Advanced Epoxy Resin/Boron Nitride Composites for High-Performance Electrotechnical Applications and Geological Instrumentation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Composites
2.3. Sample Characterization
3. Results
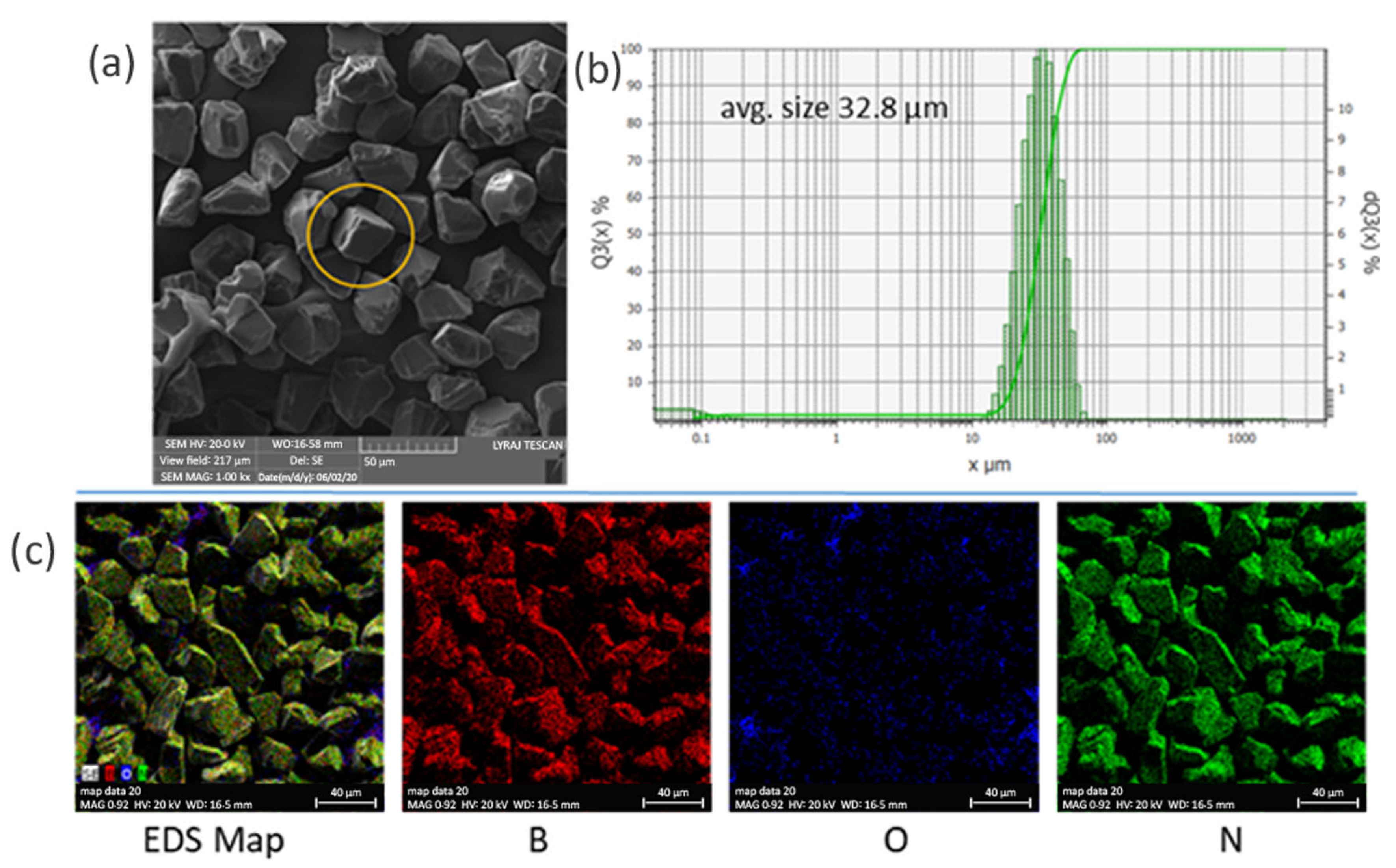
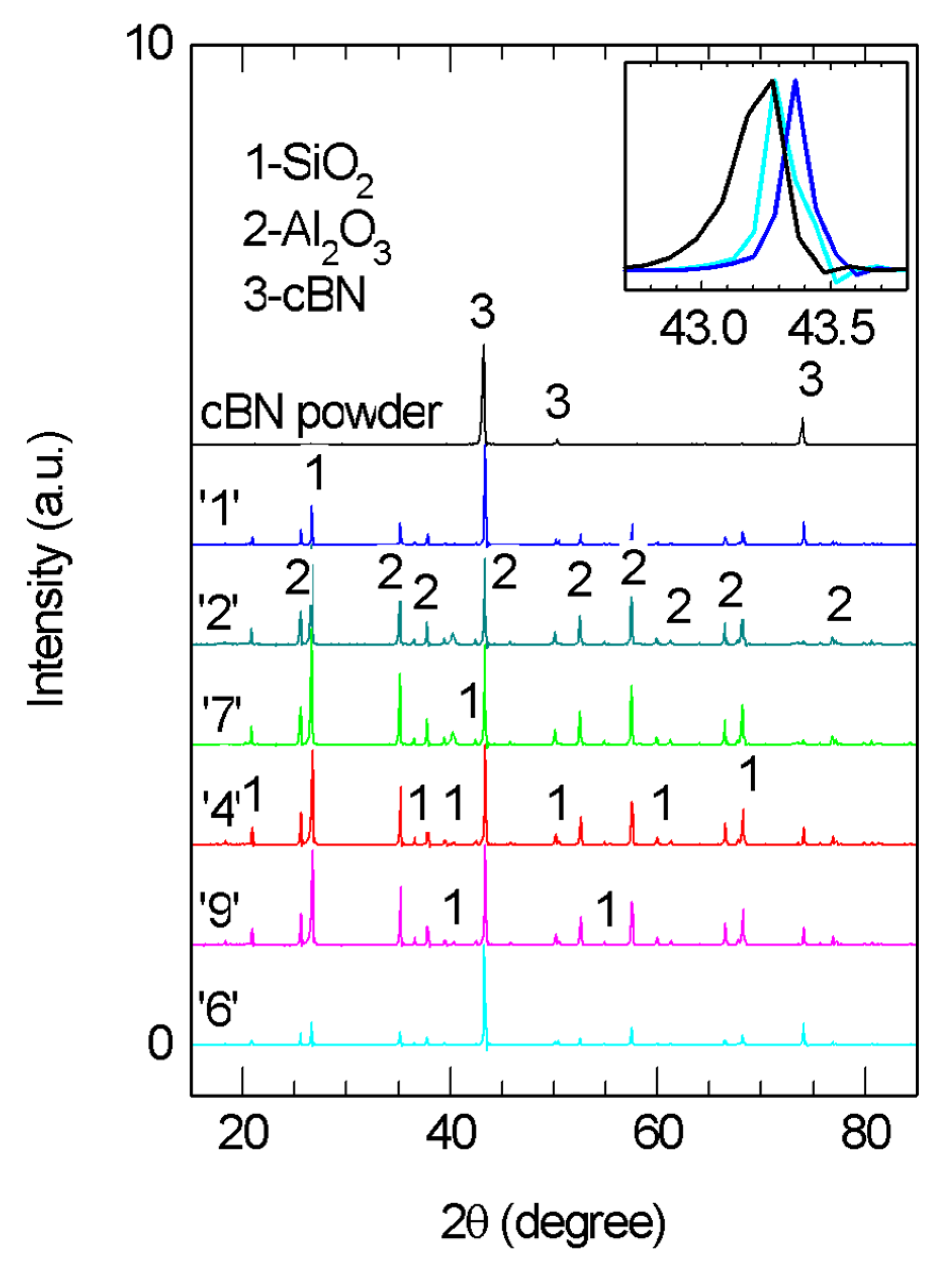
3.1. XRD of the Composites
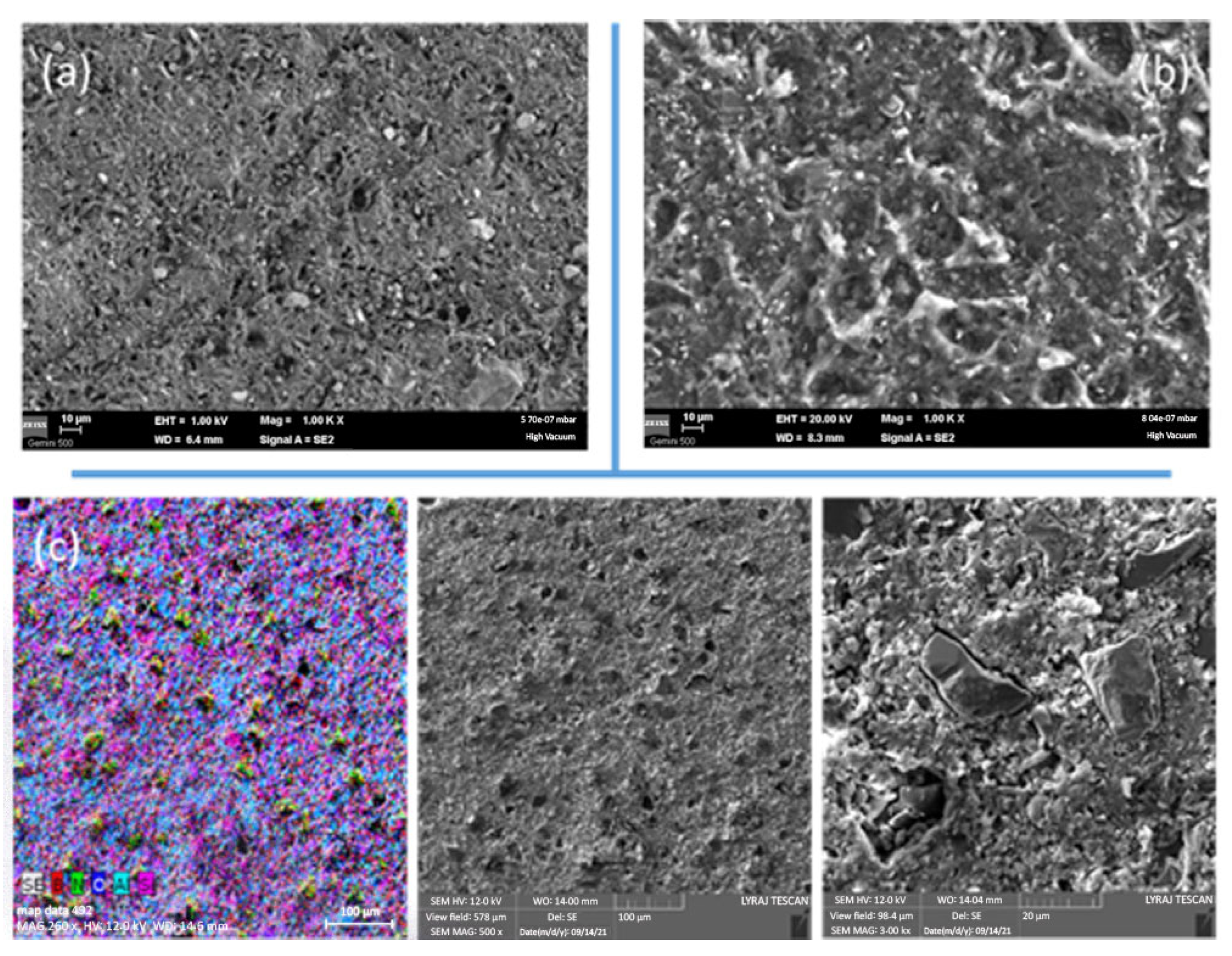
3.2. SEM Observations and Density of the Composites
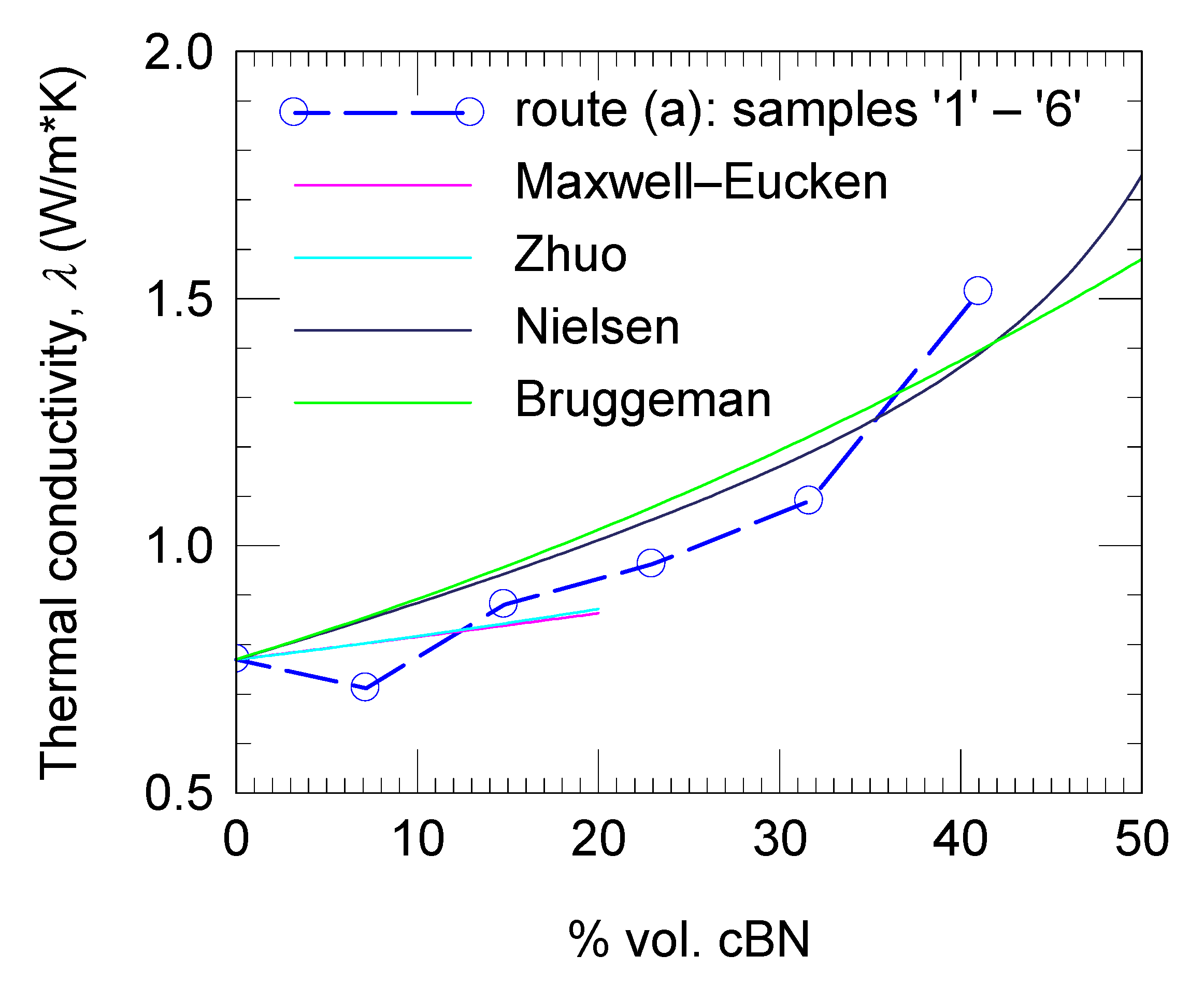
3.3. Thermal Conductivity of the Composites
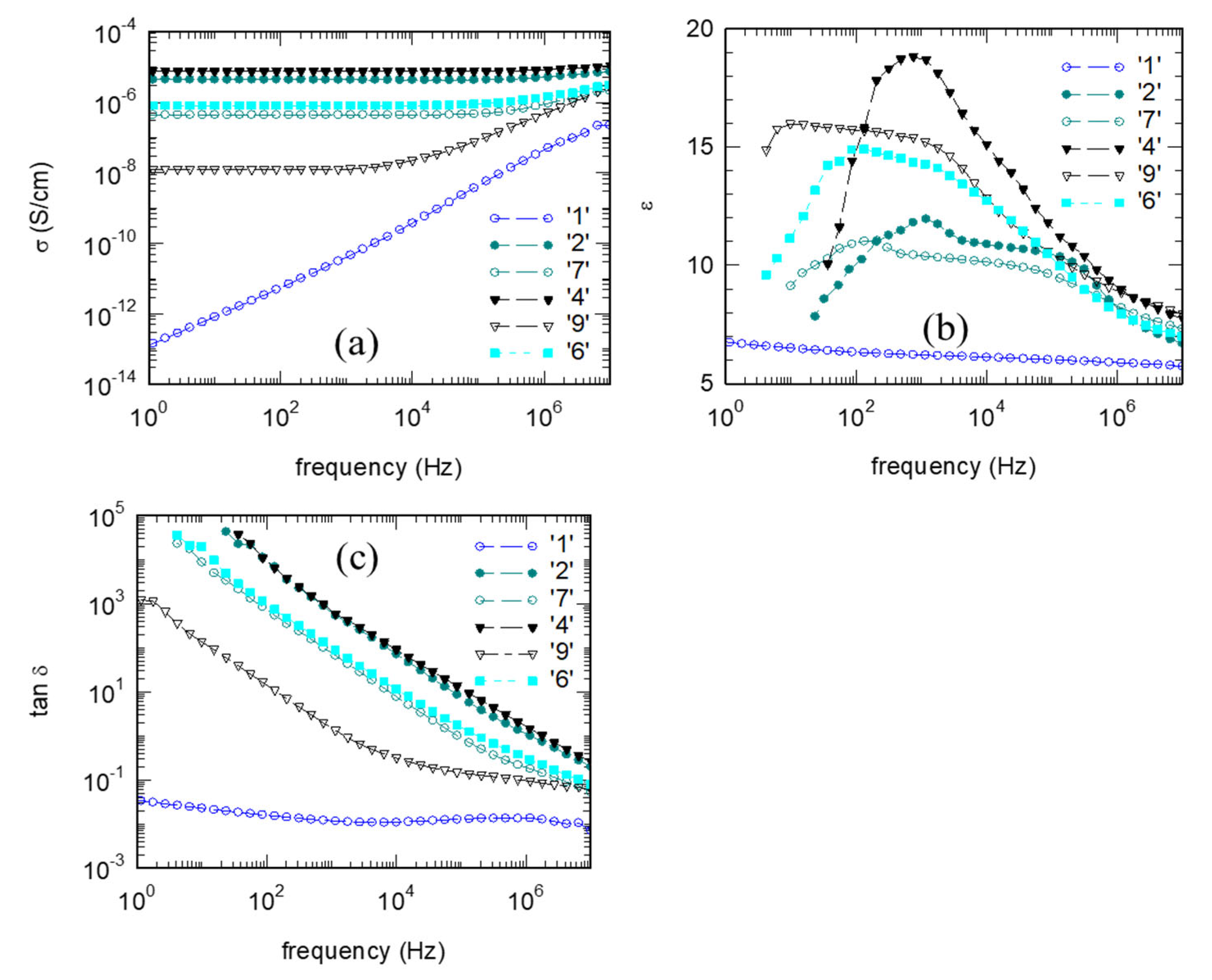
3.4. Electrical Properties of the Composites
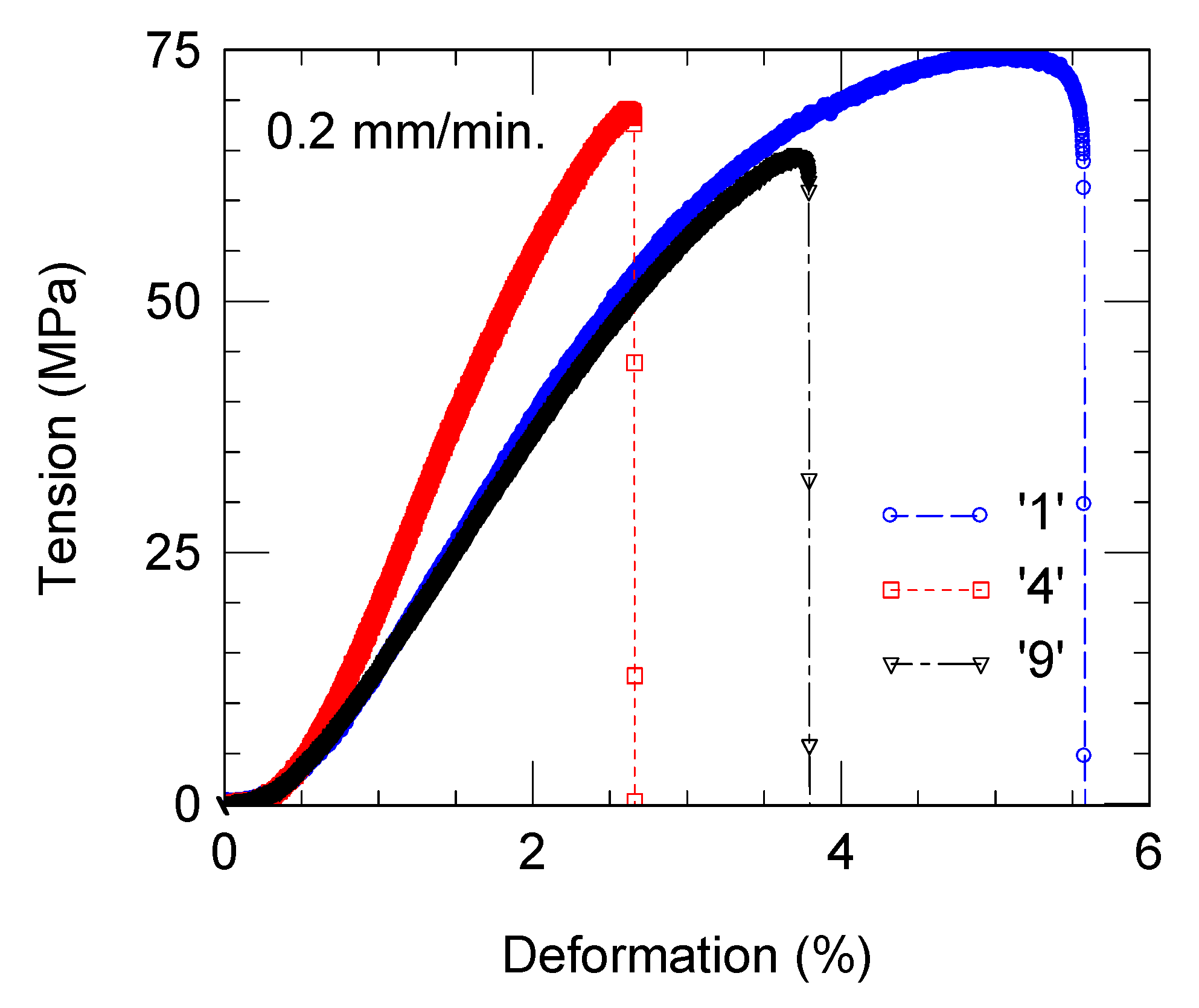
3.5. Mechanical Properties of the Composites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- He, H.; Peng, W.; Liu, J.; Chan, X.Y.; Liu, S.; Lu, L.; Le Ferrand, H. Microstructured BN Composites with Internally Designed High Thermal Conductivity Paths for 3D Electronic Packaging. Adv. Mater. 2022, 34, 2205120. [Google Scholar] [CrossRef]
- Ma, J.; Shang, T.; Ren, L.; Yao, Y.; Zhang, T.; Xie, J.; Zhang, B.; Zeng, X.; Sun, R.; Xu, J.-B.; et al. Through-Plane Assembly of Carbon Fibers into 3D Skeleton Achieving Enhanced Thermal Conductivity of a Thermal Interface Material. Chem. Eng. J. 2020, 380, 122550. [Google Scholar] [CrossRef]
- Wang, T.; Li, X.; Zhang, B.; Li, D.; Liu, J.; Zhang, G. Basic Reason for the Accumulation of Charge on the Surface of Polymer Dielectrics. Sci. China Mater. 2022, 65, 2884–2888. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, Z.; Weng, L.; Ge, S.; Jiang, D.; Huang, M.; Mulvihill, D.M.; Chen, Q.; Guo, Z.; Jazzar, A.; et al. A Roadmap Review of Thermally Conductive Polymer Composites: Critical Factors, Progress, and Prospects. Adv. Funct. Mater. 2023, 33, 2301549. [Google Scholar] [CrossRef]
- Qian, X.; Zhou, J.; Chen, G. Phonon-Engineered Extreme Thermal Conductivity Materials. Nat. Mater. 2021, 20, 1188–1202. [Google Scholar] [CrossRef]
- Lin, Y.; Li, P.; Liu, W.; Chen, J.; Liu, X.; Jiang, P.; Huang, X. Application-Driven High-Thermal-Conductivity Polymer Nanocomposites. ACS Nano 2024, 18, 3851–3870. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Yang, X.; Li, J.; Shen, Y.; Zhang, L.; Lu, R.; Wang, C.; Zheng, X.; Chen, H.; Zhang, T. Review on Polymer Composites with High Thermal Conductivity and Low Dielectric Properties for Electronic Packaging. Mater. Today Phys. 2022, 22, 100594. [Google Scholar] [CrossRef]
- Yuan, C.; Zhou, Y.; Zhu, Y.; Liang, J.; Wang, S.; Peng, S.; Li, Y.; Cheng, S.; Yang, M.; Hu, J.; et al. Polymer/Molecular Semiconductor All-Organic Composites for High-Temperature Dielectric Energy Storage. Nat. Commun. 2020, 11, 3919. [Google Scholar] [CrossRef]
- Yun, J.-H.; Yoo, Y.-J.; Kim, H.-R.; Song, Y.-M. Recent Progress in Thermal Management for Flexible/Wearable Devices. Soft Sci. 2023, 3, 12. [Google Scholar] [CrossRef]
- Zha, J.-W.; Wang, F.; Wan, B. Polymer Composites with High Thermal Conductivity: Theory, Simulation, Structure and Interfacial Regulation. Prog. Mater. Sci. 2025, 148, 101362. [Google Scholar] [CrossRef]
- Meng, X.; Yu, H.; Wang, L.; Wu, X.; Amin, B.U. Recent Progress on Fabrication and Performance of Polymer Composites with Highly Thermal Conductivity. Macromol. Mater. Eng. 2021, 306, 2100434. [Google Scholar] [CrossRef]
- Xu, Y.; Kraemer, D.; Song, B.; Jiang, Z.; Zhou, J.; Loomis, J.; Wang, J.; Li, M.; Ghasemi, H.; Huang, X.; et al. Nanostructured polymer films with metal-like thermal conductivity. Nat. Commun. 2019, 10, 1771. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Gao, B.; Wang, M.; Yuan, Z.; Shen, J.; Zhao, J.; Feng, Y. Strategies for Enhancing Thermal Conductivity of Polymer-Based Thermal Interface Materials: A Review. J. Mater. Sci. 2021, 56, 1064–1086. [Google Scholar] [CrossRef]
- Ruan, K.; Shi, X.; Guo, Y.; Gu, J. Interfacial Thermal Resistance in Thermally Conductive Polymer Composites: A Review. Compos. Commun. 2020, 22, 100518. [Google Scholar] [CrossRef]
- Naclerio, A.E.; Kidambi, P.R. A Review of Scalable Hexagonal Boron Nitride (h-BN) Synthesis for Present and Future Applications. Adv. Mater. 2022, 35, 2207374. [Google Scholar] [CrossRef]
- Wu, W.; Zheng, M.-S.; Lu, K.; Liu, F.; Song, Y.-H.; Liu, M.; Dang, Z.-M. Thermally Conductive Composites Based on Hexagonal Boron Nitride Nanosheets for Thermal Management: Fundamentals to Applications. Compos. Part A Appl. Sci. Manuf. 2023, 169, 107533. [Google Scholar] [CrossRef]
- Zaghloul, M.M.Y.; Fuseini, M. Recent Progress in Epoxy Nanocomposites: Corrosion, Structural, flame retardancy and applications—A comprehensive review. Polym. Adv. Technol. 2023, 34, 3438–3472. [Google Scholar] [CrossRef]
- Xu, T.; Zhang, K.; Cai, Q.; Wang, N.; Wu, L.; He, Q.; Wang, H.; Zhang, Y.; Xie, Y.; Yao, Y.; et al. Advances in Synthesis and Applications of Boron Nitride Nanotubes: A Review. Chem. Eng. J. 2022, 431, 134118. [Google Scholar] [CrossRef]
- Cazorla, C.; Gould, T. Polymorphism of Bulk Boron Nitride. arXiv 2018, arXiv:1812.04696v1. [Google Scholar] [CrossRef]
- Sharma, V.; Kagdada, H.L.; Jha, P.K.; Śpiewak, P.; Kurzydłowski, K.J. Thermal transport properties of boron nitride based materials: A review. Renew. Sustain. Energy Rev. 2020, 120, 109622. [Google Scholar] [CrossRef]
- Chung, S.-L.; Lin, J.-S. Thermal Conductivity of Epoxy Resin Composites Filled with Combustion Synthesized h-BN Particles. Molecules 2016, 21, 670. [Google Scholar] [CrossRef] [PubMed]
- Fan, B.; Liu, Y.; He, D.; Bai, J. Enhanced Thermal Conductivity for Mesophase Pitch-Based Carbon Fiber/Modified Boron Nitride/Epoxy Composites. Polymer 2017, 122, 71–76. [Google Scholar] [CrossRef]
- Yu, J.; Huang, X.; Wu, C.; Wu, X.; Wang, G.; Jiang, P. Interfacial Modification of Boron Nitride Nanoplatelets for Epoxy Composites with Improved Thermal Properties. Polymer 2012, 53, 471–480. [Google Scholar] [CrossRef]
- Kim, K.; Kim, J. Vertical filler alignment of boron nitride/epoxy composite for thermal conductivity enhancement via external magnetic field. Int. J. Therm. Sci. 2016, 100, 29–36. [Google Scholar] [CrossRef]
- Kim, K.; Kim, J. Fabrication of Thermally Conductive Composite with Surface Modified Boron Nitride by Epoxy Wetting Method. Ceram. Int. 2014, 40, 5181–5189. [Google Scholar] [CrossRef]
- Shao, L.; Shi, L.; Li, X.; Song, N.; Ding, P. Synergistic effect of BN and graphene nanosheets in 3D framework on the enhancement of thermal conductive properties of polymeric composites. Compos. Sci. Technol. 2016, 135, 83–91. [Google Scholar] [CrossRef]
- Cho, H.-B.; Nakayama, T.; Suematsu, H.; Suzuki, T.; Jiang, W.; Niihara, K.; Song, E.; Eom, N.S.A.; Kim, S.; Choa, Y.-H. Insulating polymer nanocomposites with high-thermal-conduction routes via linear densely packed boron nitride nanosheets. Compos. Sci. Technol. 2016, 129, 205–213. [Google Scholar] [CrossRef]
- Kim, K.; Ju, H.; Kim, J. Vertical particle alignment of boron nitride and silicon carbide binary filler system for thermal conductivity enhancement. Compos. Sci. Technol. 2016, 123, 99–105. [Google Scholar] [CrossRef]
- Muratov, D.S.; Kuznetsov, D.V.; Il’inykh, I.A.; Burmistrov, I.N.; Mazov, I.N. Thermal conductivity of polypropylene composites filled with silane-modified hexagonal BN. Compos. Sci. Technol. 2015, 111, 40–43. [Google Scholar] [CrossRef]
- Jin, W.; Zhang, W.; Gao, Y.; Liang, G.; Gu, A.; Yuan, L. Surface functionalization of hexagonal boron nitride and its effect on the structure and performance of composites. Appl. Surf. Sci. 2013, 270, 549–556. [Google Scholar] [CrossRef]
- Ahn, H.J.; Eoh, Y.J.; Park, S.D.; Kim, E.S. Thermal conductivity of polymer composites with oriented boron nitride. Thermochim. Acta 2014, 590, 138–144. [Google Scholar] [CrossRef]
- Owais, M.; Zhao, J.; Imani, A.; Wang, G.; Zhang, H.; Zhang, Z. Synergetic effect of hybrid fillers of boron nitride, graphene nanoplatelets, and short carbon fibers for enhanced thermal conductivity and electrical resistivity of epoxy nanocomposites. Compos. Part A Appl. Sci. Manuf. 2019, 117, 11–22. [Google Scholar] [CrossRef]
- Yuan, F.-Y.; Zhang, H.-B.; Li, X.; Li, X.-Z.; Yu, Z.-Z. Synergistic effect of boron nitride flakes and tetrapod-shaped ZnO whiskers on the thermal conductivity of electrically insulating phenol formaldehyde composites. Compos. Part A Appl. Sci. Manuf. 2013, 53, 137–144. [Google Scholar] [CrossRef]
- Bauccio, M. ASM Engineered Materials Reference Book, 2nd ed.; ASM International: Materials Park, OH, USA, 1994; ISBN 0871705028. [Google Scholar]
- Mizuuchi, K.; Inoue, K.; Agari, Y.; Tanaka, M.; Takeuchi, T.; Tani, J.I.; Kawahara, M.; Makino, Y.; Ito, M. Thermal Conductivity of Cubic Boron Nitride (cBN) Particle Dispersed Al Matrix Composites Fabricated by SPS. Mater. Sci. Forum 2017, 879, 2413–2418. [Google Scholar] [CrossRef]
- Mizuuchi, K.; Inoue, K.; Agari, Y.; Sugioka, M.; Tanaka, M.; Takeuchi, T.; Tani, J.-I.; Kawahara, M.; Makino, Y.; Ito, M. Effect of bimodal cBN particle size distribution on thermal conductivity of Al/cBN composite fabricated by SPS. J. Jpn. Soc. Powder Powder Metall. 2015, 62, 263–270. [Google Scholar] [CrossRef]
- Wang, P.F.; Li, Z.H.; Zhu, Y.M. Fabrication of high thermal conductive Al–cBN ceramic sinters by high temperature high pressure method. Solid State Sci. 2011, 13, 1041–1046. [Google Scholar] [CrossRef]
- Yung, K.C.; Liem, H. Enhanced Thermal Conductivity of Boron Nitride Epoxy-Matrix Composite through Multi-Modal Particle Size Mixing. J. Appl. Polym. Sci. 2007, 106, 3587–3591. [Google Scholar] [CrossRef]
- Zhao, W.; Zhao, W.; Huang, Z.; Liu, G.; Wu, B. Tribological Performances of Epoxy Resin Composite Coatings Using Hexagonal Boron Nitride and Cubic Boron Nitride Nanoparticles as Additives. Chem. Phys. Lett. 2019, 732, 136646. [Google Scholar] [CrossRef]
- Huang, Z.; Zhao, W.; Zhao, W.; Ci, X.; Li, W. Tribological and anti-corrosion performance of epoxy resin composite coatings reinforced with differently sized cubic boron nitride (CBN) particles. Friction 2021, 9, 104–118. [Google Scholar] [CrossRef]
- Sukur, E.F.; Kaybal, H.B.; Ulus, H.; Avcı, A. Tribological behavior of epoxy nanocomposites under corrosive environment: Effect of high-performance boron nitride nanoplatelet. Sigma J. Eng. Nat. Sci. 2023, 41, 385–395. [Google Scholar] [CrossRef]
- Chen, H.; Wang, Z.; Lv, M.; Cheng, X.; Zhang, Y.; Yang, Y.; Wang, J.; Chen, Y.; Lin, T.; Wu, D. Chitosan functionalized hexagonal boron nitride nanocomposite: Synthesis, characterization, and application in drug delivery. Colloids Surf. A Physicochem. Eng. Asp. 2022, 645, 128941. [Google Scholar] [CrossRef]
- Wan, Z.; Zhang, D.; Gao, C.; Lu, M.; Li, Z.; Huang, Z.; You, Y.; Wang, Z. Experimental Study on the Thermal Decomposition of Epoxy/Anhydride Thermoset Matrix in Composite Insulator Core Rods. Polym. Degrad. Stab. 2024, 224, 110697. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, R.; Ruan, K.; Ma, T.; Guo, Y.; Gu, J. Improvement of Thermal Conductivities and Simulation Model for Glass Fabrics Reinforced Epoxy Laminated Composites via Introducing Hetero-Structured BNN-30@BNNS Fillers. J. Mater. Sci. Technol. 2021, 82, 239–249. [Google Scholar] [CrossRef]
- Han, Z.; Fina, A. Thermal Conductivity of Carbon Nanotubes and Their Polymer Nanocomposites: A Review. Prog. Polym. Sci. 2011, 36, 914–944. [Google Scholar] [CrossRef]
- Fiedler, B.; Gojny, F.H.; Wichmann, M.H.G.; Nolte, M.C.M.; Schulte, K. Fundamental Aspects of Nano-Reinforced Composites. Compos. Sci. Technol. 2006, 66, 3115–3125. [Google Scholar] [CrossRef]
- Gao, J.; Dong, W.; Zhang, X.; Liu, X.; Sun, J.; Tian, Y.; Yang, Y.; Zhang, X. 3D Printed Boron Nitride-Filled Continuous Carbon Fiber/Epoxy Resin Composites with Ultra-High Thermal Conductivity and Favorable Mechanical Property. Polym. Compos. 2025, 46, 220182. [Google Scholar] [CrossRef]
- Credo, A.; Villani, M.; Popescu, M.; Riviere, N. Synchronous reluctance motors with asymmetric rotor shapes and epoxy resin for electric vehicles. In Proceedings of the IEEE Energy Conversion Congress and Exposition (ECCE), Baltimore, MD, USA, 29 September–3 October 2019; pp. 4463–4469. [Google Scholar] [CrossRef]
- Material Properties Data: Alumina (Aluminum Oxide). Archived 1 April 2010 at the Wayback Machine. Available online: www.makeitfrom.com (accessed on 3 March 2021).
- Haynes, W.M. CRC Handbook of Chemistry and Physics, 92nd ed.; CRC Press: Boca Raton, FL, USA, 2011; ISBN 1-4398-5511-0. [Google Scholar]
- Wereszczak, A.A.; Morrissey, T.G.; Volante, C.N.; Farris, P.J., Jr.; Groele, R.J.; Wiles, R.H.; Wang, H. Thermally Conductive MgO-Filled Epoxy Molding Compounds. IEEE Trans. Compon. Packag. Manuf. Technol. 2013, 3, 2156–3950. [Google Scholar] [CrossRef]
- Eucken, A. Allgemeine Gesetzmäßigkeiten für das Wärmeleitvermögen verschiedener Stoffarten und Aggregatzustände. Forsch. Auf Dem Geb. Ingenieurwesens A 1940, 11, 6–20. [Google Scholar] [CrossRef]
- Zhou, W.; Yu, D. Thermal and Dielectric Properties of the Aluminum Particle/Epoxy Resin Composites. J. Appl. Polym. Sci. 2010, 118, 3156–3166. [Google Scholar] [CrossRef]
- Nielsen, L.E. Thermal conductivity of particulate-filled polymers. J. Appl. Polym. Sci. 1973, 17, 3819–3820. [Google Scholar] [CrossRef]
- Bruggeman, D.A.G. Berechnung verschiedener physikalischer Konstanten von heterogenen Substanzen. I. Dielektrizitätskonstanten und Leitfähigkeiten der Mischkörper aus isotropen Substanzen. Ann. Phys. 1935, 416, 636–664. [Google Scholar] [CrossRef]
- Kim, Y.K.; Chung, J.Y.; Lee, J.G.; Baek, Y.K.; Shin, P.W. Synergistic effect of spherical Al2O3 particles and BN nanoplates on the thermal transport properties of polymer composites. Compos. Part A Appl. Sci. Manuf. 2017, 98, 184–191. [Google Scholar] [CrossRef]
- Yang, R.; Sheng, M.; Zhang, Y.; Gong, H.; Lin, X.; Pei, Y.; Zhang, X. Thermal and dielectric properties of epoxy-based composites filled with flake and whisker type hexagonal boron nitride materials. High Perform. Polym. 2021, 33, 417–428. [Google Scholar] [CrossRef]
- Lee Sanchez, W.A.; Huang, C.-Y.; Chen, J.-X.; Soong, Y.-C.; Chan, Y.-N.; Chiou, K.-C.; Lee, T.-M.; Cheng, C.-C.; Chiu, C.-W. Enhanced Thermal Conductivity of Epoxy Composites Filled with Al2O3/Boron Nitride Hybrids for Underfill Encapsulation Materials. Polymers 2021, 13, 147. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, Q.; Zhang, Q.; Yang, H.; Dong, J.; Cheng, J.; Tong, J.; Wen, J. High thermal conductive composite with low dielectric constant and dielectric loss accomplished through flower-like Al2O3 coated BNNs for advanced circuit substrate applications. Compos. Sci. Technol. 2021, 216, 109048. [Google Scholar] [CrossRef]


| Resin in the liquid state: | |
| Viscosity of the mixed (base and hardener) components | 2500−5500 mPas (20 °C) |
| Mixing ratio by weight | 100:10 |
| Suggested curing cycles | 48 h at 25 °C or 6 h at 80 °C |
| Resin in the cured state 24 h at RT + 15 h at 60 °C | |
| Glass transition temperature | 55−65 °C |
| Thermal conductivity (room temperature, RT = 23 ± 2 °C) | ~0.7 W/m·K |
| Electrical volume resistivity (RT) | >1013 Ω·cm |
| Linear thermal coefficient of expansion | 60–70 × 10−6 °C (Tg − 10 °C) 135–155 × 10−6 °C (Tg +10 °C) |
| Dielectric constant (RT) | 3.5−4.5 |
| Loss factor (RT) | 10−30 × 10−3 |
| Bending strength | 75−85 MPa |
| Sample | cBN Content (wt.%) (1) | Mixture Density (g/cm3) | Epoxy Base (2) + Hardener (3) (wt.%) | Preparation Route |
|---|---|---|---|---|
| ‘1’ | 0 | 1.745 | 100 | (2) + (3) |
| ‘2’ | 10 | 1.845 | 90 | method (a): [(1) + (2)] + (3) |
| ‘3’ | 20 | 1.929 | 80 | |
| ‘4’ | 30 | 2.013 | 70 | |
| ‘5’ | 40 | 2.113 | 60 | |
| ‘6’ | 50 | 2.224 | 50 | |
| ‘7’ | 10 | 1.825 | 90 | method (b): [(1) + (3)] + (2) |
| ‘8’ | 20 | 1.884 | 80 | |
| ‘9’ | 30 | 1.885 | 70 | |
| ‘10’ | 40 | 2.00 | 60 |
| Model | Formula | kf | Fitting Factor (r2) |
|---|---|---|---|
| Maxwell–Eucken | 0.856 | 0.32 | |
| Zhuo | 1.47 | 0.34 | |
| Nielsen | [54] | 2.82 | 0.89 |
| Bruggeman | 2.98 | 0.63 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caramitu, A.R.; Popescu, I.; Grigoroscuta, M.; Kuncser, A.; Ganea, P.C.; Galatanu, A.; Galatanu, M.; Aldica, G.; Badica, P.; Burdusel, M.; et al. Advanced Epoxy Resin/Boron Nitride Composites for High-Performance Electrotechnical Applications and Geological Instrumentation. Materials 2025, 18, 4860. https://doi.org/10.3390/ma18214860
Caramitu AR, Popescu I, Grigoroscuta M, Kuncser A, Ganea PC, Galatanu A, Galatanu M, Aldica G, Badica P, Burdusel M, et al. Advanced Epoxy Resin/Boron Nitride Composites for High-Performance Electrotechnical Applications and Geological Instrumentation. Materials. 2025; 18(21):4860. https://doi.org/10.3390/ma18214860
Chicago/Turabian StyleCaramitu, Alina Ruxandra, Iustina Popescu, Mihai Grigoroscuta, Andrei Kuncser, Paul Constantin Ganea, Andrei Galatanu, Magdalena Galatanu, Gheorghe Aldica, Petre Badica, Mihail Burdusel, and et al. 2025. "Advanced Epoxy Resin/Boron Nitride Composites for High-Performance Electrotechnical Applications and Geological Instrumentation" Materials 18, no. 21: 4860. https://doi.org/10.3390/ma18214860
APA StyleCaramitu, A. R., Popescu, I., Grigoroscuta, M., Kuncser, A., Ganea, P. C., Galatanu, A., Galatanu, M., Aldica, G., Badica, P., Burdusel, M., & Borș, A. M. (2025). Advanced Epoxy Resin/Boron Nitride Composites for High-Performance Electrotechnical Applications and Geological Instrumentation. Materials, 18(21), 4860. https://doi.org/10.3390/ma18214860







.jpg)

