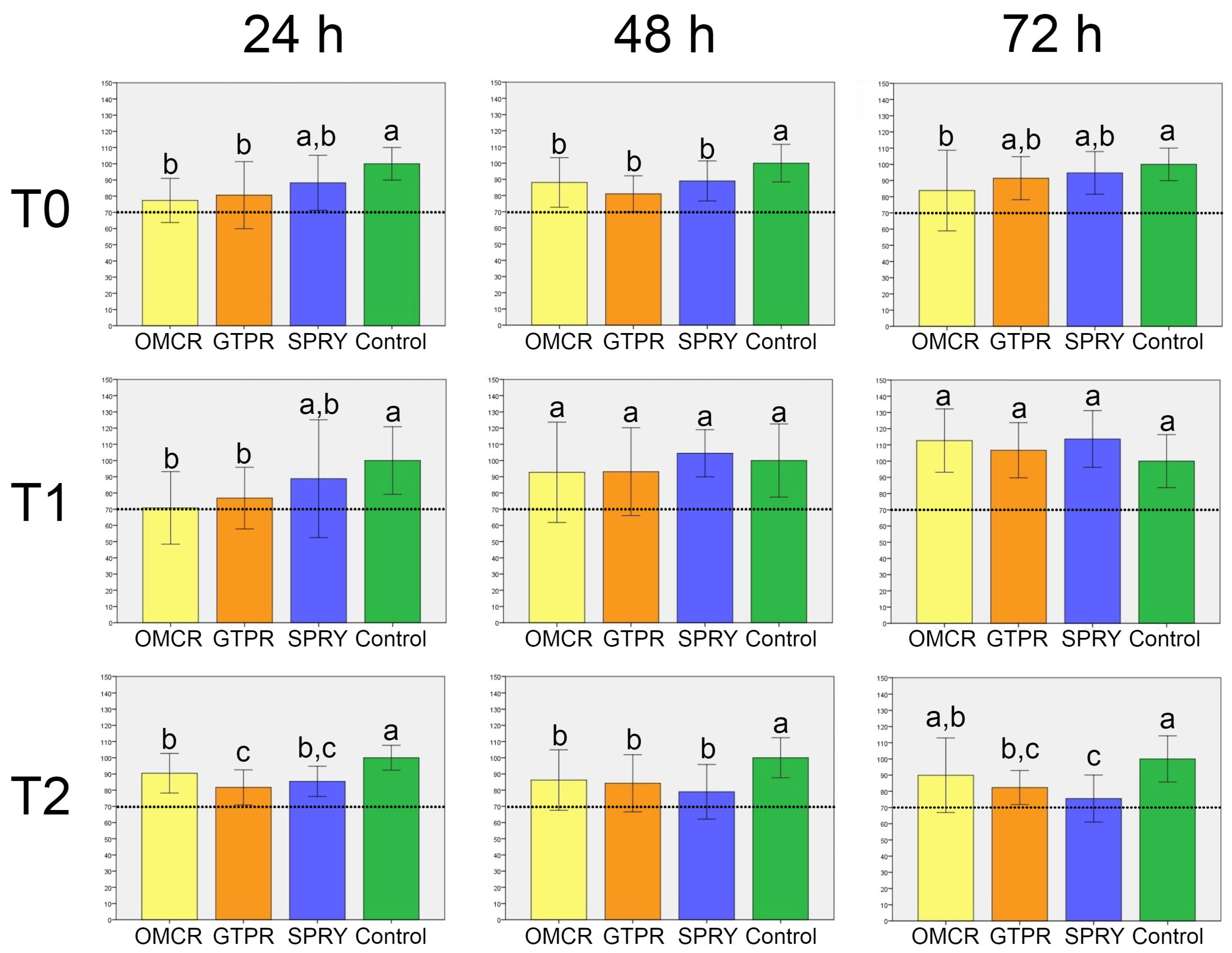
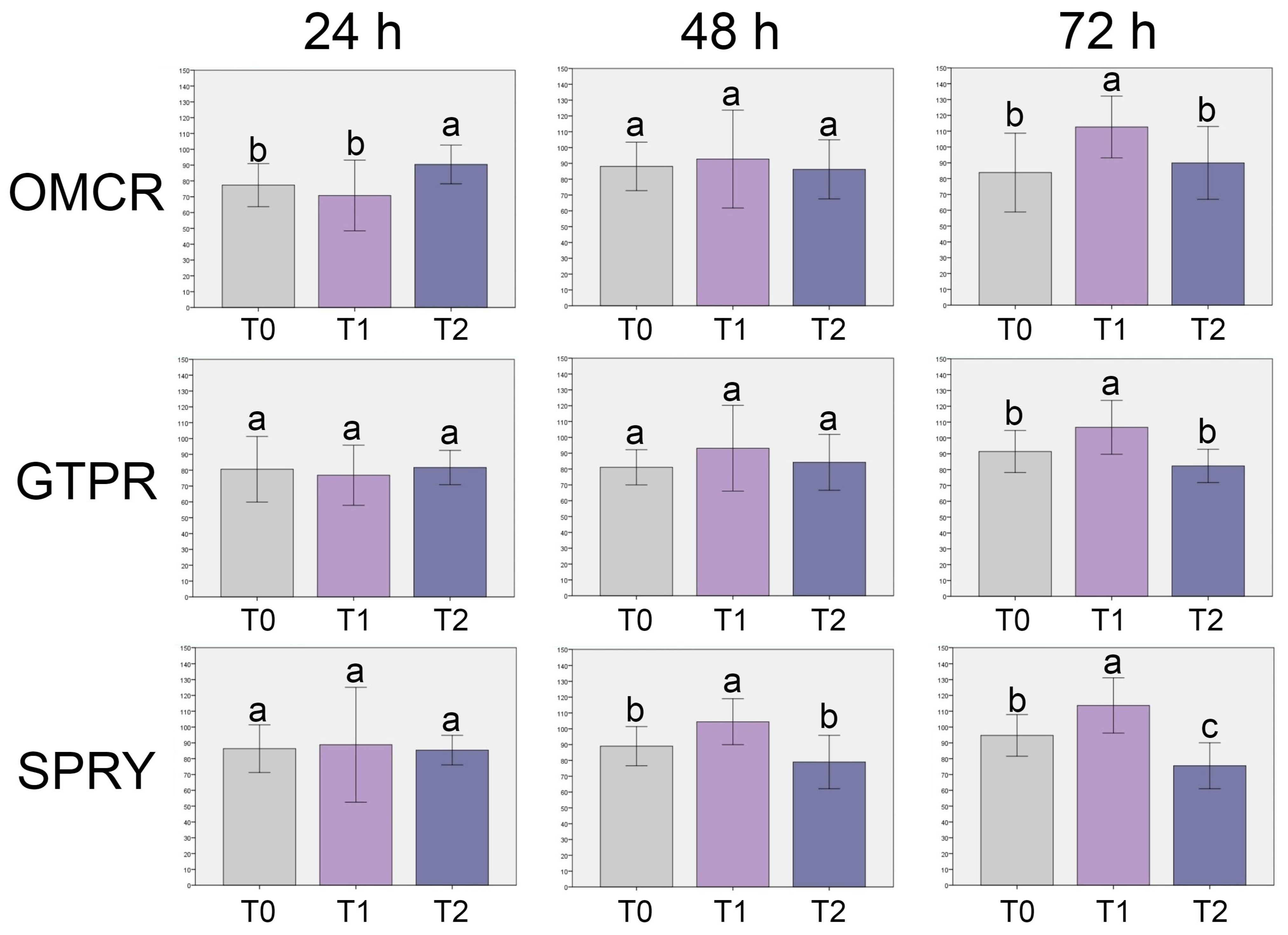
3.1. Cell Viability
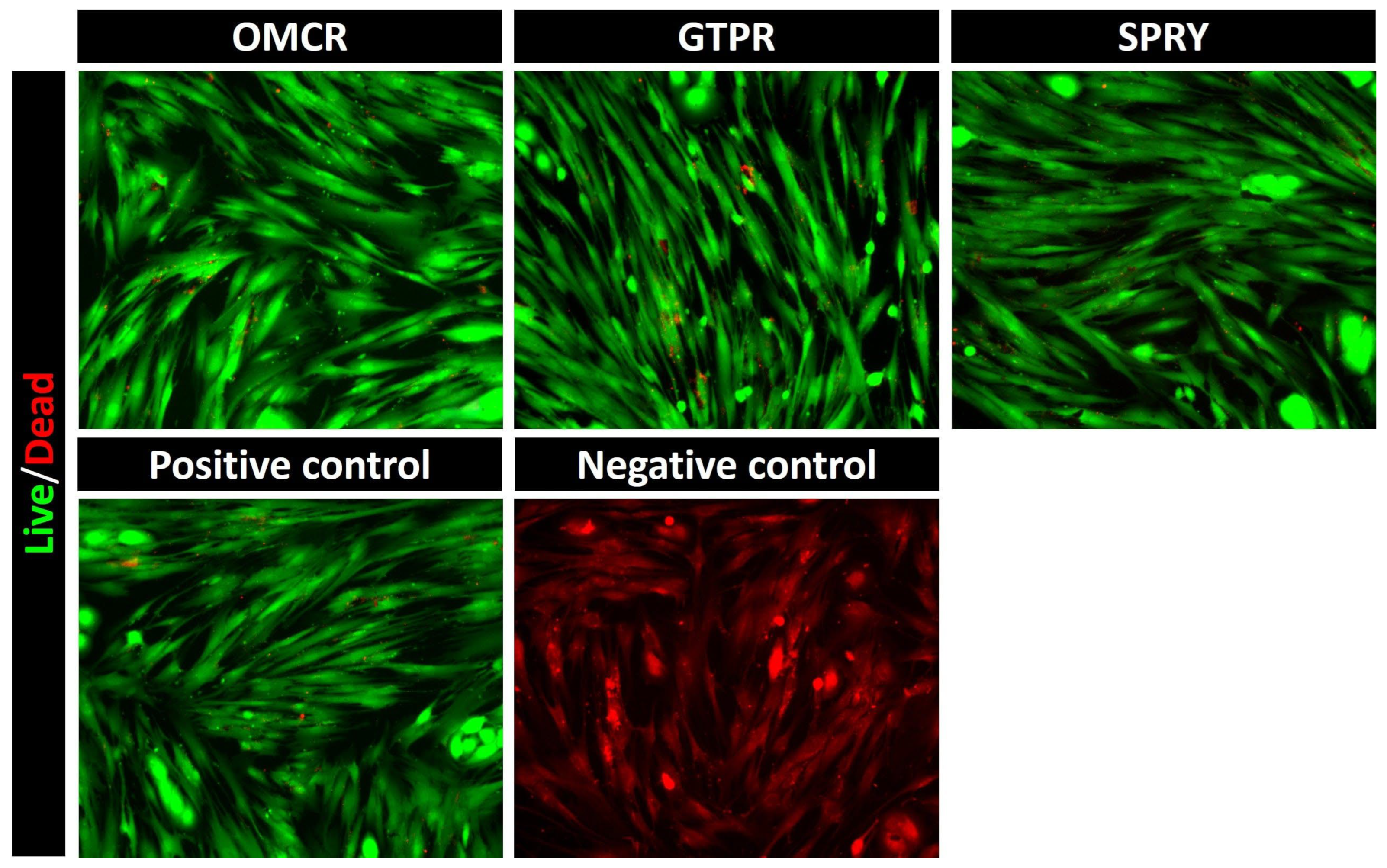
3.2. Live/Dead Cell Fluorescent Microscopy
3.3. Raman Spectroscopy
4. Discussion
5. Conclusions
- All tested materials demonstrated cell viability levels consistently above the established biocompatibility threshold. Tested light-cured composite showed the greatest variability in its biocompatibility profile, whereas the 3D printed composites exhibited a more stable biological response across all evaluation stages.
- The light-cured composite displayed greater susceptibility to chemical degradation under thermal stress compared to both tested 3D-printed composites.
- When properly processed, 3D-printed composites can offer comparable or even superior biocompatibility and chemical integrity compared to the light-cured composite. This is likely due to optimized resin formulations and post-curing protocols that promote improved polymer network organization and reduce residual monomer release, supporting their potential for clinical application in provisional and, possibly, definitive restorative procedures.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 3D | Three-dimensional |
| DC | Degree of conversion |
| DLP | Digital light processing |
| DMEM | Dulbecco’s Modified Eagle Medium |
| FDP | Fixed dental prostheses |
| FITC | Fluorescein Isothiocyanate |
| FTIR | Fourier-transform infrared spectroscopy |
| GTPR | GC Temp PRINT |
| HEMA | 2-hydroxyethyl methacrylate |
| HGF | Human gingival fibroblast |
| ISO | International Organization for Standardization |
| MMA | Methyl methacrylate |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| OD | Optical density |
| OMCR | Omnichroma |
| PBS | Phosphate-buffered saline |
| SEM/EDS | Scanning electron microscopy with energy-dispersive X-ray spectroscopy |
| SLA | Stereolithography |
| SPRY | SprintRay CROWN |
| TEGDMA | Tetraethylene glycol dimethacrylate |
| TRITC | Tetramethylrhodamine Isothiocyanate |
| UDMA | Urethane dimethacrylate |
| UV | Ultraviolet |
| XPS | X-ray photoelectron spectroscopy |
References
- Alshamrani, A.; Alhotan, A.; Kelly, E.; Ellakwa, A. Mechanical and Biocompatibility Properties of 3D-Printed Dental Resin Reinforced with Glass Silica and Zirconia Nanoparticles: In Vitro Study. Polymers 2023, 15, 2523. [Google Scholar] [CrossRef]
- Polydorou, O.; König, A.; Hellwig, E.; Kümmerer, K. Long-Term Release of Monomers from Modern Dental-Composite Materials. Eur. J. Oral Sci. 2009, 117, 68–75. [Google Scholar] [CrossRef]
- Reichl, F.X.; Durner, J.; Hickel, R.; Kunzelmann, K.H.; Jewett, A.; Wang, M.Y. Distribution and Excretion of TEGDMA in Guinea Pigs and Mice. J. Dent. Res. 2001, 80, 1412–1415. [Google Scholar] [CrossRef]
- Geurtsen, W. Biocompatibility of Resin-Modified Filling Materials. Crit. Rev. Oral Biol. Med. 2000, 11, 333–355. [Google Scholar] [CrossRef] [PubMed]
- Wiertelak-Makała, K.; Szymczak-Pajor, I.; Bociong, K.; Śliwińska, A. Considerations about Cytotoxicity of Resin-Based Composite Dental Materials: A Systematic Review. Int. J. Mol. Sci. 2024, 25, 152. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, A.; Jin, J. Photopolymerization in 3D Printing. ACS Appl. Polym. Mater. 2019, 1, 593–611. [Google Scholar] [CrossRef]
- Lottner, S.; Shehata, M.; Hickel, R.; Reichl, F.X.; Durner, J. Effects of Antioxidants on DNA Double-Strand Breaks in Human Gingival Fibroblasts Exposed to Methacrylate-Based Monomers. Dent. Mater. 2013, 29, 991–998. [Google Scholar] [CrossRef]
- Schulz, S.D.; König, A.; Steinberg, T.; Tomakidi, P.; Hellwig, E.; Polydorou, O. Human Gingival Keratinocyte Response to Substances Eluted from Silorane Composite Material Reveal Impact on Cell Behavior Reflected by RNA Levels and Induction of Apoptosis. Dent. Mater. 2012, 28, e135–e142. [Google Scholar] [CrossRef]
- Wedekind, L.; Güth, J.F.; Schweiger, J.; Kollmuss, M.; Reichl, F.X.; Edelhoff, D.; Högg, C. Elution Behavior of a 3D-Printed, Milled and Conventional Resin-Based Occlusal Splint Material. Dent. Mater. 2021, 37, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Sayed, M.E.; Shetty, M.; Alqahtani, S.M.; Al Wadei, M.H.D.; Gupta, S.G.; Othman, A.A.A.; Alshehri, A.H.; Alqarni, H.; Mobarki, A.H.; et al. Physical and Mechanical Properties of 3D-Printed Provisional Crowns and Fixed Dental Prosthesis Resins Compared to CAD/CAM Milled and Conventional Provisional Resins: A Systematic Review and Meta-Analysis. Polymers 2022, 14, 2691. [Google Scholar] [CrossRef]
- Dawood, A.; Marti, B.M.; Sauret-Jackson, V.; Darwood, A. 3D Printing in Dentistry. Br. Dent. J. 2015, 219, 521–529. [Google Scholar] [CrossRef]
- Susila, A.V.; Balasubramanian, V. Correlation of Elution and Sensitivity of Cell Lines to Dental Composites. Dent. Mater. 2016, 32, e63–e72. [Google Scholar] [CrossRef] [PubMed]
- Yoon, T.H.; Lee, Y.K.; Lim, S.; Kim, C.W. Degree of polymerization of resin composites by different light sources. J. Oral Rehabil. 2002, 29, 1165–1173. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, Q.; Meng, X.; Ye, Y.; Feng, D.; Xue, J.; Wang, H.; Huang, H.; Wang, M.; Wang, J. Rheological and Mechanical Properties of Resin-Based Materials Applied in Dental Restorations. Polymers 2021, 13, 2975. [Google Scholar] [CrossRef]
- Reymus, M.; Fabritius, R.; Kessler, A.; Hickel, R.; Edelhoff, D.; Stawarczyk, B. Fracture Load of 3D-Printed Fixed Dental Prostheses Compared with Milled and Conventionally Fabricated Ones: The Impact of Resin Material, Build Direction, Post-Curing, and Artificial Aging—An In Vitro Study. Clin. Oral Investig. 2019, 24, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Kessler, A.; Reymus, M.; Hickel, R.; Kunzelmann, K.H. Three-Body Wear of 3D-Printed Temporary Materials. Dent. Mater. 2019, 35, 1805–1812. [Google Scholar] [CrossRef]
- Scotti, C.K.; Velo, M.M.; Rizzante, F.A.; de Lima Nascimento, T.R.; Mondelli, R.F.; Bombonatti, J.F. Physical and Surface Properties of a 3D-Printed Composite Resin for a Digital Workflow. J. Prosthet. Dent. 2020, 124, 614.e1–614.e8. [Google Scholar] [CrossRef]
- Mudhaffer, S.; Althagafi, R.; Haider, J.; Satterthwaite, J.; Silikas, N. Effects of Printing Orientation and Artificial Ageing on Martens Hardness and Indentation Modulus of 3D Printed Restorative Resin Materials. Dent. Mater. 2024, 40, 1003–1014. [Google Scholar] [CrossRef]
- ISO 7405:2025; Dentistry—Evaluation of Biocompatibility of Medical Devices Used in Dentistry. International Organization for Standardization (ISO): Geneva, Switzerland, 2025.
- Blumer, L.; Schmidli, F.; Weiger, R.; Fischer, J. A Systematic Approach to Standardize Artificial Aging of Resin Composite Cements. Dent. Mater. 2015, 31, 855–863. [Google Scholar] [CrossRef]
- Morresi, A.L.; D’Amario, M.; Capogreco, M.; Gatto, R.; Marzo, G.; D’Arcangelo, C.; Monaco, A. Thermal Cycling for Restorative Materials: Does a Standardized Protocol Exist in Laboratory Testing? A Literature Review. J. Mech. Behav. Biomed. Mater. 2014, 29, 295–308. [Google Scholar] [CrossRef]
- Guerrero-Gironés, J.; López-García, S.; Pecci-Lloret, M.R.; Pecci-Lloret, M.P.; Rodríguez-Lozano, F.J.; García-Bernal, D. In Vitro Biocompatibility Testing of 3D Printing and Conventional Resins for Occlusal Devices. J. Dent. 2022, 123, 104163. [Google Scholar] [CrossRef]
- Vuković, M.; Lazarević, M.; Mitić, D.; Karisik, M.J.; Ilić, B.; Andrić, M.; Jevtić, B.; Roganović, J.; Milasin, J. Acetylsalicylic Acid (ASA) Regulation of Osteo/Odontogenic Differentiation and Proliferation of Human Dental Pulp Stem Cells (DPSCs) In Vitro. Arch. Oral Biol. 2022, 144, 105564. [Google Scholar] [CrossRef]
- ISO 10993-12:2021; Biological Evaluation of Medical Devices—Part 12: Sample Preparation and Reference Materials. International Organization for Standardization (ISO): Geneva, Switzerland, 2021.
- ISO 10993-5:2021; Biological Evaluation of Medical Devices—Part 5: Tests for in vitro cytotoxicity. International Organization for Standardization (ISO): Geneva, Switzerland, 2021.
- Bayarsaikhan, E.; Lim, J.H.; Shin, S.H.; Park, K.H.; Park, Y.B.; Lee, J.H.; Kim, J.E. Effects of Postcuring Temperature on the Mechanical Properties and Biocompatibility of Three-Dimensional Printed Dental Resin Material. Polymers 2021, 13, 1180. [Google Scholar] [CrossRef] [PubMed]
- Carey, P.R. Biochemical Applications of Raman and Resonance Raman Spectroscopies; Academic Press: New York, NY, USA, 1982; pp. xi, 262. [Google Scholar]
- Moszner, N.; Salz, U. New developments of polymeric dental composites. Prog. Polym. Sci. 2001, 26, 535–576. [Google Scholar] [CrossRef]
- Pereira, S.G.; Nunes, T.G.; Kalachandra, S.; Lemos, M.F.; Watson, T.F.; Martin, N. Effect of water sorption on the mechanical properties of resin composites. J. Mater. Sci. Mater. Med. 2005, 16, 297–303. [Google Scholar]
- Ferraro, J.R.; Nakamoto, K. Introductory Raman Spectroscopy, 2nd ed.; Elsevier: New York, NY, USA, 2003. [Google Scholar]
- Braga, L.S.; Ballester, E.R. Raman spectroscopy of dental resin composites: Filler and polymer contributions. Biomaterials 2002, 23, 2131–2137. [Google Scholar]
- Asmussen, M.S. Polymerization kinetics and spectral characterization of Bis-GMA/TEGDMA composites. Dent. Mater. 1989, 5, 330–336. [Google Scholar]
- Soares, L.E.; Rocha, R.; Martin, A.A.; Pinheiro, A.L.; Zampieri, M. Monomer Conversion of Composite Dental Resins Photoactivated by a Halogen Lamp and a LED: A FT-Raman Spectroscopy Study. Quim. Nova 2005, 28, 229–232. [Google Scholar] [CrossRef]
- Fadl, A.; Leask, A. Hiding in Plain Sight: Human Gingival Fibroblasts as an Essential, Yet Overlooked, Tool in Regenerative Medicine. Cells 2023, 12, 2021. [Google Scholar] [CrossRef]
- Frasheri, I.; Aumer, K.; Keßler, A.; Miosge, N.; Folwaczny, M. Effects of Resin Materials Dedicated for Additive Manufacturing of Temporary Dental Restorations on Human Gingival Keratinocytes. J. Esthet. Restor. Dent. 2022, 34, 1105–1112. [Google Scholar] [CrossRef]
- Kamalak, H.; Kamalak, A.; Taghizadehghalehjoughi, A. Cytotoxic Effects of New-Generation Bulk-Fill Composites on Human Dental Pulp Stem Cells. Cell. Mol. Biol. 2018, 64, 62–71. [Google Scholar] [CrossRef]
- Wellner, P.; Mayer, W.; Hickel, R.; Reichl, F.X.; Durner, J. Cytokine Release from Human Leukocytes Exposed to Silorane- and Methacrylate-Based Dental Materials. Dent. Mater. 2012, 28, 743–748. [Google Scholar] [CrossRef]
- Borelli, B.; Zarone, F.; Rivieccio, V.; Riccitiello, F.; Simeone, M.; Sorrentino, R.; Rengo, S.; Spagnuolo, G.; Procino, A. Polyacrylic Resins Regulate Transcriptional Control of Interleukin-6, gp80, and gp130 Genes in Human Gingival Fibroblasts. J. Oral Sci. 2017, 59, 87–91. [Google Scholar] [CrossRef]
- Wuersching, S.N.; Hickel, R.; Edelhoff, D.; Kollmuss, M. Initial Biocompatibility of Novel Resins for 3D Printed Fixed Dental Prostheses. Dent. Mater. 2022, 38, 1587–1597. [Google Scholar] [CrossRef] [PubMed]
- Szczesio-Wlodarczyk, A.; Sokolowski, J.; Kleczewska, J.; Bociong, K. Ageing of dental composites based on methacrylate resins—A critical review of the causes and methods of assessment. Polymers 2020, 12, 882. [Google Scholar] [CrossRef] [PubMed]
- Souza, R.O.A.; Özcan, M.; Michida, S.M.A.; de Melo, R.M.; Pavanelli, C.A.; Bottino, M.A.; Soares, L.E.S.; Martin, A.A. Conversion Degree of Indirect Resin Composites and Effect of Thermocycling on Their Physical Properties. J. Prosthodont. 2010, 19, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Pieniak, D.; Przystupa, K.; Walczak, A.; Niewczas, A.M.; Krzyżak, A.; Bartnik, G.; Gil, L.; Łonkwic, P. Hydro-Thermal Fatigue of Polymer Matrix Composite Biomaterials. Materials 2019, 12, 3650. [Google Scholar] [CrossRef]
- Pieniak, D.; Walczak, A.; Walczak, M.; Przystupa, K.; Niewczas, A.M. Hardness and Wear Resistance of Dental Biomedical Nanomaterials in a Humid Environment with Non-Stationary Temperatures. Materials 2020, 13, 1255. [Google Scholar] [CrossRef]
- Ghavami-Lahiji, M.; Firouzmanesh, M.; Bagheri, H.; Jafarzadeh Kashi, T.S.; Razazpour, F.; Behroozibakhsh, M. The Effect of Thermocycling on the Degree of Conversion and Mechanical Properties of a Microhybrid Dental Resin Composite. Restor. Dent. Endod. 2018, 43, e26. [Google Scholar] [CrossRef]
- Beltrami, R.; Colombo, M.; Rizzo, K.; Di Cristofaro, A.; Poggio, C.; Pietrocola, G. Cytotoxicity of Different Composite Resins on Human Gingival Fibroblast Cell Lines. Biomimetics 2021, 6, 26. [Google Scholar] [CrossRef]
- Kirby, S.; Pesun, I.; Nowakowski, A.; França, R. Effect of Different Post-Curing Methods on the Degree of Conversion of 3D-Printed Resin for Models in Dentistry. Polymers 2024, 16, 549. [Google Scholar] [CrossRef]
- Boussès, Y.; Brulat-Bouchard, N.; Bouchard, P.O.; Tillier, Y. A Numerical, Theoretical and Experimental Study of the Effect of Thermocycling on the Matrix–Filler Interface of Dental Restorative Materials. Dent. Mater. 2021, 37, 772–782. [Google Scholar] [CrossRef]
- Tokay, U.; Koyuturk, A.E.; Aksoy, A.; Ozmen, B. Do the Monomers Release from the Composite Resins after Artificial Aging? Microsc. Res. Tech. 2015, 78, 255–259. [Google Scholar] [CrossRef]
- Sideridou, S.A.; Tserki, V.; Papanastasiou, G. IR and Raman Studies of Photopolymerization and Degradation in Dimethacrylate Networks. Dent. Mater. 2003, 19, 598–605. [Google Scholar]
- Yoshida, K.; Greener, E.H. Resin–Filler Interactions and Raman Spectral Features in Dental Composites. J. Biomed. Mater. Res. 2001, 57, 96–102. [Google Scholar]
- Yap, H.S.; Choo, C.W. Effect of Thermocycling on the Chemical Stability of Resin Composites Monitored by Raman Spectroscopy. Dent. Mater. 2004, 20, 527–533. [Google Scholar]
- Cramer, S.R.; Stansbury, J.W.; Bowman, C.N. Monitoring Polymerization and Degradation in Dental Resins Using Raman Spectroscopy. Dent. Mater. 2006, 22, 858–867. [Google Scholar]
- Gale, M.S.; Darvell, B.W. Thermal Cycling Procedures for Laboratory Testing of Dental Restorations. J. Dent. 1999, 27, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Ferracane, J.L. Hygroscopic and Hydrolytic Effects in Dental Polymer Networks. Dent. Mater. 2006, 22, 211–222. [Google Scholar] [CrossRef]
- Schneider, L.F.J.; Cavalcante, L.M.; Silikas, N. Shrinkage Stresses Generated during Resin-Composite Applications: A Review. J. Dent. Biomech. 2010, 1, 131630. [Google Scholar] [CrossRef] [PubMed]
- Rusu, L.C.; Ardelean, L.C.; Negrutiu, M.L.; Sinescu, C.; Podoleanu, A.G. Raman Spectroscopy Applications in Dental Medicine. Rom. J. Morphol. Embryol. 2015, 56, 747–752. [Google Scholar]
- Gad, M.M.; Fouda, S.M.; Al-Harbi, F.A.; Näpänkangas, R.; Raustia, A. PMMA Denture Base Material Enhancement: A Review of Fiber, Filler, and Nanofiller Addition. Int. J. Nanomed. 2017, 12, 3801–3812. [Google Scholar] [CrossRef]
- Han, Z.; Zhu, B.; Chen, R.; Huang, Z.; Zhu, C.; Zhang, X. Effect of Silver-Supported Materials on the Mechanical and Antibacterial Properties of Reinforced Acrylic Resin Composites. Mater. Des. 2015, 65, 1245–1252. [Google Scholar] [CrossRef]
- Lee, J.-H.; Jo, J.-K.; Kim, D.-A.; Patel, K.D.; Kim, H.-W.; Lee, H.-H. Nano-Graphene Oxide Incorporated into PMMA Resin to Prevent Microbial Adhesion. Dent. Mater. 2018, 34, e63–e72. [Google Scholar] [CrossRef] [PubMed]
- Hamid, S.K.; Alghamdi, L.A.; Alshahrani, F.A.; Khan, S.Q.; Matin, A.; Gad, M.M. In Vitro Assessment of Artificial Aging on the Antifungal Activity of PMMA Denture Base Material Modified with ZrO2; Nanoparticles. Int. J. Dent. 2021, 2021, 5560443. [Google Scholar] [CrossRef] [PubMed]
- Hassanpour, M.; Narongdej, P.; Alterman, N.; Moghtadernejad, S.; Barjasteh, E. Effects of Post-Processing Parameters on 3D-Printed Dental Appliances: A Review. Polymers 2024, 16, 2795. [Google Scholar] [CrossRef]


| Name | Fabrication | Code | Matrix | Filler | Filler Load | Manufacturer |
|---|---|---|---|---|---|---|
| Omnichroma | Light-cured dental resin composite | OMCR | UDMA, TEGDMA | Spherical SiO2-ZrO2 fillers | 79 wt. % | Tokuyama, Tokyo, Japan |
| GC Temp PRINT | 3D-printed dental resin composite for temporary FDPs | GTPR | UDMA (MMA free) | Silica nanoparticles | ~20 wt. % | GC Europe, Leuven, Belgium |
| SprintRay CROWN | 3D-printed dental resin composite for permanent FDPs | SPRY | UDMA, TEGDMA, other dimethacrylates | Ceramic nanoparticles | >50 wt. % | SprintRay, Los Angeles, CA, USA |
| Raman Active Band [cm−1] | Assignment | Reference |
|---|---|---|
| 1285 | Asymmetric C–C–O stretching | [27] |
| 1605 | C=C stretching | [28] |
| 1716 | C=O stretching | [29] |
| 2880 | Symmetric CH2/CH3 stretching | [13,30] |
| 2929 | Asymmetric CH2/CH3 stretching | [28] |
| 3066 | =C–H stretching | [29] |
| Raman Active Band [cm−1] | Assignment | Reference |
|---|---|---|
| ~470 | Si–O–Si bending | [31] |
| 606 | Si–O/ring deformation | [13] |
| 878 | C–O–C stretching | [29] |
| 974 | C–O–C stretching | [29] |
| 1114 | C–O stretching of ester linkages | [28] |
| 1285 | Asymmetric C–C–O stretching | [32] |
| 1399 | CH2 scissoring/CH3 deformation | [13] |
| 1450 | CH2 scissoring/CH3 deformation | [13] |
| 1605 | C=C stretching | [28] |
| 1637 | C=C stretching | [28] |
| 1712 | C=O stretching | [30] |
| 2929 | CH2/CH3 asymmetric stretching | [30] |
| 2954 | CH2/CH3 asymmetric stretching | [30] |
| Raman Active Band [cm−1] | Assignment | Reference |
|---|---|---|
| 238 | Zr–O stretching | [31] |
| 395 | Mixed metal–oxygen | [31] |
| 517 | Si–O | [13] |
| 606 | Si–O | [13] |
| 639 | Si–O | [13] |
| 667 | Si–O | [13] |
| 737 | Si–O | [13] |
| 802 | C–O–C vibration | [29] |
| 915 | C–O–C vibration | [29] |
| 942 | C–O–C vibration | [29] |
| 1003 | Aromatic ring breathing | [30] |
| 1035 | C–O stretching | [28] |
| 1114 | C–O stretching | [28] |
| 1183 | Asymmetric C–C–O stretching | [32] |
| 1223 | Asymmetric C–C–O stretching | [32] |
| 1301 | Asymmetric C–C–O stretching | [32] |
| 1399 | CH2/CH3 deformation | [30] |
| 1449 | CH2/CH3 deformation | [30] |
| 1578 | C=C stretching | [28] |
| 1608 | C=C stretching | [28] |
| 1635 | C=C stretching | [28] |
| 1711 | C=O stretching | [13] |
| 2880 | CH2/CH3 asymmetric stretching | [30] |
| 3065 | =C–H stretching | [29] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Živković, N.; Vulović, S.; Lazarević, M.; Baraba, A.; Jakovljević, A.; Perić, M.; Mitrić, J.; Milić Lemić, A. Exploring the Biological and Chemical Properties of Emerging 3D-Printed Dental Resin Composites Compared to Conventional Light-Cured Materials. Materials 2025, 18, 5170. https://doi.org/10.3390/ma18225170
Živković N, Vulović S, Lazarević M, Baraba A, Jakovljević A, Perić M, Mitrić J, Milić Lemić A. Exploring the Biological and Chemical Properties of Emerging 3D-Printed Dental Resin Composites Compared to Conventional Light-Cured Materials. Materials. 2025; 18(22):5170. https://doi.org/10.3390/ma18225170
Chicago/Turabian StyleŽivković, Nikola, Stefan Vulović, Miloš Lazarević, Anja Baraba, Aleksandar Jakovljević, Mina Perić, Jelena Mitrić, and Aleksandra Milić Lemić. 2025. "Exploring the Biological and Chemical Properties of Emerging 3D-Printed Dental Resin Composites Compared to Conventional Light-Cured Materials" Materials 18, no. 22: 5170. https://doi.org/10.3390/ma18225170
APA StyleŽivković, N., Vulović, S., Lazarević, M., Baraba, A., Jakovljević, A., Perić, M., Mitrić, J., & Milić Lemić, A. (2025). Exploring the Biological and Chemical Properties of Emerging 3D-Printed Dental Resin Composites Compared to Conventional Light-Cured Materials. Materials, 18(22), 5170. https://doi.org/10.3390/ma18225170










