1. Introduction
The oxidation behavior of Fe–Al intermetallics (notably FeAl and related iron aluminides) is of fundamental and practical interest for high-temperature applications because these materials can form protective Al
2O
3 scales that markedly slow further oxidation [
1,
2,
3]. The formation, continuity, and crystallographic form (polymorph) of alumina are critical: a continuous, adherent α-Al
2O
3 layer provides the best long-term protection, whereas transient or non-continuous alumina permits rapid inward/outward transport of species and can lead to breakaway oxidation [
4,
5,
6,
7].
Steam (H
2O(g)) environments modify oxidation chemistry and transport in several important ways: they change the effective oxygen activity at the oxide/gas interface, enable hydroxyl-mediated transport pathways, and can promote volatile species and internal oxidation processes that are not active in dry O
2 or air. Consequently, alloys and coatings that remain protective in dry oxygen may experience morphological changes, multi-layer scale formation, or accelerated degradation in steam atmospheres—effects that become increasingly important for energy systems operating in the 600–750 °C window (e.g., ultra-supercritical steam cycles, boilers, steam turbines, nuclear claddings). Experimental and review studies document that steam commonly produces outward Fe-rich nodules, inner (Fe, Cr) or spinel layers, and can increase spallation/scale porosity compared with dry exposures [
8,
9,
10,
11,
12,
13,
14].
For Fe–Al systems, the literature shows a complex, composition- and microstructure-dependent response. Short-to-moderate exposures often produce continuous Al
2O
3 on aluminide coatings and some bulk FeAl alloys, yet long-term steam exposures may reveal: (a) development of multi-layered scales with Fe-rich outer oxides; (b) internal oxidation of Al beneath the surface; (c) Al depletion at the metal/scale interface due to interdiffusion; and (d) mechanical failure of the scale (cracking, spallation) driven by growth stresses and phase transformations [
2,
6,
9,
15,
16,
17,
18]. Alloying elements (Cr, Si, Y, reactive elements) and microstructural features (grain size, second phases, porosity, pre-existing defects or scratches) strongly influence whether protective alumina persists or the system transitions to a non-protective morphology [
6,
12,
19,
20,
21].
Despite the substantial body of work on steam oxidation of steels, Ni-base alloys, and aluminide coatings, there is a relative paucity of systematic, high-resolution, long-duration studies that document the morphological evolution of corrosion products specifically for bulk alloys whose matrix is the FeAl intermetallic phase exposed to steam at 700 °C for times approaching 2000 h. Many prior investigations focus on shorter times, on coated steels rather than bulk FeAl intermetallics, or on mixed O
2/H
2O atmospheres. Where long steam exposures have been reported, important morphologies such as nodule coalescence, multi-layer scale formation, and Al-depletion have been observed but not comprehensively correlated with high-resolution microstructural analyses across a wide time span (hours → thousands of hours) [
3,
9,
10,
11,
13,
22,
23,
24,
25,
26].
The present study fills this gap by performing a time-series analysis of oxide morphology and composition on a FeAl-based alloy exposed to H2O(g) at 700 °C for exposure times up to 2000 h. We combine surface SEM/EDS, where appropriate, XRD phase analysis, to classify oxide morphologies as a function of exposure time; identify transitions from protective alumina-dominated scales to complex multi-layered or Fe-rich morphologies; and assess the role of alloy chemistry and initial microstructure (including scratches/defects) in governing scale integrity and protectiveness.
These results provide mechanistic insight relevant to material selection and coating design for long-term service in steam environments, and supply a detailed dataset (morphology classes, composition profiles, and kinetic fingerprints) that can support modeling efforts and practical guidelines for Fe-Al-based materials and aluminide coatings in power-plant and related high-temperature steam applications.
2. Materials and Methods
The tests were conducted on samples made of the Fe40Al5Cr0.2TiB alloy, the chemical composition of which is presented in
Table 1. ARMCO iron (technically pure), ARO aluminum (99.995 wt%), aluminothermic chromium obtained by the Kroll method, and amorphous boron (technically pure) were used for melting. Melts were carried out in a Balzers VSG-2 induction vacuum furnace (Balzers, Lichtenstein). Due to the fact that the as-cast material was brittle and had a coarse-grained structure and significant segregation of chemical composition, heat treatment was applied. Homogenizing annealing was carried out at 1050 °C for 72 h, as previous studies had shown that these are the parameters at which microsegregation of chemical composition is removed [
27,
28].
Corrosion tests at 700 °C in a flowing water vapor environment were performed on a test stand designed for isothermal oxidation corrosion. Oxidation times were 1, 50, 100, 300, 1000, and 2000 h. Oxidation in a steam environment was conducted in a furnace consisting of a tubular reactor divided into two zones. The first zone was the furnace’s corrosion chamber, while the second zone was the steam superheater. A steam generator was mounted on the front panel of the furnace, with a capacity of 200 mL/h during the test. During the test, steam was continuously supplied to the steam superheater and then to the corrosion chamber. Distilled water was used to generate the steam. Observations of the oxide layer surface of the Fe40Al5Cr0.2TiB intermetallic alloy samples after oxidation for initial oxidation times (1 h, 50 h, and 100 h) were performed using a ZEISS SUPRA 35 high-resolution scanning electron microscope (Carl Zeiss, Oberkochen, Germany). Observations of the surface state after oxidation for 300, 1000, and 2000 h were performed using a Hitachi S-3400N scanning electron microscope (SEM) (Hitachi High-Tech Corp., Tokyo, Japan) with secondary electron (SE) and backscattered electron (BSE) detectors, and a Hitachi S-4200 microscope with secondary electron (SE) detectors (Hitachi High-Tech Corp., Tokyo, Japan). The chemical composition of the oxidation products was determined using a Thermo Noran (System Six) energy-dispersive X-ray spectrometer (EDS) (Waltham, MA, USA) at an electron beam acceleration voltage of 15 keV, coupled to a Hitachi S-3400N microscope. Details of the scale morphology were also observed using a TESCAN S-8000 BrightBeam™ SEM at a voltage of 5 kV (TESCAN, Brno, Czech Republic). Surface development was measured using a non-contact optical profilometer with a MicroProf 3000 (FRT GmbH, Bergisch Gladbach, Germany) white light aberration head.
3. Results
3.1. Surface Morphology and Oxide Evolution (SEM/EDS/XRD)
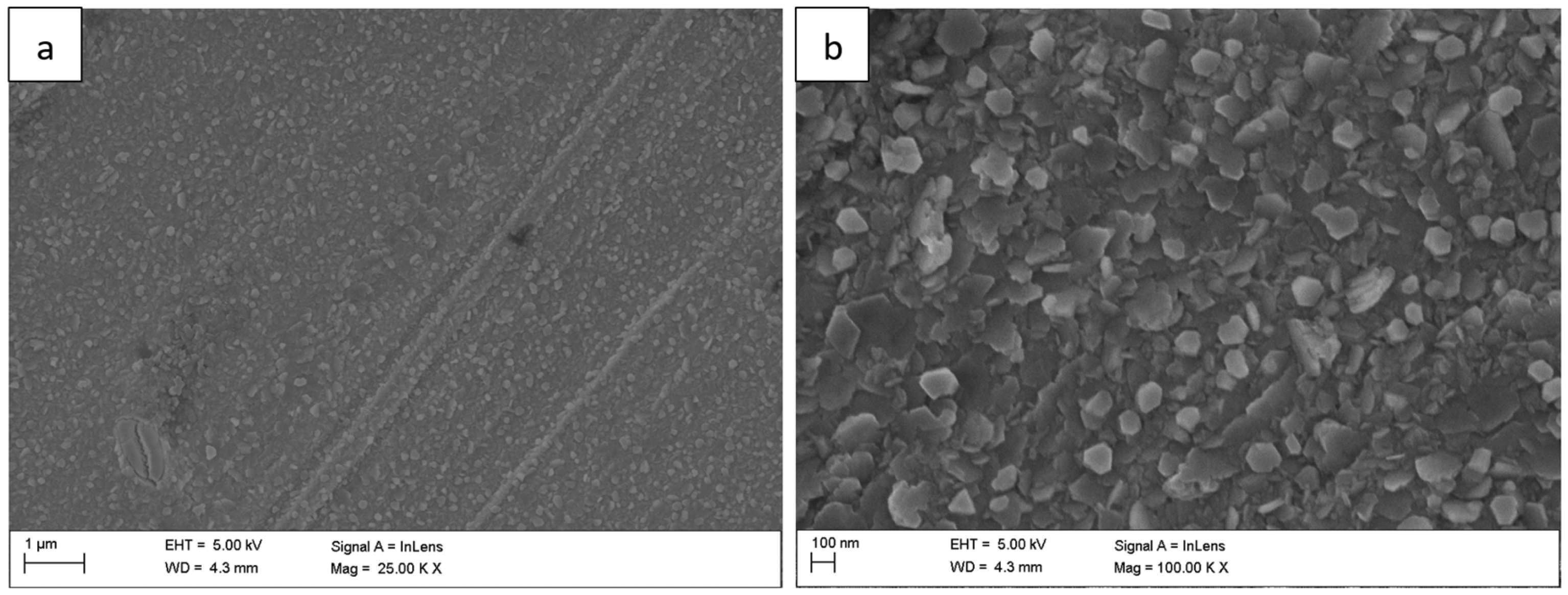
The surface morphology of the Fe40Al5Cr0,2TiB alloy after oxidation in water vapor at 700 °C evolved significantly with exposure time. After 1 h (
Figure 1), a relatively continuous, fine-grained oxide layer covered the surface, consisting mainly of needle-like structures (whiskers) and small agglomerates (flakes). These elongated oxides grew perpendicularly to the substrate, forming a columnar morphology typical of the metastable θ-Al
2O
3 polymorph [
1,
2,
5]. As the exposure time increased to 50 h (
Figure 2) and 100 h (
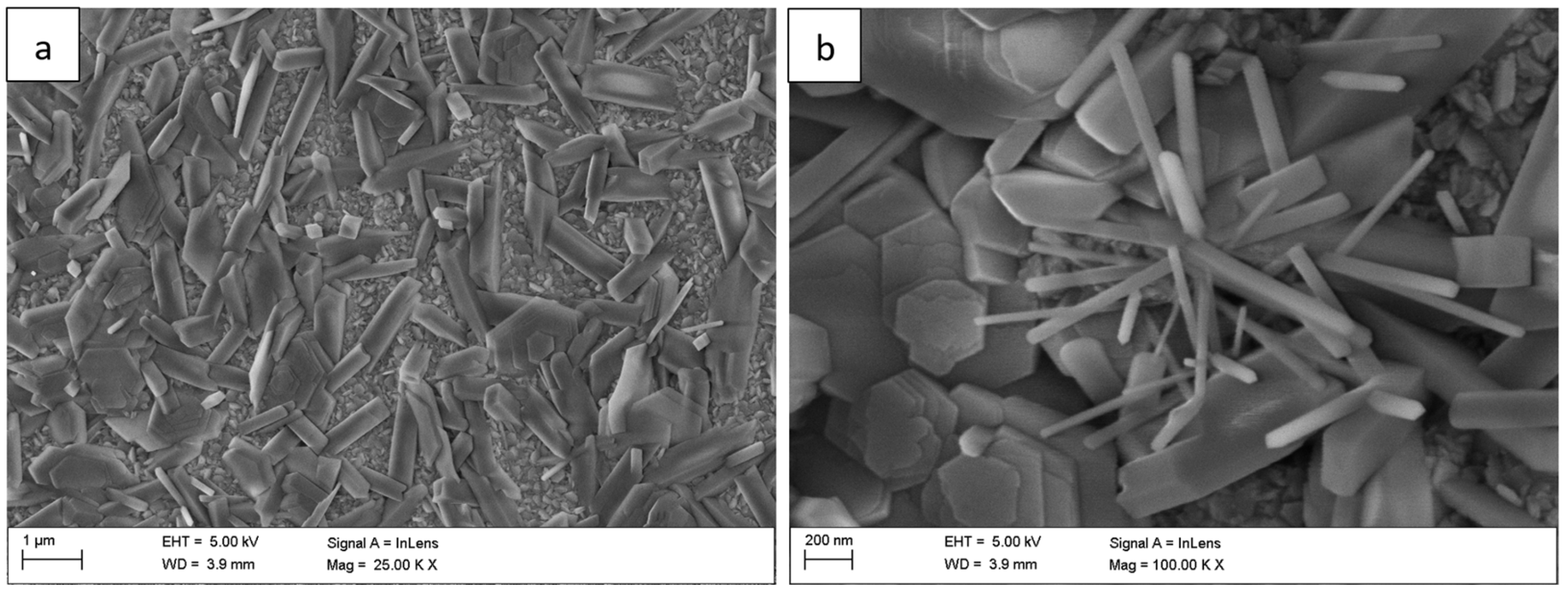
Figure 3), whisker density decreased, and the oxide became denser, forming clustered grains with a partially flattened morphology.
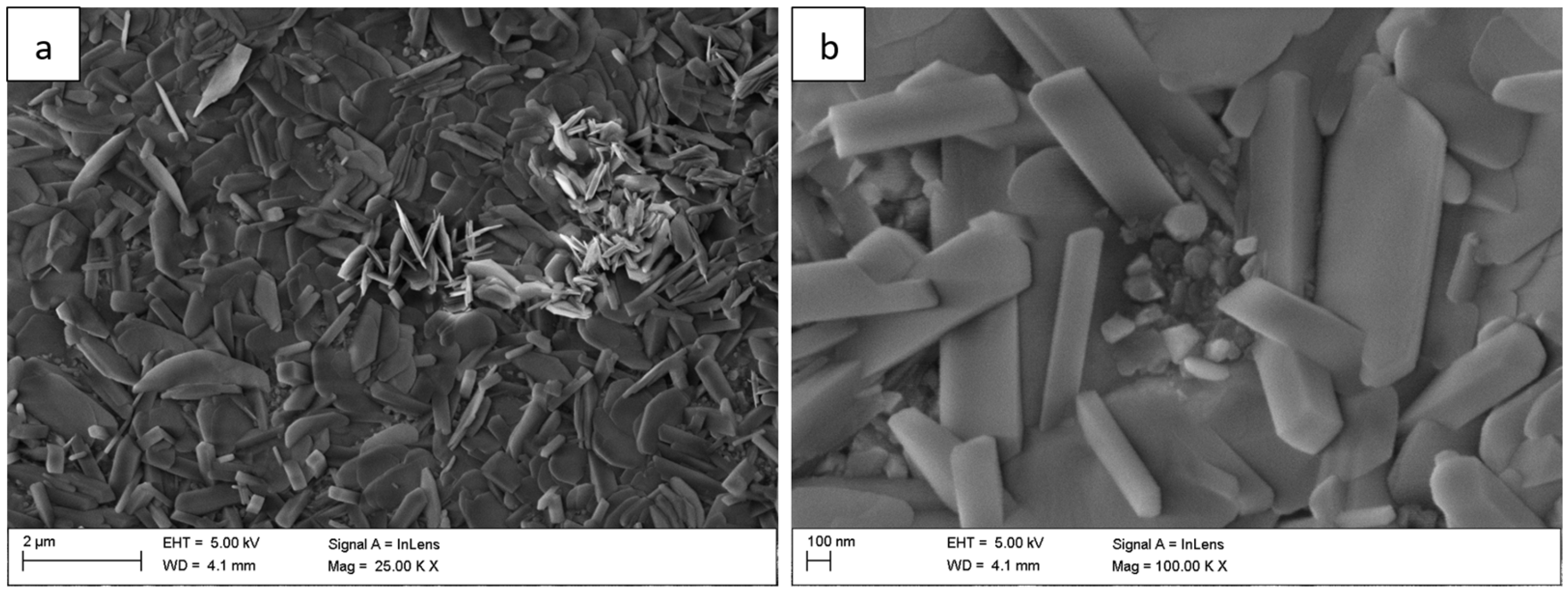
For long-term oxidation (≥1000 h,
Figure 4 and
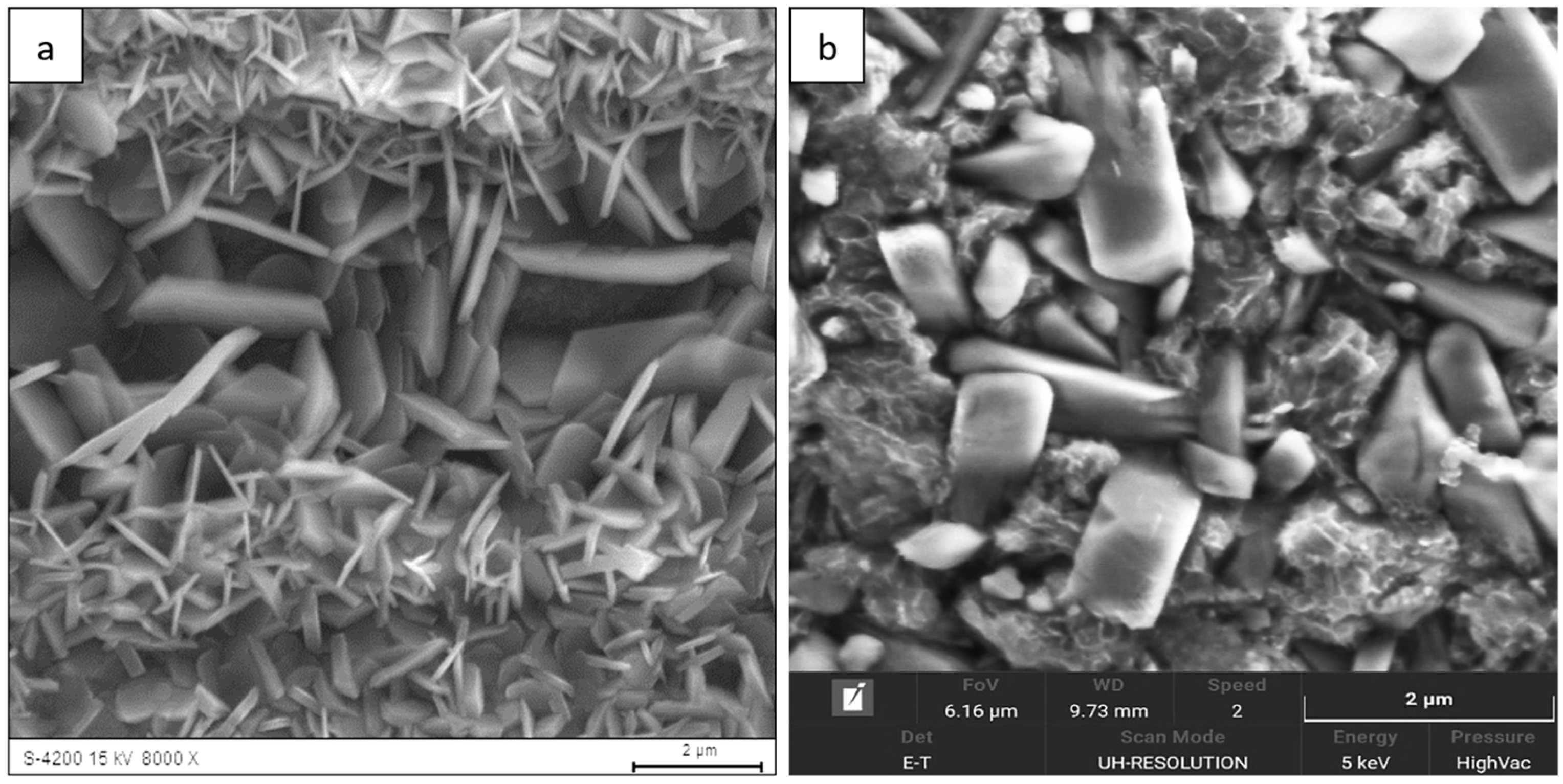
Figure 5), the whisker-like features gradually transformed into compact, globular clusters distributed heterogeneously across the surface. After 2000 h (
Figure 6), the oxide layer exhibited coarse granular agglomerates and locally sintered regions, indicating a transformation toward the stable α-Al
2O
3 structure [
3,
4,
8]. The morphological transition from fine whiskers to dense nodules corresponds to the thermally activated θ → α phase transformation, which is associated with decreasing defect density and a reduction in internal energy per unit volume [
5].
Similar morphological evolution has been reported for FeAl and FeCrAl alloys oxidized in dry air or steam at 750–950 °C [
13,
29]. Notably, the present results confirm that prolonged oxidation even at 700 °C in high H
2O(g) activity promotes the nucleation and growth of α-Al
2O
3, likely due to local temperature gradients and Al diffusion from the substrate. This observation aligns with the findings of Cebulski et al. [
6] and Grabke [
1], who indicated that the presence of Cr and Ti additions facilitates alumina stabilization at lower temperatures by altering interfacial energy and vacancy concentrations.
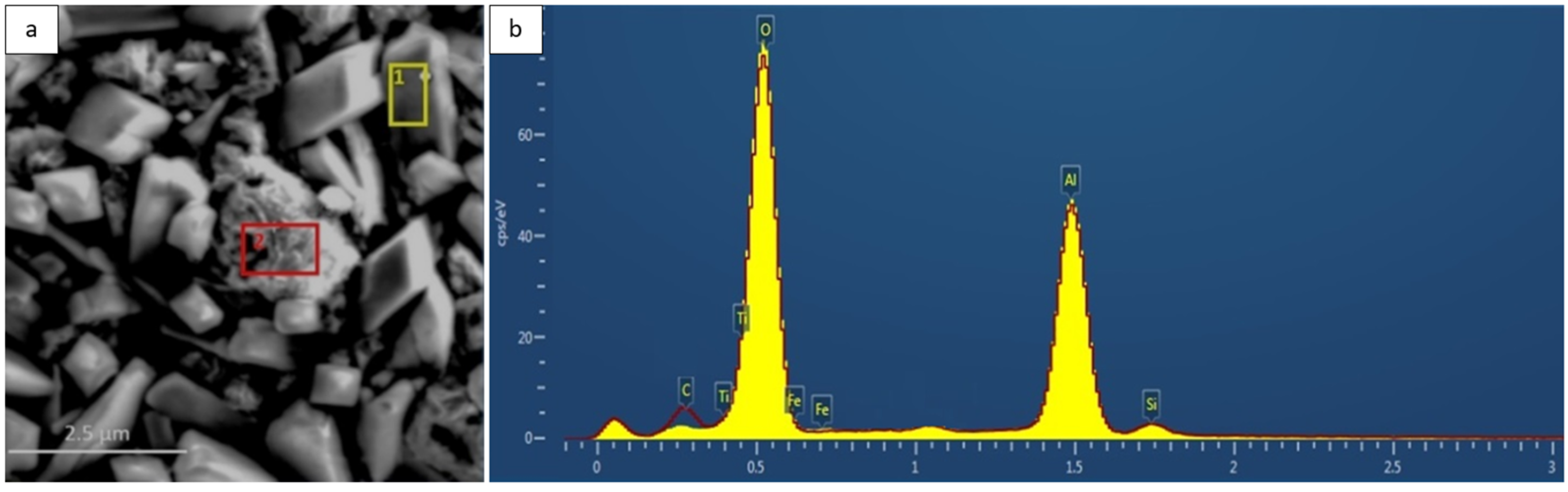
EDS analysis revealed that the oxide layer contained predominantly aluminum and oxygen, with trace amounts of Fe, Cr, and Ti (
Figure 7). The presence of these substrate elements in the outer scale results from the relatively deep excitation volume during EDS measurements and local diffusion processes at the metal/oxide interface [
10,
11]. In some areas, minor Si contamination was observed, originating from the furnace lining material.
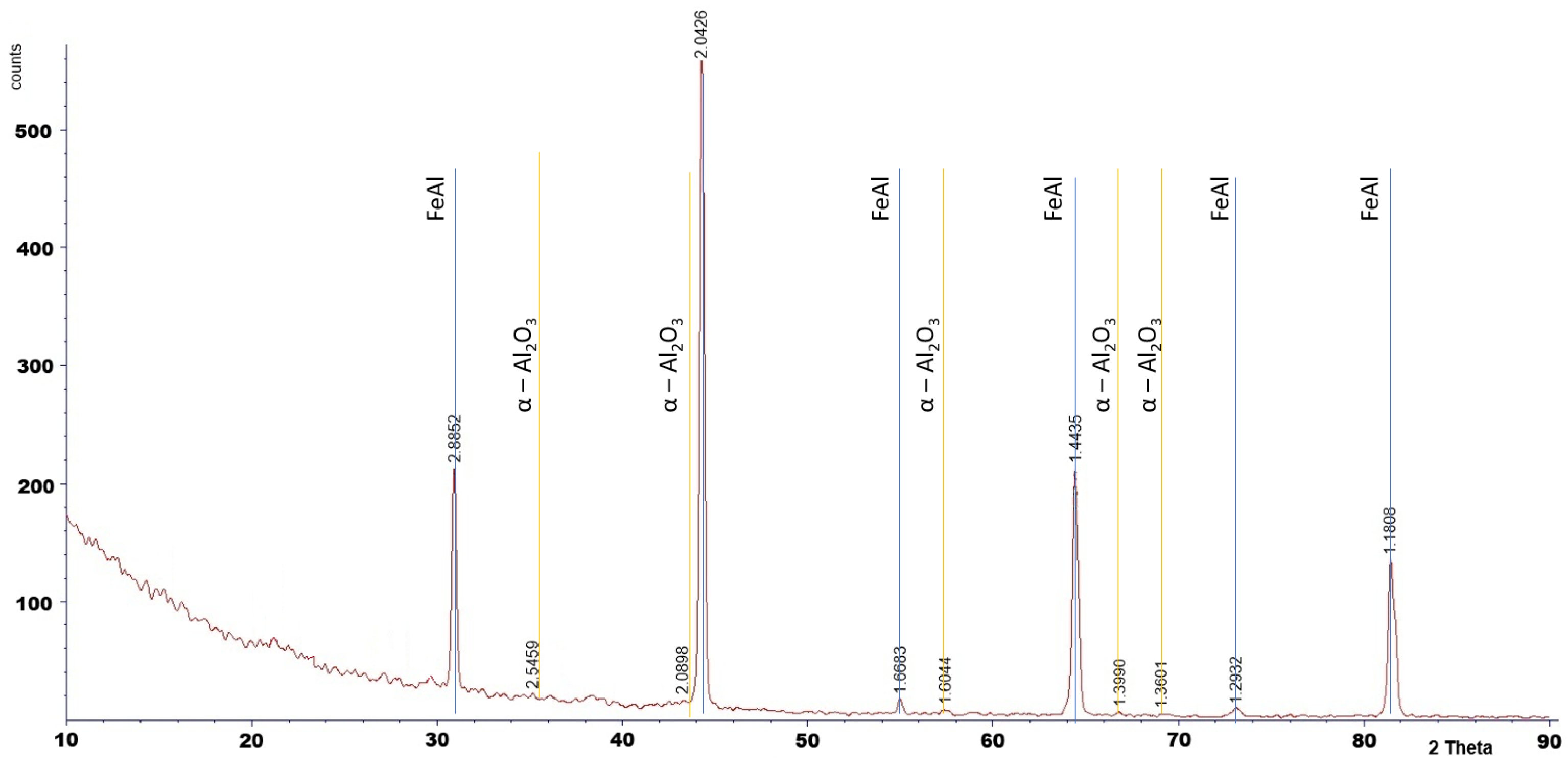
Phase analysis by XRD confirmed the presence of α-Al
2O
3 after 2000 h of oxidation (
Figure 8).
The XRD analysis was performed only for the specimen oxidized for 2000 h. For shorter oxidation times, the oxide layers were too thin to produce detectable diffraction peaks, as XRD is a bulk-sensitive technique. Phase identification for shorter exposures was therefore supported by SEM observations and literature reports.
The transition of whisker morphology (θ-Al
2O
3) into granular α-Al
2O
3 was also observed for NiAl alloys at 1000–1100 °C [
5,
30] and for FeCrAl coatings at 850–900 °C [
8,
16]. In this study, the same evolution occurred at 700 °C, suggesting that water vapor may accelerate the stabilization of α-Al
2O
3 through hydroxyl-assisted diffusion mechanisms, as reported in previous studies [
18,
23].
3.2. Surface Topography (Optical Profilometry)
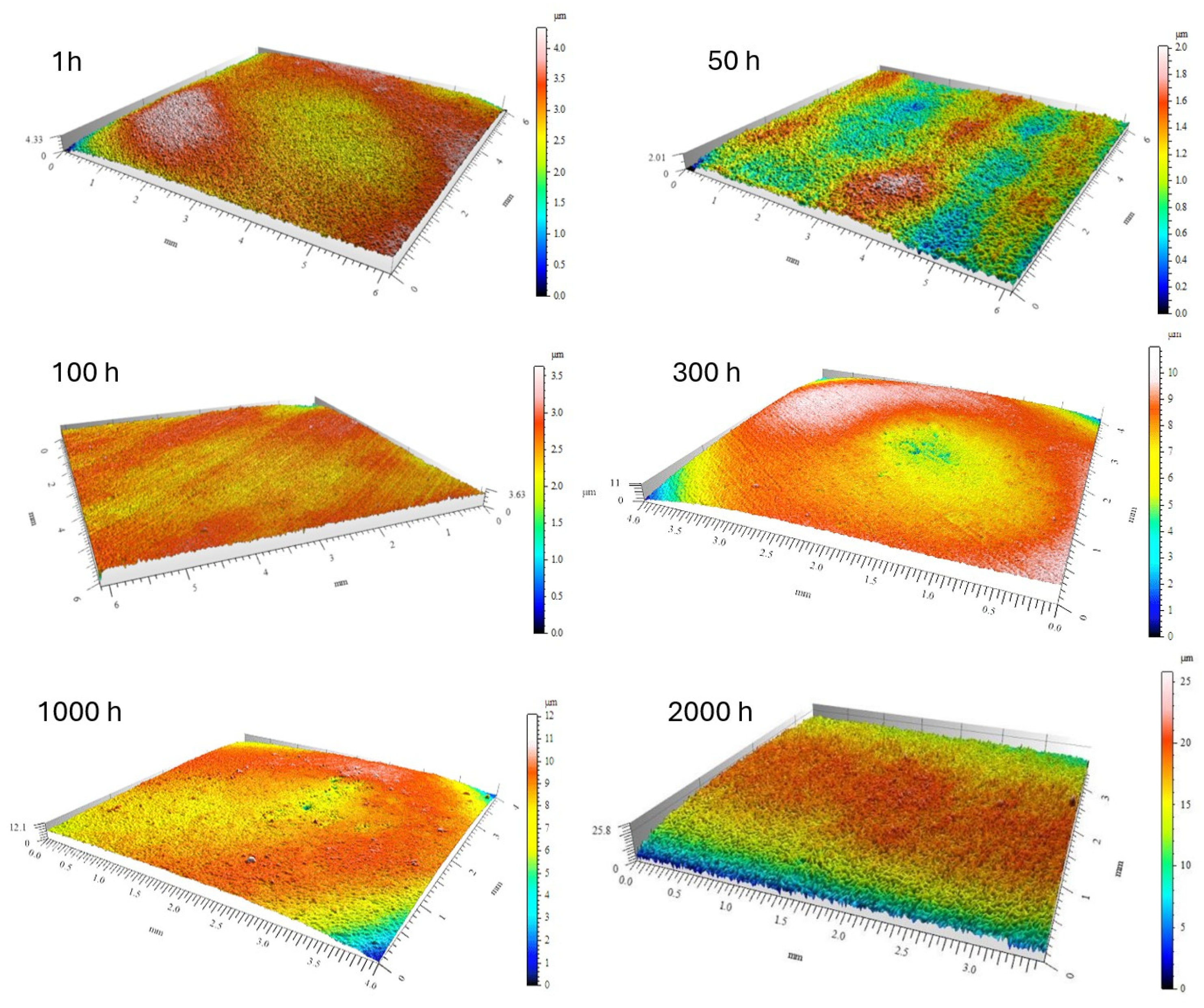
The evolution of surface roughness parameters with oxidation time, determined by optical profilometry, is summarized in
Table 2. The mean roughness (R
a) and maximum height (R
z) showed an initial slight decrease after 50–100 h of exposure (R
a ≈ 0.09 µm, Rz ≈ 0.57 µm), suggesting that fine-grained alumina layers partially smoothed the surface (
Figure 9 and
Figure 10). However, after 2000 h, both R
a and R
z increased markedly to 0.83 µm and 5.79 µm, respectively, reflecting the formation of coarse α-Al
2O
3 nodules and localized delamination zones.
The correlation between the increasing roughness and the observed SEM morphologies indicates that long-term oxidation in steam promotes thickening and sintering of the oxide scale, leading to the development of surface asperities and cracks [
4,
19,
20]. These changes are consistent with models of alumina scale growth under stress accumulation and void formation beneath the oxide layer [
10,
26].
The increase in Ra and Rz parameters is therefore a quantitative signature of the morphological transition from a fine protective θ-Al2O3 whisker network to a coarse α-Al2O3 granular scale. The combination of SEM, EDS, and profilometry analyses clearly demonstrates that steam not only accelerates the θ → α transformation but also modifies the topography of the oxide, leading to increased roughness.
4. Analysis of the Results and Conclusions
The morphology of the oxide layer formed during steam oxidation, observed by scanning electron microscopy (SEM), at the initial stage of oxidation consists of flake-like regions. With increasing oxidation time, oxide structures in the form of needle-shaped whiskers growing from the substrate toward the oxidizing atmosphere were identified on the surface. Prolonged oxidation (2000 h) resulted in the formation of oxides with a granular morphology. Their nucleation and growth depend on local oxidation conditions, as indicated by their nonuniform and dispersed distribution over the surface. The whisker-shaped oxides, which crystallized during the early stages of oxidation, gradually transformed with increasing exposure time, disappearing and giving rise to more compact, flat crystallites.
The presence of aluminum oxide on the surface of the Fe40Al5Cr0.2TiB alloy in the form of fine needle-like structures was also reported in oxidation studies conducted by Nowak K. and Kupka M. [
31], although this morphology appeared only during oxidation at a higher temperature of 800 °C for 200 h. At 900 °C, the needle-like oxide morphology was more pronounced. A similar needle-shaped oxide morphology was also observed in [
32] during oxidation at 750 °C and 950 °C for 550 h. Comparable oxide morphologies have been reported not only for FeAl-based intermetallic alloys but also for NiAl alloys, which, like FeAl, crystallize in the B2 structure. In the case of NiAl, however, the needle-like aluminum oxide morphology was observed during oxidation at 1000 °C [
33,
34].
These observations indicate that both the morphology of corrosion products forming on FeAl-based intermetallic alloys and the kinetics of the oxidation process depend on the oxidation temperature and exposure time. All the above-mentioned researchers have noted that the needle-like oxide morphology is characteristic of the θ-Al
2O
3 phase. The morphology of oxides formed on the Fe40Al5Cr0.2TiB alloy obtained in this study can therefore be associated with the allotropic form of aluminum oxide present on the alloy surface. X-ray diffraction (XRD) analysis identified the presence of the α-Al
2O
3 phase. Importantly, the stable α-Al
2O
3 phase is generally recognized as a high-temperature modification of aluminum oxide, typically forming on the surface of oxidized materials at temperatures above 900 °C [
31,
35,
36,
37].
Author Contributions
Conceptualization, J.C. and M.P.; methodology, J.C., M.P. and J.W.; validation, D.P. and M.S.; formal analysis, J.G.; investigation, D.P., M.S. and J.W.; data curation, M.P.; writing—original draft preparation, J.C., M.P., D.P., A.S., J.G., M.S. and J.W.; writing—review and editing, J.C., M.P., D.P., A.S., J.G., M.S. and J.W.; visualization, D.P. and M.P.; supervision, A.S. All authors have read and agreed to the published version of the manuscript.
Funding
The project was co-financed by the European Union within the program “The European Funds for Śląsk (Silesia) 2021–2027”.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
Author Dorota Pasek was employed by the company Promobil s.c. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Grabke, H.J. The Oxidation of NiAl and FeAl. Intermetallics 1999, 7, 1153–1158. [Google Scholar] [CrossRef]
- Xu, C.-H.; Gao, W.; He, Y.-D. High temperature oxidation behaviour of FeAl intermetallics—Oxide scales formed in ambient atmosphere. Scr. Mater. 2000, 42, 975–980. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhao, Q.; Lu, J.; Liang, Z.; Yuan, Y.; Gu, Y.; Huang, J. Steam Oxidation of Scratched β-FeAl Coatings on P92 Steel. Mater. Corros. 2022, 74, 197–208. [Google Scholar] [CrossRef]
- Smialek, J.L. Oxide Morphology and a Spalling Model for NiAl. Metall. Trans. A 1978, 9, 309–320. [Google Scholar] [CrossRef]
- Doychak, J.; Smialek, J.L.; Mitchell, T.E. Transient Oxidation of Single-Crystal β-NiAl. Metall. Trans. A 1989, 20, 499–518. [Google Scholar] [CrossRef]
- Cebulski, J.; Pasek, D.; Sozańska, M.; Popczyk, M.; Gabor, J.; Swinarew, A. Oxidation of the Alloy Based on the Intermetallic Phase FeAl in the Temperature Range of 700–1000 °C in Air and Possibilities of Practical Application. Materials 2025, 18, 1835. [Google Scholar] [CrossRef] [PubMed]
- Brumm, M.W.; Grabke, H.J. Oxidation Behaviour of NiAl—II. Cavity Formation beneath the Oxide Scale on NiAl of Different Stoichiometries. Corros. Sci. 1993, 34, 547–561. [Google Scholar] [CrossRef]
- Agüero, A.; Baráibar, I.; Gutiérrez, M.; Tuurna, S.; Toivonen, A.; Penttilä, S.; Auerkari, P. Steam Oxidation of Aluminide-Coated and Uncoated TP347HFG Stainless Steel under Atmospheric and Ultra-Supercritical Steam Conditions at 700 °C. Coatings 2020, 10, 839. [Google Scholar] [CrossRef]
- Yuan, J.; Wu, X.; Wang, W.; Zhu, S.; Wang, F. Investigation on the Enhanced Oxidation of Ferritic/Martensitic Steel P92 in Pure Steam. Materials 2014, 7, 2772–2783. [Google Scholar] [CrossRef]
- Qi, W.; Qiao, Y.; Ru, W.; Wang, X.; Zhang, X.; Zheng, T.; Du, S.; Wang, P.; Yang, K. High-Temperature Steam Oxidation and Surface Microstructure Evolution of Fe13Cr6Al(1–4)Mo0.15Y Alloys. Metals 2024, 14, 1229. [Google Scholar] [CrossRef]
- Lu, J.; Huang, J.; Wang, J.; Yang, Z.; Gu, Y. Long-term Degradation Behavior of Slurry Aluminide Coating on Super304H Stainless Steel at 650 °C. Corros. Sci. 2021, 178, 109054. [Google Scholar] [CrossRef]
- Yang, Z.; Lu, J.; Zhang, P.; Le, M.; Zhou, Y.; Huang, J.; Yuan, Y.; Gu, Y. Oxidation Performance and Degradation Mechanism of Aluminide Coatings—Steam Studies and Microstructural Evolution. Surf. Coat. Technol. 2020, 391, 125700. [Google Scholar] [CrossRef]
- Rutkowski, B.; Baran, K.; Błoniarz, R.; Kozieł, T. The Morphology and Microstructure of Oxide Scale Grown on Austenitic Steel during Steam Oxidation at 700 °C for 500 h. Materials 2021, 14, 3821. [Google Scholar] [CrossRef] [PubMed]
- Cebulski, J. Application of FeAl interemetallic phase matrix based alloys in the turbine components of a turbocharger. Metalurgija 2015, 54, 154–156. [Google Scholar]
- Milewska, A.; Hierro, M.P.; Trilleros, J.A.; Bolívar, F.J.; Pérez, F.J. Coating Design Using Computational Codes for Steam—FeAl Coatings, Oxidation, Steam, Steel P92, Thermo-Calc. Mater. Sci. Forum 2004, 461–464, 321–326. [Google Scholar] [CrossRef]
- Taghipour, M.; Eslami, A.; Bahrami, A. High-Temperature Oxidation Behaviour of Aluminide Coatings on HP-MA Steel. Surf. Coat. Technol. 2022, 434, 128181. [Google Scholar] [CrossRef]
- Senderowski, C. Synthesis, Properties and Applications of Intermetallics, Ceramic and Cermet Coatings. Materials 2022, 15, 8408. [Google Scholar] [CrossRef] [PubMed]
- Dudziak, T.; Jura, K.; Polkowska, A.; Deodeshmukh, V.; Warmuzek, M.; Witkowska, M.; Ratuszek, W.; Chruściel, K. Steam Oxidation Resistance of Advanced Steels and Ni-Base Alloys—1000 h at 700 °C. Oxid. Met. 2018, 89, 755–779. [Google Scholar] [CrossRef]
- Rebak, R.B.; Gupta, V.K.; Larsen, M. Oxidation Characteristics of Two FeCrAl Alloys in Air and Steam. JOM 2018, 70, 1484–1492. [Google Scholar] [CrossRef]
- Kopec, M. Recent Advances in the Deposition of Aluminide Coatings and High-Temperature Performance. Coatings 2024, 14, 630. [Google Scholar] [CrossRef]
- Li, Q. Influence of Pre-Oxidation on High-Temperature Oxidation of Aluminide Coatings. Front. Mater. 2021, 8, 679682. [Google Scholar] [CrossRef]
- Pint, B.A.; Zhang, Y.; Walker, L.R.; Wright, I.G. Long-Term Performance of Aluminide Coatings on Fe-Base Alloys. Surf. Coat. Technol. 2007, 202, 637–642. [Google Scholar] [CrossRef]
- Montealegre, M.; González-Carrasco, J.; Morris-Muñoz, M.; Chao, J.; Morris, D. Oxidation Behaviour of ODS FeAlCr Intermetallic Alloys—Role of Dispersoids and Microstructure. Intermetallics 2005, 8, 439–446. [Google Scholar] [CrossRef]
- Smialek, J.L. A Deterministic Interfacial Cyclic Oxidation Spalling Model. Acta Mater. 2003, 51, 469–483. [Google Scholar] [CrossRef]
- Agüero, A. Aluminide Coatings by Means of Slurry Application: A Low-Cost Route. Coatings 2024, 14, 1243. [Google Scholar] [CrossRef]
- Zhou, Y.; Lu, J.; Shen, M.; Yuan, Y.; Gu, Y.; Yang, Z.; Huang, J.; Zhao, Q. Low-Temperature Formation and Steam Oxidation of β-FeAl Coating on P92 Steel. Corros. Sci. 2019, 163, 108227. [Google Scholar] [CrossRef]
- Cebulski, J.; Lalik, S. Changes in the structure of alloy on the matrix of FeAl intermetallic phase after primary crystallization and homogenizing treatment. J. Mater. Process. Technol. 2005, 162–163, 4–8. [Google Scholar] [CrossRef]
- Barcik, J.; Cebulski, J. Plastify of alloy based on the matrix of intermetallic FeAl phase. Arch. Metall. 2000, 45, 315–330. [Google Scholar]
- Reszka, K.; Morgiel, J.; Żurek, Z.; Jaron, A. Characterization of alumina scale formed on FeCrAl steel. Arch. Metall. Mater. 2014, 59, 77–81. [Google Scholar] [CrossRef]
- Swadźba, R.; Swadźba, L.; Wiedermann, J.; Hetmańczyk, M.; Witala, B. Characterization of Alumina Scales Grown on a 2nd Generation Single Crystal Ni Superalloy During Isothermal Oxidation at 1050, 1100 and 1150 °C. Oxid. Met. 2014, 82, 195–208. [Google Scholar] [CrossRef]
- Nowak, K.; Kupka, M. High-temperature oxidation behaviour of B2 FeAl based alloy with Cr, Zr and B additions. Mater. Chem. Phys. 2012, 132, 902–908. [Google Scholar] [CrossRef]
- Janda, D.; Fietzek, H.; Galetz, M.; Heilmaier, M. The effect of micro-alloying with Zr and Nb on the oxidation behavior of Fe3Al and FeAl alloys. Intermetallics 2013, 41, 51–57. [Google Scholar] [CrossRef]
- Tolpygo, V.K.; Clarke, D.R. Microstructural study of the theta-alpha transformation in alumina scales formed on nickel-aluminides. Mater. High Temp. 2000, 17, 59–70. [Google Scholar] [CrossRef]
- El-Awadi, G.A.; Abdel-Samad, S.; Ezzat, S. Elshazly. Hot corrosion behavior of Ni based Inconel 617 and Inconel 738 superalloys. Appl. Surf. Sci. 2016, 37, 224–230. [Google Scholar] [CrossRef]
- Kulak, K.; Kupka, M.; Dercz, G. Oxidation of multicomponent iron aluminide based on FeAl phase. Mater. Eng. 2011, 4, 510–513. [Google Scholar]
- Pasek, D. Utlenianie Stopu na Osnowie Fazy Międzymetalicznej FeAl w Powietrzu I Parze Wodnej [Oxidation of FeAl Intermetallic-Based Alloy in Air and Steam]. Ph.D. Thesis, Silesian University of Technology, Gliwice, Poland, 2021. [Google Scholar]
- Fornalczyk, A.; Cebulski, J.; Pasek, D. The morphology of corrosion products in FeAl alloys after heatresistance tests at different temperatures. Solid State Phenom. 2015, 227, 409–412. [Google Scholar] [CrossRef]
Figure 1.
Surface morphology of the Fe40Al5Cr0.2TiB alloy after oxidation in water vapor at 700 °C for 1 h: (a) low-magnification SEM image showing general oxide coverage on the alloy surface; (b) high-magnification SEM image showing the morphology of fine, needle-like oxide.
Figure 1.
Surface morphology of the Fe40Al5Cr0.2TiB alloy after oxidation in water vapor at 700 °C for 1 h: (a) low-magnification SEM image showing general oxide coverage on the alloy surface; (b) high-magnification SEM image showing the morphology of fine, needle-like oxide.
Figure 2.
Surface morphology of the Fe40Al5Cr0.2TiB alloy after oxidation in water vapor at 700 °C for 50 h: (a) low-magnification SEM image illustrating the formation of a continuous oxide layer; (b) high-magnification SEM image showing partial coalescence and morphology of fine, needle-like oxide whiskers.
Figure 2.
Surface morphology of the Fe40Al5Cr0.2TiB alloy after oxidation in water vapor at 700 °C for 50 h: (a) low-magnification SEM image illustrating the formation of a continuous oxide layer; (b) high-magnification SEM image showing partial coalescence and morphology of fine, needle-like oxide whiskers.
Figure 3.
Surface morphology of the Fe40Al5Cr0.2TiB alloy after oxidation in water vapor at 700 °C for 100 h: (a) low-magnification SEM image showing the progressive densification of the oxide scale; (b) high-magnification image revealing clustered grains with partially flattened morphology.
Figure 3.
Surface morphology of the Fe40Al5Cr0.2TiB alloy after oxidation in water vapor at 700 °C for 100 h: (a) low-magnification SEM image showing the progressive densification of the oxide scale; (b) high-magnification image revealing clustered grains with partially flattened morphology.
Figure 4.
Surface morphology of the Fe40Al5Cr0.2TiB alloy after oxidation in water vapor at 700 °C for 300 h: (a) low-magnification image showing heterogeneous distribution of oxide clusters; (b) high-magnification image presenting the transition from whisker-like to compact granular structures.
Figure 4.
Surface morphology of the Fe40Al5Cr0.2TiB alloy after oxidation in water vapor at 700 °C for 300 h: (a) low-magnification image showing heterogeneous distribution of oxide clusters; (b) high-magnification image presenting the transition from whisker-like to compact granular structures.
Figure 5.
Surface morphology of the Fe40Al5Cr0.2TiB alloy after oxidation in water vapor at 700 °C for 1000 h: (a) low-magnification image of the surface showing growing granular regions; (b) high-magnification image showing morphology oxide.
Figure 5.
Surface morphology of the Fe40Al5Cr0.2TiB alloy after oxidation in water vapor at 700 °C for 1000 h: (a) low-magnification image of the surface showing growing granular regions; (b) high-magnification image showing morphology oxide.
Figure 6.
Surface morphology of the Fe40Al5Cr0.2TiB alloy after oxidation in water vapor at 700 °C for 2000 h: (a) low-magnification SEM image showing coarse granular agglomerates distributed over the surface; (b) high-magnification SEM image showing sintered α-Al2O3 nodules and locally compacted regions.
Figure 6.
Surface morphology of the Fe40Al5Cr0.2TiB alloy after oxidation in water vapor at 700 °C for 2000 h: (a) low-magnification SEM image showing coarse granular agglomerates distributed over the surface; (b) high-magnification SEM image showing sintered α-Al2O3 nodules and locally compacted regions.
Figure 7.
(a) Surface microstructure of the Fe40Al5Cr0.2TiB alloy after oxidation in water vapor at 700 °C for 2000 h; two EDS analysis points are indicated (red and yellow). (b) EDS spectra from the corresponding areas shown in (a): the red line represents the spectrum from the red-marked area, and the yellow line represents the spectrum from the yellow-marked area. The spectra overlap due to the very similar chemical composition of both regions (Al and O with minor Fe, Cr, and Ti).
Figure 7.
(a) Surface microstructure of the Fe40Al5Cr0.2TiB alloy after oxidation in water vapor at 700 °C for 2000 h; two EDS analysis points are indicated (red and yellow). (b) EDS spectra from the corresponding areas shown in (a): the red line represents the spectrum from the red-marked area, and the yellow line represents the spectrum from the yellow-marked area. The spectra overlap due to the very similar chemical composition of both regions (Al and O with minor Fe, Cr, and Ti).
Figure 8.
X-Ray analysis of the Fe40Al5Cr0.2TiB alloy after oxidation in water vapor at 700 °C for 2000 h.
Figure 8.
X-Ray analysis of the Fe40Al5Cr0.2TiB alloy after oxidation in water vapor at 700 °C for 2000 h.
Figure 9.
Three-dimensional image of the surface of the Fe40Al5Cr0.2TiB alloy oxidized in a steam environment at 700 °C for different times.
Figure 9.
Three-dimensional image of the surface of the Fe40Al5Cr0.2TiB alloy oxidized in a steam environment at 700 °C for different times.
Figure 10.
Waviness profiles on selected cross-sections of the sample surfaces after oxidation in a steam environment at 700 °C after different times.
Figure 10.
Waviness profiles on selected cross-sections of the sample surfaces after oxidation in a steam environment at 700 °C after different times.
Table 1.
Chemical composition of Fe40Al5Cr0.2TiB alloy.
Table 1.
Chemical composition of Fe40Al5Cr0.2TiB alloy.
| Compound | Fe | Al | Cr | Ti | B |
|---|
| % at. | 54.80 | 40.10 | 4.86 | 0.18 | 0.06 |
Table 2.
Summary of roughness parameters after oxidation.
Table 2.
Summary of roughness parameters after oxidation.
| Parameter | 1 h | 50 h | 100 h | 300 h | 1000 h | 2000 h |
|---|
| Ra | 0.119 µm | 0.0847 µm | 0.0921 µm | 0.133 µm | 0.175 µm | 0.83 µm |
| Rz | 0.711 µm | 0.486 µm | 0.570 µm | 0.785 µm | 1.19 µm | 5.79 µm |
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).