Nanocarrier-Based Delivery Systems for Natural Compounds Across Research Stages
Abstract
1. Introduction
- to classify and compare the main types of nanocarriers used for phytochemical delivery;
- to describe in detail the formulation techniques, encapsulation efficiencies, and release profiles achieved;
- to analyze tissue-targeting strategies, both passive and active, and their impact on selective drug accumulation;
- to summarize the most promising preclinical results, with a focus on pharmacodynamic and pharmacokinetic outcomes in cancer, inflammation, neurodegeneration, and infection models;
- to discuss the main barriers to clinical translation, including regulatory issues, long-term safety, and production scalability;
- to propose perspectives for future development in light of emerging trends and unmet clinical needs.
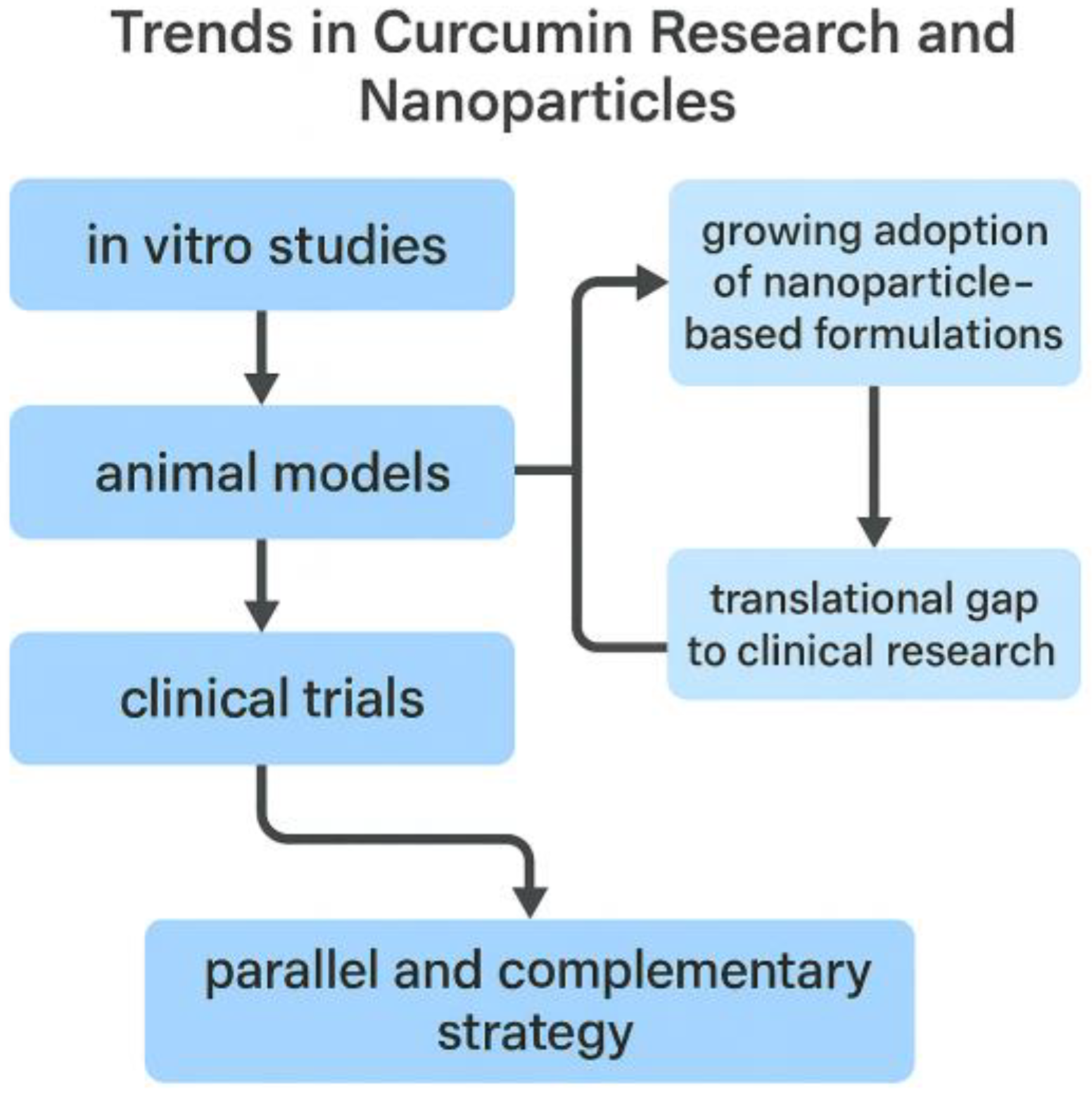
2. Trends in Curcumin Research and the Emerging Role of Nanoparticle-Based Delivery
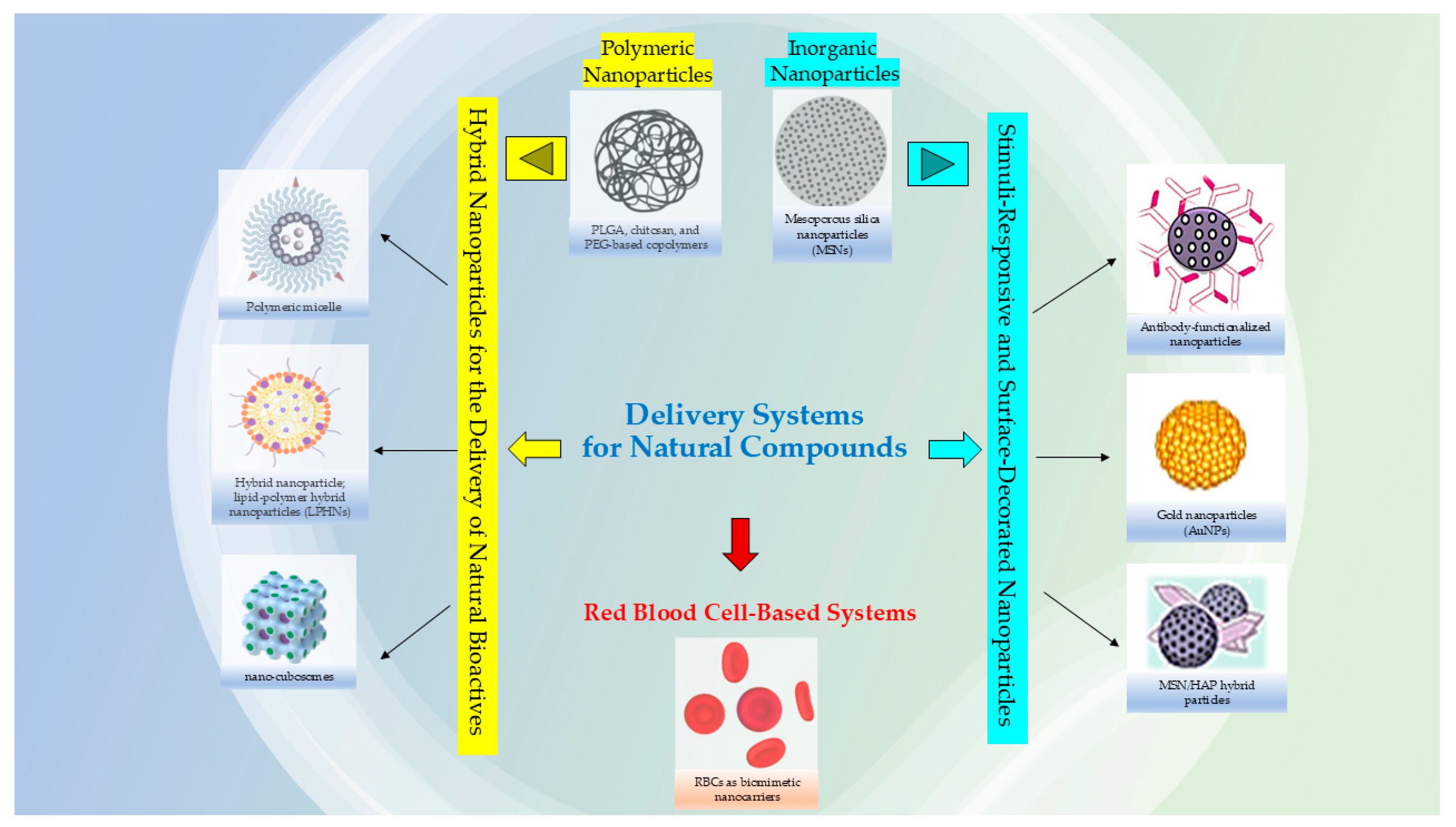
3. Advanced Nanomaterials for the Delivery of Natural Bioactives
3.1. Polymeric Nanoparticles for the Delivery of Natural Bioactives
3.2. Inorganic Nanoparticles for the Delivery of Natural Bioactives
3.3. Hybrid Nanoparticles for the Delivery of Natural Bioactives
3.4. Stimuli-Responsive and Surface-Decorated Nanoparticles
3.5. Biomimetic Nanocarriers: Red Blood Cell–Based Systems
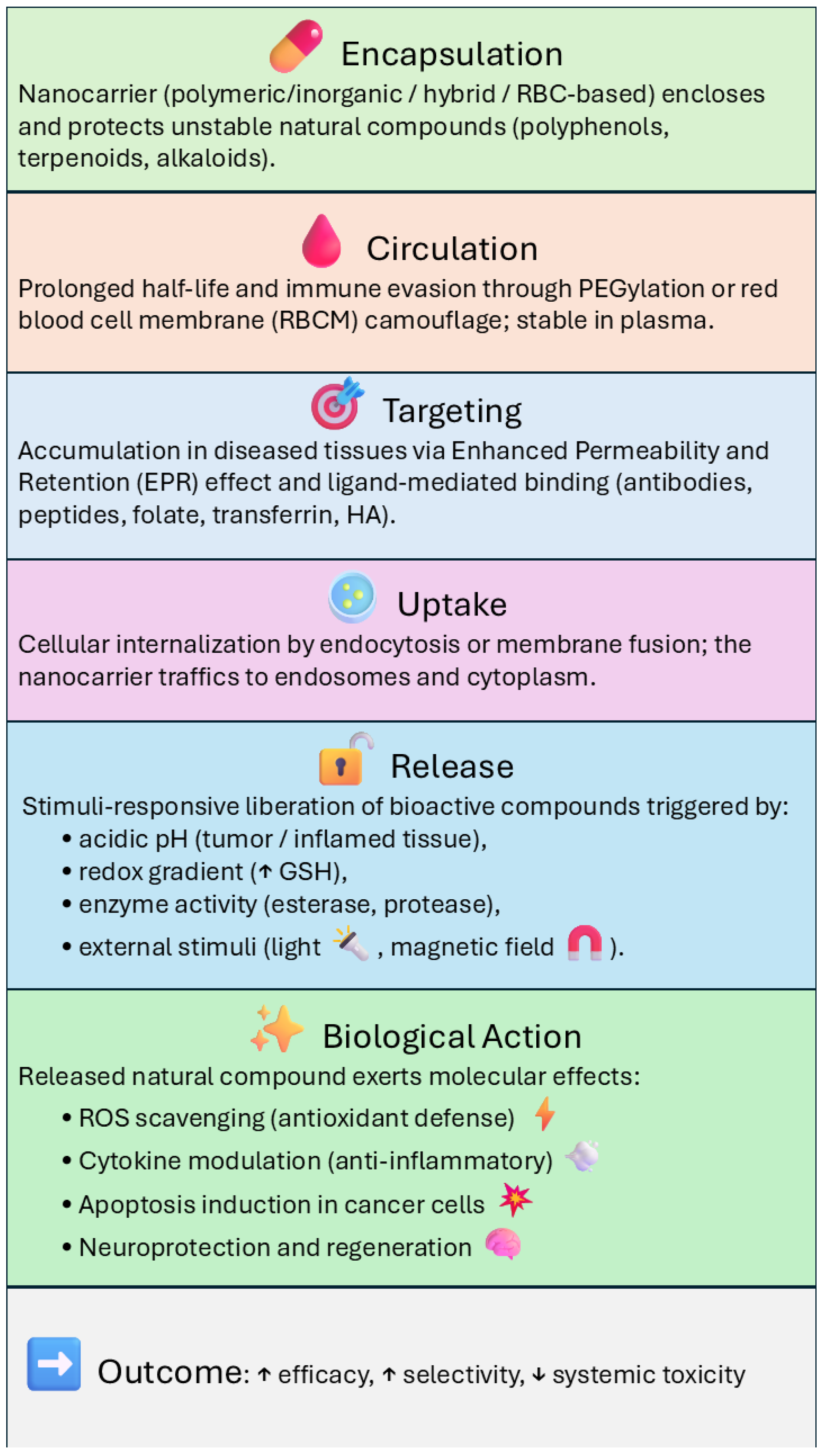
4. Targeting Strategies and Release Mechanisms for Nanoparticle-Based Delivery of Natural Bioactives
4.1. Passive Targeting
4.2. Active Targeting
4.3. Controlled and Stimuli-Responsive Release
4.4. Combination of Targeting and Stimuli-Responsiveness
5. Preclinical Applications of Nanoparticle-Based Delivery of Natural Bioactives
5.1. Anticancer Applications
5.2. Neuroprotective Effects
5.3. Anti-Inflammatory and Antioxidant Therapies
5.4. Antimicrobial Applications
5.5. Pharmacokinetics, Biodistribution, and Safety Profiles
5.6. Preclinical Pharmacokinetics and Lipid Disorders
5.7. Stem Cell Differentiation Through Scaffold Architecture
6. Clinical Translation of Nanoparticle-Based Delivery of Natural Bioactives: Barriers and Perspectives
6.1. Regulatory and Standardization Challenges
6.2. Safety and Long-Term Toxicity
6.3. Manufacturing and Scalability
6.4. Clinical Design and Biomarker Selection
6.5. Intellectual Property and Commercialization
6.6. Emerging Clinical Evidence and Future Perspectives
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABC | Accelerated blood clearance |
| AKT | v-Akt murine thymoma viral oncogene homolog |
| ALP | Alkaline phosphatase |
| AMPK | AMP-activated protein kinase |
| AuNPs | Gold nanoparticles |
| BBB | Blood–brain barrier |
| BOIN (TITE-BOIN) | Time-to-event Bayesian Optimal Interval (dose-escalation design) |
| Cmax | Maximum plasma concentration |
| CMC | Carboxymethyl chitosan |
| CNS | Central nervous system |
| CRISPR | Clustered Regularly Interspaced Short Palindromic Repeats |
| EGCG | Epigallocatechin gallate |
| EMA | European Medicines Agency |
| EPR | Enhanced permeability and retention |
| EVs | Extracellular vesicles |
| FDA | Food and Drug Administration |
| FA | Folic acid |
| GNA | Gambogenic acid |
| GMP | Good manufacturing practice |
| GSH | Glutathione |
| HAP | Hydroxyapatite |
| LNPs | Lipid nanoparticles (for nucleic-acid delivery) |
| LPHNs | Lipid–polymer hybrid nanoparticles |
| mRNA | Messenger RNA |
| mTOR | Mechanistic target of rapamycin |
| MTD | Maximum tolerated dose |
| MEW | Melt electrospinning writing |
| MSNs | Mesoporous silica nanoparticles |
| NIR | Near-infrared |
| NLCs | Nanostructured lipid carriers |
| OS | Overall survival |
| PCL | Poly(ε-caprolactone) |
| PBLG | Poly(γ-benzyl-L-glutamate) |
| PBPK | Physiologically based pharmacokinetic |
| PEG | Polyethylene glycol |
| PHPMA | Poly(hydroxypropyl methacrylamide) |
| PDI | Polydispersity index |
| PD | Pharmacodynamics |
| PK | Pharmacokinetics |
| PLGA | Poly(lactic-co-glycolic acid) |
| PFS | Progression-free survival |
| ROS | Reactive oxygen species |
| RBC | Red blood cell |
| RBCM-NPs | Red blood cell membrane–coated nanoparticles |
| RBC-EVs | Red blood cell–derived extracellular vesicles |
| RC-Lips | Red blood cell–mimicking liposomes |
| RP2D | Recommended Phase II dose |
| RT | Radiotherapy |
| TMZ | Temozolomide |
| TPGS | d-α-Tocopheryl polyethylene glycol 1000 succinate |
| SLN/SLNs | Solid lipid nanoparticle(s) |
| t½ (t1/2) | Terminal half-life |
| Tmax | Time to Cmax |
| YAP | Yes-associated protein |
References
- Ma, C.; Peng, Y.; Li, H.; Chen, W. Organ-on-a-Chip: A New Paradigm for Drug Development. Trends Pharmacol. Sci. 2021, 42, 119–133. [Google Scholar] [CrossRef]
- Elmowafy, M.; Shalaby, K.; Elkomy, M.H.; Alsaidan, O.A.; Gomaa, H.A.M.; Abdelgawad, M.A.; Mostafa, E.M. Polymeric Nanoparticles for Delivery of Natural Bioactive Agents: Recent Advances and Challenges. Polymers 2023, 15, 1123. [Google Scholar] [CrossRef]
- Khalil, N.M.; do Nascimento, T.C.F.; Casa, D.M.; Dalmolin, L.F.; de Mattos, A.C.; Hoss, I.; Romano, M.A.; Mainardes, R.M. Pharmacokinetics of Curcumin-Loaded PLGA and PLGA–PEG Blend Nanoparticles after Oral Administration in Rats. Colloids Surf. B Biointerfaces 2013, 101, 353–360. [Google Scholar] [CrossRef]
- Xie, X.; Tao, Q.; Zou, Y.; Zhang, F.; Guo, M.; Wang, Y.; Wang, H.; Zhou, Q.; Yu, S. PLGA Nanoparticles Improve the Oral Bioavailability of Curcumin in Rats: Characterizations and Mechanisms. J. Agric. Food Chem. 2011, 59, 9280–9289. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Feng, R.; Sun, M.; Guo, C.; Gao, Y.; Li, L.; Zhai, G. Curcumin-Loaded PLGA-PEG-PLGA Triblock Copolymeric Micelles: Preparation, Pharmacokinetics and Distribution in Vivo. J. Colloid. Interface Sci. 2011, 354, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Sapino, S.; Ugazio, E.; Gastaldi, L.; Miletto, I.; Berlier, G.; Zonari, D.; Oliaro-Bosso, S. Mesoporous Silica as Topical Nanocarriers for Quercetin: Characterization and in Vitro Studies. Eur. J. Pharm. Biopharm. 2015, 89, 116–125. [Google Scholar] [CrossRef]
- Antunes Filho, S.; dos Santos, M.S.; dos Santos, O.A.L.; Backx, B.P.; Soran, M.-L.; Opriş, O.; Lung, I.; Stegarescu, A.; Bououdina, M. Biosynthesis of Nanoparticles Using Plant Extracts and Essential Oils. Molecules 2023, 28, 3060. [Google Scholar] [CrossRef]
- Ghosh, S.; Solanki, R.; Bhatia, D.; Sankaranarayanan, S. Nanomaterials for Delivery of Medicinal Plant Extracts and Phytochemicals: Potential Applications and Future Perspectives. Plant Nano Biol. 2025, 12, 100161. [Google Scholar] [CrossRef]
- Backx, B.P.; dos Santos, M.S.; dos Santos, O.A.L.; Filho, S.A. The Role of Biosynthesized Silver Nanoparticles in Antimicrobial Mechanisms. Curr. Pharm. Biotechnol. 2021, 22, 762–772. [Google Scholar] [CrossRef] [PubMed]
- Awlqadr, F.H.; Majeed, K.R.; Altemimi, A.B.; Hassan, A.M.; Qadir, S.A.; Saeed, M.N.; Faraj, A.M.; Salih, T.H.; Abd Al-Manhel, A.J.; Najm, M.A.A.; et al. Nanotechnology-Based Herbal Medicine: Preparation, Synthesis, and Applications in Food and Medicine. J. Agric. Food Res. 2025, 19, 101661. [Google Scholar] [CrossRef]
- Zazuli, Z.; Hartati, R.; Rowa, C.R.; Asyarie, S.; Satrialdi. The Potential Application of Nanocarriers in Delivering Topical Antioxidants. Pharmaceuticals 2025, 18, 56. [Google Scholar] [CrossRef] [PubMed]
- Karnwal, A.; Jassim, A.Y.; Mohammed, A.A.; Sharma, V.; Al-Tawaha, A.R.M.S.; Sivanesan, I. Nanotechnology for Healthcare: Plant-Derived Nanoparticles in Disease Treatment and Regenerative Medicine. Pharmaceuticals 2024, 17, 1711. [Google Scholar] [CrossRef]
- Lv, Y.; Li, W.; Liao, W.; Jiang, H.; Liu, Y.; Cao, J.; Lu, W.; Feng, Y. Nano-Drug Delivery Systems Based on Natural Products. Int. J. Nanomed. 2024, 19, 541–569. [Google Scholar] [CrossRef]
- Sharmila, A.; Bhadra, P.; Kishore, C.; Selvaraj, C.I.; Kavalakatt, J.; Bishayee, A. Nanoformulated Terpenoids in Cancer: A Review of Therapeutic Applications, Mechanisms, and Challenges. Cancers 2025, 17, 3013. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Su, J.; Kamal, Z.; Guo, P.; Wu, X.; Lu, L.; Wu, H.; Qiu, M. Odorranalectin Modified PEG–PLGA/PEG–PBLG Curcumin-Loaded Nanoparticle for Intranasal Administration. Drug Dev. Ind. Pharm. 2020, 46, 899–909. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; He, Y.; Zhou, G.; Li, X.; Feng, A.; Zheng, W. Target Challenging-Cancer Drug Delivery to Gastric Cancer Tissues with a Fucose Graft Epigallocatechin-3-Gallate-Gold Particles Nanocomposite Approach. J. Photochem. Photobiol. B 2018, 183, 147–153. [Google Scholar] [CrossRef]
- Shukla, R.; Chanda, N.; Zambre, A.; Upendran, A.; Katti, K.; Kulkarni, R.R.; Nune, S.K.; Casteel, S.W.; Smith, C.J.; Vimal, J.; et al. Laminin Receptor Specific Therapeutic Gold Nanoparticles (198 AuNP-EGCg) Show Efficacy in Treating Prostate Cancer. Proc. Natl. Acad. Sci. USA 2012, 109, 12426–12431. [Google Scholar] [CrossRef]
- Yi, X.; Bao, F.; Fu, S.; Yang, Y.; Xu, Y. Preparation of Mesoporous Silica/Hydroxyapatite Loaded Quercetin Nanoparticles and Research on Its Antibacterial Properties. Med. Eng. Phys. 2024, 126, 104160. [Google Scholar] [CrossRef]
- Gao, W.; Fan, X.; Bi, Y.; Zhou, Z.; Yuan, Y. Preparation of NIR-Responsive Gold Nanocages as Efficient Carrier for Controlling Release of EGCG in Anticancer Application. Front. Chem. 2022, 10, 926002. [Google Scholar] [CrossRef]
- Kumah, E.A.; Fopa, R.D.; Harati, S.; Boadu, P.; Zohoori, F.V.; Pak, T. Human and Environmental Impacts of Nanoparticles: A Scoping Review of the Current Literature. BMC Public Health 2023, 23, 1059. [Google Scholar] [CrossRef]
- Ma, X.; Tian, Y.; Yang, R.; Wang, H.; Allahou, L.W.; Chang, J.; Williams, G.; Knowles, J.C.; Poma, A. Nanotechnology in Healthcare, and Its Safety and Environmental Risks. J. Nanobiotechnol. 2024, 22, 715. [Google Scholar] [CrossRef]
- Safarpour, F.; Kharaziha, M.; Emadi, R. Inspiring Biomimetic System Based on Red Blood Cell Membrane Vesicles for Effective Curcumin Loading and Release. Int. J. Pharm. 2022, 613, 121419. [Google Scholar] [CrossRef]
- Tang, Q.; Dong, M.; Xu, Z.; Xue, N.; Jiang, R.; Wei, X.; Gu, J.; Li, Y.; Xin, R.; Wang, J.; et al. Red Blood Cell-Mimicking Liposomes Loading Curcumin Promote Diabetic Wound Healing. J. Control. Release 2023, 361, 871–884. [Google Scholar] [CrossRef]
- Ventola, C.L. Progress in Nanomedicine: Approved and Investigational Nanodrugs. Pharm. Ther. 2017, 42, 742–755. [Google Scholar]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer Nanomedicine: Progress, Challenges and Opportunities. Nat. Rev. Cancer 2017, 17, 20–37. [Google Scholar] [CrossRef] [PubMed]
- Eichholz, K.F.; Hoey, D.A. Mediating Human Stem Cell Behaviour via Defined Fibrous Architectures by Melt Electrospinning Writing. Acta Biomater. 2018, 75, 140–151. [Google Scholar] [CrossRef]
- Saber, M.M.; Al-mahallawi, A.M.; Nassar, N.N.; Stork, B.; Shouman, S.A. Targeting Colorectal Cancer Cell Metabolism through Development of Cisplatin and Metformin Nano-Cubosomes. BMC Cancer 2018, 18, 822. [Google Scholar] [CrossRef]
- Álvarez-Bermúdez, O.; Adam-Cervera, I.; Landfester, K.; Muñoz-Espí, R. Morphology Control of Polymer–Inorganic Hybrid Nanomaterials Prepared in Miniemulsion: From Solid Particles to Capsules. Polymers 2024, 16, 2997. [Google Scholar] [CrossRef]
- Tiboni, M.; Elmowafy, E.; El-Derany, M.O.; Benedetti, S.; Campana, R.; Verboni, M.; Potenza, L.; Palma, F.; Citterio, B.; Sisti, M.; et al. A Combination of Sugar Esters and Chitosan to Promote in Vivo Wound Care. Int. J. Pharm. 2022, 616, 121508. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.; Sarolia, J.; Vyas, B.; Wagh, P.; Ankur, K.; Kumar, M.A. PLGA Nanoparticles for Nose to Brain Delivery of Clonazepam: Formulation, Optimization by 32 Factorial Design, In Vitro and In Vivo Evaluation. Curr. Drug Deliv. 2021, 18, 805–824. [Google Scholar] [CrossRef] [PubMed]
- Formica, M.L.; Real, D.A.; Picchio, M.L.; Catlin, E.; Donnelly, R.F.; Paredes, A.J. On a Highway to the Brain: A Review on Nose-to-Brain Drug Delivery Using Nanoparticles. Appl. Mater. Today 2022, 29, 101631. [Google Scholar] [CrossRef]
- Biswas, U.K.; Bose, A.; Ghosh, B.; Sharma, S. An Insight into Chemically Modified Chitosan and Their Biological, Pharmaceutical, and Medical Applications: A Review. Int. J. Biol. Macromol. 2025, 303, 140612. [Google Scholar] [CrossRef]
- Gagliardi, A.; Giuliano, E.; Venkateswararao, E.; Fresta, M.; Bulotta, S.; Awasthi, V.; Cosco, D. Biodegradable Polymeric Nanoparticles for Drug Delivery to Solid Tumors. Front. Pharmacol. 2021, 12, 601626. [Google Scholar] [CrossRef] [PubMed]
- Garay, R.P.; El-Gewely, R.; Armstrong, J.K.; Garratty, G.; Richette, P. Antibodies against Polyethylene Glycol in Healthy Subjects and in Patients Treated with PEG-Conjugated Agents. Expert. Opin. Drug Deliv. 2012, 9, 1319–1323. [Google Scholar] [CrossRef]
- Hoang Thi, T.T.; Pilkington, E.H.; Nguyen, D.H.; Lee, J.S.; Park, K.D.; Truong, N.P. The Importance of Poly(Ethylene Glycol) Alternatives for Overcoming PEG Immunogenicity in Drug Delivery and Bioconjugation. Polymers 2020, 12, 298. [Google Scholar] [CrossRef] [PubMed]
- Alberg, I.; Kramer, S.; Schinnerer, M.; Hu, Q.; Seidl, C.; Leps, C.; Drude, N.; Möckel, D.; Rijcken, C.; Lammers, T.; et al. Polymeric Nanoparticles with Neglectable Protein Corona. Small 2020, 16, e1907574. [Google Scholar] [CrossRef]
- Behzadi, S.; Serpooshan, V.; Sakhtianchi, R.; Müller, B.; Landfester, K.; Crespy, D.; Mahmoudi, M. Protein Corona Change the Drug Release Profile of Nanocarriers: The “Overlooked” Factor at the Nanobio Interface. Colloids Surf. B Biointerfaces 2014, 123, 143–149. [Google Scholar] [CrossRef]
- Qi, W.; Chen, J.; Rui, S.; Li, S.; Ding, Y.; Feng, S.; Liu, Z.; Liu, Q.; Wang, S.; Zhao, Q. Variable Pore Size of Mesoporous Silica in Improving Physical Stability and Oral Bioavailability of Insoluble Drugs. Int. J. Pharm. 2025, 674, 125394. [Google Scholar] [CrossRef]
- Pérez-Moreno, A.M.; Aranda, C.J.; Torres, M.J.; Mayorga, C.; Paris, J.L. Immunomodulatory Potential of Rapamycin-Loaded Mesoporous Silica Nanoparticles: Pore Size-Dependent Drug Loading, Release, and in Vitro Cellular Responses. Drug Deliv. Transl. Res. 2024, 14, 3467–3476. [Google Scholar] [CrossRef]
- Benkő, F.; Kristó, K.; Sovány, T. Mesoporous Silica Nanoparticles as Drug Delivery Systems. Pharmaceuticals 2025, 18, 1392. [Google Scholar] [CrossRef]
- Iranshahy, M.; Hanafi-Bojd, M.Y.; Aghili, S.H.; Iranshahi, M.; Nabavi, S.M.; Saberi, S.; Filosa, R.; Nezhad, I.F.; Hasanpour, M. Curcumin-Loaded Mesoporous Silica Nanoparticles for Drug Delivery: Synthesis, Biological Assays and Therapeutic Potential—A Review. RSC Adv. 2023, 13, 22250–22267. [Google Scholar] [CrossRef]
- Hartono, S.B.; Hadisoewignyo, L.; Yang, Y.; Meka, A.K.; Antaresti; Yu, C. Amine Functionalized Cubic Mesoporous Silica Nanoparticles as an Oral Delivery System for Curcumin Bioavailability Enhancement. Nanotechnology 2016, 27, 505605. [Google Scholar] [CrossRef]
- Adefegha, S.A.; Ogundare, P.O.; Oboh, G.; Esatbeyoglu, T.; Papenbrock, J. An Overview on the Mechanisms of Encapsulation of Polyphenols Used to Help Fight Diabetes Mellitus. Discov. Food 2025, 5, 185. [Google Scholar] [CrossRef]
- Zhang, Q.; Tong, J.; Zhou, W.; Zhong, Z.; Hu, Q.; Ma, Q.; Long, H.; Wu, S.; Shi, X.; Ye, Q. Antibacterial and Antioxidant Chitosan Nanoparticles Improve the Preservation Effect for Donor Kidneys in Vitro. Carbohydr. Polym. 2022, 287, 119326. [Google Scholar] [CrossRef]
- Rizvi, S.Z.H.; Shah, F.A.; Khan, N.; Muhammad, I.; Ali, K.H.; Ansari, M.M.; Din, F.U.; Qureshi, O.S.; Kim, K.-W.; Choe, Y.-H.; et al. Simvastatin-Loaded Solid Lipid Nanoparticles for Enhanced Anti-Hyperlipidemic Activity in Hyperlipidemia Animal Model. Int. J. Pharm. 2019, 560, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Uchôa, A.F.C.; Formiga, A.L.D.; Alves, Á.E.F.; Cardoso, A.L.M.R.; Pereira, G.M.d.A.; Carvalho, L.M.M.; da Silva, L.F.A.; Pereira, P.S.d.S.; de Souza, P.H.O.; Jales, S.T.L.; et al. Optimization and Functionalization of Copaiba Oil-Loaded Nanostructured Lipid Carriers to Improve Cytotoxicity against Breast Cancer Cells. J. Drug Deliv. Sci. Technol. 2025, 105, 106575. [Google Scholar] [CrossRef]
- Pottoo, F.H.; Ibrahim, A.M.; Alammar, A.; Alsinan, R.; Aleid, M.; Alshehhi, A.; Alshehri, M.; Mishra, S.; Alhajri, N. Thymoquinone: Review of Its Potential in the Treatment of Neurological Diseases. Pharmaceuticals 2022, 15, 408. [Google Scholar] [CrossRef] [PubMed]
- Shaddel, R.; Rashidinejad, A.; Karimkhani, M.M.; Tarhan, O.; Jafari, S.M. Nanodelivery Systems of Thymoquinone for Improving Its Bioavailability and Efficiency in the Food and Biomedical Applications. Ind. Crops Prod. 2025, 234, 121555. [Google Scholar] [CrossRef]
- Aboelnaga, N.; Eltaher, S.S.; Saif, N.A.; Loay, O.; Abd El- Rahman, A.; Haroun, A.; Hakim, C.; Elsayed, M.; Attallah, N.E.; El-Geneidy, H.B.; et al. Nano-Boosted Thymoquinone: Moving beyond Antibiotics to Inhibit Staphylococcus aureus Biofilms. Biofouling 2025, 41, 676–695. [Google Scholar] [CrossRef]
- Ghasemian, S.; Mehri, S.; Tabeshpour, J.; Zirak, M.R.; Nezhadhosein, F.; Hosseinzadeh, H. Protective Effect of Thymoquinone against Acrylamide-Induced Hepatotoxicity in the Rat: Role of MAPK and Apoptosis Pathways. Naunyn Schmiedebergs Arch. Pharmacol. 2025, 1–12. [Google Scholar] [CrossRef]
- Andreani, T.; Cheng, R.; Elbadri, K.; Ferro, C.; Menezes, T.; dos Santos, M.R.; Pereira, C.M.; Santos, H.A. Natural Compounds-Based Nanomedicines for Cancer Treatment: Future Directions and Challenges. Drug Deliv. Transl. Res. 2024, 14, 2845–2916. [Google Scholar] [CrossRef]
- Anwar, D.M.; Hedeya, H.Y.; Ghozlan, S.H.; Ewas, B.M.; Khattab, S.N. Surface-Modified Lipid-Based Nanocarriers as a Pivotal Delivery Approach for Cancer Therapy: Application and Recent Advances in Targeted Cancer Treatment. Beni Suef Univ. J. Basic. Appl. Sci. 2024, 13, 106. [Google Scholar] [CrossRef]
- Tang, H.; Zhao, W.; Yu, J.; Li, Y.; Zhao, C. Recent Development of PH-Responsive Polymers for Cancer Nanomedicine. Molecules 2018, 24, 4. [Google Scholar] [CrossRef]
- Liu, Y.; Si, L.; Jiang, Y.; Jiang, S.; Zhang, X.; Li, S.; Chen, J.; Hu, J. Design of PH-Responsive Nanomaterials Based on the Tumor Microenvironment. Int. J. Nanomed. 2025, 20, 705–721. [Google Scholar] [CrossRef]
- Gaddimath, S.; Payamalle, S.; Channabasavana Hundi Puttaningaiah, K.P.; Hur, J. Recent Advances in PH and Redox Responsive Polymer Nanocomposites for Cancer Therapy. J. Compos. Sci. 2024, 8, 28. [Google Scholar] [CrossRef]
- Tapponi, S.; Yusuf, A.; Alsaafin, F.; Hussain, Z. Breaking Barriers with PH-Responsive Nanocarriers: A New Frontier in Precision Oncology. Int. J. Pharm. 2025, 682, 125931. [Google Scholar] [CrossRef] [PubMed]
- Mashaqbeh, H.; Obaidat, R.; Alsmadi, M.M.; Bardaweel, S.; Hailat, N. Characterization and Optimization of Colon Specific Nanosponges Immobilized Polymeric Microbeads Formulation for the Combined Delivery of 5-Fluorouracil and Curcumin. J. Drug Deliv. Sci. Technol. 2024, 99, 105968. [Google Scholar] [CrossRef]
- Xu, X.; Ye, L.; Bao, C.; Hong, W.; Wang, K.; Qiu, S.; Xu, Y.; Piao, J.; Yao, Q. Glutathione-Responsive FA-CMC-GNA Nanoparticles: A Novel Approach for Enhanced Delivery of Gambogenic Acid in Lung Cancer Treatment. Adv. Compos. Hybrid. Mater. 2025, 8, 159. [Google Scholar] [CrossRef]
- Ziegler, R.; Ilyas, S.; Mathur, S.; Goya, G.F.; Fuentes-García, J.A. Remote-Controlled Activation of the Release through Drug-Loaded Magnetic Electrospun Fibers. Fibers 2024, 12, 48. [Google Scholar] [CrossRef]
- Antonelli, A.; Bianchi, M.; Fear, E.; Giorgi, L.; Rossi, L. Management of Fibromyalgia: Novel Nutraceutical Therapies Beyond Traditional Pharmaceuticals. Nutrients 2025, 17, 530. [Google Scholar] [CrossRef]
- De Castro, F.; Stefàno, E.; Fanizzi, F.P.; Di Corato, R.; Abdalla, P.; Luchetti, F.; Nasoni, M.G.; Rinaldi, R.; Magnani, M.; Benedetti, M.; et al. Compatibility of Nucleobases Containing Pt(II) Complexes with Red Blood Cells for Possible Drug Delivery Applications. Molecules 2023, 28, 6760. [Google Scholar] [CrossRef] [PubMed]
- Slavu, L.M.; Antonelli, A.; Scarpa, E.S.; Abdalla, P.; Wilhelm, C.; Silvestri, N.; Pellegrino, T.; Scheffler, K.; Magnani, M.; Rinaldi, R.; et al. Optimization of Magnetic Nanoparticles for Engineering Erythrocytes as Theranostic Agents. Biomater. Sci. 2023, 11, 3252–3268. [Google Scholar] [CrossRef]
- Nguyen, D.B.; Tran, H.T.; Kaestner, L.; Bernhardt, I. The Relation Between Extracellular Vesicles Released from Red Blood Cells, Their Cargo, and the Clearance by Macrophages. Front. Physiol. 2022, 13, 783260. [Google Scholar] [CrossRef]
- Usman, W.M.; Pham, T.C.; Kwok, Y.Y.; Vu, L.T.; Ma, V.; Peng, B.; Chan, Y.S.; Wei, L.; Chin, S.M.; Azad, A.; et al. Efficient RNA Drug Delivery Using Red Blood Cell Extracellular Vesicles. Nat. Commun. 2018, 9, 2359. [Google Scholar] [CrossRef]
- Biagiotti, S.; Canonico, B.; Tiboni, M.; Abbas, F.; Perla, E.; Montanari, M.; Battistelli, M.; Papa, S.; Casettari, L.; Rossi, L.; et al. Efficient and Highly Reproducible Production of Red Blood Cell-Derived Extracellular Vesicle Mimetics for the Loading and Delivery of RNA Molecules. Sci. Rep. 2024, 14, 14610. [Google Scholar] [CrossRef]
- Ghoshal, B.; Jhunjhunwala, S. A Game of Hide-and-Seek: How Extracellular Vesicles Evade the Immune System. Drug Deliv. Transl. Res. 2025, 15, 2624–2642. [Google Scholar] [CrossRef]
- Joshi, U.; George, L.B.; Highland, H. Red Blood Cell Extracellular Vesicles: New Frontiers in Hematological Biomarker Discovery. Front. Med. 2025, 12, 1644077. [Google Scholar] [CrossRef]
- Gan, Y.; Li, C.; Peng, X.; Wu, S.; Li, Y.; Tan, J.P.K.; Yang, Y.Y.; Yuan, P.; Ding, X. Fight Bacteria with Bacteria: Bacterial Membrane Vesicles as Vaccines and Delivery Nanocarriers against Bacterial Infections. Nanomedicine 2021, 35, 102398. [Google Scholar] [CrossRef] [PubMed]
- Scarpa, E.-S.; Giordani, C.; Antonelli, A.; Petrelli, M.; Balercia, G.; Silvetti, F.; Pieroni, A.; Sabbatinelli, J.; Rippo, M.R.; Olivieri, F.; et al. The Combination of Natural Molecules Naringenin, Hesperetin, Curcumin, Polydatin and Quercetin Synergistically Decreases SEMA3E Expression Levels and DPPIV Activity in In Vitro Models of Insulin Resistance. Int. J. Mol. Sci. 2023, 24, 8071. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Pan, J.; Yuan, R.; Chen, M.; Guo, X.; Zhou, S. Shell-Sheddable Polymeric Micelles Alleviate Oxidative Stress and Inflammation for Enhanced Ischemic Stroke Therapy. Nano Lett. 2023, 23, 6544–6552. [Google Scholar] [CrossRef]
- Kim, M.S.; Haney, M.J.; Zhao, Y.; Mahajan, V.; Deygen, I.; Klyachko, N.L.; Inskoe, E.; Piroyan, A.; Sokolsky, M.; Okolie, O.; et al. Development of Exosome-Encapsulated Paclitaxel to Overcome MDR in Cancer Cells. Nanomedicine 2016, 12, 655–664. [Google Scholar] [CrossRef]
- Semmler-Behnke, M.; Kreyling, W.G.; Lipka, J.; Fertsch, S.; Wenk, A.; Takenaka, S.; Schmid, G.; Brandau, W. Biodistribution of 1.4- and 18-nm Gold Particles in Rats. Small 2008, 4, 2108–2111. [Google Scholar] [CrossRef] [PubMed]
- Geiser, M.; Kreyling, W.G. Deposition and Biokinetics of Inhaled Nanoparticles. Part. Fibre Toxicol. 2010, 7, 2. [Google Scholar] [CrossRef]
- Abdelmonem, M.; Al-Mokaddem, A.K.; Zakaria, M.Y. TPGS-Functionalized Nanocarriers with Improved Flavonoid Oral Bioavailability and Therapeutic Action: Pharmacokinetic and Mechanistic Insights in Diabetes-Induced Retinopathy. Eur. J. Pharm. Biopharm. 2025, 216, 114851. [Google Scholar] [CrossRef]
- Shabestari, S.M.; Pourmadadi, M.; Abdouss, H.; Ghanbari, T.; Abdouss, M.; Rahdar, A.; Cambón, A.; Taboada, P. PH-Responsive Chitosan-Sodium Alginate Nanocarriers for Curcumin Delivery against Brain Cancer. Colloids Surf. B Biointerfaces 2025, 255, 114875. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, J.; Li, C.; Qiao, H.; Hussain, Z. Functionalization of Curcumin Nanomedicines: A Recent Promising Adaptation to Maximize Pharmacokinetic Profile, Specific Cell Internalization and Anticancer Efficacy against Breast Cancer. J. Nanobiotechnol. 2023, 21, 106. [Google Scholar] [CrossRef]
- Hu, C.-M.J.; Zhang, L.; Aryal, S.; Cheung, C.; Fang, R.H.; Zhang, L. Erythrocyte Membrane-Camouflaged Polymeric Nanoparticles as a Biomimetic Delivery Platform. Proc. Natl. Acad. Sci. USA 2011, 108, 10980–10985. [Google Scholar] [CrossRef]
- Göran Ronquist, K. Extracellular Vesicles and Energy Metabolism. Clin. Chim. Acta 2019, 488, 116–121. [Google Scholar] [CrossRef]
- Sil, S.; Dagur, R.S.; Liao, K.; Peeples, E.S.; Hu, G.; Periyasamy, P.; Buch, S. Strategies for the Use of Extracellular Vesicles for the Delivery of Therapeutics. J. Neuroimmune Pharmacol. 2020, 15, 422–442. [Google Scholar] [CrossRef] [PubMed]
- Asadujjaman, M.; Nam, Y.R.; Lee, D.-E.; Choi, J.U.; Kim, S.H.; Jang, D.-J.; Jee, J.-P. Fusion Nanoparticle System of Extracellular Vesicles and Docetaxel-Loaded Liposomes: An Innovative Therapeutic Strategy to Enhance Anticancer Efficacy. J. Pharm. Investig. 2025, 1–20. [Google Scholar] [CrossRef]
- Kumar, P.; Mehta, D.; Bissler, J.J. Physiologically Based Pharmacokinetic Modeling of Extracellular Vesicles. Biology 2023, 12, 1178. [Google Scholar] [CrossRef] [PubMed]
- Di Buduo, C.A.; Wray, L.S.; Tozzi, L.; Malara, A.; Chen, Y.; Ghezzi, C.E.; Smoot, D.; Sfara, C.; Antonelli, A.; Spedden, E.; et al. Programmable 3D Silk Bone Marrow Niche for Platelet Generation Ex Vivo and Modeling of Megakaryopoiesis Pathologies. Blood 2015, 125, 2254–2264. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Zheng, S.; Shan, S.; Cai, J.; Liu, Y.; Chen, W.; He, X.; Zhao, C. Delivery of Natural Small Molecules Through Nanocarriers for Cancer Treatment. Food Front. 2025, 6, 1303–1322. [Google Scholar] [CrossRef]
- Dhupal, M.; Chowdhury, D. Phytochemical-Based Nanomedicine for Advanced Cancer Theranostics: Perspectives on Clinical Trials to Clinical Use. Int. J. Nanomed. 2020, 15, 9125–9157. [Google Scholar] [CrossRef]
- Baumann, A.; Tuerck, D.; Prabhu, S.; Dickmann, L.; Sims, J. Pharmacokinetics, Metabolism and Distribution of PEGs and PEGylated Proteins: Quo Vadis? Drug Discov. Today 2014, 19, 1623–1631. [Google Scholar] [CrossRef]
- Karim, A.; Rehman, A.; Feng, J.; Noreen, A.; Assadpour, E.; Kharazmi, M.S.; Lianfu, Z.; Jafari, S.M. Alginate-Based Nanocarriers for the Delivery and Controlled-Release of Bioactive Compounds. Adv. Colloid. Interface Sci. 2022, 307, 102744. [Google Scholar] [CrossRef]
- VandenBerg, M.A.; Dong, X.; Smith, W.C.; Tian, G.; Stephens, O.; O’Connor, T.F.; Xu, X. Learning from the Future: Towards Continuous Manufacturing of Nanomaterials. AAPS Open 2025, 11, 7. [Google Scholar] [CrossRef]
- Gimondi, S.; Ferreira, H.; Reis, R.L.; Neves, N.M. Microfluidic Devices: A Tool for Nanoparticle Synthesis and Performance Evaluation. ACS Nano 2023, 17, 14205–14228. [Google Scholar] [CrossRef]
- Huang, S.; Xu, D.; Zhang, L.; Hao, L.; Jia, Y.; Zhang, X.; Cheng, T.; Chen, J. Therapeutic Effects of Curcumin Liposomes and Nanocrystals on Inflammatory Osteolysis: In Vitro and in Vivo Comparative Study. Pharmacol. Res. 2023, 192, 106778. [Google Scholar] [CrossRef]
- Hao, M.; Zhang, C.; Wang, T.; Hu, H. Pharmacological Effects, Formulations, and Clinical Research Progress of Curcumin. Front. Pharmacol. 2025, 16, 1509045. [Google Scholar] [CrossRef] [PubMed]
- Amaroli, A.; Panfoli, I.; Bozzo, M.; Ferrando, S.; Candiani, S.; Ravera, S. The Bright Side of Curcumin: A Narrative Review of Its Therapeutic Potential in Cancer Management. Cancers 2024, 16, 2580. [Google Scholar] [CrossRef]
- Holdhoff, M.; Sahebjam, S.; Huang, P.; Kamson, D.O.; Iacoboni, M.; Dobson-Brown, T.; Schreck, K.C.; Kleinberg, L.; Redmond, K.J.; Croog, V.J.; et al. Liposomal Curcumin and Standard Radiation and Temozolomide for Newly Diagnosed High-Grade Gliomas: A Phase 1/2 Study. J. Clin. Oncol. 2025, 43, 16. [Google Scholar] [CrossRef]
- Bailly, A.-L.; Correard, F.; Popov, A.; Tselikov, G.; Chaspoul, F.; Appay, R.; Al-Kattan, A.; Kabashin, A.V.; Braguer, D.; Esteve, M.-A. In Vivo Evaluation of Safety, Biodistribution and Pharmacokinetics of Laser-Synthesized Gold Nanoparticles. Sci. Rep. 2019, 9, 12890. [Google Scholar] [CrossRef] [PubMed]
- Caputo, F.; Favre, G.; Borchard, G.; Calzolai, L.; Fisicaro, P.; Frejafon, E.; Günday-Türeli, N.; Koltsov, D.; Minelli, C.; Nelson, B.C.; et al. Toward an International Standardisation Roadmap for Nanomedicine. Drug Deliv. Transl. Res. 2024, 14, 2578–2588. [Google Scholar] [CrossRef]
- Musazzi, U.M.; Franzè, S.; Condorelli, F.; Minghetti, P.; Caliceti, P. Feeding Next-Generation Nanomedicines to Europe: Regulatory and Quality Challenges. Adv. Healthc. Mater. 2023, 12, e2301956. [Google Scholar] [CrossRef] [PubMed]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and Challenges of Liposome Assisted Drug Delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef]
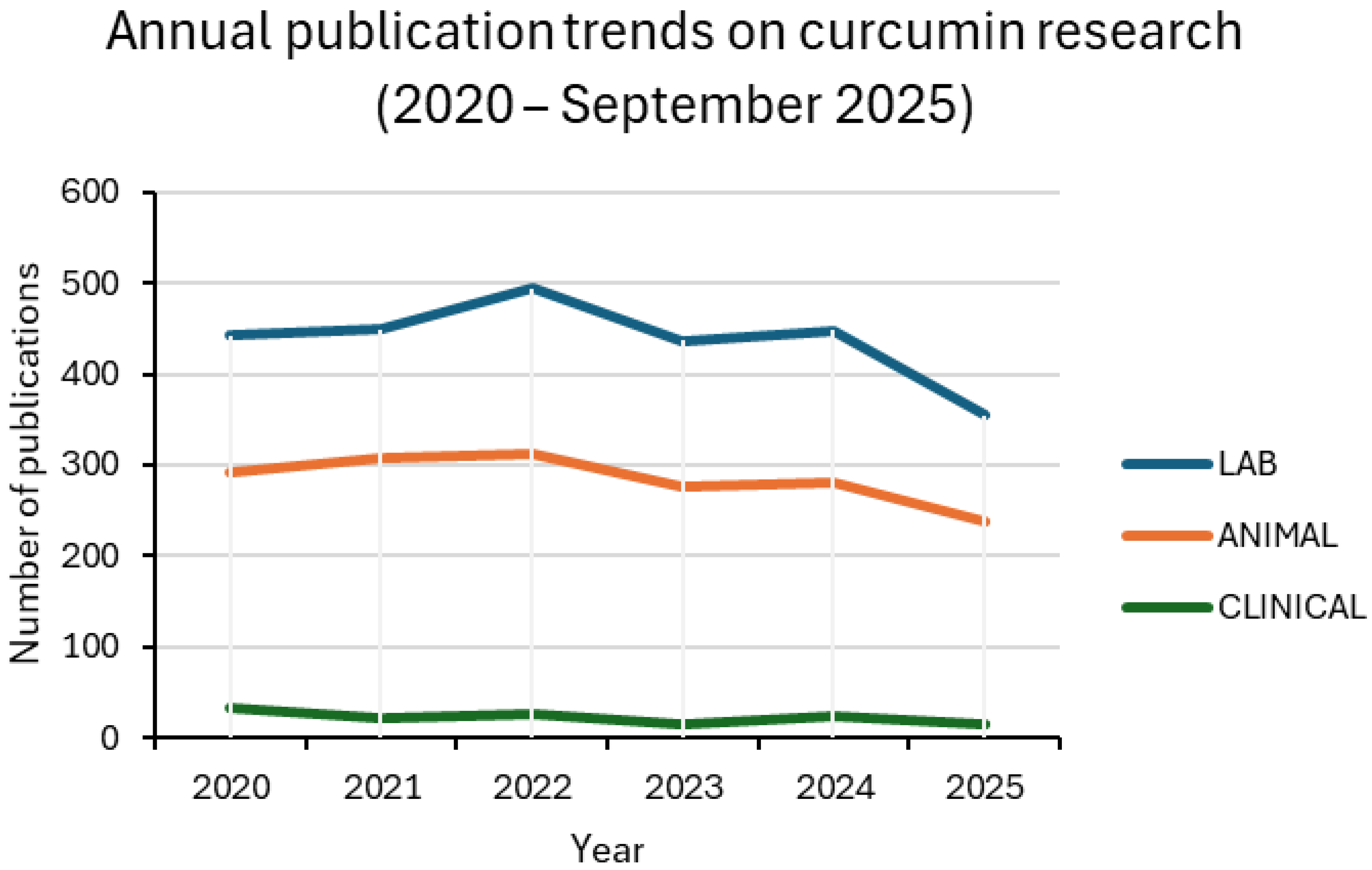
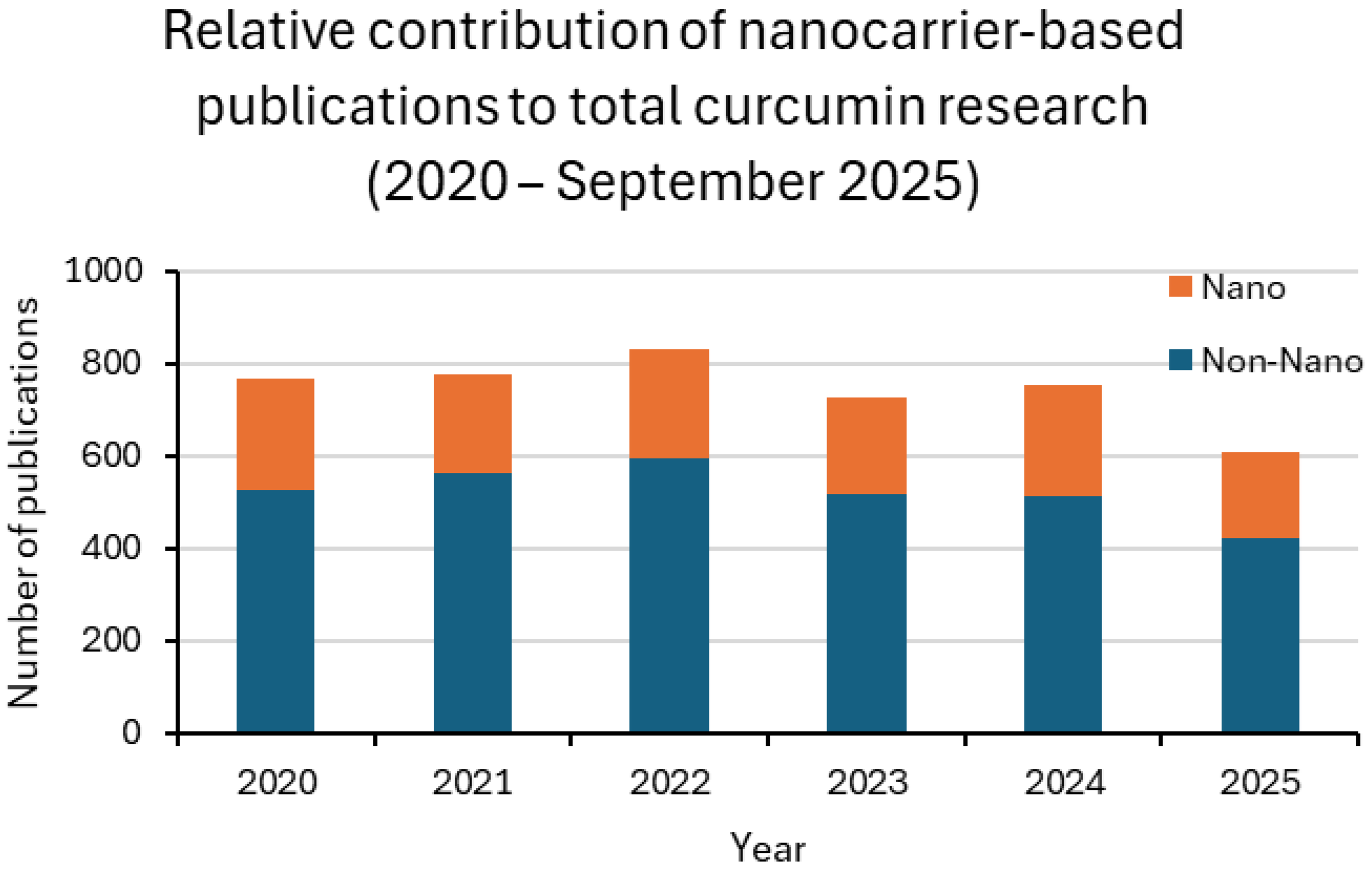

| Year | LAB_NANO/ LAB (%) | ANIMAL_NANO/ ANIMAL (%) | CLINICAL_NANO/ CLINICAL (%) |
|---|---|---|---|
| 2020 | 28.7 | 37.2 | 18.8 |
| 2021 | 28.3 | 28.2 | 9.5 |
| 2022 | 27.3 | 31.3 | 15.4 |
| 2023 | 28.8 | 29.0 | 20.0 |
| 2024 | 31.0 | 35.0 | 20.0 |
| 2025 | 31.9 | 30.1 | 7.1 |
| System | Natural Compound (s) | Main Findings | Preclinical Application | Reference |
|---|---|---|---|---|
| RBCM vesicles (RBCM-Cur) | Curcumin | High entrapment efficiency; tunable size, zeta potential, and release via sonication. | Potential systemic anti-inflammatory therapy | [22] |
| RBC-mimicking liposomes (RC-Lips) | Curcumin | Prolonged circulation; macrophage polarization to M2 phenotype; accelerated wound closure. | Diabetic wound healing, tissue repair | [23] |
| RBC-derived extracellular vesicles | Cas9 mRNA, gRNAs, oligonucleotides | Efficient nucleic acid delivery in human cells and xenograft mouse models; no cytotoxicity. | Gene editing and RNA-based therapeutics | [64] |
| RBC-EVs (soft extrusion method) | miR-210 | Homogeneous vesicles; efficient delivery to endothelial cells; improved angiogenic activity. | Regenerative medicine, vascular repair | [65] |
| RBC-EVs | — | Highlighted role as carriers and diagnostic biomarkers in inflammatory/hematological disorders. | Biomarker discovery, theranostic potential | [67] |
| Nanocarrier/Formulation | Natural Compound | Experimental Model | Key Outcomes | Reference |
|---|---|---|---|---|
| PLGA and PLGA–PEG nanoparticles (emulsion solvent evaporation) | Curcumin | Rodent models (oral administration) | ↑ Bioavailability 15.6-fold (PLGA) and 55.4-fold (PLGA–PEG) vs. free suspension; ↑ Cmax, Tmax, t1/2, AUC; ↓ clearance; significant tumor growth inhibition | [3] |
| FU–CMC–EGCG gold nanocomposites | Epigallocatechin gallate (EGCG) | Gastric cancer cells; in vivo gastric cancer model | ~89% tumor cell apoptosis at 20 mg/L; selective effect (no toxicity in HaCaT cells); superior anticancer activity in vivo | [16] |
| 198Au–EGCG radioactive gold nanoparticles | Epigallocatechin gallate (EGCG) | Prostate cancer models (theranostic approach) | >70% retention in tumor; prolonged survival; dual imaging + therapy; biocompatible synthesis; safe Hg-198 decay product (<1000-fold below EPA threshold) | [17] |
| Technology | Efficiency/ Yield/ Throughput | Initial Cost/ Investment | Product Uniformity/ Reproducibility | Compatible Carrier Types/ Application Notes |
|---|---|---|---|---|
| Batch Manufactory | Moderate, but with limited scalability; difficulty scaling linearly with volume (each batch requires adjustment) | Relatively low for small systems; costs increase with scale. | Batch-to-batch variability, wider distributions | Lipids (liposomes, SLN), polymers, emulsions; often used in industrial laboratories [86]. |
| Continuous manufacturing | High productivity, continuous integrated process | High initial investment, maintenance costs | Better uniformity, tighter control of process parameters | Polymeric nanoparticles, LNPs for mRNA therapy; e.g., VandenBerg et al. [87]. |
| Microfluidics/ continuous flow on a microscopic scale | Low absolute productivity per channel, but potential for parallelization | Medium–high (sophisticated devices, microchip, precise pumps) | Excellent dimensional uniformity, low PDI, reproducible | Lipid, polymeric particles, hybrid nanocarriers; e.g., Gimondi et al. [88]. |
| Compound/Formulation | Nanocarrier Type | Clinical/Translational Setting | Key Outcomes | Reference |
|---|---|---|---|---|
| Curcumin liposomes | Liposomal formulation | Post-arthroplasty inflammatory osteolysis (preclinical/early translational) | Reduced osteolysis, improved anti-inflammatory response | [89] |
| Curcumin formulations | Nanoparticles, micelles, liposomes (review) | Multiple indications (oncology, inflammation, metabolic diseases) | Improved bioavailability; identified translational barriers (heterogeneous response, limited endpoints) | [90] |
| Curcumin delivery systems | Nanoparticles, hydrogels | General clinical use (narrative review) | Highlighted therapeutic potential, need for advanced delivery, and awareness of drug–drug interactions | [91] |
| Liposomal Curcumin (LipoCurc™) | Liposomal nanoparticle | High-grade gliomas (Phase I/II trial, NCT05768919) | Dose-escalation with RT + TMZ; endpoints: MTD, RP2D, safety; preliminary results show tolerability and early efficacy | [92] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antonelli, A.; Palma, F. Nanocarrier-Based Delivery Systems for Natural Compounds Across Research Stages. Materials 2025, 18, 4960. https://doi.org/10.3390/ma18214960
Antonelli A, Palma F. Nanocarrier-Based Delivery Systems for Natural Compounds Across Research Stages. Materials. 2025; 18(21):4960. https://doi.org/10.3390/ma18214960
Chicago/Turabian StyleAntonelli, Antonella, and Francesco Palma. 2025. "Nanocarrier-Based Delivery Systems for Natural Compounds Across Research Stages" Materials 18, no. 21: 4960. https://doi.org/10.3390/ma18214960
APA StyleAntonelli, A., & Palma, F. (2025). Nanocarrier-Based Delivery Systems for Natural Compounds Across Research Stages. Materials, 18(21), 4960. https://doi.org/10.3390/ma18214960






