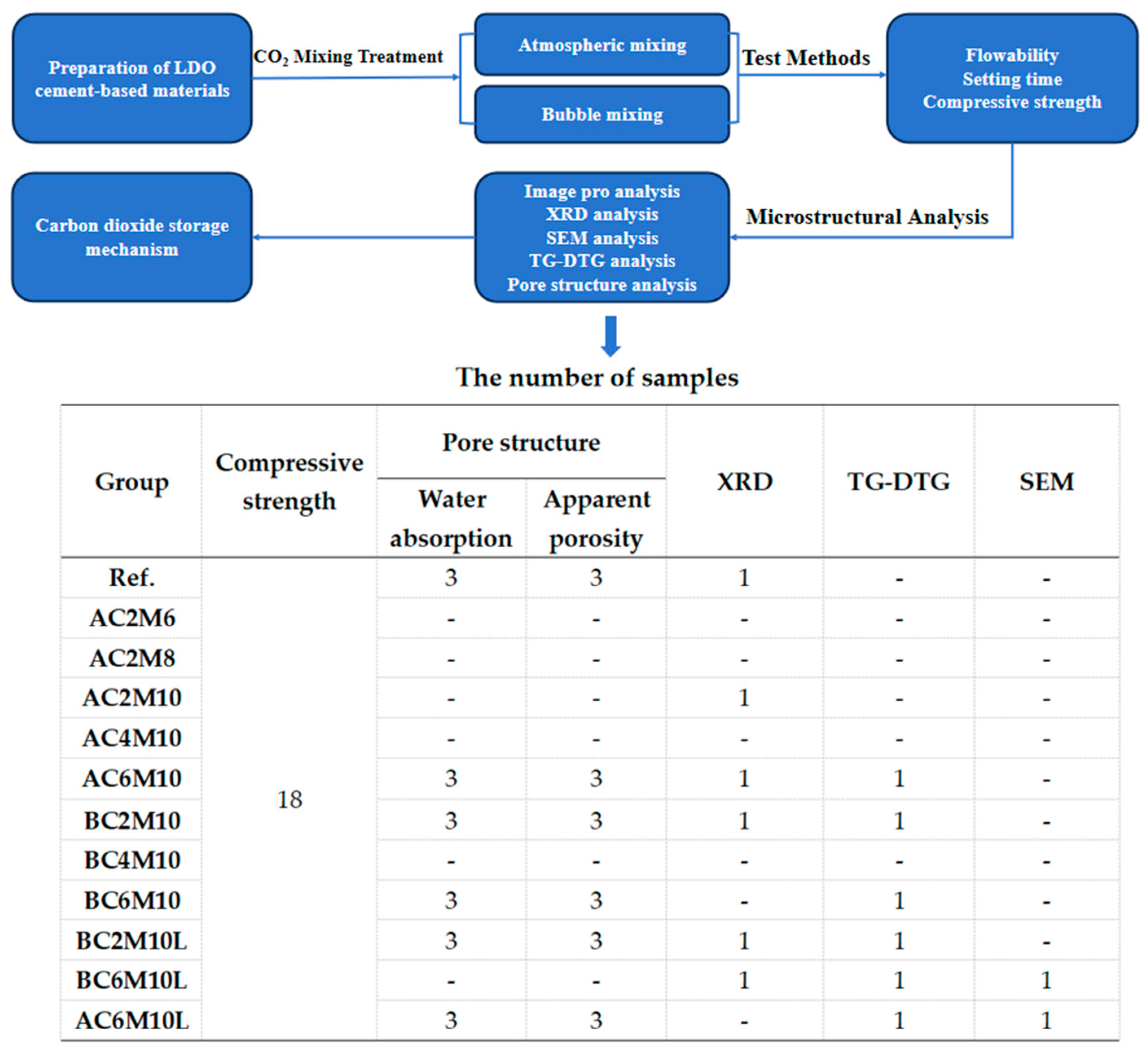
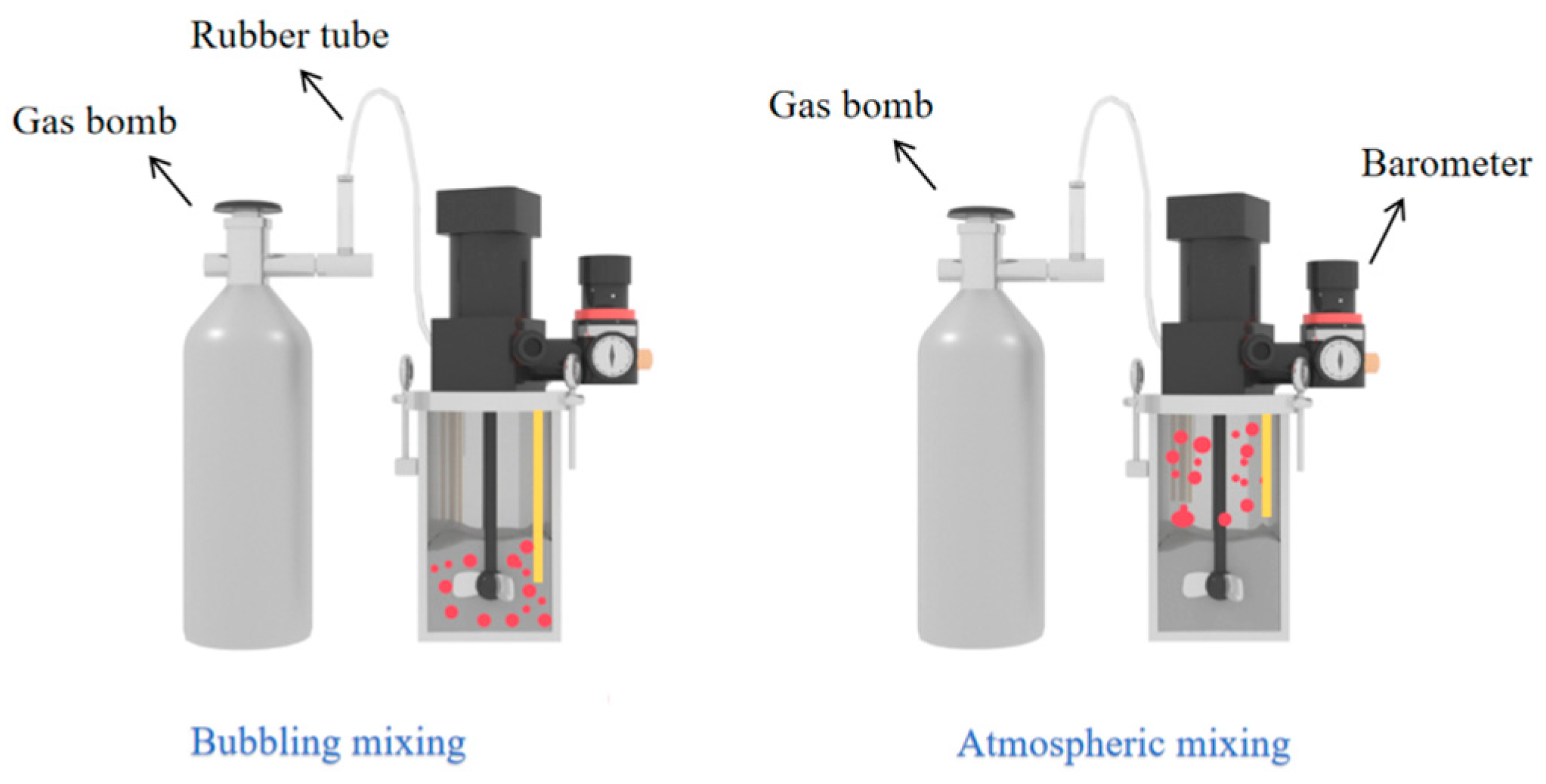
1. Introduction
Global warming has risen as one of the most pressing challenges worldwide. CO
2 emissions resulting from human production activities are the major cause and must be mitigated to alleviate the environmental burden [
1,
2,
3]. With the increasing demand for urbanization over the past few decades, the construction industry has emitted 8% CO
2, largely due to the extensive use of Portland cement as a binder [
4]. Consequently, the urgent need to reduce carbon emissions in the construction industry has drawn attention from both academic and industrial sectors.
Nowadays, carbon capture, utilization, and storage (CCUS) technology is a cutting-edge approach aimed at industrial decarbonization [
5]. Generally, this technology captures CO
2 generated during industrial production, compresses it under high pressure, and either stores it underground or utilizes it for product manufacturing to reduce CO
2 emissions [
6,
7]. It has been recognized that CCUS technology can be an effective tool to mitigate the carbon footprint of the cement industry. Carbonation curing, for instance, has been reported as one of the major approaches for the implementation of CCUS technology in the construction industry [
8]. Cement and concrete can be used as a carbon sink due to ca-bearing hydration products, which favor carbonation by generating calcium carbonate and calcium silicate hydrates (C-S-H) [
9]. The reactions can be detailed in the following equations [
10]:
Concrete subjected to carbonation curing is normally reported with great improvement on early-age strength enhancement and microstructure refinement, as the reaction degrees of the clinker phases can be largely improved under carbonation curing [
11]. Despite these benefits, there are shortcomings that need to be addressed. For instance, as carbonation curing requires a relatively long duration, the intensive demands for the carbonation chambers remain challenging for its execution [
12]. Meanwhile, for the hardened cement concrete, carbonation curing may lead to a gradient difference in terms of the reaction of cement clinker, which leads to an uneven distribution of carbonated phases [
13]. This has the potential to cause undesirable performance variations in cement-based materials.
In addition to carbonation curing, another approach for CCUS in cement-based materials can be the in situ carbonation of cement paste, namely carbonation mixing. Compared to carbonation curing, it has the advantage of reduced time consumption in concrete production, as it is performed during the initial mixing process [
14]. Moreover, it eliminates the need for specialized carbonation curing chambers, making it more feasible for industrial applications. Carbonation mixing can also evenly distribute the carbonated phases through the mixing process with the obtaining of more homogeneity of the cement matrix. However, carbonation mixing involves various mixing parameters, which can significantly impact the mechanical properties and carbon capture capacity of concrete. Wang et al. [
15] quantified the optimal proportion of CO
2 injection during the mixing stage (1.6 wt.%) and observed a 36.5% increase in the 3-day compressive strength of the cement paste and a 20% increase in the 28-day strength. These studies have shown that carbonation mixing can possibly enhance the mechanical properties of cement concrete, further underscoring its practical potential. Nonetheless, the carbon capture efficiency of cement pastes by carbonation mixing is reported to be lower than that of long-term carbonation curing [
16]. Improving this efficiency is a challenge to industry and academia for reducing the carbon footprint of the cement or concrete industry.
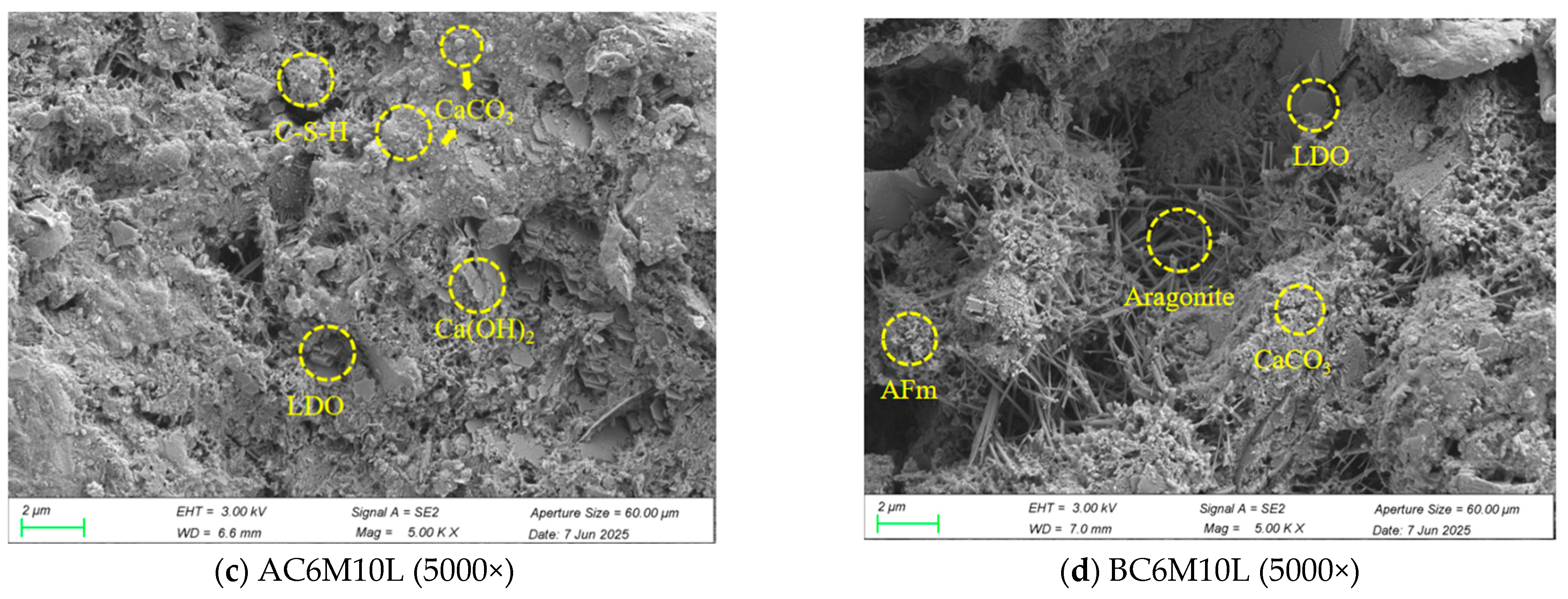
Layered double oxide (LDO) is the calcined product of layered double hydroxides (LDH) and has recently been studied for improving the performance of cementitious materials. In the work by Khalkhal et al. [
17], CaAl-LDH was applied as a hardening accelerator in concrete. The specimens containing CaAl-LDH showed a 61% increase in early compressive strength and a 71% increase in flexural strength. Chen et al. [
18] studied the influence of LDO on the physical and mechanical properties of cement-based materials and found the mechanical properties of cement mortar with 0.5% and 1% of LDO increased accordingly. Other than its benefits for strength enhancement, it should be highlighted that LDO can reconstruct its structure to form LDH through cement hydration due to its “memory effect”. LDH has been widely reported with desirable capacity for anion capture [
19]. Carbonate ions, in particular, are commonly captured within this structure. Chi utilizes the anion exchange capacity of CaAl-LDH and applies it to the immobilization of heavy metals, resulting in a reduction in the leaching rates of Pb
2+ and Mn
2+ in cement paste by 7% and 53%, respectively [
20]. Duan et al. [
21] found that with the addition of Mg
3Al-CO
3 hydrotalcite, especially calcined hydrotalcite (LDO), the carbonation depth of concrete was significantly reduced. Meis et al. [
22] studied the influence of LDHs’ particle size on the CO
2 adsorption performance, revealing that decreasing the particle size can lead to a significant increase in CO
2 adsorption capacity. These findings demonstrate the innovative application of LDH materials, revealing that CaAl-LDO not only readily participates in the formation of cement hydration products compared to MgAl-LDH, but also serves as an additional calcium source to promote calcium carbonate formation, thereby significantly enhancing the CO
2 storage capacity of cement-based materials.
The application of LDO specifically for in situ carbonation in cement has not yet been systematically investigated, making this a novel and valuable area of study. This study systematically investigates the synergistic effects of key carbonation mixing parameters, including mixing time, CO2 injection duration, and mixing methods in combination with LDO on the properties of cement paste. By evaluating their combined impact on mechanical properties, microstructure, and most critically, CO2 storage capacity, this study aims to provide a novel and practical strategy for improving carbonation mixing technology in cement-based materials.
4. Conclusions
CO2 mixing is one of the effective methods for reducing the carbon footprint of cement concrete. Minerals with great carbonate binding capacity are of great interest to promote the carbon capture of cementitious materials. Owing to the memory effect of LDO, it is already acknowledged for desirable anion capture and adsorption capacity in various literatures. In this paper, we investigated CO2 mixing in cement pastes, where LDO was introduced to promote the carbon capture capacity. Different mixing parameters were systematically studied to provide a comprehensive understanding of the relationship among the performance of cementitious materials, CO2 mixing parameters, and LDO. Based on the results, the following conclusions can be drawn:
- (1)
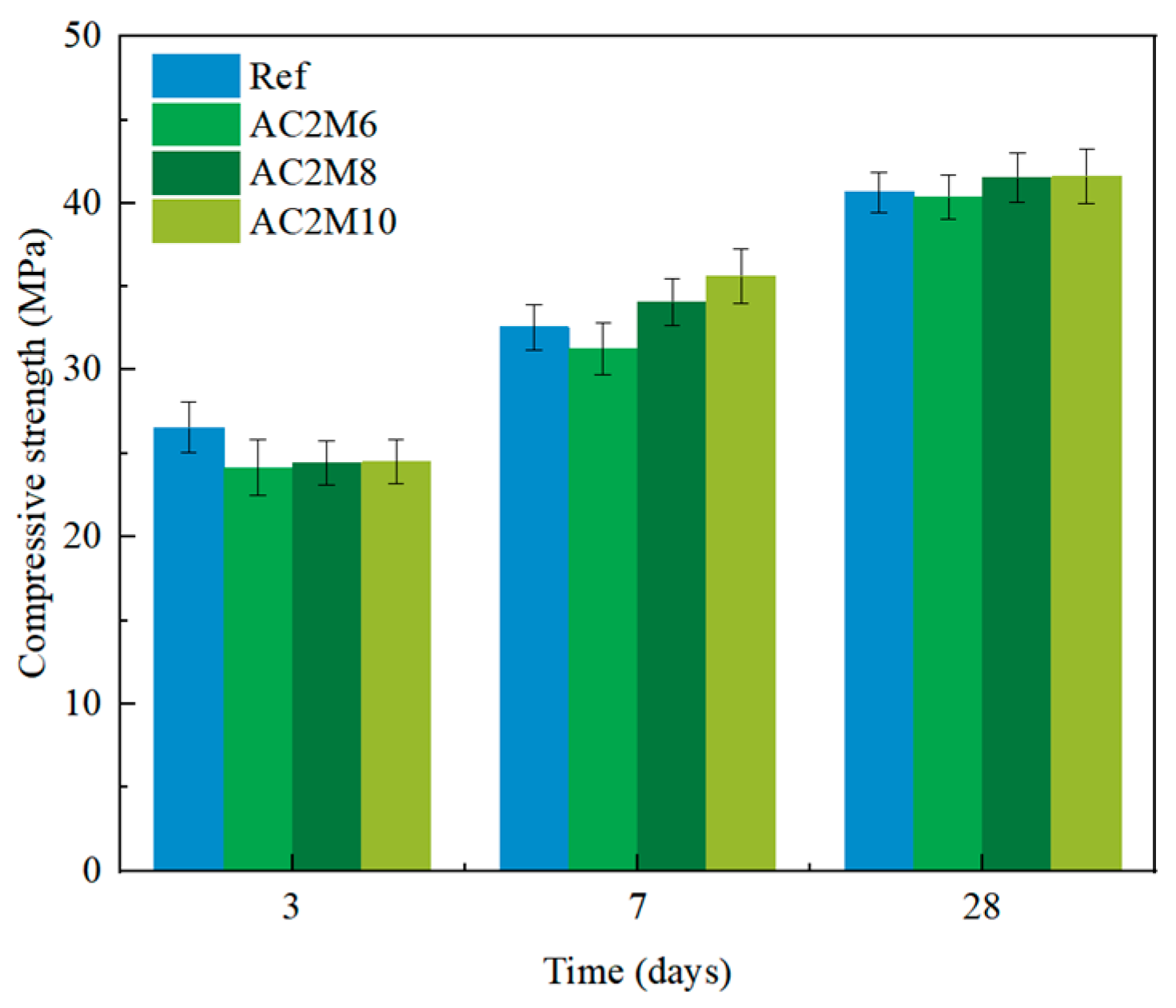
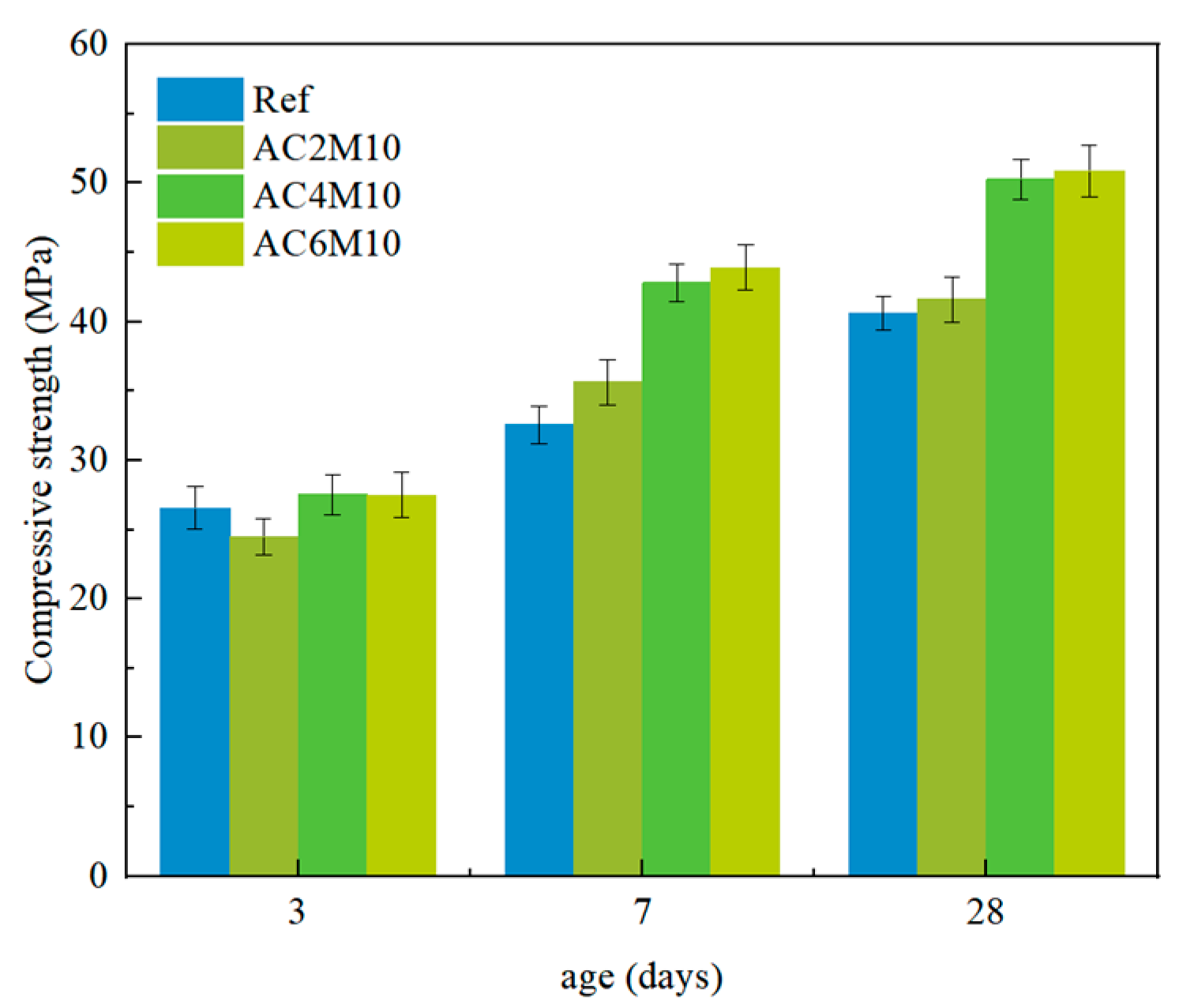
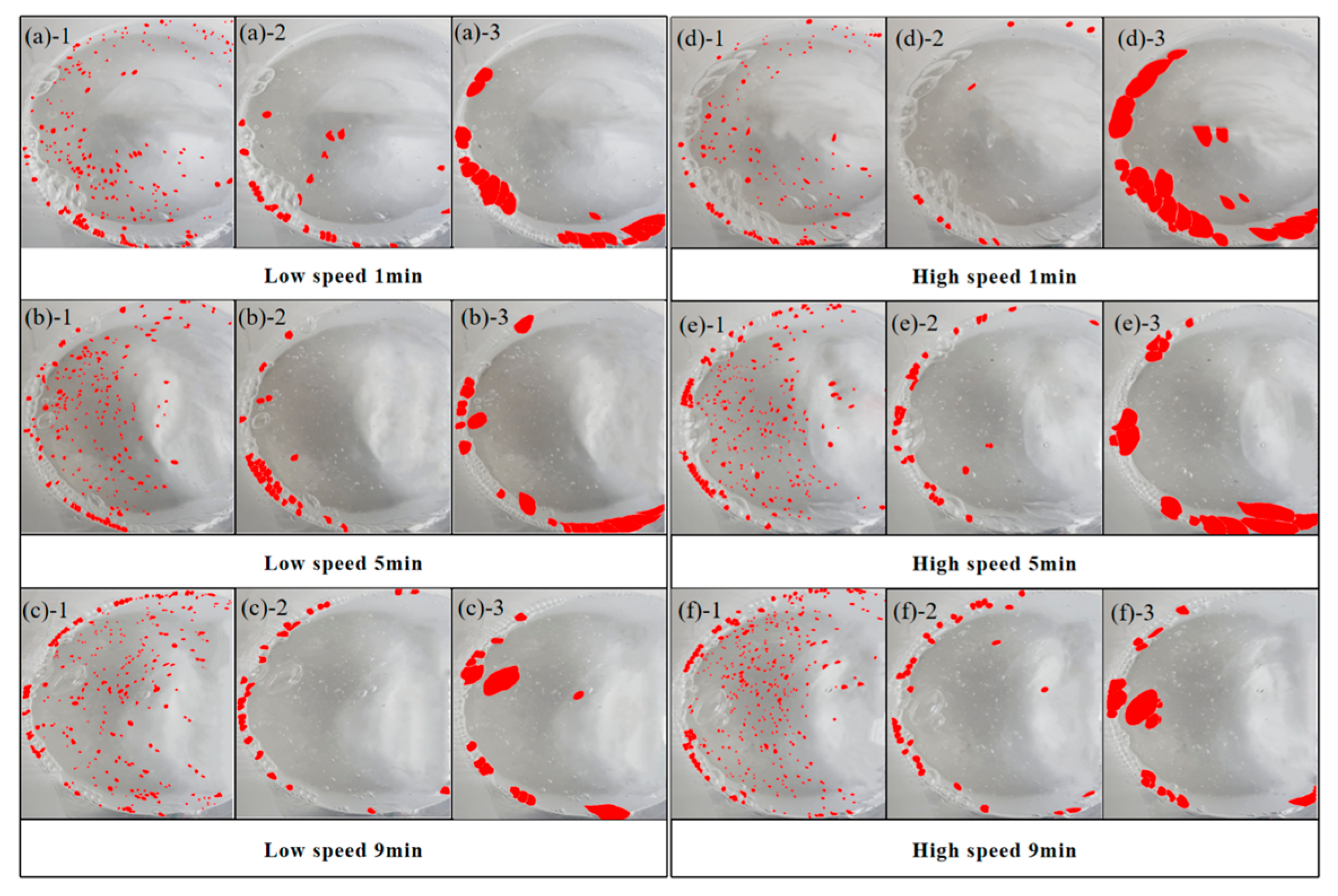
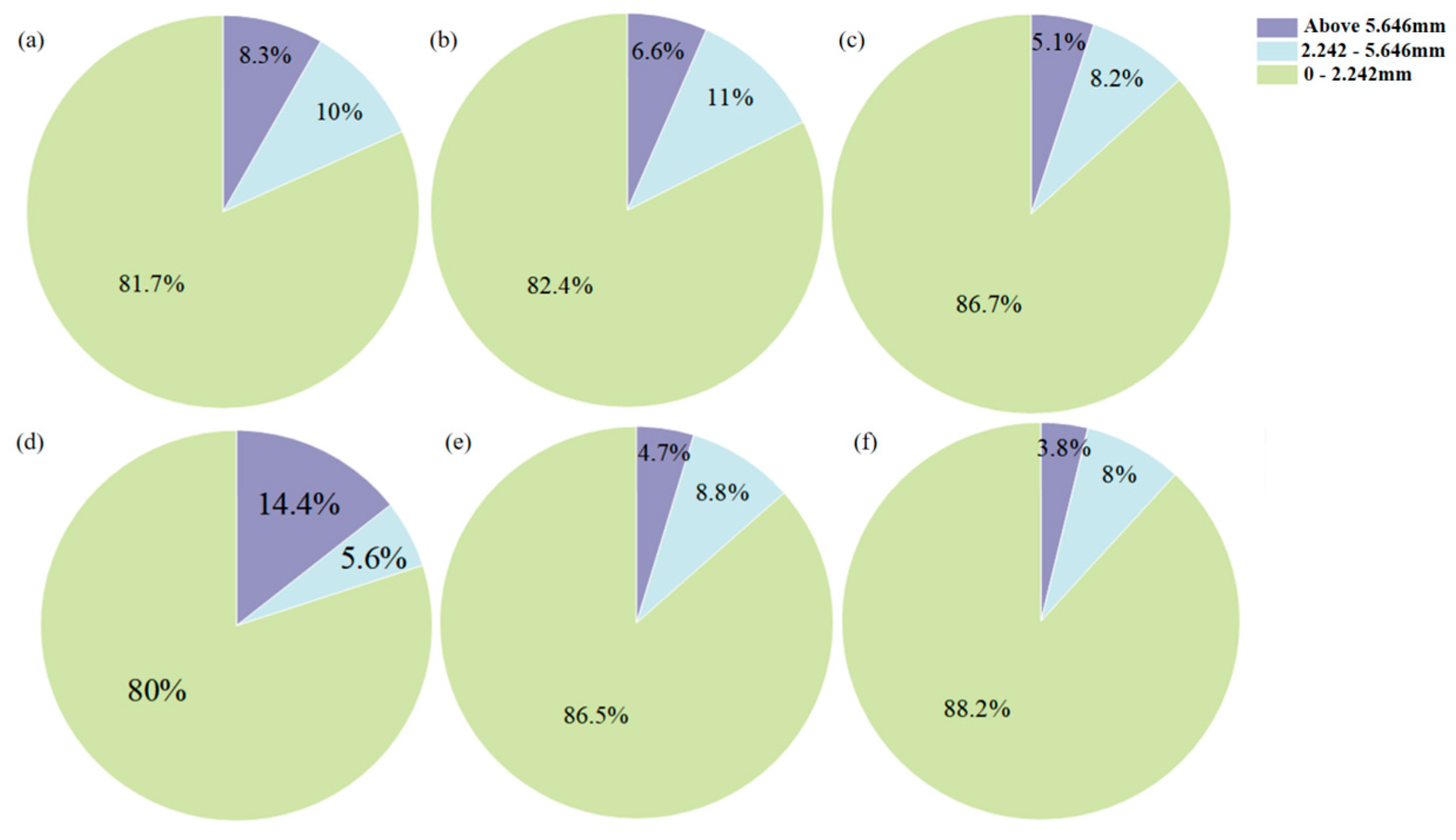
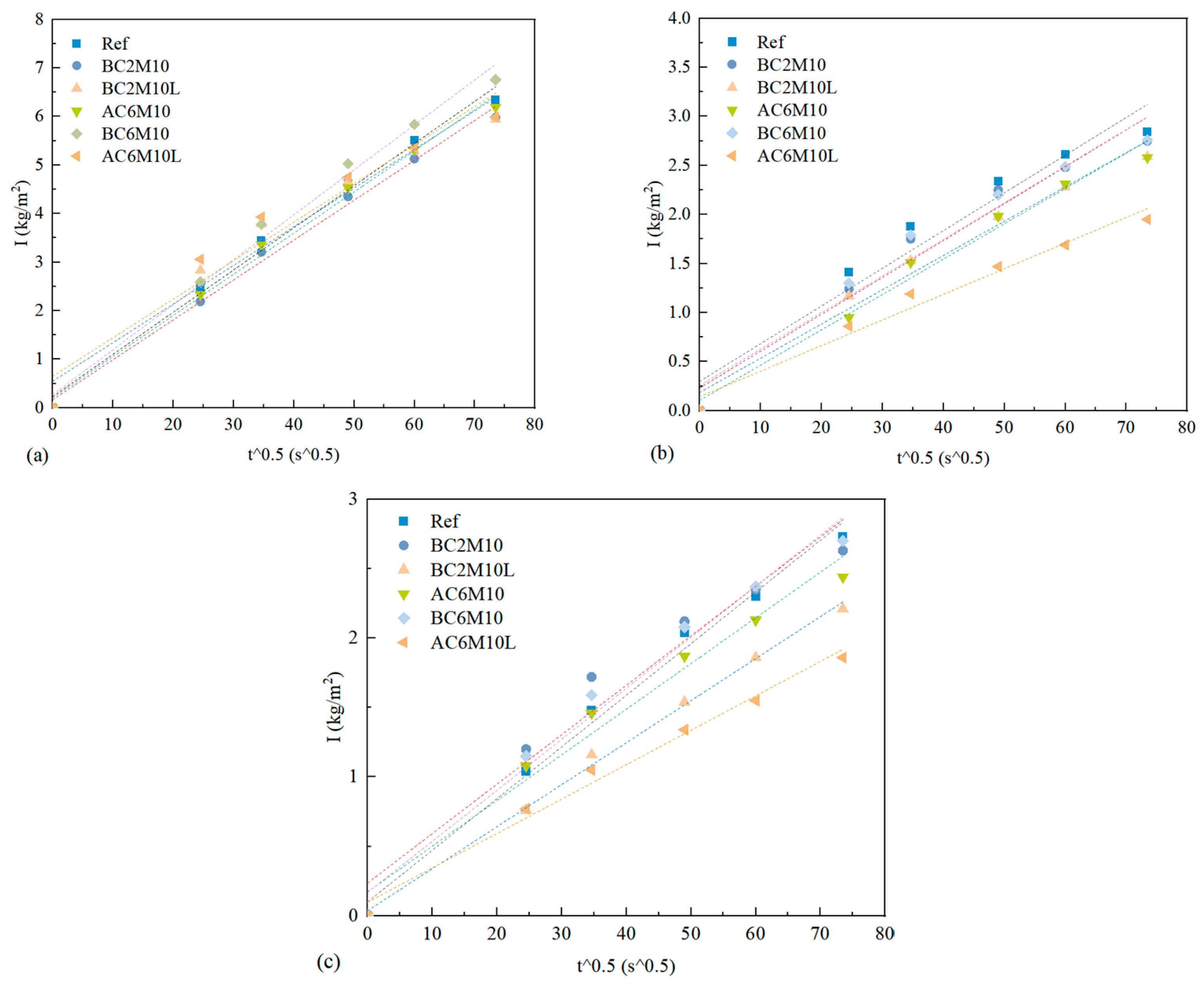
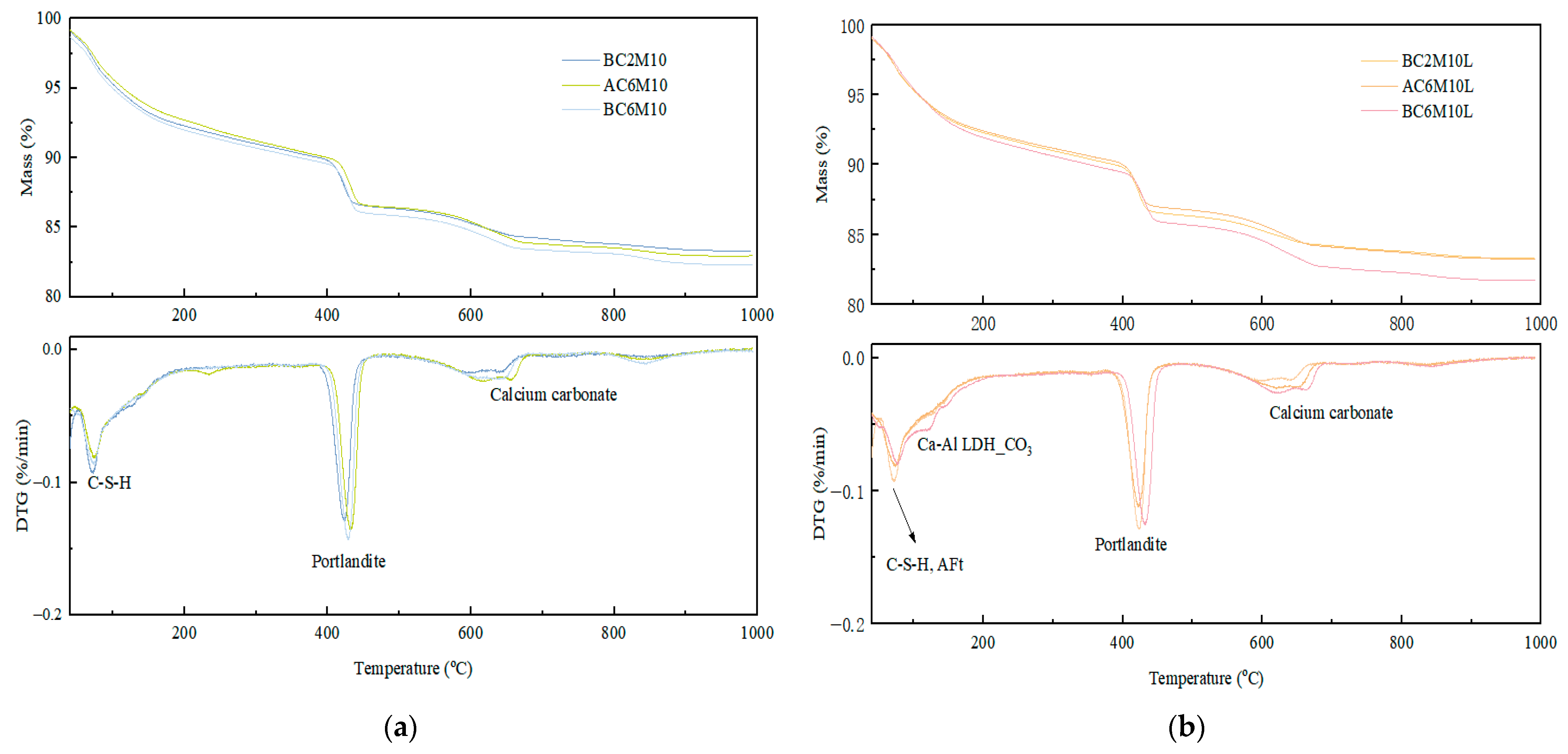
In atmospheric CO2 mixing, extending the CO2 injection duration significantly improves the compressive strength. In contrast, extending CO2 injection duration leads to a decrease in strength in bubble mixing. While the reaction mechanism remains similar, as indicated from XRD results, the loss in strength in bubble mixing is attributed to the capture of gas bubbles in the matrix, resulting in a more porous microstructure compared to the ones by atmospheric mixing, as evidenced by the higher water adsorption coefficient in bubble-mixed samples.
- (2)
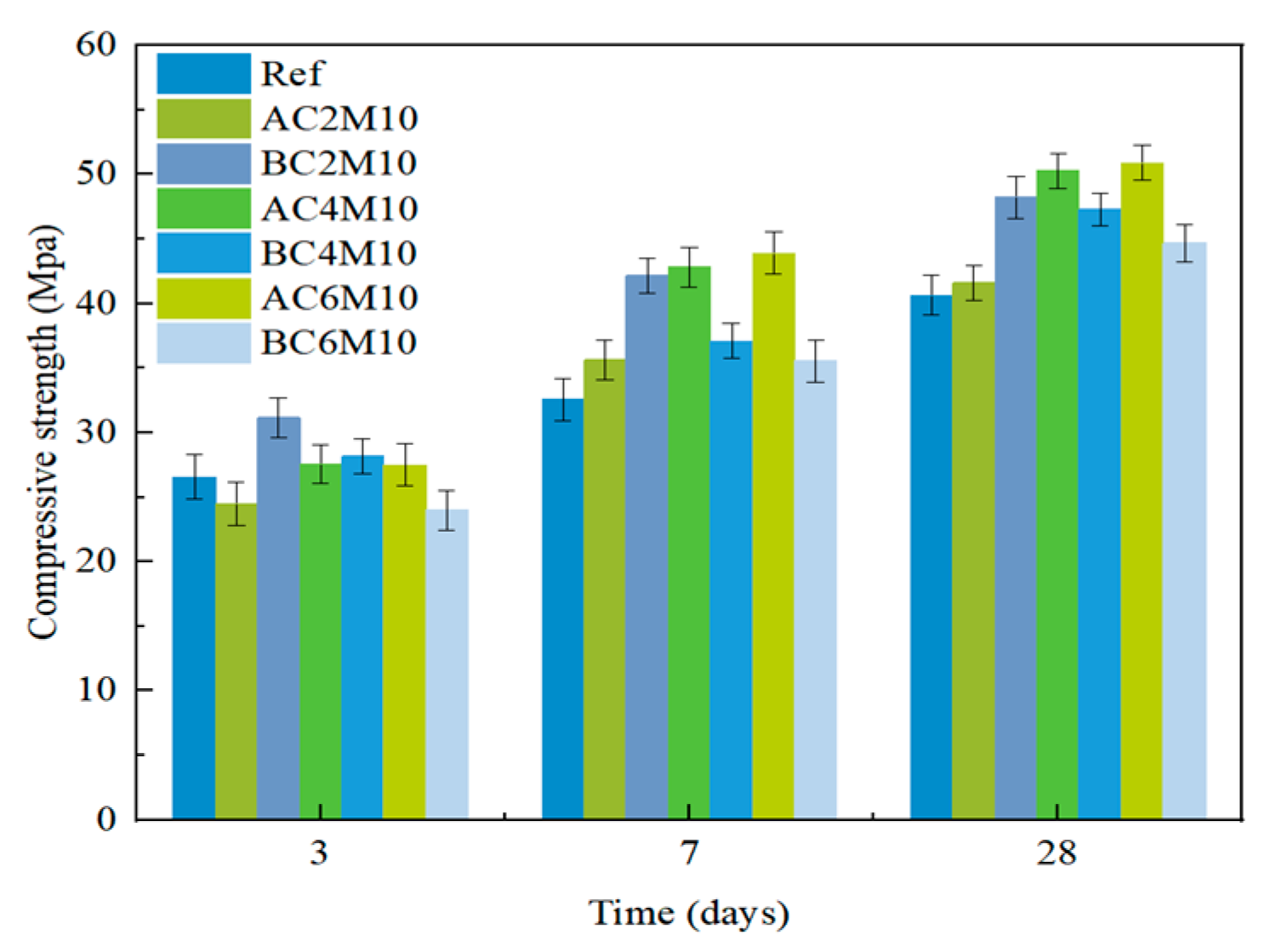
Bubble mixing is more efficient in carbon capture for cementitious materials, compared to atmospheric mixing, as a consequence of increased contacting surface area between CO2 and pastes. This can be validated by the significant early strength improvement by comparing BC2M10 with AC2M10 and can also be supported by the TGA.
- (3)
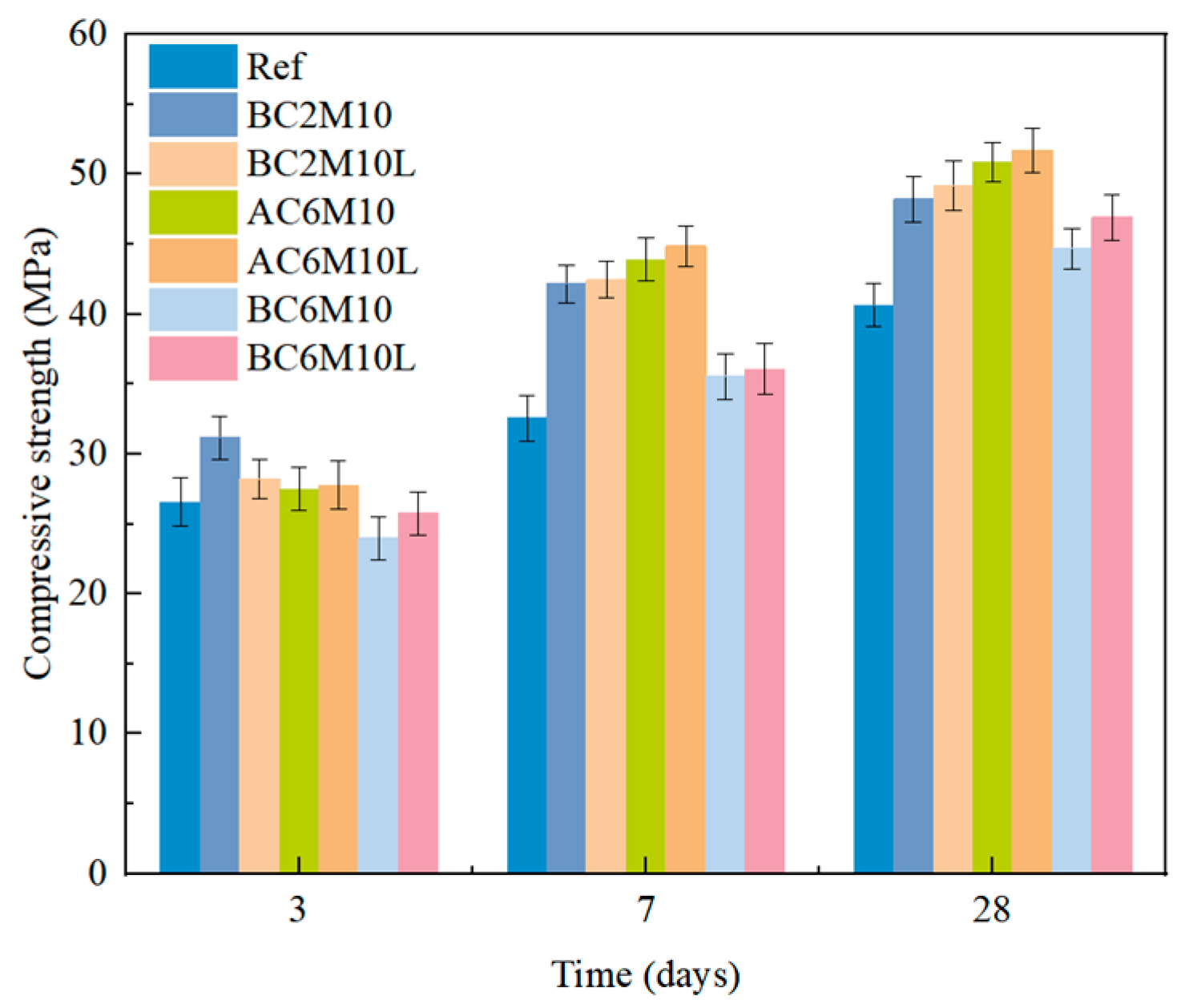
The introduction of LDO is evidenced with a promoted carbon capture capacity of cement pastes through carbonation mixing, regardless of the mixing methods. Under the same mixing parameters, the carbon capture capacity can be improved at most by 34.01% with the introduction of LDO, highlighting its significant environmental benefits.
In our current research, LDO shows its outstanding performance in improving the carbon storage capacity of cementitious materials. LDO with different Ca/Al ratios on the performance of cementitious materials, including their dispersion, macroscale properties, and microstructure, as well as carbon capture capacity, are of great interest, meriting significant attention for future research. It is important to note that this study utilized laboratory-synthesized LDO with a controlled particle size. Consequently, potential challenges related to its large-scale, cost-effective production and uniform dispersion during industrial-scale concrete mixing were not investigated. Furthermore, the long-term durability and stability of LDO within the cement matrix under various environmental conditions remain to be verified. Atmospheric mixing and bubble mixing technology can be applied in the field of prefabricated buildings. During mixing, engineered nozzles can uniformly inject carbon dioxide into the concrete, ensuring thorough mixing with the raw materials. Furthermore, concrete mixer trucks can be equipped with an auxiliary CO2 cylinder. A specialized gas distribution system integrated into the mixing blades would disperse CO2 uniformly throughout the concrete during transit. This facilitates homogeneous carbonation reactions throughout the mixture, effectively implementing both techniques.