Ligand-Mediated, Temperature-Tuned Synthesis of CsPbBr3 Nanosheets for Ordered Superlattice Assembly
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
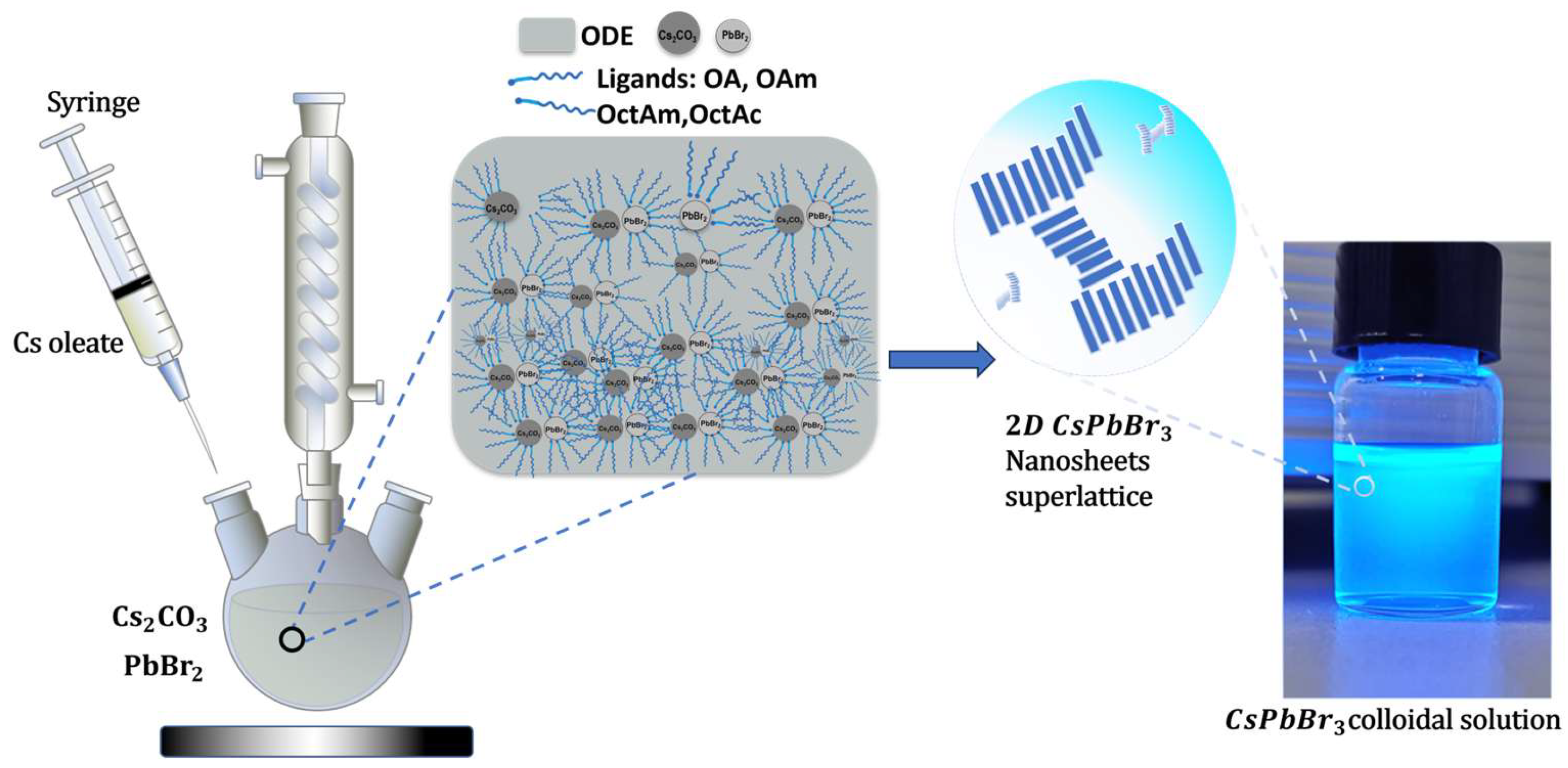
2.2. Methods
2.2.1. Cesium Oleate Precursor Synthesis
2.2.2. Synthesis of CsPbBr3 Nanosheets
2.2.3. Isolation and Purification of Nanosheets
2.3. Characterization
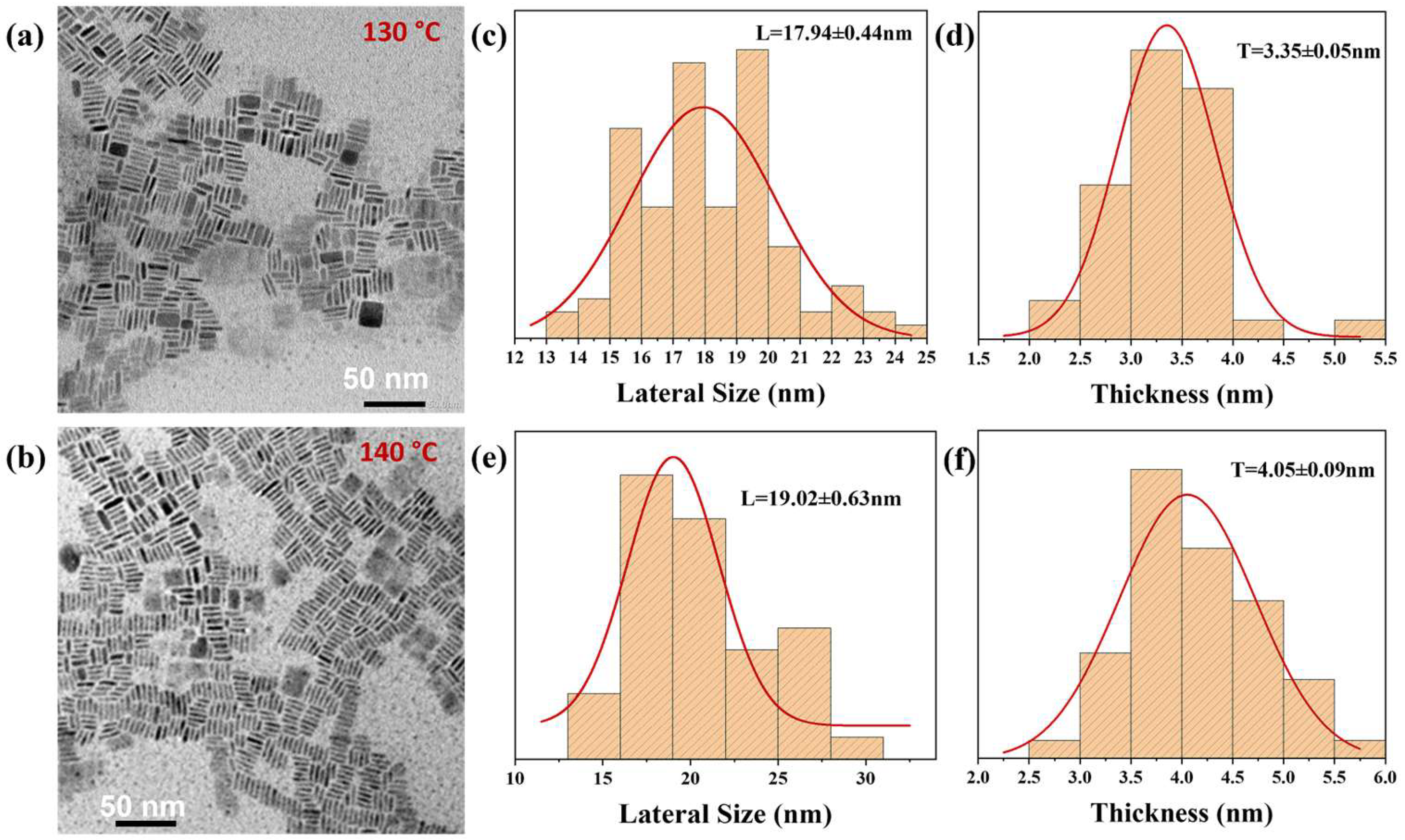
2.3.1. Characterization of Material Morphology
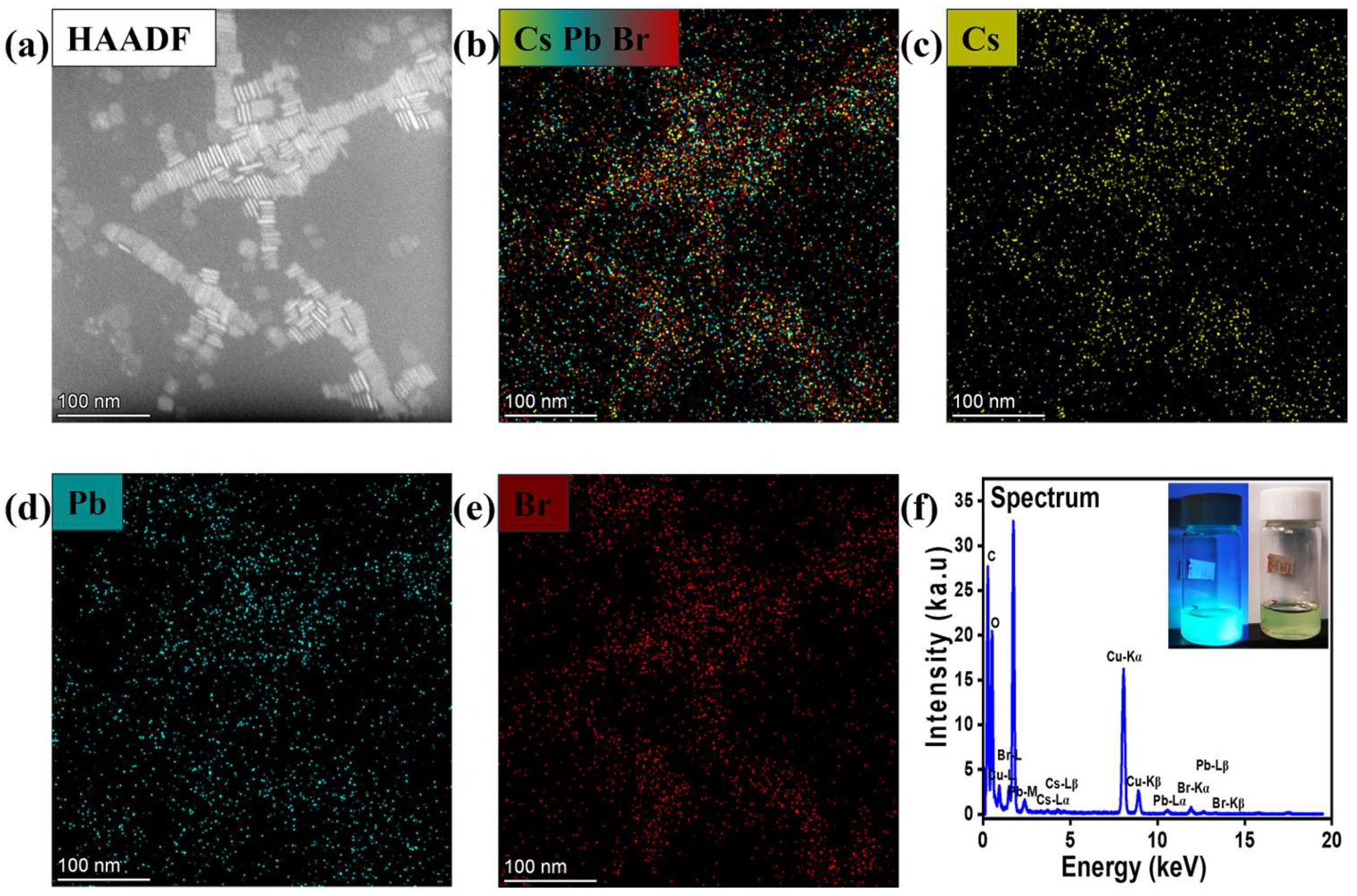
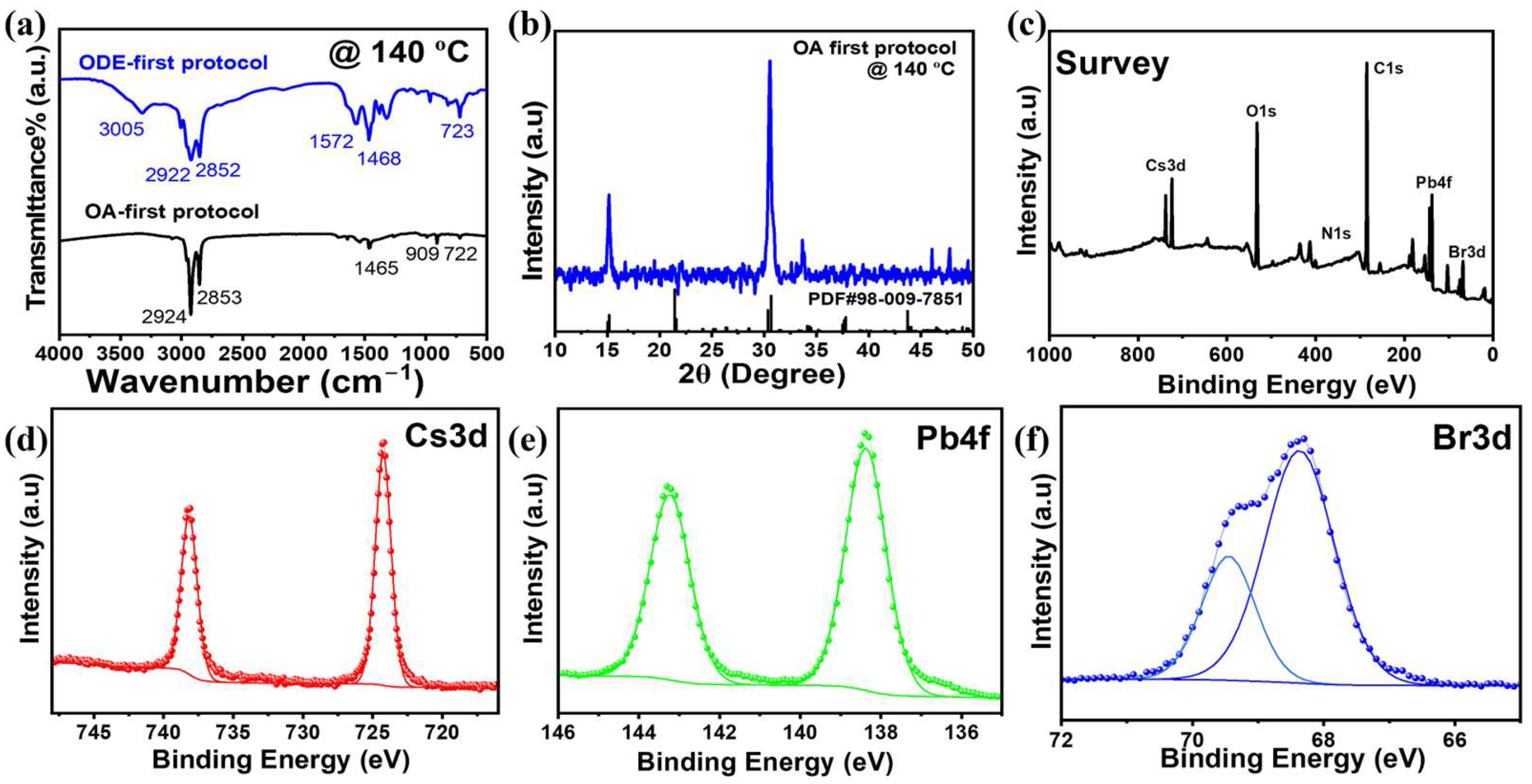
2.3.2. Structural and Surface Characterization
2.3.3. Fluorescence Spectrum Characterization
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Swarnkar, A.; Chulliyil, R.; Ravi, V.K.; Irfanullah, M.; Chowdhury, A.; Nag, A. Colloidal CsPbBr3 perovskite nanocrystals: Luminescence beyond traditional quantum dots. Angew. Chem. 2015, 127, 15644–15648. [Google Scholar] [CrossRef]
- Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Krieg, F.; Caputo, R.; Hendon, C.H.; Yang, R.X.; Walsh, A.; Kovalenko, M.V. Nanocrystals of cesium lead halide perovskites (CsPbX3, X= Cl, Br, and I): Novel optoelectronic materials showing bright emission with wide color gamut. Nano Lett. 2015, 15, 3692–3696. [Google Scholar] [CrossRef]
- Qiu, L.; Si, G.; Bao, X.; Liu, J.; Guan, M.; Wu, Y.; Qi, X.; Xing, G.; Dai, Z.; Bao, Q. Interfacial engineering of halide perovskites and two-dimensional materials. Chem. Soc. Rev. 2023, 52, 212–247. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Ran, C.; Gao, W.; Li, M.; Xia, Y.; Huang, W. Metal Halide Perovskite for next-generation optoelectronics: Progresses and prospects. eLight 2023, 3, 3. [Google Scholar] [CrossRef]
- Li, C.; Chen, C.; Gao, W.; Dong, H.; Zhou, Y.; Wu, Z.; Ran, C. Wide-Bandgap Lead Halide Perovskites for Next-Generation Optoelectronics: Current Status and Future Prospects. ACS Nano 2024, 18, 35130–35163. [Google Scholar] [CrossRef]
- Stoumpos, C.C.; Cao, D.H.; Clark, D.J.; Young, J.; Rondinelli, J.M.; Jang, J.I.; Hupp, J.T.; Kanatzidis, M.G. Ruddlesden–Popper hybrid lead iodide perovskite 2D homologous semiconductors. Chem. Mater. 2016, 28, 2852–2867. [Google Scholar] [CrossRef]
- Fu, Y.; Zhu, H.; Chen, J.; Hautzinger, M.P.; Zhu, X.-Y.; Jin, S. Metal halide perovskite nanostructures for optoelectronic applications and the study of physical properties. Nat. Rev. Mater. 2019, 4, 169–188. [Google Scholar] [CrossRef]
- Li, W.; Li, T.; Tong, Y.; Qi, H.; Zhang, Y.; Guo, Y.; Wang, H.; Wang, H.; Wang, K.; Wang, H. Fabrication of highly luminescent quasi two-dimensional CsPbBr3 perovskite films in high humidity air for light-emitting diodes. ACS Appl. Mater. Interfaces 2023, 15, 36602–36610. [Google Scholar] [CrossRef]
- Tyagi, D.; Laxmi, V.; Basu, N.; Reddy, L.; Tian, Y.; Ouyang, Z.; Nayak, P.K. Recent advances in two-dimensional perovskite materials for light-emitting diodes. Discov. Nano 2024, 19, 109. [Google Scholar] [CrossRef]
- Sun, S.; Yuan, D.; Xu, Y.; Wang, A.; Deng, Z. Ligand-mediated synthesis of shape-controlled cesium lead halide perovskite nanocrystals via reprecipitation process at room temperature. ACS Nano 2016, 10, 3648–3657. [Google Scholar] [CrossRef]
- Imran, M.; Di Stasio, F.; Dang, Z.; Canale, C.; Khan, A.H.; Shamsi, J.; Brescia, R.; Prato, M.; Manna, L. Colloidal synthesis of strongly fluorescent CsPbBr3 nanowires with width tunable down to the quantum confinement regime. Chem. Mater. 2016, 28, 6450–6454. [Google Scholar] [CrossRef]
- Liu, Q.; Li, H.; Wang, X.; He, J.; Luo, X.; Wang, M.; Liu, J.; Liu, Y. Synthesis and properties of size-adjustable CsPbBr3 nanosheets for potential photocatalysis. Materials 2024, 17, 2563. [Google Scholar] [CrossRef] [PubMed]
- Akkerman, Q.A.; D’innocenzo, V.; Accornero, S.; Scarpellini, A.; Petrozza, A.; Prato, M.; Manna, L. Tuning the optical properties of cesium lead halide perovskite nanocrystals by anion exchange reactions. J. Am. Chem. Soc. 2015, 137, 10276–10281. [Google Scholar] [CrossRef] [PubMed]
- Kostopoulou, A.; Konidakis, I.; Stratakis, E. Two-dimensional metal halide perovskites and their heterostructures: From synthesis to applications. Nanophotonics 2023, 12, 1643–1710. [Google Scholar] [CrossRef] [PubMed]
- Shamsi, J.; Urban, A.S.; Imran, M.; De Trizio, L.; Manna, L. Metal halide perovskite nanocrystals: Synthesis, post-synthesis modifications, and their optical properties. Chem. Rev. 2019, 119, 3296–3348. [Google Scholar] [CrossRef]
- Otero-Martínez, C.; Ye, J.; Sung, J.; Pastoriza-Santos, I.; Pérez-Juste, J.; Xia, Z.; Rao, A.; Hoye, R.L.; Polavarapu, L. Colloidal metal-halide perovskite nanoplatelets: Thickness-controlled synthesis, properties, and application in light-emitting diodes. Adv. Mater. 2022, 34, 2107105. [Google Scholar] [CrossRef]
- He, J.; Li, H.; Liu, C.; Wang, X.; Zhang, Q.; Liu, J.; Wang, M.; Liu, Y. Hot-Injection Synthesis of Cesium Lead Halide Perovskite Nanowires with Tunable Optical Properties. Materials 2024, 17, 2173. [Google Scholar] [CrossRef]
- Shamsi, J.; Dang, Z.; Bianchini, P.; Canale, C.; Di Stasio, F.; Brescia, R.; Prato, M.; Manna, L. Colloidal synthesis of quantum confined single crystal CsPbBr3 nanosheets with lateral size control up to the micrometer range. J. Am. Chem. Soc. 2016, 138, 7240–7243. [Google Scholar] [CrossRef]
- Hu, Z.; O’Neill, R.; Lesyuk, R.; Klinke, C. Colloidal two-dimensional metal chalcogenides: Realization and application of the structural anisotropy. Acc. Chem. Res. 2021, 54, 3792–3803. [Google Scholar] [CrossRef]
- Liang, Z.; Zhao, S.; Xu, Z.; Qiao, B.; Song, P.; Gao, D.; Xu, X. Shape-controlled synthesis of all-inorganic CsPbBr3 perovskite nanocrystals with bright blue emission. ACS Appl. Mater. Interfaces 2016, 8, 28824–28830. [Google Scholar] [CrossRef]
- Vale, B.R.; Socie, E.; Burgos-Caminal, A.; Bettini, J.; Schiavon, M.A.; Moser, J.-E. Exciton, biexciton, and hot exciton dynamics in CsPbBr3 colloidal nanoplatelets. J. Phys. Chem. Lett. 2019, 11, 387–394. [Google Scholar] [CrossRef]
- Shukla, A.; Kaur, G.; Babu, K.J.; Ghorai, N.; Goswami, T.; Kaur, A.; Ghosh, H.N. Effect of confinement on the exciton and biexciton dynamics in perovskite 2D-nanosheets and 3D-nanocrystals. J. Phys. Chem. Lett. 2020, 11, 6344–6352. [Google Scholar] [CrossRef]
- Sheng, X.; Chen, G.; Wang, C.; Wang, W.; Hui, J.; Zhang, Q.; Yu, K.; Wei, W.; Yi, M.; Zhang, M. Polarized optoelectronics of CsPbX3 (X= Cl, Br, I) perovskite nanoplates with tunable size and thickness. Adv. Funct. Mater. 2018, 28, 1800283. [Google Scholar] [CrossRef]
- Boles, M.A.; Engel, M.; Talapin, D.V. Self-assembly of colloidal nanocrystals: From intricate structures to functional materials. Chem. Rev. 2016, 116, 11220–11289. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, X.; Liu, X. Self-assembly of colloidal inorganic nanocrystals: Nanoscale forces, emergent properties and applications. Chem. Soc. Rev. 2021, 50, 2074–2101. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Chen, Q.; Chun, J.; Fichthorn, K.; De Yoreo, J.; Zheng, H. Nanoparticle assembly and oriented attachment: Correlating controlling factors to the resulting structures. Chem. Rev. 2023, 123, 3127–3159. [Google Scholar] [CrossRef]
- Glotzer, S.C. Shape matters. Nature 2012, 481, 450–452. [Google Scholar] [CrossRef]
- Yu, S.; Zhang, C.; Yang, H. Two-dimensional metal nanostructures: From theoretical understanding to experiment. Chem. Rev. 2023, 123, 3443–3492. [Google Scholar] [CrossRef]
- Basera, P.; Traoré, B.; Even, J.; Katan, C. Interfacial engineering to modulate surface dipoles, work functions and dielectric confinement of halide perovskites. Nanoscale 2023, 15, 11884–11897. [Google Scholar] [CrossRef]
- Song, T.; Ma, Q.-X.; Wang, Q.; Zhang, H.-L. Design of two-dimensional halide perovskite composites for optoelectronic applications and beyond. Mater. Adv. 2022, 3, 756–778. [Google Scholar] [CrossRef]
- Zhang, Q.; Diao, F.; Xue, X.; Sheng, X.; Barba, D.; Wang, Y. Self-assembly of CsPbBr3 nanocubes into 2D nanosheets. ACS Appl. Mater. Interfaces 2021, 13, 44777–44785. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Siron, M.; Lu, D.; Yang, J.; Dos Reis, R.; Cui, F.; Gao, M.; Lai, M.; Lin, J.; Kong, Q. Self-assembly of two-dimensional perovskite nanosheet building blocks into ordered Ruddlesden–Popper perovskite phase. J. Am. Chem. Soc. 2019, 141, 13028–13032. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Grünwald, M. Orientational order in self-assembled nanocrystal superlattices. J. Am. Chem. Soc. 2019, 141, 1980–1988. [Google Scholar] [CrossRef]
- Kagan, C.R.; Lifshitz, E.; Sargent, E.H.; Talapin, D.V. Building devices from colloidal quantum dots. Science 2016, 353, aac5523. [Google Scholar] [CrossRef]
- Frank, K.; Henke, N.A.; Lampe, C.; Lorenzen, T.; März, B.; Sun, X.; Haas, S.; Gutowski, O.; Dippel, A.-C.; Mayer, V. Antisolvent controls the shape and size of anisotropic lead halide perovskite nanocrystals. Nat. Commun. 2024, 15, 8952. [Google Scholar] [CrossRef]
- Kovalenko, M.V.; Protesescu, L.; Bodnarchuk, M.I. Properties and potential optoelectronic applications of lead halide perovskite nanocrystals. Science 2017, 358, 745–750. [Google Scholar] [CrossRef]
- Chen, X.; Wang, X.; Fang, D. A review on C1s XPS-spectra for some kinds of carbon materials. Fuller. Nanotub. Carbon Nanostructures 2020, 28, 1048–1058. [Google Scholar] [CrossRef]
- Fang, D.; He, F.; Xie, J.; Xue, L. Calibration of binding energy positions with C1s for XPS results. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2020, 35, 711–718. [Google Scholar] [CrossRef]
- Hu, L.; Wang, C.; Kennedy, R.M.; Marks, L.D.; Poeppelmeier, K.R. The role of oleic acid: From synthesis to assembly of perovskite nanocuboid two-dimensional arrays. Inorg. Chem. 2015, 54, 740–745. [Google Scholar] [CrossRef]
- Jurow, M.J.; Lampe, T.; Penzo, E.; Kang, J.; Koc, M.A.; Zechel, T.; Nett, Z.; Brady, M.; Wang, L.-W.; Alivisatos, A.P. Tunable anisotropic photon emission from self-organized CsPbBr3 perovskite nanocrystals. Nano Lett. 2017, 17, 4534–4540. [Google Scholar] [CrossRef]
- Shi, Z.; Yang, Y.; Sun, X.-Y.; Lang, F.; Lin, L. Improvement in optical properties of Cs 4 PbBr 6 nanocrystals using aprotic polar purification solvent. RSC Adv. 2021, 11, 16453–16460. [Google Scholar] [CrossRef]
- Zhang, X.; Bai, X.; Wu, H.; Zhang, X.; Sun, C.; Zhang, Y.; Zhang, W.; Zheng, W.; Yu, W.W.; Rogach, A.L. Water-assisted size and shape control of CsPbBr3 perovskite nanocrystals. Angew. Chem. Int. Ed. 2018, 57, 3337–3342. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Saidaminov, M.I.; Dursun, I.; Yang, H.; Murali, B.; Alarousu, E.; Yengel, E.; Alshankiti, B.A.; Bakr, O.M.; Mohammed, O.F. Zero-dimensional Cs4PbBr6 perovskite nanocrystals. J. Phys. Chem. Lett. 2017, 8, 961–965. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Liang, R.; Ge, M.; Sun, G.; Zhang, Y.; Xing, G. Surface passivation using 2D perovskites toward efficient and stable perovskite solar cells. Adv. Mater. 2022, 34, 2105635. [Google Scholar] [CrossRef] [PubMed]
- Iwanaga, M.; Hayashi, T. Exciton-relaxation dynamics in lead halides. J. Lumin. 2003, 102, 663–668. [Google Scholar] [CrossRef]
- Guo, Y.; Yaffe, O.; Hull, T.D.; Owen, J.S.; Reichman, D.R.; Brus, L.E. Dynamic emission Stokes shift and liquid-like dielectric solvation of band edge carriers in lead-halide perovskites. Nat. Commun. 2019, 10, 1175. [Google Scholar] [CrossRef]
- Mastrikov, Y.A.; Chuklina, N.; Sokolov, M.; Popov, A.; Gryaznov, D.; Kotomin, E.; Maier, J. Small radius electron and hole polarons in Pb X 2 (X= F, Cl, Br) crystals: A computational study. J. Mater. Chem. C 2021, 9, 16536–16544. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdalla, Z.; Liu, C.; Kareem, S.; Wang, X.; Tang, Z.; Liu, Y. Ligand-Mediated, Temperature-Tuned Synthesis of CsPbBr3 Nanosheets for Ordered Superlattice Assembly. Materials 2025, 18, 4885. https://doi.org/10.3390/ma18214885
Abdalla Z, Liu C, Kareem S, Wang X, Tang Z, Liu Y. Ligand-Mediated, Temperature-Tuned Synthesis of CsPbBr3 Nanosheets for Ordered Superlattice Assembly. Materials. 2025; 18(21):4885. https://doi.org/10.3390/ma18214885
Chicago/Turabian StyleAbdalla, Zahir, Chengqi Liu, Shefiu Kareem, Xiaoqian Wang, Zisheng Tang, and Yong Liu. 2025. "Ligand-Mediated, Temperature-Tuned Synthesis of CsPbBr3 Nanosheets for Ordered Superlattice Assembly" Materials 18, no. 21: 4885. https://doi.org/10.3390/ma18214885
APA StyleAbdalla, Z., Liu, C., Kareem, S., Wang, X., Tang, Z., & Liu, Y. (2025). Ligand-Mediated, Temperature-Tuned Synthesis of CsPbBr3 Nanosheets for Ordered Superlattice Assembly. Materials, 18(21), 4885. https://doi.org/10.3390/ma18214885






