One-Pot Preparation of Easily Dispersible Hexagonal Mg(OH)2 Modified with THPS and Its Flame-Retardant EVA Copolymer
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
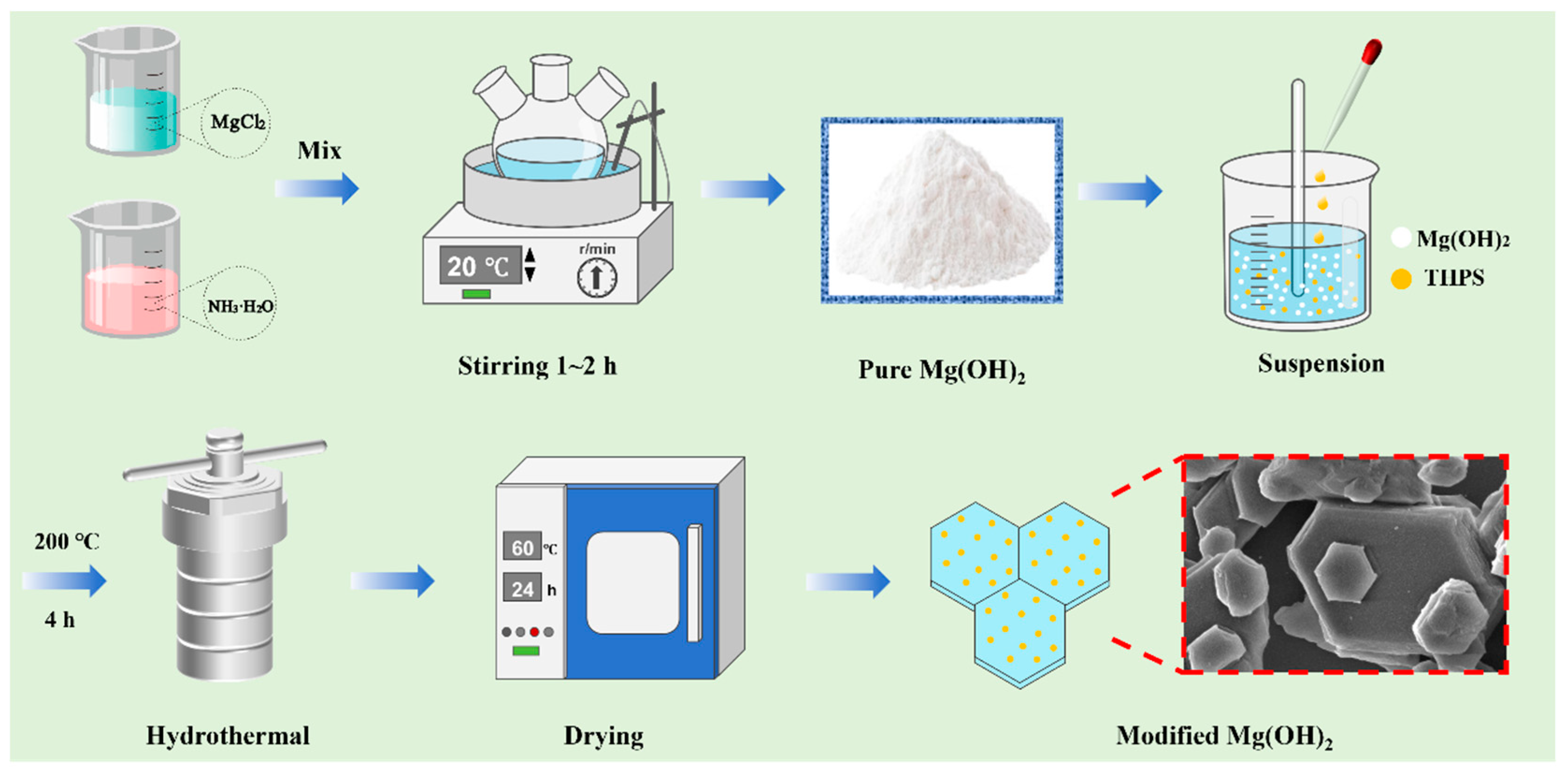
2.2. Synthesis of MH
2.3. Preparation of Modified Mg(OH)2
2.4. Preparation of EVA Composites
2.5. Characterization
3. Results and Discussion
3.1. Optimal Modified Conditions for MH
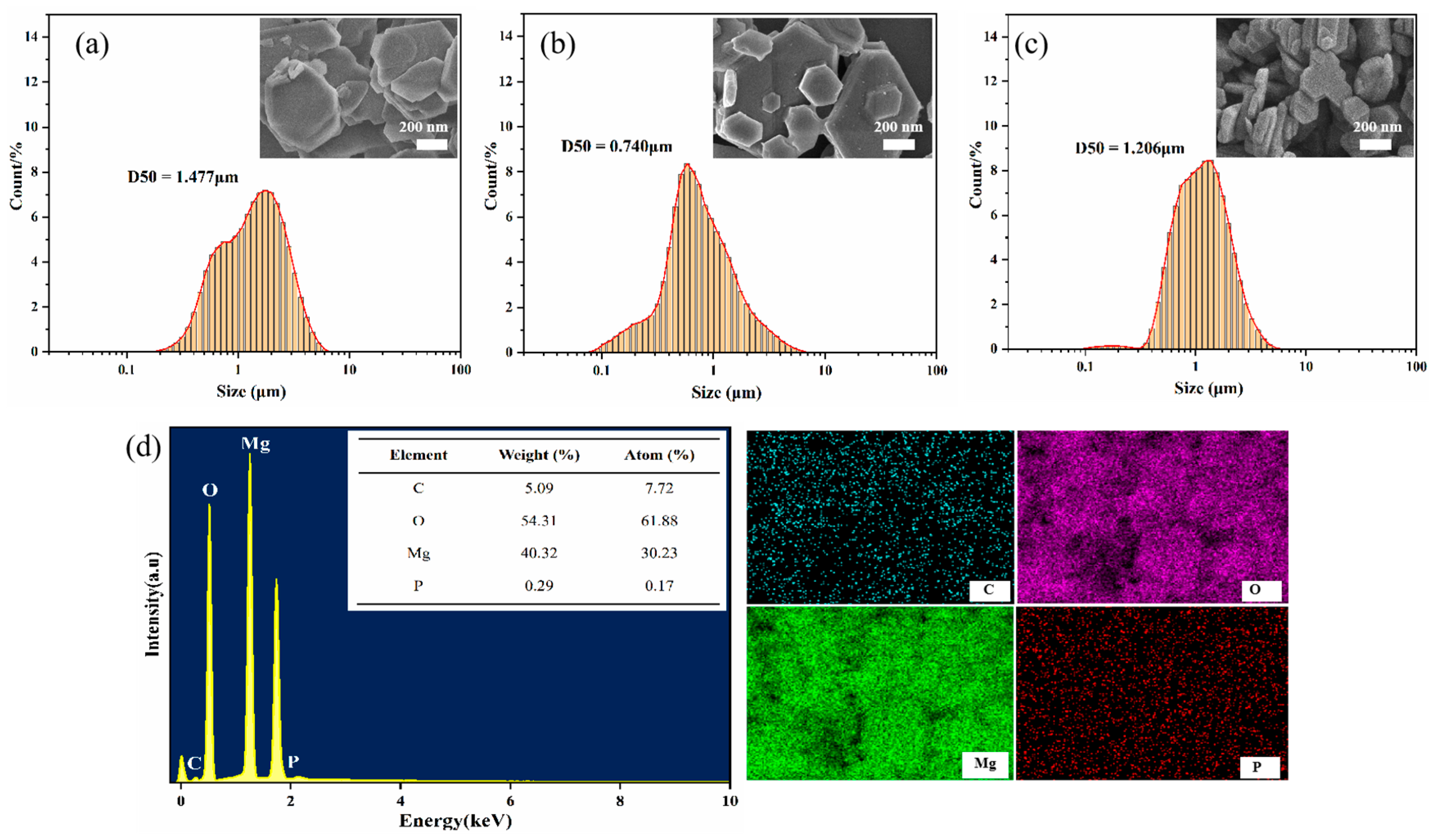
3.1.1. Morphology
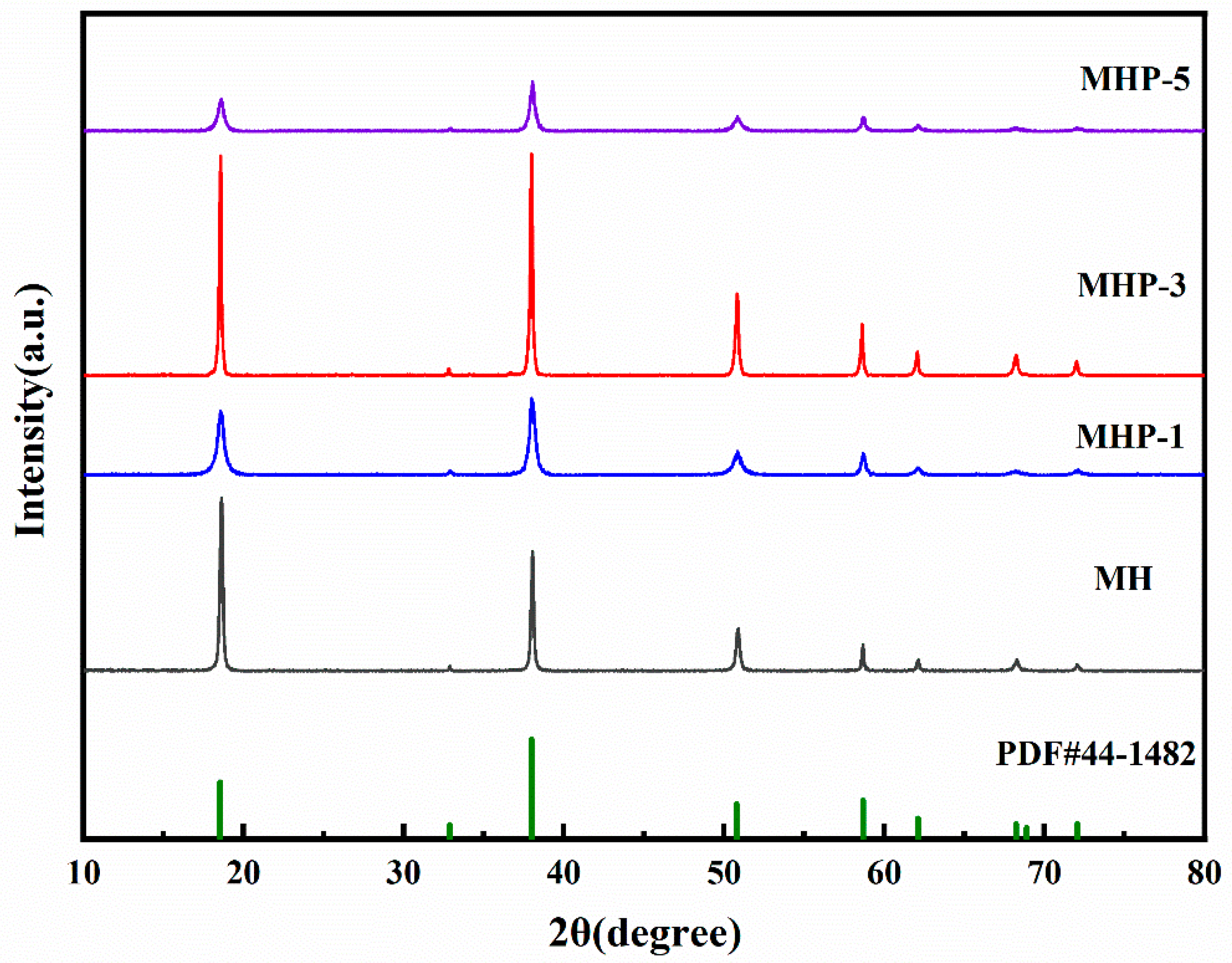
3.1.2. Crystal Structure
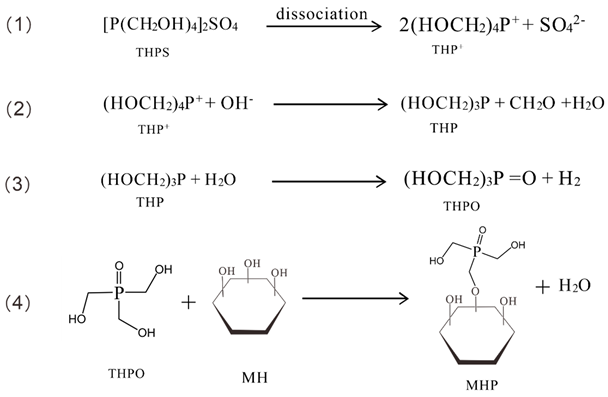
3.2. Modification Reaction Mechanism
3.3. Thermal Analysis of Modified MH
3.4. Flame-Retardant Properties of EVA Composites
3.4.1. Fire Retardancy
3.4.2. Flame Retardancy Mechanism Analysis
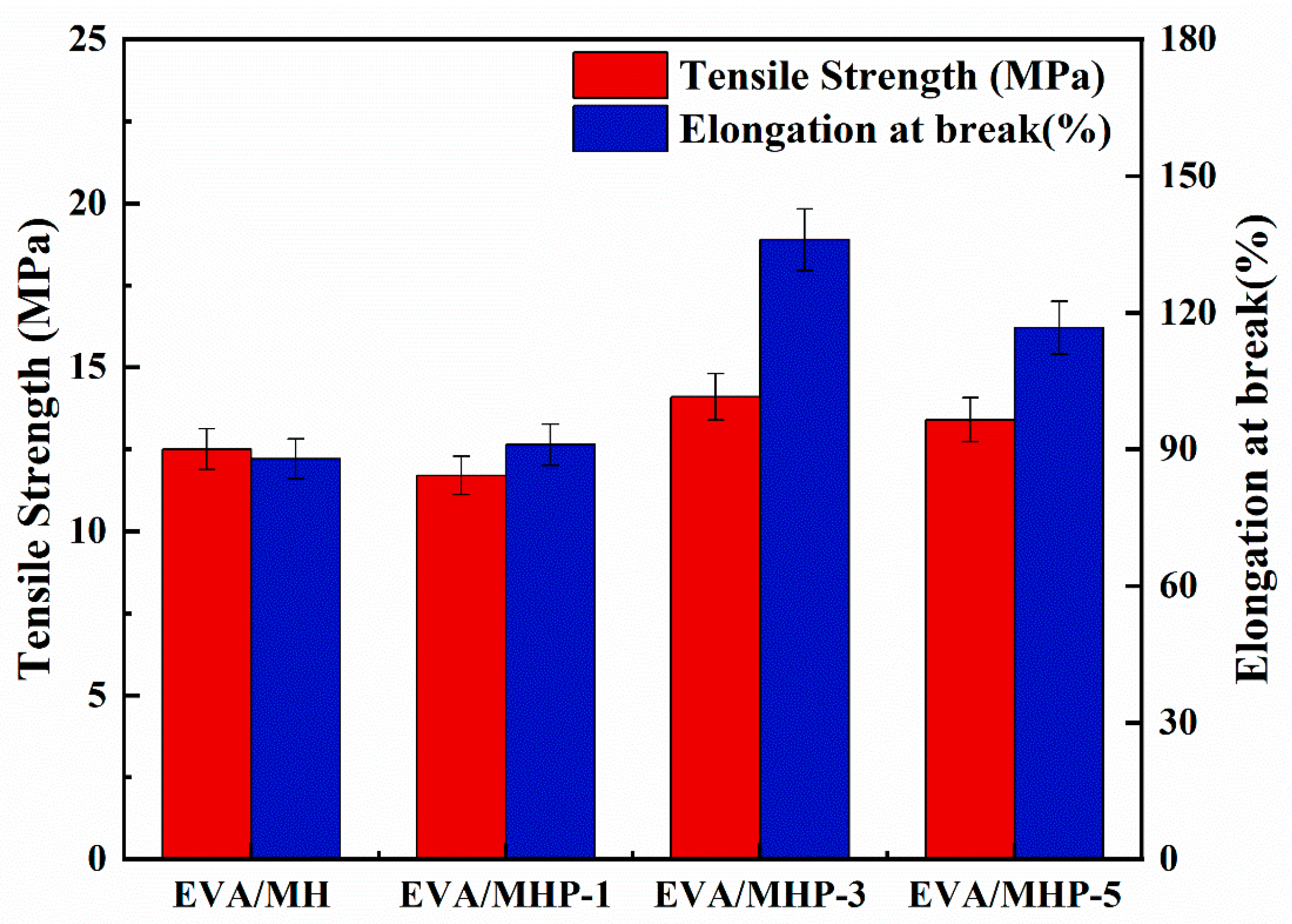
3.4.3. Mechanical Performance of MHP
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, Y.M.; Deng, C.; Wang, Y.Z. A novel high-temperature-resistant polymeric material for cables and insulated wires via the ceramization of mica-based ceramifiable EVA composites. Compos. Sci. Technol. 2016, 132, 116–122. [Google Scholar] [CrossRef]
- Gu, H.; Lv, T.; Zhang, Q.; Chi, D.; Zhang, Y.; Cheng, Z.; Xie, Z.; Xu, Y.; Zhang, D.; Liu, Y. Smart reconfigurable electromagnetic metamaterials based on thermadapt shape memory Poly(ethylene–vinyl acetate). Chem. Eng. J. 2024, 491, 152106. [Google Scholar] [CrossRef]
- Huo, Y.L.; Liu, T.N.; Lu, D.; Han, X.Y.; Sun, H.Y.; Chen, Z.T.; Li, Y.Z.; Huang, J.G.; Yang, Y.Z. Enhancing mechanical properties and crack resistance of high-strength SHCC/ECC for durable transportation through ethylene-vinyl acetate polymer modification. Case Stud. Constr. Mater. 2024, 21, e03878. [Google Scholar] [CrossRef]
- Ji, J.T.; Ni, D.H.; Shi, Y.Q.; Yang, Z.Y.; Ma, M.; Zhu, S.F.; Wang, X. A simple method for preparing flame retardancy EVA/POE-g-MAH composites with high tensile strength by modified magnesium hydroxide and aluminum hydroxide. J. Appl. Polym. Sci. 2024, 141, e55505. [Google Scholar] [CrossRef]
- Zhang, C.; Xie, H.; Du, Y.; Li, X.; Zhou, W.; Wu, T.; Qu, J. Shelter Forest Inspired Superhydrophobic Flame-Retardant Composite with Root-Soil Interlocked Micro/Nanostructure Enhanced Mechanical, Physical, and Chemical Durability. Adv. Funct. Mater. 2023, 33, 2213398. [Google Scholar] [CrossRef]
- Bi, X.; Song, K.; Zhang, H.; Pan, Y.-T.; He, J.; Wang, D.-Y.; Yang, R. Dimensional change of red phosphorus into nanosheets by metal–organic frameworks with enhanced dispersion in flame retardant polyurea composites. Chem. Eng. J. 2024, 482, 148997. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Y.; Cheng, C.; Lyu, S.; Zhu, Z. Preparation of phosphorus-doped chitosan derivative and its applications in polylactic acid: Crystallization, flame retardancy, anti-dripping and mechanical properties. Int. J. Biol. Macromol. 2024, 265, 130648. [Google Scholar] [CrossRef]
- Fu, Z.; Ma, Z.; Liu, J.; Li, C.; Liu, C.; Wang, Q.; Song, L.; Yu, Q.; Cheng, G.; Han, Y.; et al. Phosphorus-containing active esters modified dicyclopentadiene epoxy resins with simultaneously improved flame retardancy, thermal stability, and dielectric properties. Chem. Eng. J. 2024, 482, 148998. [Google Scholar] [CrossRef]
- Hua, Y.; Liu, J.; Zhang, J.; Liu, Z.; Hu, G.; Yang, Y.; Sui, Y.; Sun, J.; Gu, X.; Zhang, S. A compound with boron and phosphorus towards epoxy resin with excellent flame retardancy, smoke suppression, transparency, and dielectric properties. Chem. Eng. J. 2024, 483, 149212. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.; Yang, S.; Chen, X.; Chen, K.; Zhou, G.; Liu, X.; Xu, L.; Huo, S.; Song, P.; et al. Multifunctional phosphorus-containing imidazoliums endowing one-component epoxy resins with superior thermal latency, heat resistance, mechanical properties, and fire safety. Chem. Eng. J. 2024, 485, 149852. [Google Scholar] [CrossRef]
- Cui, Y.; Jiao, Y.; Zhang, G.; Huo, Z.; Sun, J.; Qu, H.; Xu, J. Biomass-derived polyelectrolyte fire retardant: Synergistic phosphorus-nitrogen doping for enhanced epoxy resin flame retardancy and smoke suppression. Polym. Degrad. Stab. 2025, 234, 111207. [Google Scholar] [CrossRef]
- Lu, J.; Tu, H.; Gu, G. Synthesis of brominated flame retardants with different brominated structures and study on flame retardancy of polystyrene resin. React. Funct. Polym. 2023, 193, 105769. [Google Scholar] [CrossRef]
- Maga, D.; Aryan, V.; Beard, A. Toward Sustainable Fire Safety: Life Cycle Assessment of Phosphinate-Based and Brominated Flame Retardants in E-Mobility and Electronic Devices. ACS Sustain. Chem. Eng. 2024, 12, 3652–3658. [Google Scholar] [CrossRef]
- Wang, B.T.; Ye, R.F.; Guo, Z.H.; Li, J.; Fang, Z.P.; Ran, S.Y. Thermal stability and fire safety of polycarbonate flame retarded by the brominated flame retardant and a non-antimony synergistic agent. J. Polym. Res. 2023, 30, 240. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Dai, X.R.; Luo, P.; Sinha, T.K.; Kim, J.K.; Li, H. Lightweight, Elastomeric, and Flame-Retardant Foams from Expanded Chlorinated Polymers. Macromol. Mater. Eng. 2019, 304, 1900145. [Google Scholar] [CrossRef]
- Shi, X.-H.; Xie, W.-M.; Shi, H.; Wu, S.-J.; Liu, Q.-Y.; Wang, D.-Y. Preparation of phosphorus-containing organic-hybrid layered double hydroxide as a flame retardant for thermoplastic polyurethane. Appl. Clay Sci. 2024, 258, 107489. [Google Scholar] [CrossRef]
- Gao, Y.J.; Li, M.X.; Cui, M.L.; Cheng, X.W.; Wu, C.; Liu, W.; Guan, J.P. Polyaniline/chitosan coating as the novel and sustainable flame retardant and UV protection route for silk fabric. Prog. Org. Coat. 2024, 186, 108036. [Google Scholar] [CrossRef]
- Mapossa, A.B.; dos Anjos, E.G.R.; Sundararaj, U. Boosting Flame Retardancy of Polypropylene/Calcium Carbonate Composites with Inorganic Flame Retardants. Materials 2024, 17, 4553. [Google Scholar] [CrossRef]
- Zhou, D.; Luo, Q.; Nie, G.; Dong, M.; Du, X.; Liu, H.; Wu, Z.; Li, J. Preparation of high-quality zinc borate flame retardant: The existence mechanism and synergistic coupling separation of chloride ions in zinc borate. Sep. Purif. Technol. 2024, 344, 27198. [Google Scholar] [CrossRef]
- Han, S.S.; Li, Q.S.; Ma, N.; Liu, D.Y.; Sui, G.X.; Araby, S. Supramolecular-Wrapped α-Zirconium Phosphate Nanohybrid for Fire Safety and Reduced Toxic Emissions of Thermoplastic Polyurethane. ACS Appl. Polym. Mater. 2024, 6, 1376–1388. [Google Scholar] [CrossRef]
- Gao, L.; Bao, Y.; Tang, P.; Liu, C.; Zhang, W. Highly flame retardant of cotton fabric with a sandwich-like CoMnAl-LDH/bio-waterborne polyurethane/phytic acid nanocoating. Cellulose 2024, 31, 8369–8385. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Z.; Qi, X.L.; Wang, D.Y. Recent Progress on Metal-Organic Framework and Its Derivatives as Novel Fire Retardants to Polymeric Materials. Nanomicro Lett. 2020, 12, 173. [Google Scholar] [CrossRef] [PubMed]
- Balducci, G.; Diaz, L.B.; Gregory, D.H. Recent progress in the synthesis of nanostructured magnesium hydroxide. CrystEngComm 2017, 19, 6067–6084. [Google Scholar] [CrossRef]
- Zhang, S.; Bu, X.X.; Gu, X.Y.; Sun, J.; Li, H.F.; Tang, W.F. Improving the mechanical properties and flame retardancy of ethylene-vinyl acetate copolymer by introducing bis [3-(triethoxysilyl) propyl] tetrasulfide modified magnesium hydroxide. Surf. Interface Anal. 2017, 49, 607–614. [Google Scholar] [CrossRef]
- Bi, Q.Q.; Yao, D.W.; Yin, G.Z.; You, J.Q.; Liu, X.Q.; Wang, N.; Wang, D.Y. Surface engineering of magnesium hydroxide via bioinspired iron-loaded polydopamine as green and efficient strategy to epoxy composites with improved flame retardancy and reduced smoke release. React. Funct. Polym. 2020, 155, 104690. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, H.; Zhang, B.X.; Mo, C.; Dai, Y.Q.; Fan, C.Y.; Xu, M. Effect of different surface modifiers on the flame retardancy of ethylene-vinyl acetate copolymer/polyethylene/magnesium hydroxide composite systems. J. Appl. Polym. Sci. 2024, 141, e56061. [Google Scholar] [CrossRef]
- Wang, Z.Z.; Qu, B.J.; Fan, W.C.; Huang, P. Combustion characteristics of halogen-free flame-retarded polyethylene containing magnesium hydroxide and some synergists. J. Appl. Polym. Sci. 2001, 81, 206–214. [Google Scholar] [CrossRef]
- Shi, X.Y.; Chen, H.; Sun, Y.Z.; Song, X.F. Simulation of the Influence Mechanism of Hydrothermal Modification on Magnesium Hydroxide. Cryst. Res. Technol. 2023, 58, 2300006. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, W.; Hu, M.; Tang, Y.; Xu, G.; Hou, X.; Zhou, Y. The bond-forming wet modification of two-dimensional Mg(OH)2 nanoflakes by silane coupling agent for EVA resin with enhanced performance. Polym. Compos. 2025, 46, 11557–11571. [Google Scholar] [CrossRef]
- Liu, T.T.; Wang, F.; Li, G.; Liu, P.; Gao, C.; Ding, Y.F.; Zhang, S.M.; Kong, X.R.; Yang, M.S. Magnesium hydroxide nanoparticles grafted by DOPO and its flame retardancy in ethylene-vinyl acetate copolymers. J. Appl. Polym. Sci. 2021, 138, e49607. [Google Scholar] [CrossRef]
- Li, P.; Li, L.J.; Ji, L.M.; Dang, L.; Lan, S.J.; Zhu, D.H. Flame retardancy and smoke suppression effect of an organic-inorganic hybrid containing phosphaphenanthrene-modified magnesium hydroxide/zinc borate on epoxy resin. J. Appl. Polym. Sci. 2023, 140, e54575. [Google Scholar] [CrossRef]
- Kim, H.H.; Sim, M.J.; Lee, J.C.; Cha, S.H. The effects of chemical structure for phosphorus-nitrogen flame retardants on flame retardant mechanisms. J. Mater. Sci. 2023, 58, 6850–6864. [Google Scholar] [CrossRef]
- Wang, P.; Cai, Z.S. Highly efficient flame-retardant epoxy resin with a novel DOPO-based triazole compound: Thermal stability, flame retardancy and mechanism. Polym. Degrad. Stab. 2017, 137, 138–150. [Google Scholar] [CrossRef]
- Markwart, J.C.; Battig, A.; Zimmermann, L.; Wagner, M.; Fischer, J.; Schartel, B.; Wurm, F.R. Systematically Controlled Decomposition Mechanism in Phosphorus Flame Retardants by Precise Molecular Architecture: P-O vs P-N. ACS Appl. Polym. Mater. 2019, 1, 1118–1128. [Google Scholar] [CrossRef]
- Noh, J.; Kang, I.; Choi, J.; Fatima, H.; Yoo, P.J.; Oh, K.W.; Park, J. Surface modification of magnesium hydroxide nanoparticles with hexylphosphoric acid to improve thermal stabilities of polyethylene composites. Polym. Bull. 2016, 73, 2855–2866. [Google Scholar] [CrossRef]
- Dun, L.; Ouyang, Z.; Sun, Q.H.; Yue, X.J.; Wu, G.D.; Li, B.H.; Kang, W.D.; Wang, Y.H. A Simple and Efficient Magnesium Hydroxide Modification Strategy for Flame-Retardancy Epoxy Resin. Polymers 2024, 16, 1471. [Google Scholar] [CrossRef]
- Li, S.Y.; Wang, C.F.; Wang, G.D.; Wang, Y.L.; Han, Z.D. Polycarbosilane/Divinylbenzene-Modified Magnesium Hydroxide to Enhance the Flame Retardancy of Ethylene-Vinyl Acetate Copolymer. Polymers 2023, 15, 4440. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, M.Y.; Liang, N.X.; Wang, Y.R.; Li, Z.; Li, X. Polyphosphazene functionalized magnesium hydroxide core-shell architecture fire retardant: Toward a fire-safe multifunctional styrene-butadiene-styrene asphalt. Fuel 2024, 370, 131822. [Google Scholar] [CrossRef]
- Yao, M.; Wu, H.J.; Liu, H.; Zhou, Z.X.; Wang, T.; Jiao, Y.H.; Qu, H.Q. In-situ growth of boron nitride for the effect of layer-by-layer assembly modified magnesium hydroxide on flame retardancy, smoke suppression, toxicity and char formation in EVA. Polym. Degrad. Stab. 2021, 183, 109417. [Google Scholar] [CrossRef]
- Guo, G.; Sun, J.; Zhao, C.; Liu, Y.; Liu, C.M. Phosphorus-containing polymers from THPS. VII. Synthesis of phosphorus-containing trialkynes and their metal-free 1,3-dipolar cycloaddition reaction with azidated soybean-oil. Green. Chem. 2016, 18, 1278–1286. [Google Scholar] [CrossRef]
- Conlette, O. Impacts of Tetrakis-hydroxymethyl Phosphonium Sulfate (THPS) Based Biocides on the Functional Group Activities of Some Oil Field Microorganisms Associated with Corrosion and Souring. Br. Microbiol. Res. J. 2014, 4, 1463–1475. [Google Scholar] [CrossRef]
- Tan, Z.W.; Sun, J.; Wu, C.Y.; Qiu, J.J.; Liu, C.M. Phosphorus-containing polymers from THPS. IV: Synthesis and properties of phosphorus-containing polybenzoxazines as a green route for recycling toxic phosphine (PH(3)) tail gas. J. Hazard. Mater. 2017, 322, 540–550. [Google Scholar] [CrossRef]
- Romano, S.; Trespi, S.; Achermann, R.; Battaglia, G.; Raponi, A.; Marchisio, D.; Mazzotti, M.; Micale, G.; Cipollina, A. The Role of Operating Conditions in the Precipitation of Magnesium Hydroxide Hexagonal Platelets Using NaOH Solutions. Cryst. Growth Des. 2023, 23, 6491–6505. [Google Scholar] [CrossRef]
- Cheng, X.-W.; Xuan, K.; Guan, J.-P.; Liu, W.; Chen, G. Construction of silane-modified nanoscale magnesium hydroxide as an inorganic flame-retardant coating for silk textiles. Colloids Surf. A Physicochem. Eng. Asp. 2023, 676, 132287. [Google Scholar] [CrossRef]
- GB/T 2406.2-2009; Plastics—Determination of Burning Behaviour by Oxygen Index—Part 2: Ambient-Temperature Test. Standardization Administration of China: Beijing, China, 2009.
- GB/T 2408-2021; Plastics—Determination of Burning Characteristics—Horizontal and Vertical Test. Standardization Administration of China: Beijing, China, 2021.
- ASTM E662-18; Standard Test Method for Specific Optical Density of Smoke Generated by Solid Materials. ASTM International: West Conshohocken, PA, USA, 2018.
- ASTM D638-22; Standard Test Method for Tensile Properties of Plastics. ASTM International: West Conshohocken, PA, USA, 2022.
- Du, Z.; Yang, E.-H.; Unluer, C. Investigation of the properties of Mg(OH)2 extracted from magnesium-rich brine via the use of an industrial by-product. Cem. Concr. Compos. 2024, 152, 105658. [Google Scholar] [CrossRef]
- Kumari, L.; Li, W.Z.; Vannoy, C.H.; Leblanc, R.M.; Wang, D.Z. Synthesis, characterization and optical properties of Mg(OH)2 micro-/nanostructure and its conversion to MgO. Ceram. Int. 2009, 35, 3355–3364. [Google Scholar] [CrossRef]
- Du, Z.; Unluer, C. Modification of magnesium hydroxide for improved performance in CO2 sequestration. Cem. Concr. Res. 2024, 177, 107418. [Google Scholar] [CrossRef]
- Jia, B.; Gao, L. Morphology Transformation of Nanoscale Magnesium Hydroxide: From Nanosheets to Nanodisks. J. Am. Ceram. Soc. 2006, 89, 3881–3884. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, T.; Fang, H.; Li, S.; Yao, Y.; He, Y. A Novel Preparation of Nano-sized Hexagonal Mg(OH)2. Procedia Eng. 2015, 102, 388–394. [Google Scholar] [CrossRef]
- Wang, P.; Li, C.; Gong, H.; Wang, H.; Liu, J. Morphology control and growth mechanism of magnesium hydroxide nanoparticles via a simple wet precipitation method. Ceram. Int. 2011, 37, 3365–3370. [Google Scholar] [CrossRef]
- Dawson, P.; Hadfield, C.; Wilkinson, G. The polarized infra-red and Raman spectra of Mg(OH)2 and Ca(OH)2. J. Phys. Chem. Solids 1973, 34, 1217–1225. [Google Scholar] [CrossRef]
- Bhatte, K.D.; Sawant, D.N.; Deshmukh, K.M.; Bhanage, B.M. Additive free microwave assisted synthesis of nanocrystalline Mg(OH)2 and MgO. Particuology 2012, 10, 384–387. [Google Scholar] [CrossRef]
- Wurm, A.; Kuhn, J.; Kugel, K.; Putzer, D.; Arora, R.; Coraca-Huber, D.C.; Zelger, P.; Badzoka, J.; Kappacher, C.; Huck, C.W.; et al. Raman microscopic spectroscopy as a diagnostic tool to detect Staphylococcus epidermidis in bone grafts. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 280, 121570. [Google Scholar] [CrossRef]
- Panikar, S.S.; Deodhar, B.S.; Sawant, D.K.; Klaassen, J.J.; Deng, J.; Durig, J.R. Raman and infrared spectra, r(0) structural parameters, and vibrational assignments of (CH(3))(2)PX where X.=H., CN, and Cl. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 103, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Carauta, A.N.; de Souza, V.; Hollauer, E.; Tellez, S.C. Vibrational study of dialkylphosphonates: Di-n-propyl- and di-i-propylphosphonates by semiempirical and ab initio methods. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2004, 60, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Ghazaryan, V.V.; Zakharov, B.A.; Petrosyan, A.M.; Boldyreva, E.V. L-Argininium phosphite-a new candidate for NLO materials. Acta Crystallogr. C Struct. Chem. 2015, 71, 415–421. [Google Scholar] [CrossRef]
- Jiang, W.; Hua, X.; Han, Q.; Yang, X.; Lu, L.; Wang, X. Preparation of lamellar magnesium hydroxide nanoparticles via precipitation method. Powder Technol. 2009, 191, 227–230. [Google Scholar] [CrossRef]
- Li, P.; Jin, H.; Zhong, G.; Ji, H.; Li, Z.; Yang, J. Electrochemistry of P–C Bonds in Phosphorus–Carbon Based Anode Materials. ACS Appl. Mater. Interfaces 2022, 14, 18506–18512. [Google Scholar] [CrossRef]
- Puziy, A.M.; Poddubnaya, O.I.; Socha, R.P.; Gurgul, J.; Wisniewski, M. XPS and NMR studies of phosphoric acid activated carbons. Carbon 2008, 46, 2113–2123. [Google Scholar] [CrossRef]
- Zheng, T.; Xia, W.; Guo, J.; Liu, Y. Modified magnesium hydroxide encapsulated by melamine cyanurate in flame-retardant polyamide-6. J. Polym. Res. 2020, 27, 258. [Google Scholar] [CrossRef]
- Jiang, Z.; Chen, Z.; An, Y.; Wang, S.; Li, T.; Yang, W.; Lu, H.; Wei, C. Flammability Performance of Ceramifiable Polydimethylsiloxane (PDMS) Composites with Needle-Like Wollastonite. J. Appl. Polym. Sci. 2025, 142, e56664. [Google Scholar] [CrossRef]
- Zhao, H.B.; Liu, B.W.; Wang, X.L.; Chen, L.; Wang, X.L.; Wang, Y.Z. A flame-retardant-free and thermo-cross-linkable copolyester: Flame-retardant and anti-dripping mode of action. Polymer 2014, 55, 2394–2403. [Google Scholar] [CrossRef]

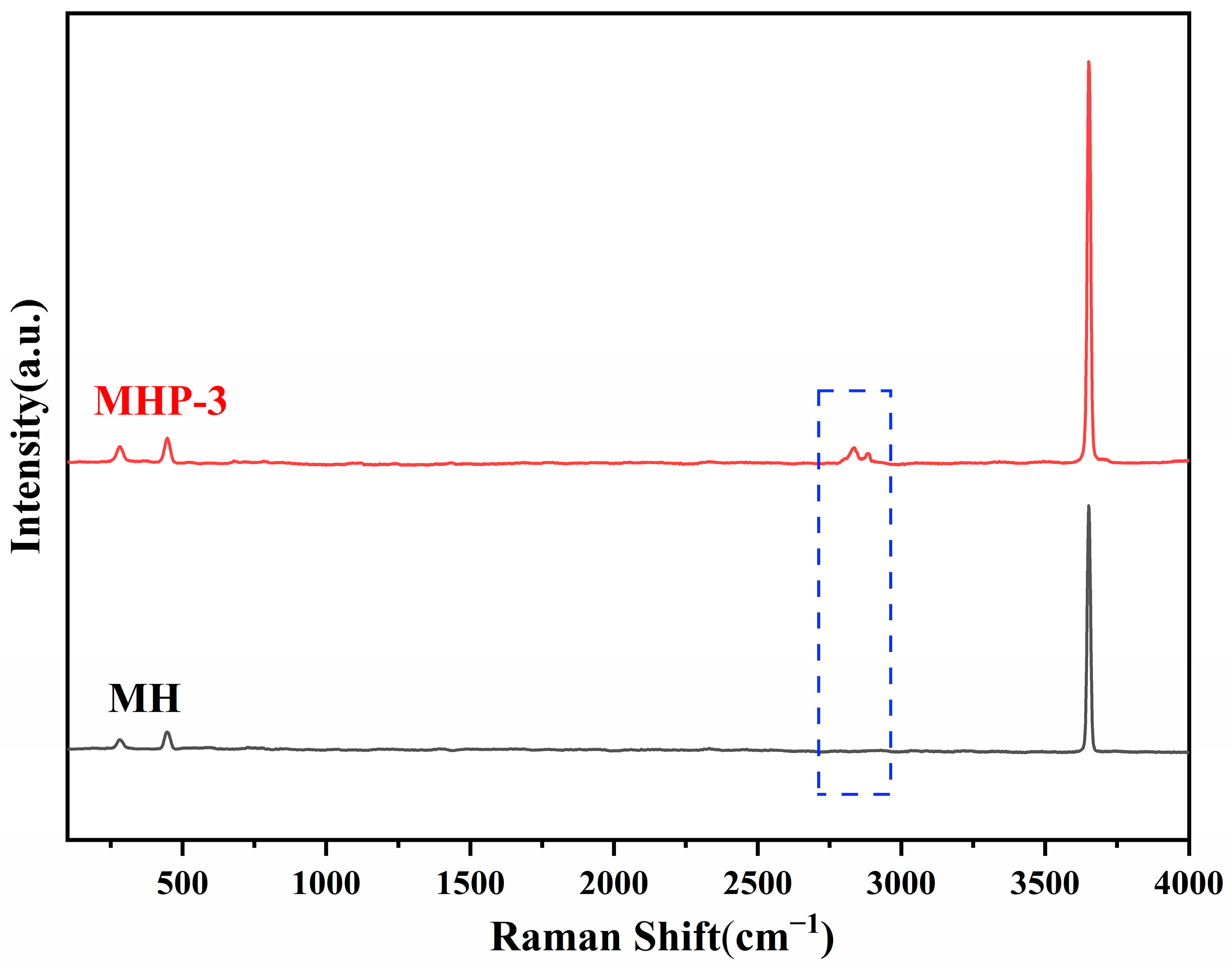
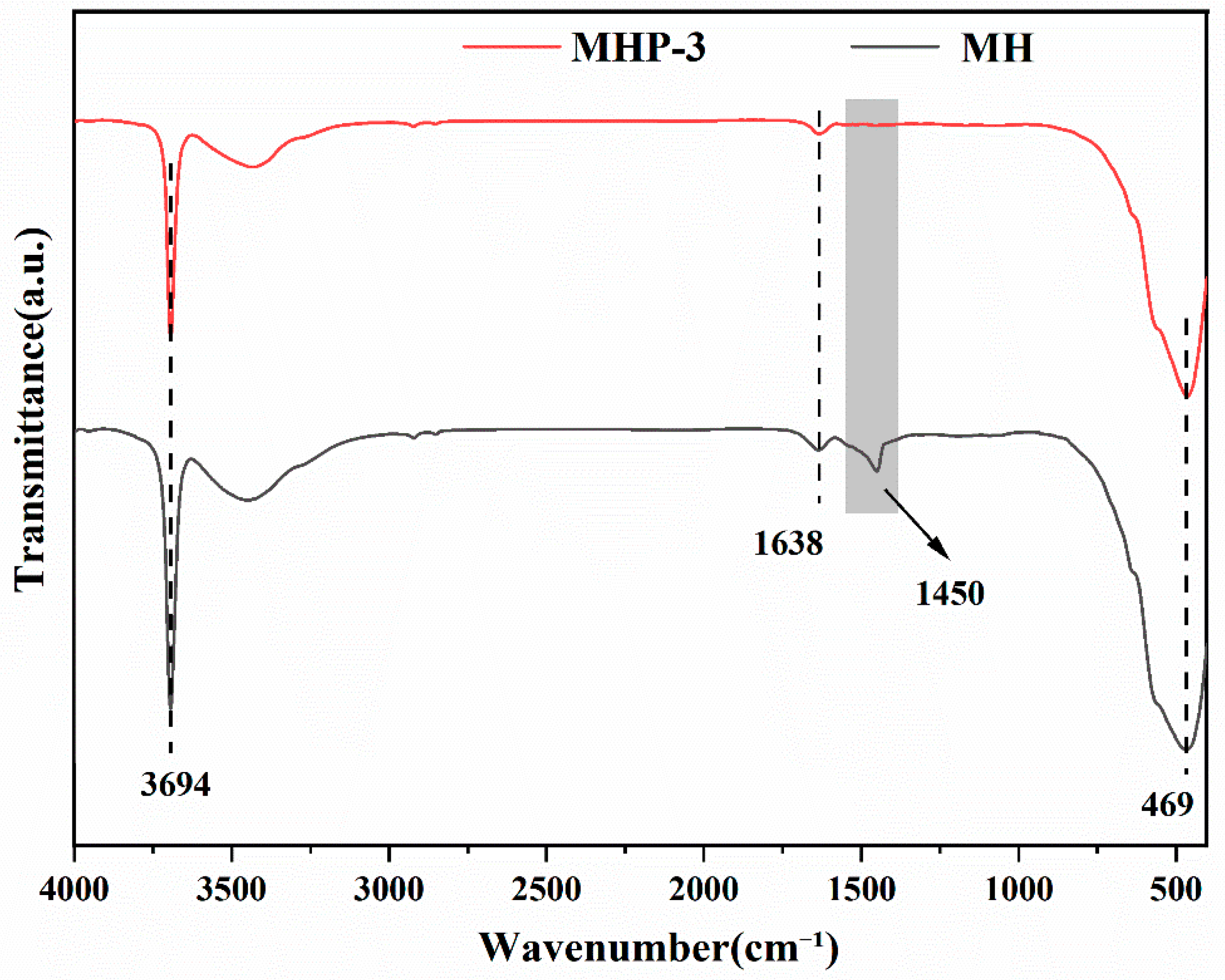
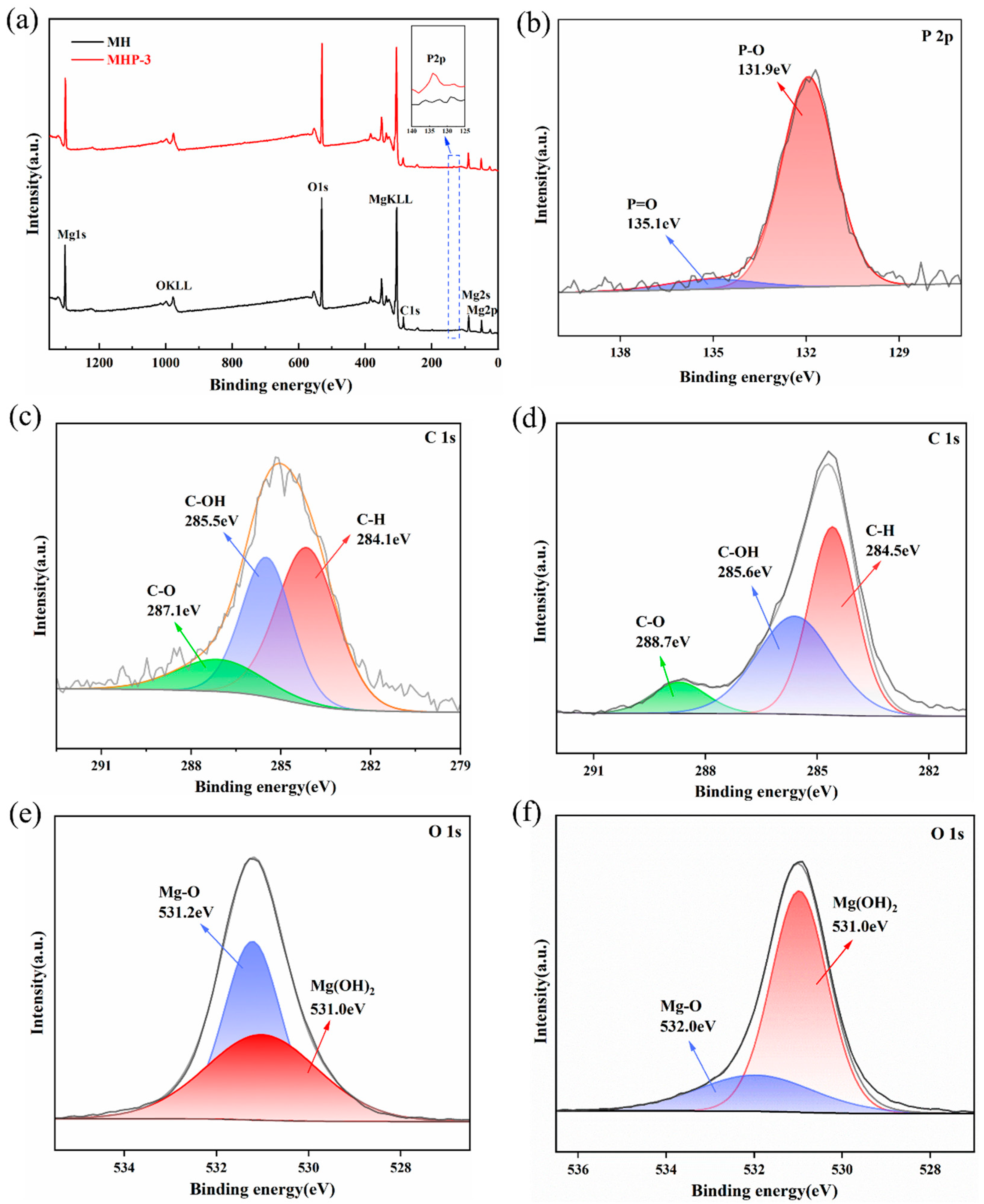
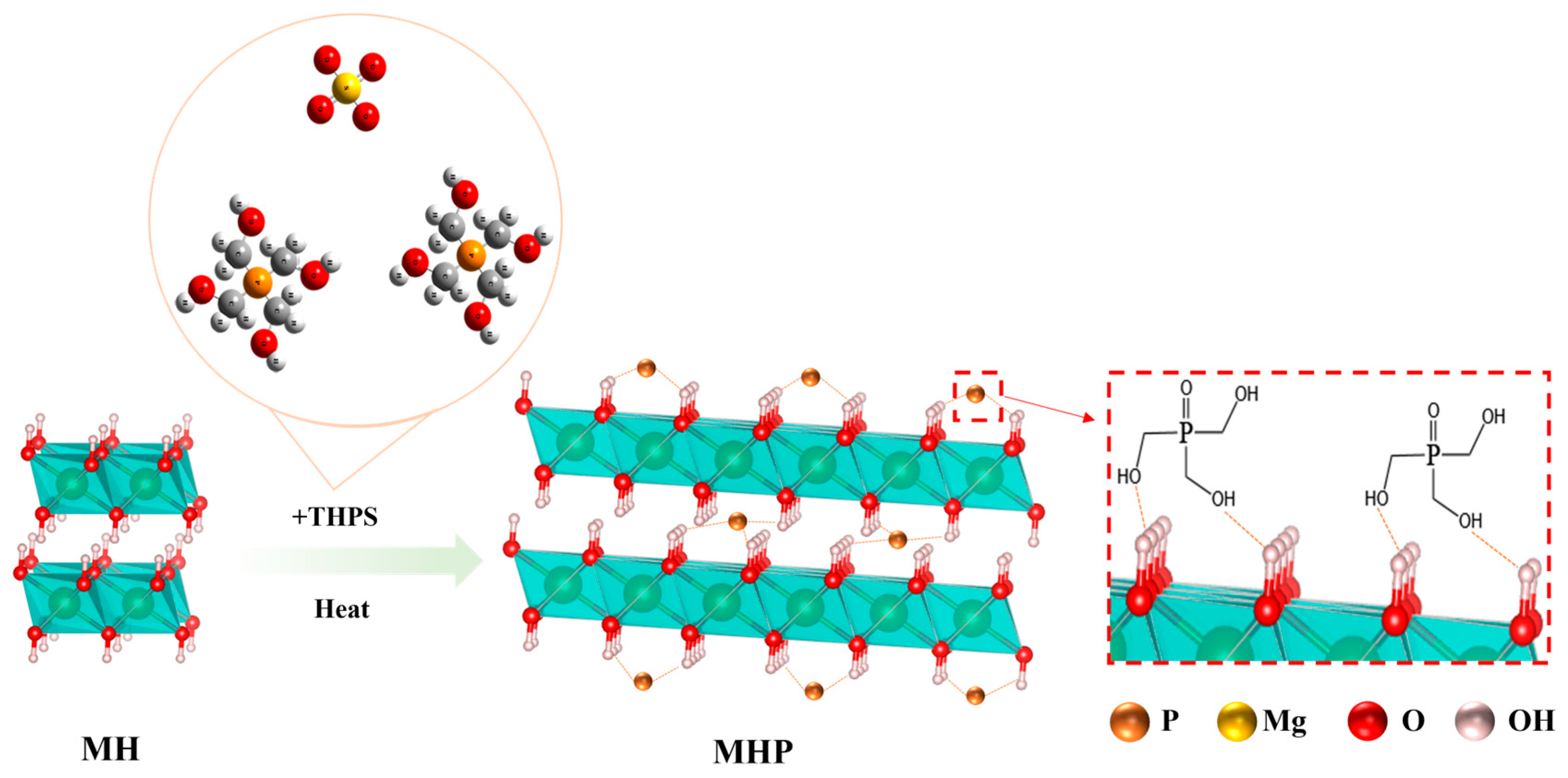

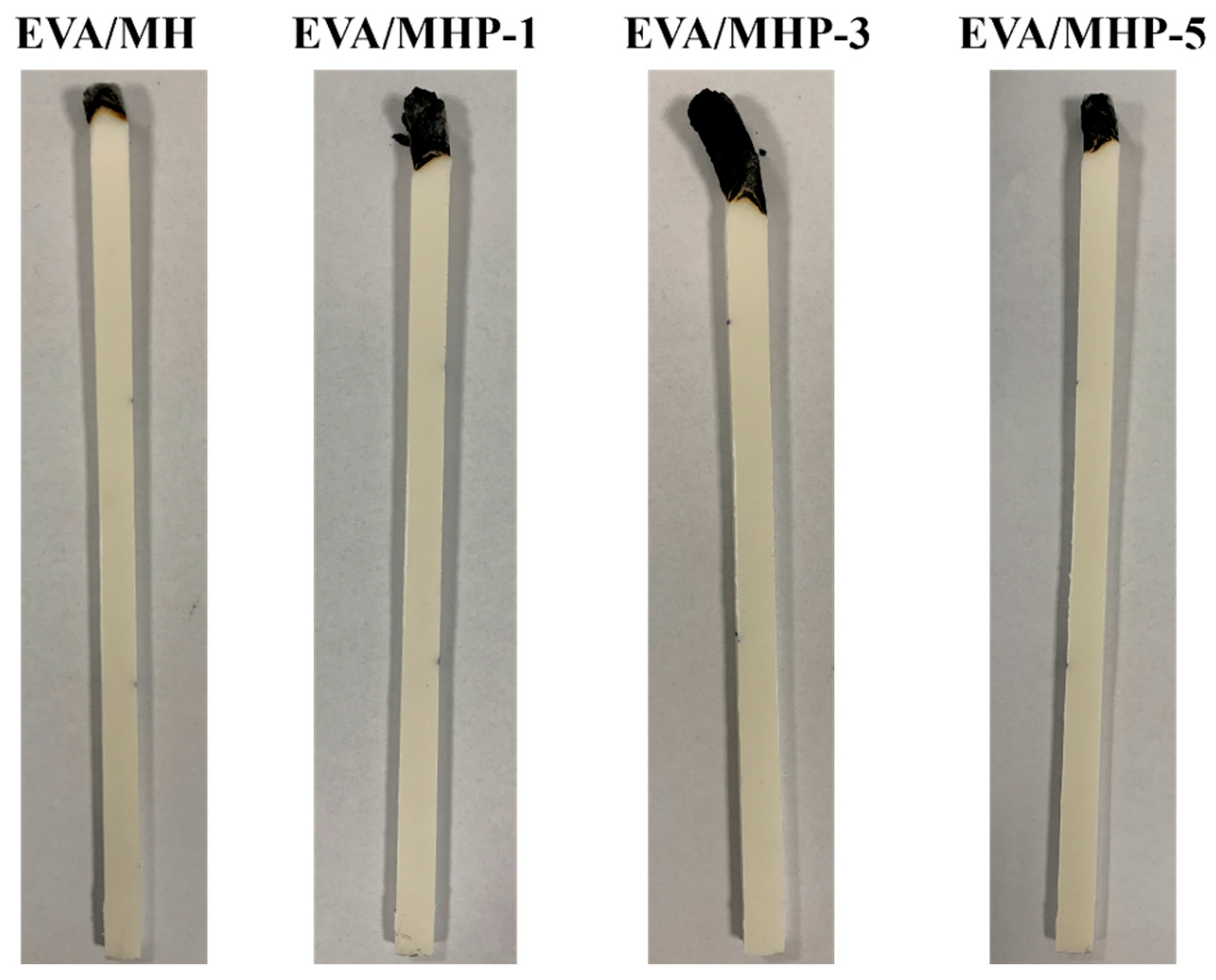
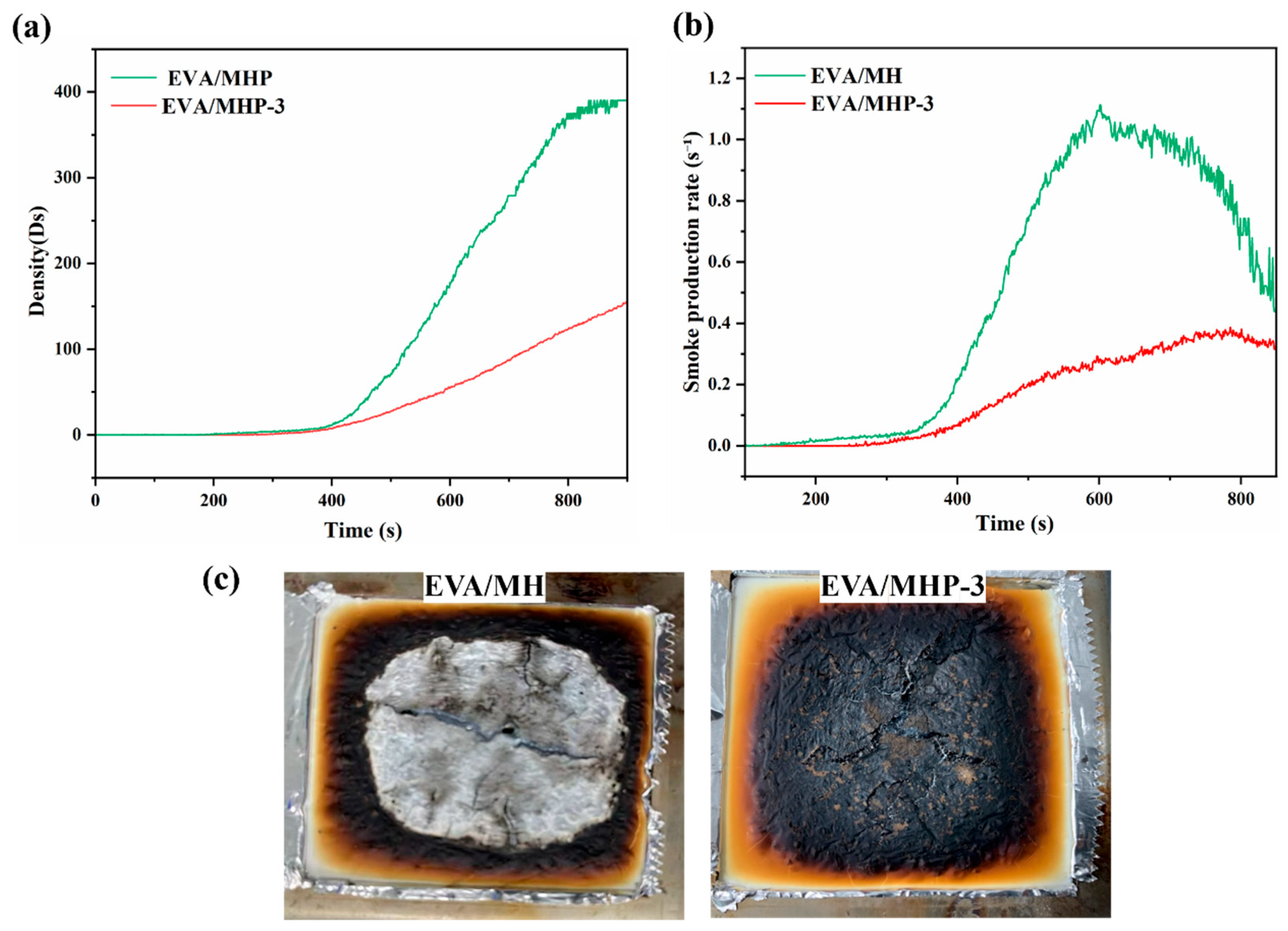
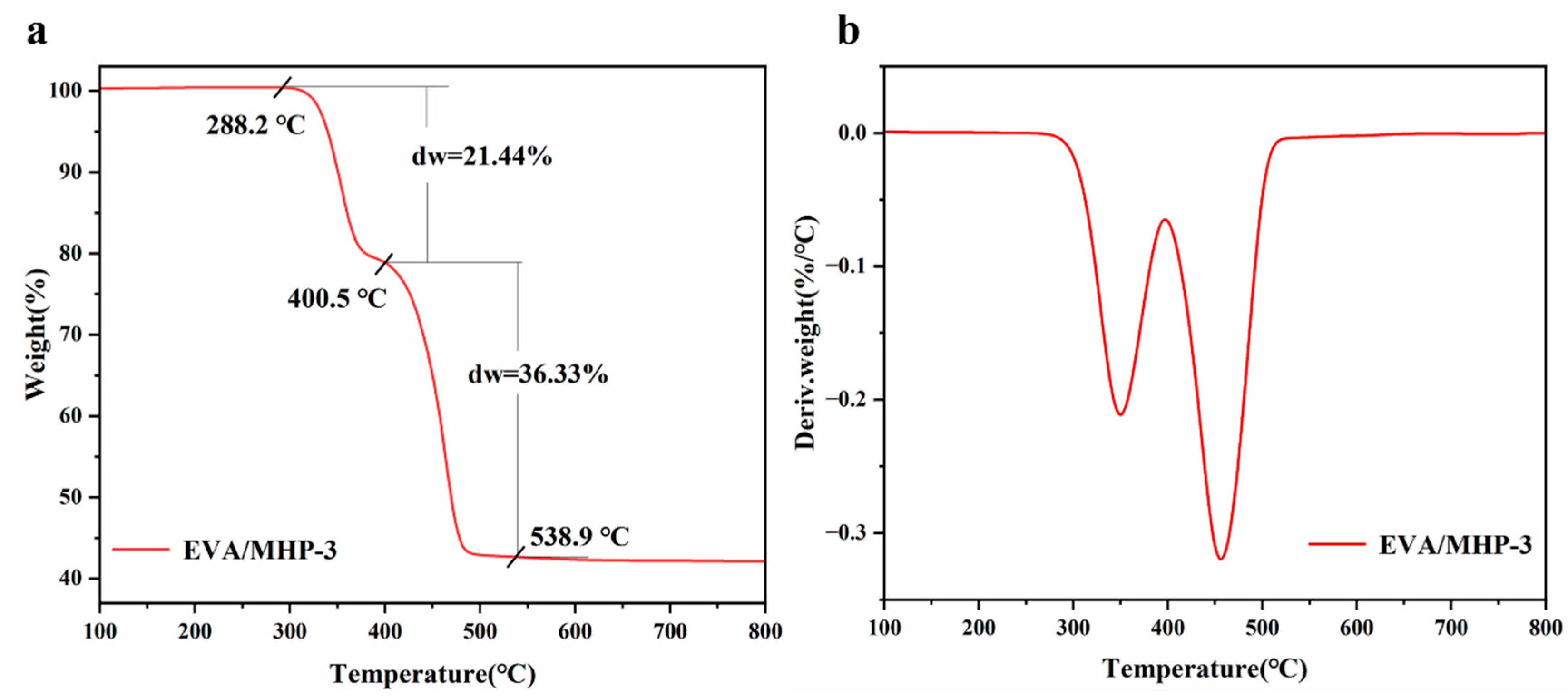
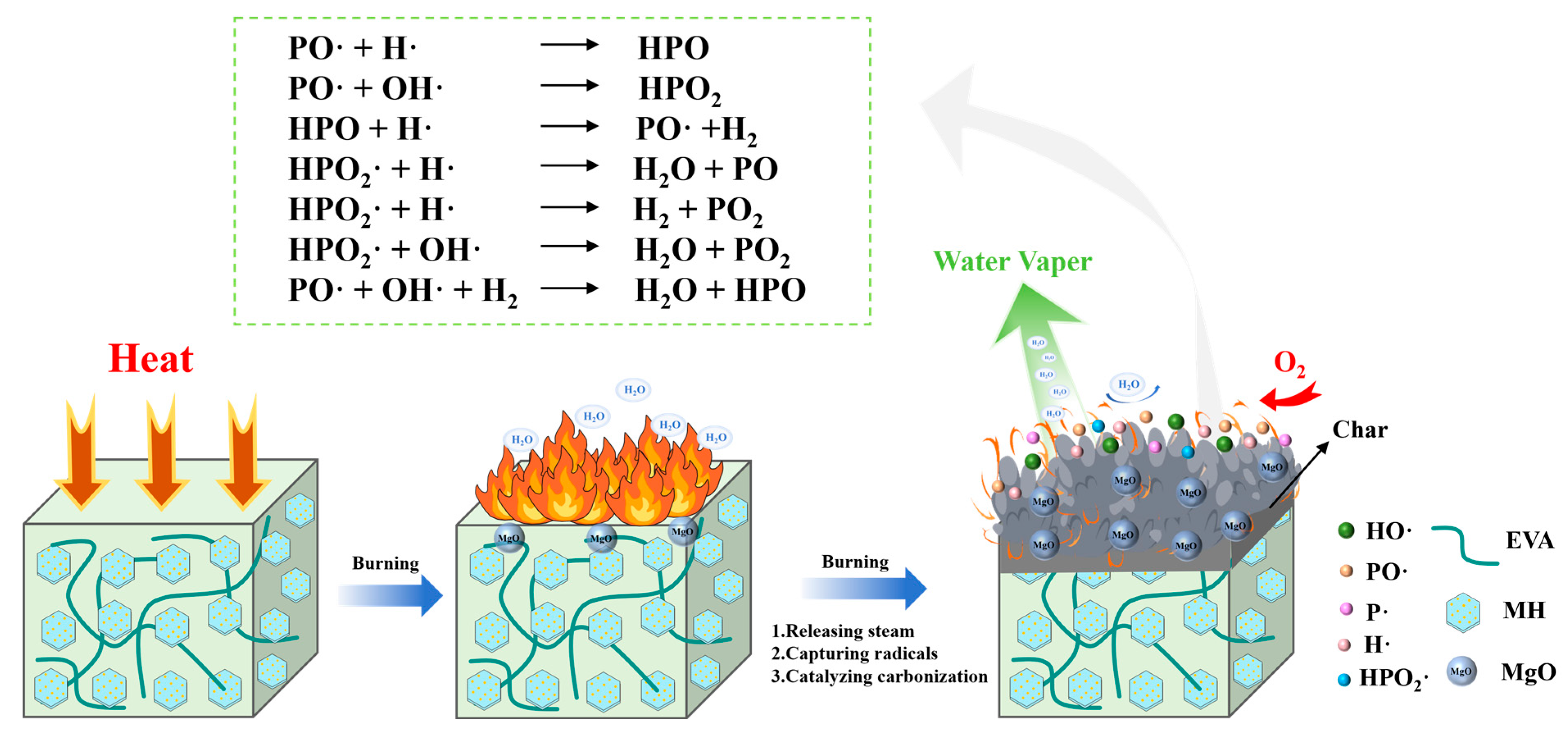
| Samples | Temperature (°C) | I(001)/I(101) | Grain Size/nm | |
|---|---|---|---|---|
| (001) | (101) | |||
| MH | 60 | 0.71 | 17.04 | 21.92 |
| MH | 200 | 1.71 | 34.61 | 34.62 |
| MHP-1 | 200 | 0.97 | 16.15 | 19.97 |
| MHP-3 | 200 | 0.99 | 45.26 | 43.97 |
| MHP-5 | 200 | 0.79 | 17.42 | 22.95 |
| Samples | T5% (°C) | Tmax (°C) | Residue at 800 °C (%) |
|---|---|---|---|
| MH | 353.0 | 376.2 | 68.2 |
| MHP-1 | 344.7 | 387.6 | 67.0 |
| MHP-3 | 352.2 | 383.6 | 68.4 |
| MHP-5 | 345.4 | 366.1 | 67.4 |
| Samples | Vertical Burning Test | |||
|---|---|---|---|---|
| t1/t2 (s) | Dripping/Ignition of Cotton | Rating | LOI (%) | |
| EVA | >30 | YES/YES | NR | 19.8 |
| EVA/MH | >30 | NO/NO | NR | 23.9 |
| EVA/MHP-1 | 6.4/5.6 | NO/NO | V-0 | 29.0 |
| EVA/MHP-3 | 2.3/3.4 | NO/NO | V-0 | 31.3 |
| EVA/MHP-5 | 5.2/4.3 | NO/NO | V-0 | 30.5 |
| Samples | Tensile Strength (MPa) | StdDev (MPa) | Elongation at Break (%) | StdDev (%) |
|---|---|---|---|---|
| EVA/MH | 12.5 | 0.2 | 87.9 | 4.8 |
| EVA/MHP-1 | 11.7 | 0.3 | 91 | 9.7 |
| EVA/MHP-3 | 14.1 | 0.4 | 136 | 0.4 |
| EVA/MHP-5 | 13.4 | 0.4 | 116.7 | 15.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Xu, H.; Chen, J. One-Pot Preparation of Easily Dispersible Hexagonal Mg(OH)2 Modified with THPS and Its Flame-Retardant EVA Copolymer. Materials 2025, 18, 4847. https://doi.org/10.3390/ma18214847
Liu X, Xu H, Chen J. One-Pot Preparation of Easily Dispersible Hexagonal Mg(OH)2 Modified with THPS and Its Flame-Retardant EVA Copolymer. Materials. 2025; 18(21):4847. https://doi.org/10.3390/ma18214847
Chicago/Turabian StyleLiu, Xia, Haihui Xu, and Jinyang Chen. 2025. "One-Pot Preparation of Easily Dispersible Hexagonal Mg(OH)2 Modified with THPS and Its Flame-Retardant EVA Copolymer" Materials 18, no. 21: 4847. https://doi.org/10.3390/ma18214847
APA StyleLiu, X., Xu, H., & Chen, J. (2025). One-Pot Preparation of Easily Dispersible Hexagonal Mg(OH)2 Modified with THPS and Its Flame-Retardant EVA Copolymer. Materials, 18(21), 4847. https://doi.org/10.3390/ma18214847






