Guerbet Alcohols, Ideal Substrates for the Sustainable Production of Branched Esters
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Experimental Procedure
2.3. Recovery and Reuse of the Biocatalysts
2.4. Density and Viscosity Determination
3. Results and Discussion
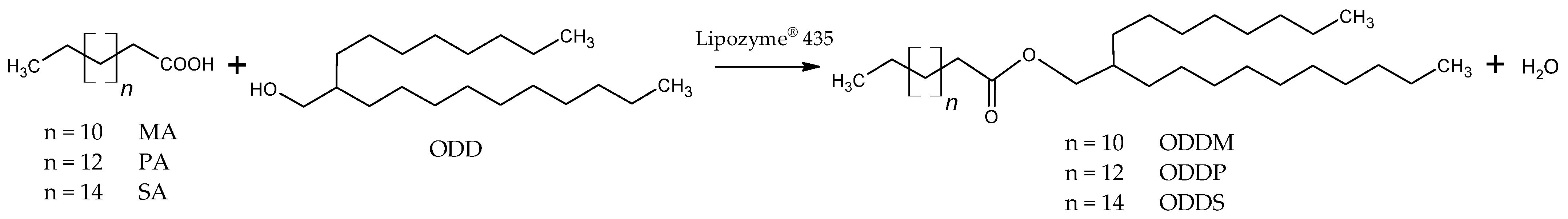
3.1. Influence of Enzyme Concentration
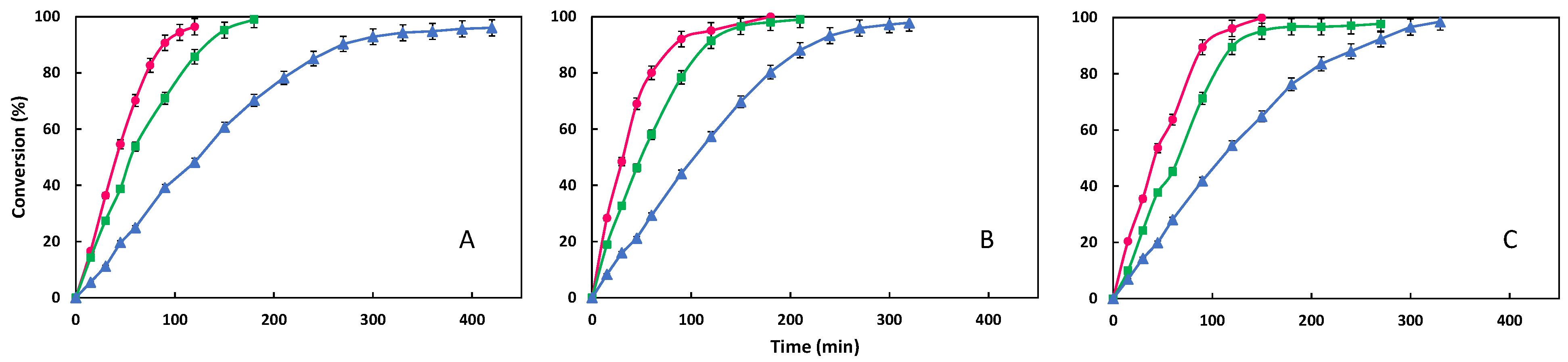
3.2. Influence of Temperature
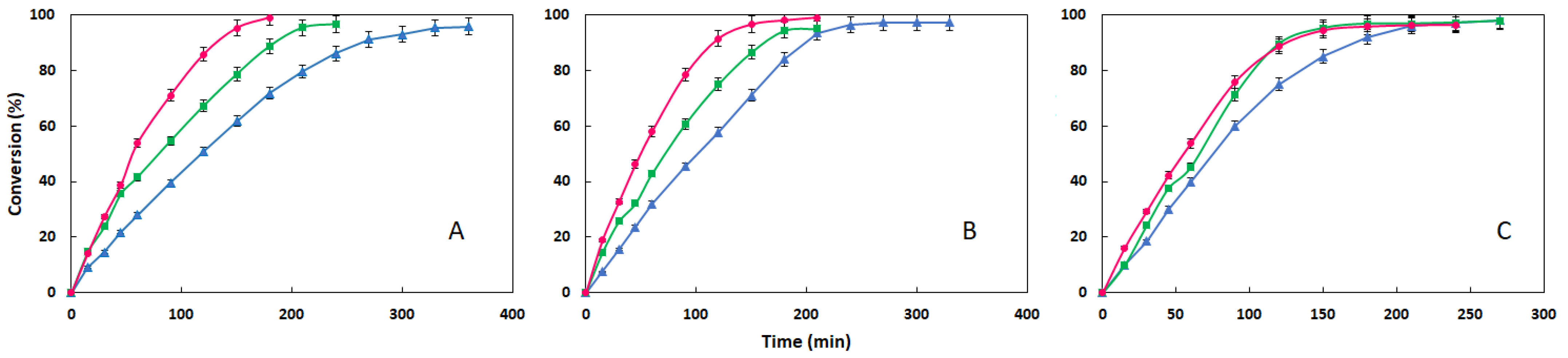
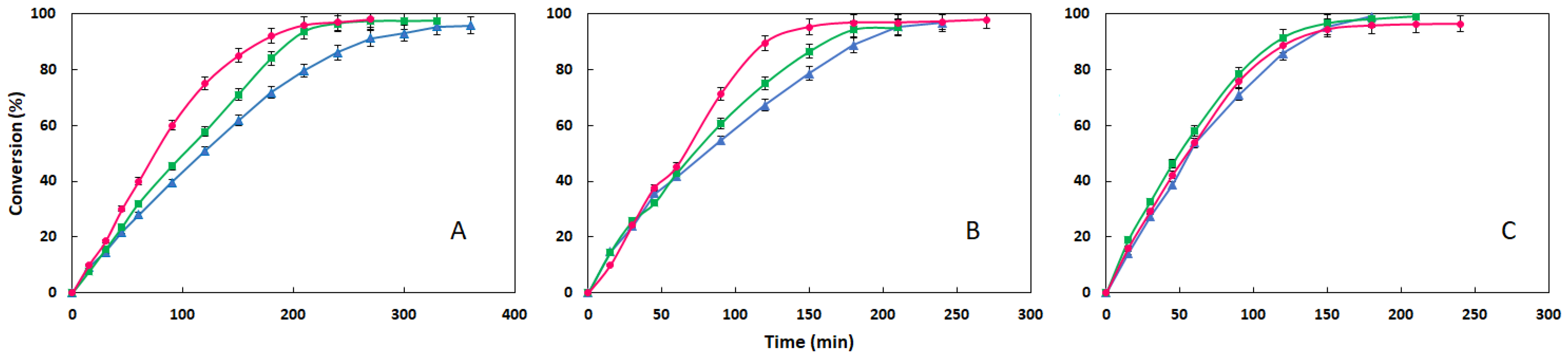
3.3. Comparison of the Three Esters at Different Temperature: Influence of Acid Moiety
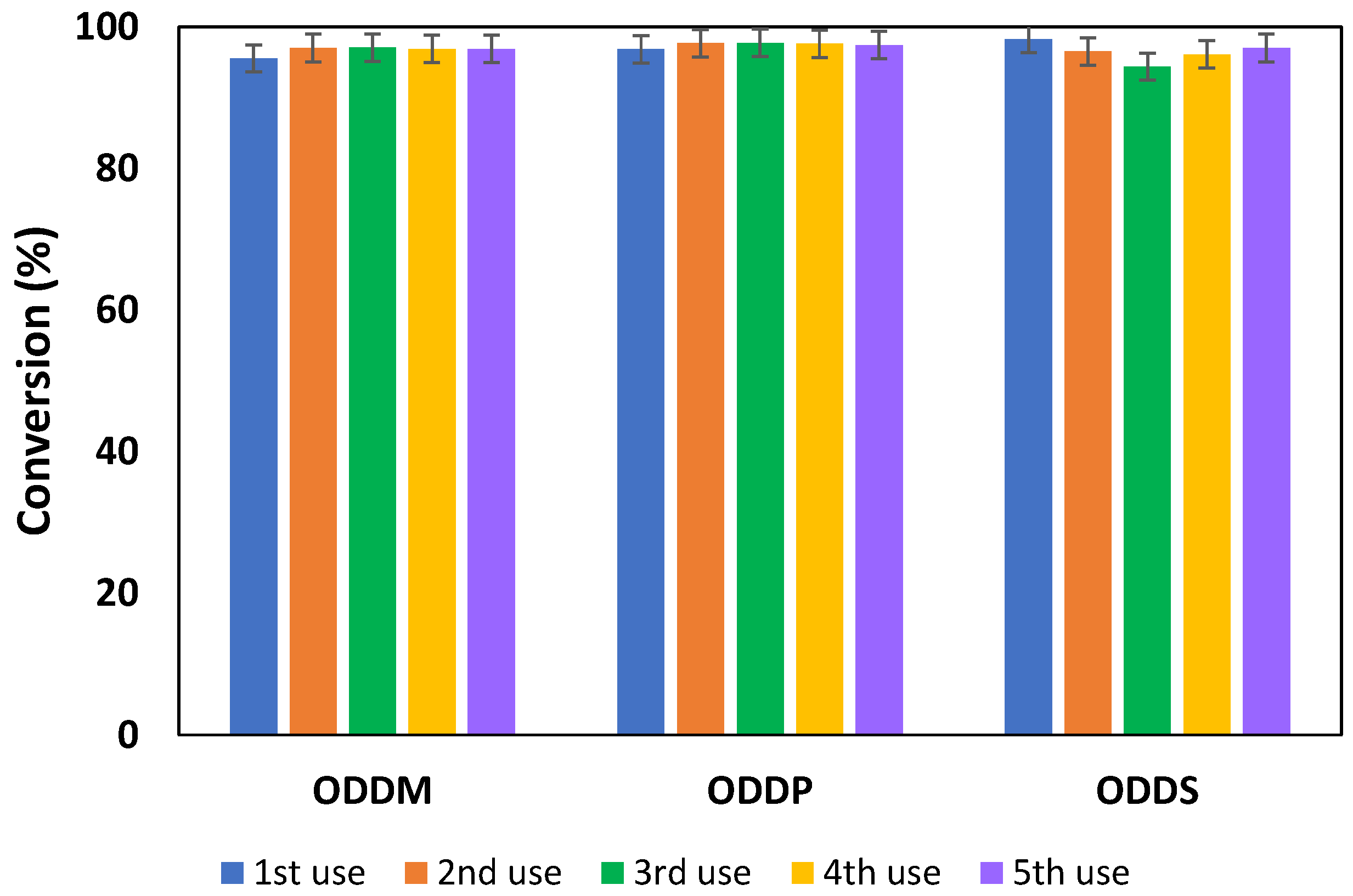
3.4. Study of Enzyme Reuse
3.5. Study of Environmental Sustainability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AE | Atom economy |
| AV | Acid Value |
| CalB | Candida antarctica lipase B |
| cEF | Complete E-factor |
| CME | Carbon mass efficiency |
| D4 | Octamethylcyclotetrasiloxane |
| D5 | Decamethylcyclopentasiloxane |
| EF | E-factor |
| EHS | Environmental, health and safety |
| EPA | Environmental Protection Agency |
| GCI | Green Chemistry Institute |
| LCA | Life cycle assessment |
| MA | Myristic acid |
| ODD | 2-octyl-1-dodecanol |
| ODDM | 2-octyl-1-dodecanoyl myristate |
| ODDP | 2-octyl-1-dodecanoyl palmitate |
| ODDS | 2-octyl-1-dodecanoyl stearate |
| PA | Palmitic acid |
| PBT | Persistent, bioaccumulative and toxic |
| PMI | Process mass intensity |
| SA | Stearic acid |
| vPvB | Very persistent and very bioaccumulative |
References
- Van Reeth, I. Silicones—A key ingredient in cosmetic and toiletry formulations. In Handbook of Cosmetic Science and Technology; CRC Press: Boca Raton, FL, USA, 2014; Volume 4, pp. 321–329. [Google Scholar]
- O’Lenick, A.J. Silicones for Personal Care, 2nd ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2008. [Google Scholar]
- Berthiaume, M.D. Silicones in cosmetics. In Principles of Polymer Science and Technology in Cosmetics and Personal Care; Marcel Dekker, Inc.: New York, NY, USA, 1999; Volume 22. [Google Scholar]
- Garaud, J.L. Les silicones—50 ans d’innovation en cosmétique. L’actual. Chim. 2008, 323–324, 29–34. [Google Scholar]
- ECHA. UK-CA’s Report on the Identification of PBT and vPvB Substance Results of Evaluation of PBT/vPvB Properties of D4. Extract of PBT Information for D4. 2015. Available online: https://echa.europa.eu/documents/10162/17233/annex_2_uk-cas_pbt_report_on_d4_20150205_en.pdf/6ad91f5c-19fb-411f-b23e-4cb736aab044 (accessed on 7 November 2025).
- ECHA. UK-CA’s Report on the Identification of PBT and vPvB Substance Results of Evaluation of PBT/vPvB Properties of D5. Extract of PBT Information for D5. 2015. Available online: https://echa.europa.eu/documents/10162/17233/annex_3_uk-cas_pbt_report_on_d5_20150205_en.pdf/4ede40da-d500-404b-a531-6c27d57aed85 (accessed on 7 November 2025).
- COMMISSION REGULATION (EU) 2018/35 of 10 January 2018 Amending Annex XVII to Regulation (EC) No 1907/2006. Available online: https://eur-lex.europa.eu/eli/reg/2018/35/oj (accessed on 7 November 2025).
- Gabriëls, D.; Yesid Hernández, W.; Sels, B.; Van Der Voort, P.; Verberckmoes, A. Review of catalytic systems and thermodynamics for the Guerbet condensation reaction and challenges for biomass valorization. Catal. Sci. Technol. 2015, 5, 3876–3902. [Google Scholar] [CrossRef]
- Gryglewicz, S. Enzyme catalysed synthesis of some adipic esters. J. Mol. Catal. B Enzym. 2001, 15, 9–13. [Google Scholar] [CrossRef]
- He, X.L.; Chen, B.Q.; Tan, T.W. Enzymatic synthesis of 2-ethylhexyl esters of fatty acids by immobilized lipase from Candida sp.99–125. J. Mol. Catal. B Enzym. 2002, 18, 333–339. [Google Scholar] [CrossRef]
- Gryglewicz, S. Lipase catalysed synthesis of sebacic and phthalic esters. Enzym. Microb. Technol. 2003, 33, 952–957. [Google Scholar] [CrossRef]
- Tan, T.; Chen, B.Q.; Ye, H. Enzymatic synthesis of 2-ethylhexyl palmitate by lipase immobilized on fabric membranes in the batch reactor. Biochem. Eng. J. 2006, 29, 41–45. [Google Scholar] [CrossRef]
- Verma, M.; Chauhan, G.; Kanwar, S. Purification and characterization of a low molecular mass alkaliphilic lipase of Bacillus cereus MTCC 8372. Acta Microbiol. Immunol. Hung. 2008, 55, 327–342. [Google Scholar] [CrossRef]
- Richetti, A.; Leite, S.G.F.; Antunes, O.A.C.; Lerin, L.A.; Dallago, R.M.; Emmerich, D.; di Luccio, M.; Oliveira, J.V.; Treichel, H.; de Oliveira, D. Assessment of process variables on 2-ethylhexyl palmitate production using Novozym 435 as catalyst in a solvent-free system. Bioprocess Biosyst. Eng. 2010, 33, 331–337. [Google Scholar] [CrossRef]
- Richetti, A.; Leite, S.G.F.; Antunes, O.A.C.; de Souza, A.L.F.; Lerin, L.A.; Dallago, R.M.; Paroul, N.; di Luccio, M.; Oliveira, J.V.; Treichel, H.; et al. Optimization of 2-ethylhexyl palmitate production using Lipozyme RM IM as catalyst in a solvent-free system. Appl. Biochem. Biotechnol. 2010, 260, 2498–2508. [Google Scholar] [CrossRef]
- Akerman, C.O.; Hagstrom, A.E.V.; Mollaahmad, M.A.; Karlsson, S.; Hatti-Kaul, R. Biolubricant synthesis using immobilized lipase: Process optimisation of trimethylolpropane oleate production. Process Biochem. 2011, 46, 2225–2231. [Google Scholar] [CrossRef]
- Brenneis, R.; Baeck, B. Esterification of fatty acids using Candida antarctica lipase A in water-abundant systems. Biotechnol Lett. 2012, 34, 1459–1463. [Google Scholar] [CrossRef]
- Tao, Y.; Chen, B.; Liu, L.; Tan, T. Synthesis of trimethylolpropane esters with immobilized lipase from Candida sp. 99–125. J. Mol. Catal. B Enzym. 2012, 74, 151–155. [Google Scholar] [CrossRef]
- Tao, Y.; Cui, C.; Shen, H.; Liu, L.; Chen, B.; Tan, T. Enhancing trimethylolpropane esters synthesis through lipase immobilized on surface hydrophobic modified support and appropriate substrate feeding methods. Enzym. Microb. Technol. 2014, 58–59, 60–67. [Google Scholar] [CrossRef]
- Trivedi, J.; Aila, M.; Sharma, C.D.; Gupta, P.; Kaul, S. Clean synthesis of biolubricant range esters using novel liquid lipase enzyme in solvent free medium. SpringerPlus 2015, 4, 165. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Y.; Zhang, Y.; Zheng, L.; Huang, H.; Wang, Z. Synthesis of 2-ethylhexyl palmitate catalyzed by enzyme under microwave. Appl. Biochem. Biotechnol. 2018, 185, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, T.; Choi, N.; Kim, B.H.; Oh, S.W.; Kim, I.H. Synthesis of diethylhexyl adipate by Candida antarctica lipase-catalyzed esterification. Process Biochem. 2019, 78, 58–62. [Google Scholar] [CrossRef]
- Lee, A.; Kim, H.; Choi, N.; Yoon, S.W.; Kim, Y.; Kim, H.R.; Kim, I.H. Preparation of diisononyl adipate in a solvent-free system via an immobilized lipase-catalyzed esterification. Enzym. Microb. Technol. 2019, 131, 109340. [Google Scholar] [CrossRef]
- Zhu, W.; Liang, F.; Hou, H.; Chen, Y.; Liu, X.; Zhu, X. Enzymatic synthesis of a polyol ester from levulinic acid and trimethylolpropane and its tribological behavior as potential biolubricant basestock. Polymers 2020, 12, 2256. [Google Scholar] [CrossRef]
- Murcia, M.D.; Serrano-Arnaldos, M.; Ortega-Requena, S.; Máximo, F.; Bastida, J.; Montiel, M.C. Optimization of a sustainable biocatalytic process for the synthesis of ethylhexyl fatty acids esters. Catal. Today 2020, 346, 98–105. [Google Scholar] [CrossRef]
- Serrano-Arnaldos, M.; Ortega-Requena, S.; Sánchez, J.A.; Hernández, A.; Montiel, M.C.; Máximo, F.; Bastida, J. Sustainable synthesis of branched-chain diesters. J. Biotechnol. 2021, 325, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Montiel, M.C.; Asensi, M.; Gimeno-Martos, S.; Máximo, F.; Bastida, J. Sustainable biocatalytic procedure for obtaining new branched acid esters. Materials 2021, 14, 6847. [Google Scholar] [CrossRef]
- He, C.; Guo, Z.; Deng, Z.; Li, S.; Zhang, X. Enzyme-catalyzed preparation of polyol ester lubricants and performance researchbased on pelargonic acid, oleic acid and trimethylolpropane. Biochem. Eng. J. 2022, 187, 108641. [Google Scholar] [CrossRef]
- Máximo, F.; Asensi, M.; Serrano-Arnaldos, M.; Ortega-Requena, S.; Montiel, C.; Bastida, J. Biocatalytic intensified process for the synthesis of neopentyl glycol dicaprylate/dicaprate. Sustain. Chem. Pharm. 2022, 30, 100882. [Google Scholar] [CrossRef]
- Montiel, C.; Gimeno-Martos, S.; Ortega-Requena, S.; Serrano-Arnaldos, M.; Máximo, F.; Bastida, J. Green production of a high-value branched-chain diester: Optimization based on operating conditions and economic and sustainability criteria. Appl. Sci. 2023, 13, 6177. [Google Scholar] [CrossRef]
- Montiel, M.C.; Gómez, M.; Murcia, M.D.; Ortega-Requena, S.; Máximo, F.; Bastida, J. Sustainable Biocatalytic Synthesis of a Second-Generation Biolubricant. Sustainability 2024, 16, 1615. [Google Scholar] [CrossRef]
- Máximo, F.; Bastida, J.; Montiel, C.; Gómez, M.; Murcia, M.D.; Barqueros, C.; Ortega-Requena, S. Branched saturated esters and diesters: Sustainable synthesis of excellent biolubricants. Catal. Today 2024, 429, 114509. [Google Scholar] [CrossRef]
- O’Lenick, A.J. Guerbet Chemistry. J. Surfactants Deterg. 2001, 4, 311–315. [Google Scholar] [CrossRef]
- Waykole, C.S.; Mali, S.N.; Mahale, D.D.; Pratapa, A.P. Guerbet alcohol esters: Practical synthesis and applications. J. Indian Chem. Soc. 2022, 99, 100304. [Google Scholar] [CrossRef]
- Knothe, G. Synthesis, applications, and characterization of Guerbet compounds and their derivatives. Lipid Technol. 2002, 14, 102–104. [Google Scholar]
- Ultrus Prospector. Technical Information of ISOFOL 20. Available online: https://www.ulprospector.com/es/eu/PersonalCare/Detail/2262/68432/ISOFOL-20 (accessed on 7 November 2025).
- ASTM D974-02e1; Standard Test Method for Acid and Base Number by Color-Indicator Titration. ASTM International: West Conshohocken, PA, USA, 2002.
- Wang, X.; Chen, Y.; Ma, Y.; Jin, Q.; Wang, X. Lipozyme 435-catalyzed synthesis of eicosapentaenoyl ethanolamidein a solvent-free system. J. Mol. Catal. B Enzym. 2015, 122, 233–239. [Google Scholar] [CrossRef]
- Yu, D.; Zhang, X.; Wang, T.; Geng, H.; Wang, L.; Jiang, L.; Elfalleh, W. Immobilized Candida antarctica lipase B (CALB) on functionalized MCM-41: Stability and catalysis of transesterification of soybean oil and phytosterol. Food Biosci. 2021, 40, 100906. [Google Scholar] [CrossRef]
- Serrano-Arnaldos, M.; Montiel, M.C.; Ortega-Requena, S.; Máximo, F.; Bastida, J. Development and economic evaluation of an eco-friendly biocatalytic synthesis of emollient esters. Bioprocess Biosyst. Eng. 2020, 43, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Roschangar, F.; Sheldon, R.A.; Senanayake, C.H. Overcoming barriers to green chemistry in the pharmaceutical industry—The Green Aspiration Level TM concept. Green Chem. 2015, 17, 752. [Google Scholar] [CrossRef]
- Sheldon, R.A. The E factor 25 years on: The rise of green chemistry and sustainability. Green Chem. 2017, 19, 18. [Google Scholar] [CrossRef]
- Jimenez-Gonzalez, C.; Lund, C. Green metrics in pharmaceutical development. Curr. Opin. Green Sustain. Chem. 2022, 33, 100564. [Google Scholar] [CrossRef]
- de María, P.B. Biocatalysis, sustainability, and industrial applications: Show me the metrics. Curr. Opin. Green Sustain. Chem. 2021, 31, 100514. [Google Scholar] [CrossRef]
- Hessel, V.; Escribà-Gelonch, M.; Bricout, J.; Tran, N.N.; Anastasopoulou, A.; Ferlin, F.; Valentini, F.; Lanari, D.; Vaccaro, L. Quantitative sustainability assessment of flow chemistry—From simple metrics to holistic assessment. ACS Sustain. Chem. Eng. 2021, 9, 9508–9540. [Google Scholar] [CrossRef]
- Lima-Ramos, J.; Tufvesson, P.; Woodley, J.M. Application of environmental and economic metrics to guide the development of biocatalytic processes. Green Process Synth. 2014, 3, 195–213. [Google Scholar] [CrossRef]
- Sheldon, R.A. Metrics of green chemistry and sustainability: Past, present, and future. ACS Sustain. Chem. Eng. 2018, 6, 32–48. [Google Scholar] [CrossRef]
- Sheldon, R.A. The E factor at 30: A passion for pollution prevention. Green Chem. 2023, 25, 1704–1728. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Woodley, J.M. Role of biocatalysis in sustainable chemistry. Chem. Rev. 2018, 118, 801–838. [Google Scholar] [CrossRef] [PubMed]
| Trade Name | Supplier |
|---|---|
| INCI name: octyldodecyl myristate | |
| PARYOL MIRISTIL 8–12 | A&A Fratelli Parodi, Genoa, Italy |
| Bernel Ester 2014 | Alzo International, Freehold, NJ, USA |
| Dermol 2014 | |
| Wickenol 142 | |
| Octyldodecyl Myristate | Blue Sun International, Miami, FL, USA |
| Corum 5024 | CORUM, La Chaux-de-Fonds, Switzerland |
| ERCAREL ODM V | ErcaWilmar, São Paulo, Brazil |
| HEST ODM | Ethox Chemicals, Greenville, SC, USA |
| MOD MB | Gattefossé, Saint-Priest, France |
| ODM 100KC | KCI, San Antonio, TX, USA |
| ODM | Kokyu Alcohol Kogyo, Nagoya, Japan |
| NIKKOL ODM-100 | NIKKOL GROUP (Nikko Chemicals), Tokyo, Japan |
| Natura-tec Ultrafeel ODM | Natura-Tec, Fremont, CA, USA |
| PaesterTM ODM | Patech Fine Chemicals, Elmsford, NY, USA |
| RITAMOLLIENT ODDM | RITA Corporation, Crystal Lake, IL, USA |
| Saboderm ODM | Sabo, Levata, Italy |
| DUB MOD | Stearinerie Dubois, Boulogne-Billancourt, France |
| INCI name: octyldodecyl palmitate | |
| Corum 5025 | CORUM, La Chaux-de-Fonds, Switzerland |
| INCI name: octyldodecyl stearate | |
| Corum 5026 | CORUM, La Chaux-de-Fonds, Switzerland |
| HEST ODS | Ethox Chemicals, Greenville, SC, USA |
| CeraphylTM ODS ester | Ashland, Wilmington, DE, USA |
| Ester | Amount of Lipase (g) | Average Rate at 30 min (min−1) | Time to Conversion up 95% (min) |
|---|---|---|---|
| ODDM | 0.25 | 0.373 | 390 |
| 0.5 | 0.914 | 150 | |
| 1 | 1.213 | 120 | |
| ODDP | 0.25 | 0.533 | 270 |
| 0.5 | 1.091 | 150 | |
| 1 | 1.614 | 120 | |
| ODDS | 0.25 | 0.476 | 300 |
| 0.5 | 0.808 | 150 | |
| 1 | 1.185 | 120 |
| Ester | Temperature (°C) | Average Rate at 30 min (min−1) | Time to Conversion up 95% (min) |
|---|---|---|---|
| ODDM | 70 | 0.490 | 360 |
| 80 | 0.794 | 210 | |
| 90 | 0.914 | 150 | |
| ODDP | 70 | 0.521 | 240 |
| 80 | 0.857 | 210 | |
| 90 | 1.091 | 150 | |
| ODDS | 70 | 0.617 | 210 |
| 80 | 0.808 | 180 | |
| 90 | 0.977 | 180 |
| Ester | Temperature (°C) | Dynamic Viscosity (cp) | Cinematic Viscosity (mm2/s) |
|---|---|---|---|
| ODDM | 70 | 5.975 | 7.269 |
| 80 | 4.794 | 5.879 | |
| 90 | 3.929 | 4.857 | |
| ODDP | 70 | 6.880 | 8.365 |
| 80 | 5.492 | 6.730 | |
| 90 | 4.479 | 5.533 | |
| ODDS | 70 | 8.492 | 10.330 |
| 80 | 6.851 | 8.402 | |
| 90 | 5.576 | 6.893 |
| Green Metric | Formula | ODDM | ODDP | ODDS |
|---|---|---|---|---|
| Atom Economy (AE) (%) | 96.57 | 96.76 | 97.00 | |
| E-Factor (EF) | 0.041 | 0.032 | 0.010 | |
| Complete E-factor (cEF) | 0.076 | 0.067 | 0.041 | |
| Carbon Mass Efficiency (CME) (%) | 96.70 | 97.55 | 99.58 | |
| Process Mass Intensity (PMI) | 1.076 | 1.071 | 1.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montiel, M.C.; Máximo, F.; Gómez, M.; Murcia, M.D.; Ortega-Requena, S.; Bastida, J. Guerbet Alcohols, Ideal Substrates for the Sustainable Production of Branched Esters. Materials 2025, 18, 5129. https://doi.org/10.3390/ma18225129
Montiel MC, Máximo F, Gómez M, Murcia MD, Ortega-Requena S, Bastida J. Guerbet Alcohols, Ideal Substrates for the Sustainable Production of Branched Esters. Materials. 2025; 18(22):5129. https://doi.org/10.3390/ma18225129
Chicago/Turabian StyleMontiel, María Claudia, Fuensanta Máximo, María Gómez, María Dolores Murcia, Salvadora Ortega-Requena, and Josefa Bastida. 2025. "Guerbet Alcohols, Ideal Substrates for the Sustainable Production of Branched Esters" Materials 18, no. 22: 5129. https://doi.org/10.3390/ma18225129
APA StyleMontiel, M. C., Máximo, F., Gómez, M., Murcia, M. D., Ortega-Requena, S., & Bastida, J. (2025). Guerbet Alcohols, Ideal Substrates for the Sustainable Production of Branched Esters. Materials, 18(22), 5129. https://doi.org/10.3390/ma18225129









