Study on the Thermal and Rheological Properties of Nano-TiO2-Modified Double Phase Change Asphalt
Abstract
1. Introduction
2. Experiment and Materials
2.1. Raw Materials
2.2. Preparation of Composite PCMs and Double PCMs
2.3. Preparation of Double PCM Asphalt
2.4. Testing and Characterization
2.4.1. Heat Storage Performance
2.4.2. Thermal Conductivity
2.4.3. Microscopic Properties
2.4.4. Thermal Stability
2.4.5. Phase Change Durability
2.4.6. Rheological Properties of Double Phase Change Asphalt
2.4.7. Temperature Regulation Performance of Double PCM Asphalt
3. Results and Discussion
3.1. Research on the Adsorption Ratio of PCMs
3.2. Research on the Dosage of Nano-TiO2
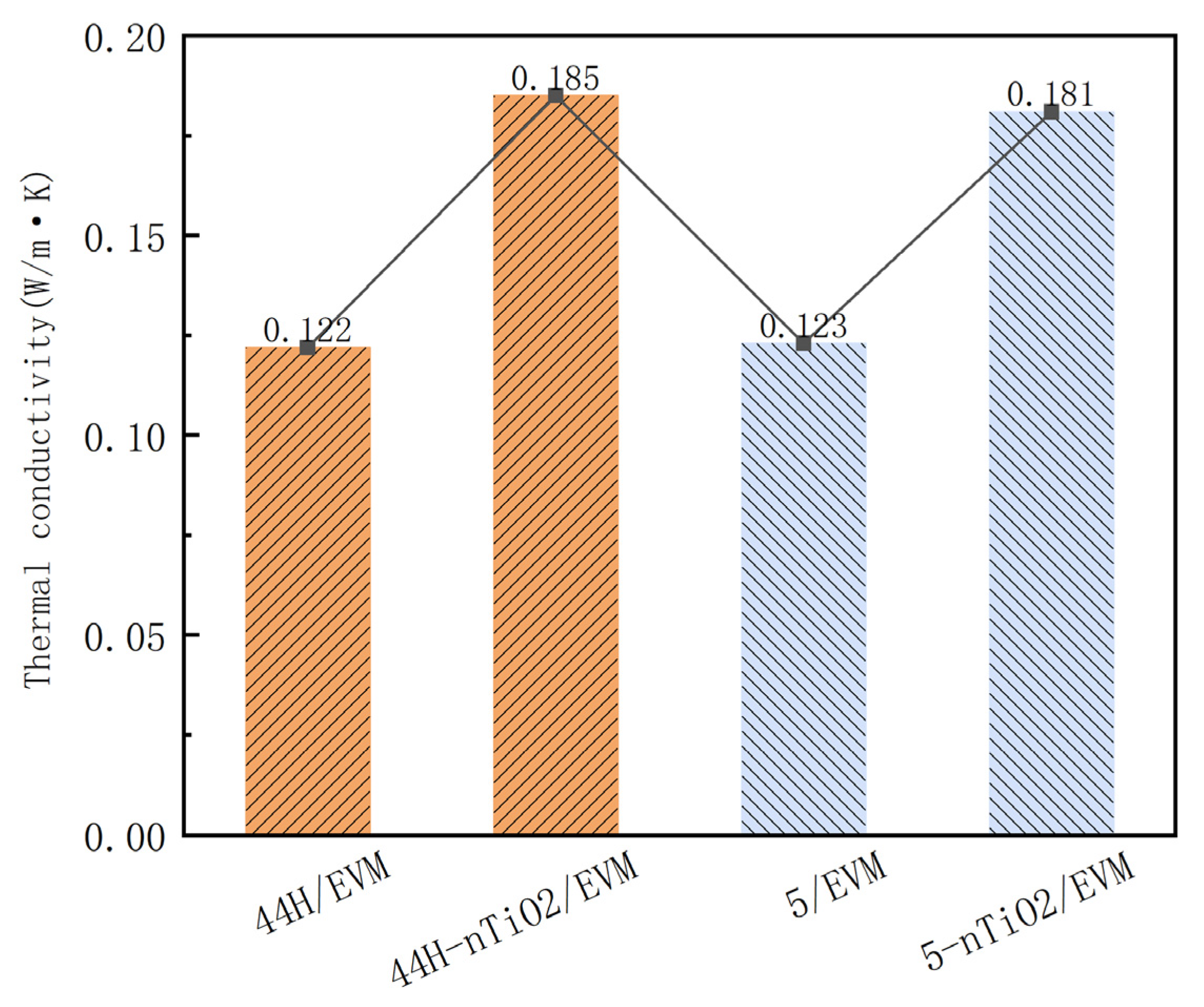
3.3. Analysis of Thermal Conductivity of the CPCMs
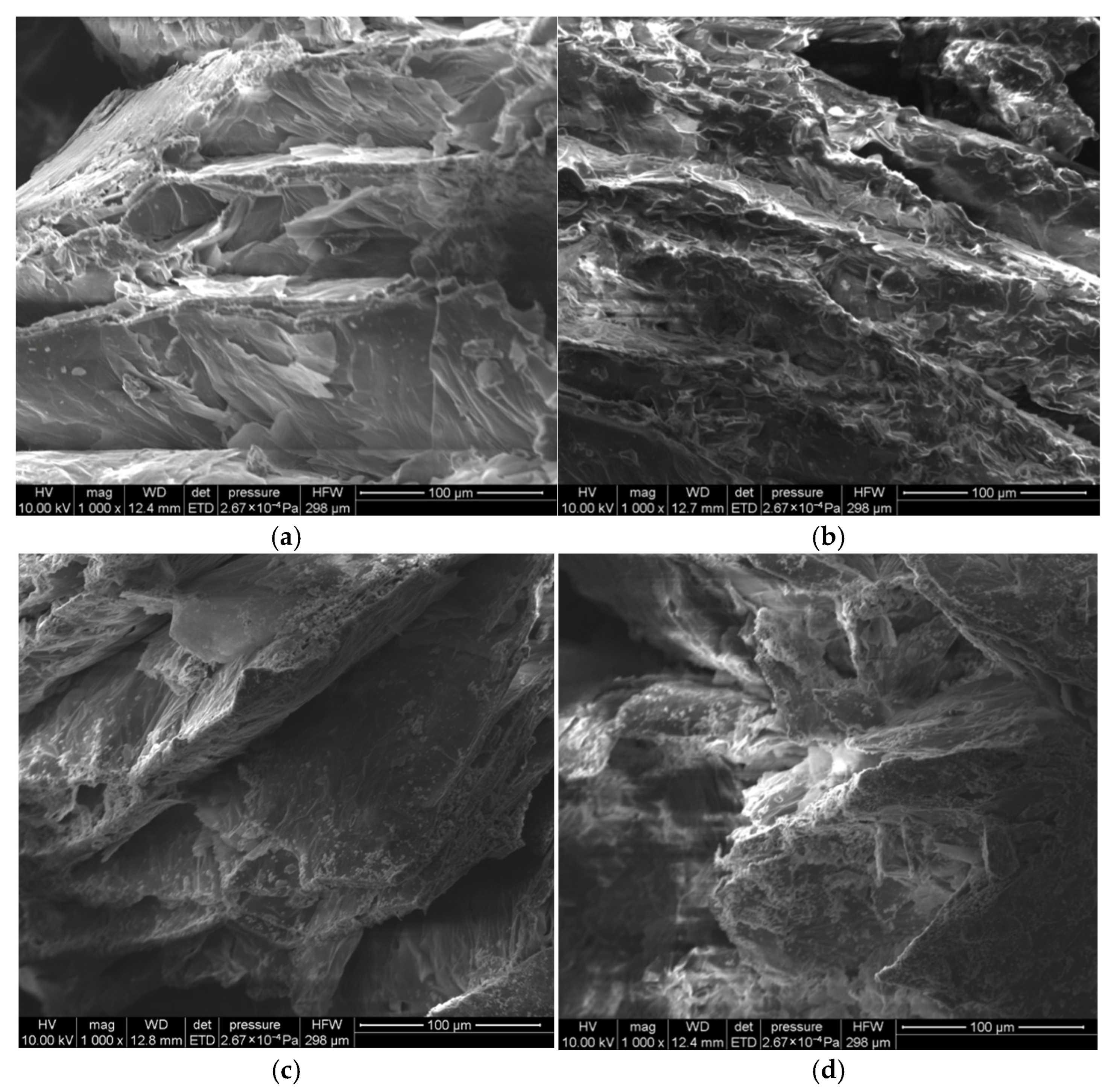
3.4. Microscopic Morphology Analysis
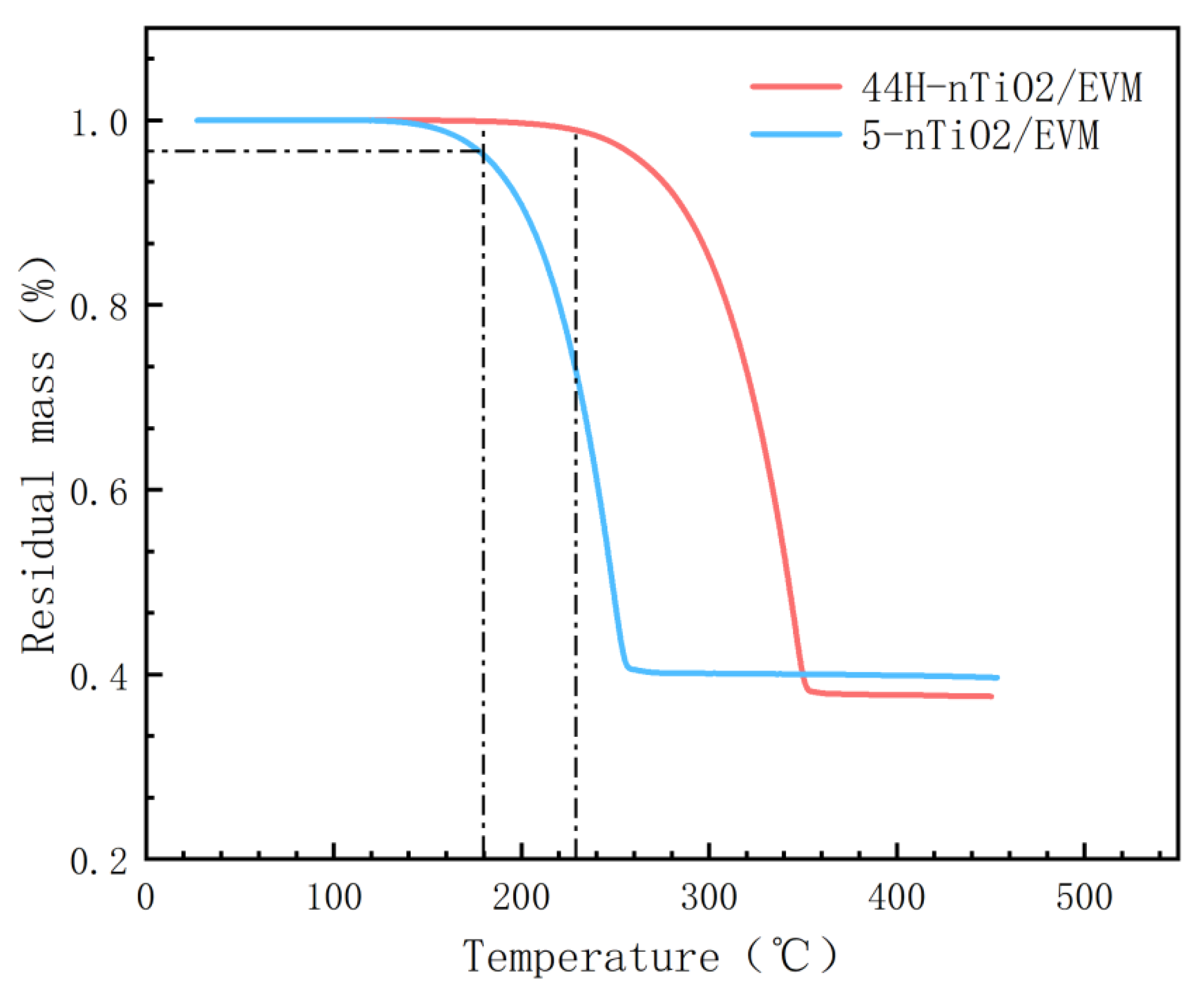
3.5. Thermal Stability Analysis
3.6. Phase Change Durability Analysis
3.7. Analysis of Thermal Storage Performance of Double PCM Asphalt
3.8. Analysis of the Rheological Properties of the Double PCM Asphalt
3.8.1. High-Temperature Performance Analysis
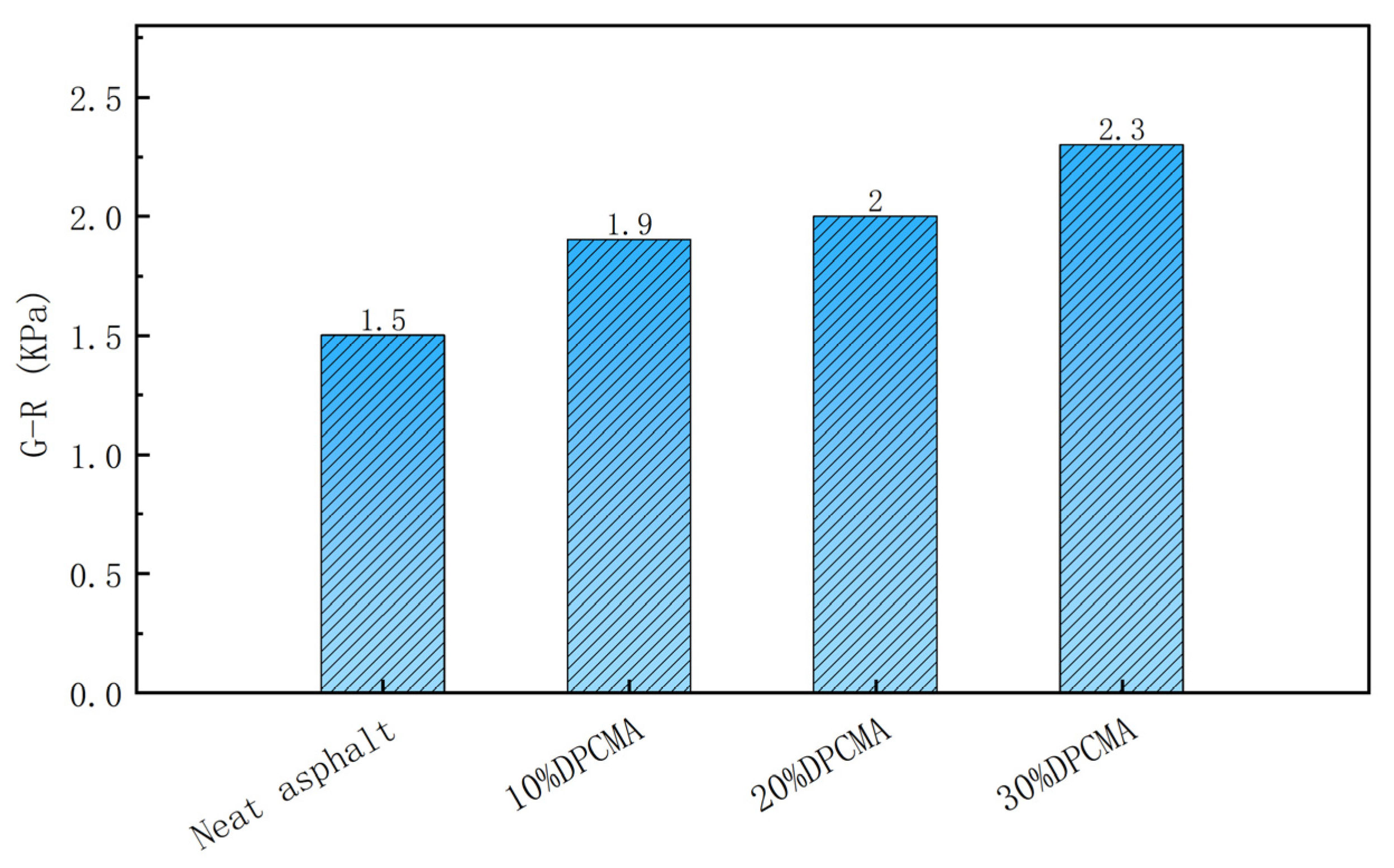
3.8.2. Analysis of Fatigue Resistance Performance
3.8.3. Analysis of Low-Temperature Crack Resistance Performance
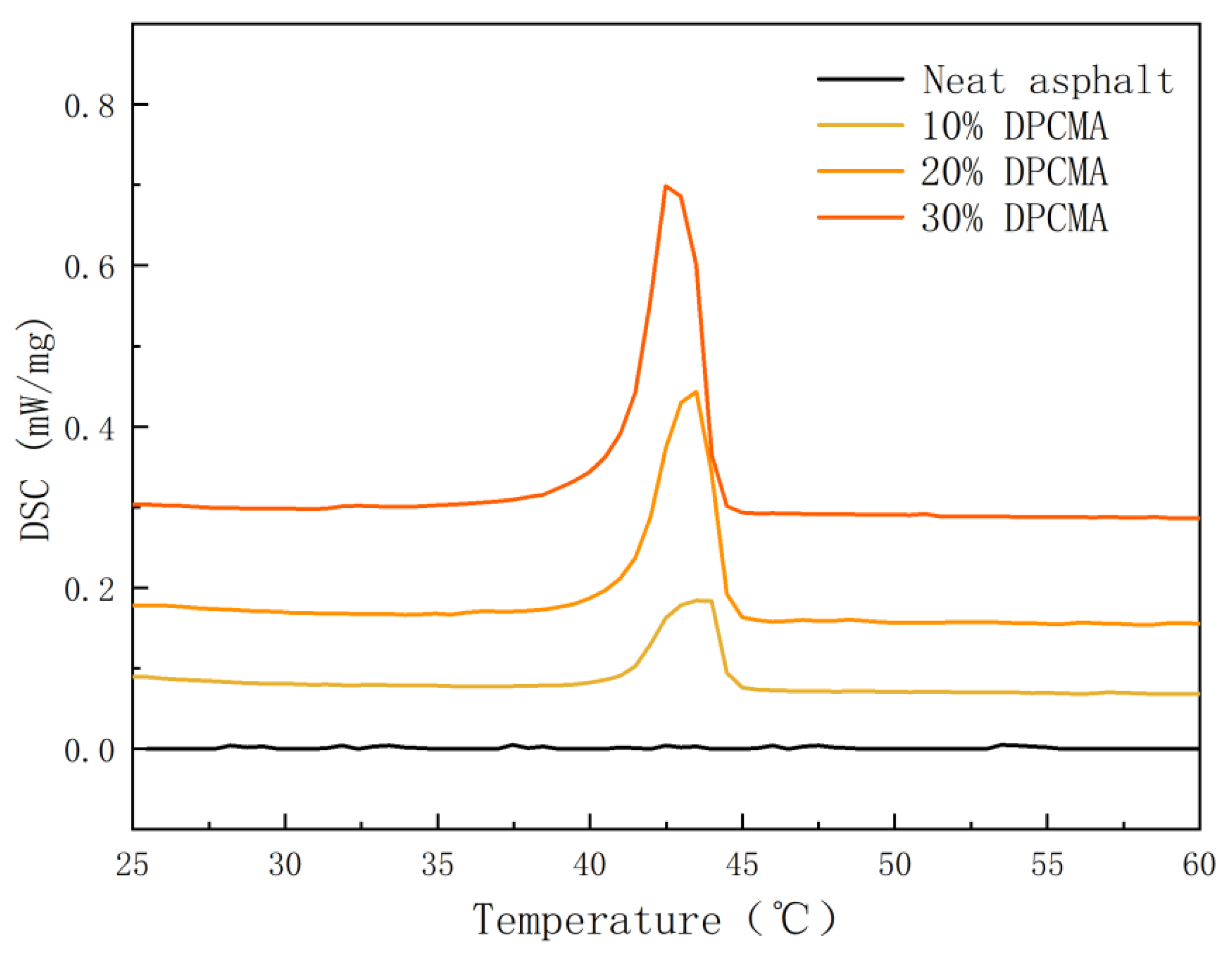
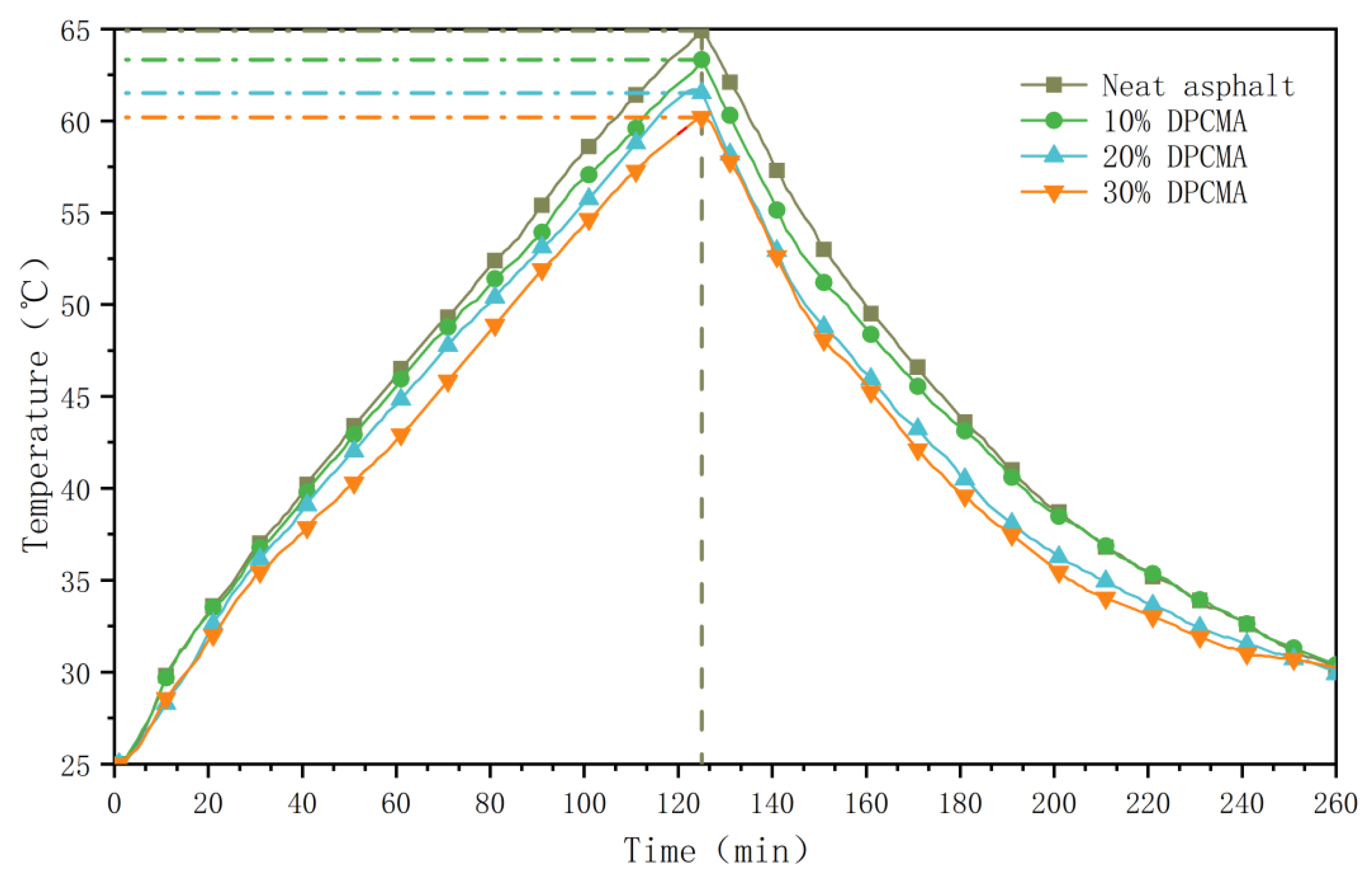
3.9. Analysis of the Temperature Regulation Performance of the Double PCM Asphalt
4. Conclusions
- (1)
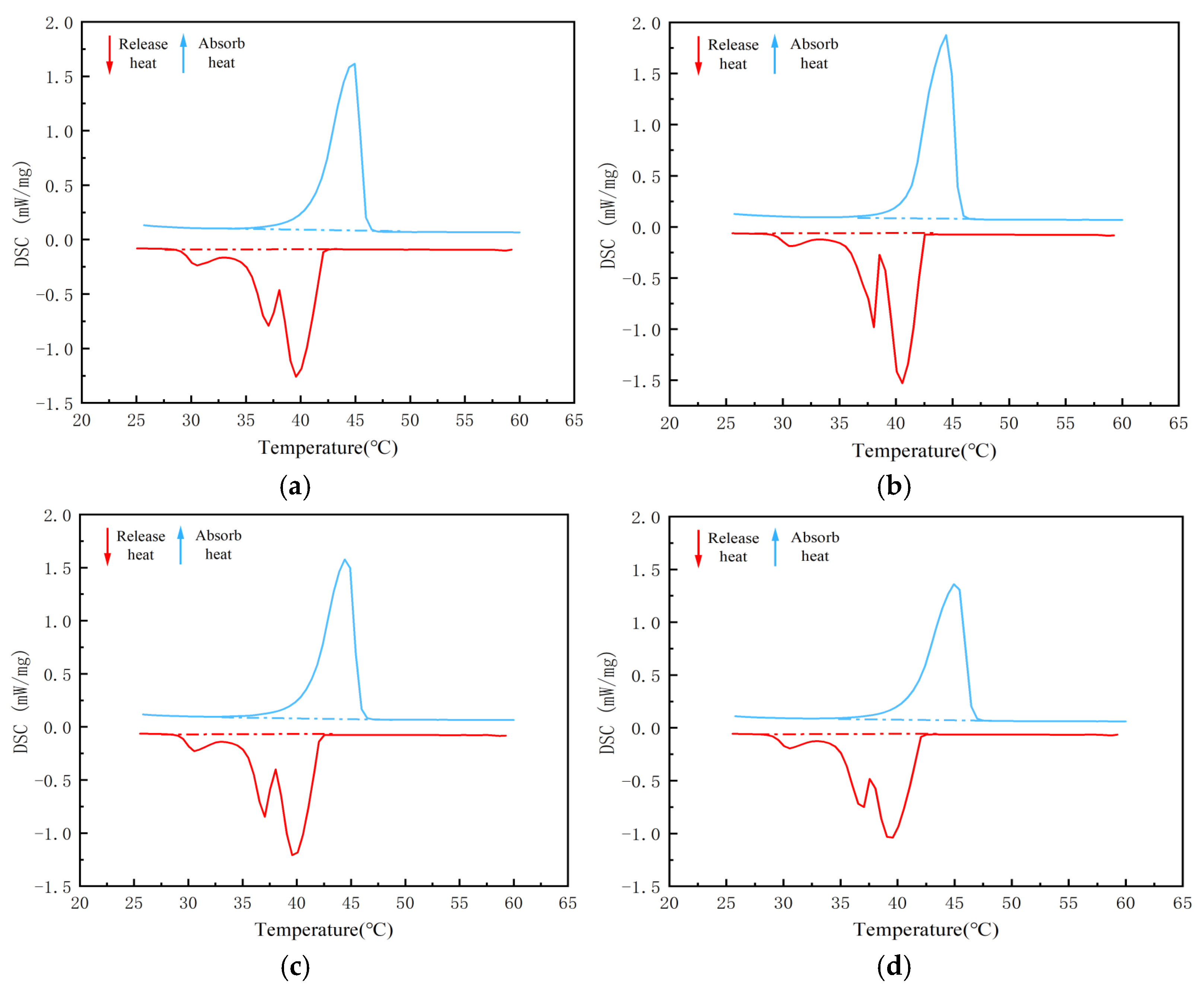
- When the dosage of nano-TiO2 is 2%, it can effectively increase the thermal conductivity of the composite PCM. The thermal conductivity growth rates of the high- and low-temperature composite PCMs are 51.6% and 47.2%, respectively. In addition, the addition of nano-TiO2 makes the phase change temperature range of the CPCMs more concentrated and does not affect the adsorption and phase change enthalpy values of the CPCMs. The phase transition enthalpy values of the high/low-temperature CPCMs 5-nTiO2/EVM and 44H-nTiO2/EVM are 106.6 J/g and 150.8 J/g, respectively. Moreover, at a high temperature of 180 °C, the remaining masses of the two are 96.2% and 99.9%, respectively.
- (2)
- With the increase in the dosage of double PCM, the temperature regulation performance of the double phase change asphalt also improves accordingly. When the dosage of the double PCM is 10%, 20%, and 30%, it can reduce the high-temperature extreme value of asphalt by 1.5 °C, 3.4 °C, and 4.7 °C and increase the low-temperature extreme value by 0.8 °C, 2.1 °C, and 3.0 °C. When the dosage of the double PCM is 20%, compared with the original asphalt, the heating rate of the double PCM decreases by 8.5%, and the cooling rate decreases by 5.6%.
- (3)
- The high-temperature and medium-temperature performance of the double PCM asphalt is somewhat lower than that of the original asphalt, but the influence on the medium-temperature fatigue resistance is not significant. In terms of low-temperature crack resistance, the performance improves when the dosage is 10%, but it decreases when the dosage is 30%. When the dosage is 20%, the performance of the double PCM asphalt is closest to that of the original asphalt. Furthermore, when the dosage of the double PCM increases from 10% to 20%, the growth in the temperature regulation effect of the double PCM is greater than that when the dosage of the double PCM increases from 20% to 30%. Therefore, in order to enhance the temperature regulation effect while reducing the negative impact of the double PCM on the high-temperature performance of asphalt, and to ensure that the medium-temperature fatigue resistance and low-temperature crack resistance of the double phase change asphalt do not differ significantly from those of the original asphalt, the experimental results show that 20% is a relatively reasonable dosage of the double PCM.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, M.; Liang, M.; Jiao, Y.; Zhao, W.; Duan, Y.; Liu, H. A review of phase change materials in asphalt binder and asphalt mixture. Constr. Build. Mater. 2020, 258, 119565. [Google Scholar] [CrossRef]
- Guo, M.; Zhang, R.; Du, X.; Liu, P. A state-of-the-art review on the functionality of ultra-thin overlays towards a future low carbon road maintenance. Engineering 2024, 32, 82–98. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, H.; You, Z.; Hossiney, N. Application of phase change material in asphalt mixture—A review. Constr. Build. Mater. 2020, 263, 120219. [Google Scholar] [CrossRef]
- Zhao, X.; Shen, A.; Ma, B. Temperature adaptability of asphalt pavement to high temperatures and significant temperature differences. Adv. Mater. Sci. Eng. 2018, 2018, 9436321. [Google Scholar] [CrossRef]
- Ding, T.; Wei, S.; Ren, X.; Guo, M.; Yu, D. Multiscale evaluation on temperature adjusting performance and road performance of asphalt mixture containing dual phase change materials. Constr. Build. Mater. 2024, 417, 135302. [Google Scholar] [CrossRef]
- Manning, B.J.; Bender, P.R.; Cote, S.A.; Lewis, R.A.; Sakulich, A.R.; Mallick, R.B. Assessing the feasibility of incorporating phase change material in hot mix asphalt. Sustain. Cities Soc. 2015, 19, 11–16. [Google Scholar] [CrossRef]
- Farah, S.; Farouk, F.; Pascal, H.B. Phase change materials (PCM) for cooling applications in buildings: A review. Energy Build. 2016, 129, 396–431. [Google Scholar] [CrossRef]
- Athukorallage, B.; Dissanayaka, T.; Senadheera, S.; James, D. Performance analysis of incorporating phase change materials in asphalt concrete pavements. Constr. Build. Mater. 2018, 164, 419–432. [Google Scholar] [CrossRef]
- Kakar, M.R.; Refaa, Z.; Worlitschek, J. Use of microencapsulated phase change materials in bitumen to mitigate the thermal distresses in asphalt pavements. In RILEM 252-CMB-Symposium on Chemo Mechanical Characterization of Bituminous Materials; Springer International Publishing: Cham, Switzerland, 2018; pp. 129–135. [Google Scholar]
- Zhang, D.; Chen, M.; Wu, S.; Liu, Q.; Wan, J. Preparation of expanded graphite/polyethylene glycol composite phase change material for thermoregulation of asphalt binder. Constr. Build. Mater. 2018, 169, 513–521. [Google Scholar] [CrossRef]
- Amin, M.; Putra, N.; Kosasih, E.A.; Prawiro, E.; Luanto, R.A.; Mahlia, T. Thermal properties of beeswax/graphene phase change material as energy storage for building applications. Appl. Therm. Eng. 2017, 112, 273–280. [Google Scholar] [CrossRef]
- Prabhu, B.; Gurusamy, P.; Arunkumar, T. Solar photovoltaic cooling using Paraffin phase change material: Comprehensive assessment. Renew. Sustain. Energy Rev. 2024, 197, 114372. [Google Scholar] [CrossRef]
- Nazir, H.; Batool, M.; Osorio, F.J.B.; Isaza-Ruiz, M.; Xu, X.; Vignarooban, K.; Phelan, P.; Inamuddin; Kannan, A.M. Recent developments in phase change materials for energy storage applications: A review. Int. J. Heat Mass Transf. 2019, 129, 491–523. [Google Scholar] [CrossRef]
- Vasu, A.; Hagos, F.Y.; Mamat, R.; Kaur, J.; Noor, M. The effect of thermal cyclic variation on the thermophysical property degradation of paraffin as a phase changing energy storage material. Appl. Therm. Eng. 2019, 149, 22–33. [Google Scholar] [CrossRef]
- Du, Y.; Liu, P.; Wang, J.; Wang, H.; Hu, S.; Tian, J.; Li, Y. Laboratory investigation of phase change effect of polyethylene glycolon on asphalt binder and mixture performance. Constr. Build. Mater. 2019, 212, 1–9. [Google Scholar] [CrossRef]
- Chen, M.Z.; Hong, J.; Wu, S.P.; Lu, W.; Xu, G.J. Optimization of phase change materials used in asphalt pavement rutting. Adv. Mater. Res. 2011, 219–220, 1375–1378. [Google Scholar] [CrossRef]
- Sarı, A.; Sarı, H.; Önal, A. Thermal properties and thermal reliability of eutectic mixtures of some fatty acids as latent heat storage materials. Energy Convers. Manag. 2004, 45, 365–376. [Google Scholar] [CrossRef]
- Chen, M.; Zheng, S.; Wu, S.; Xu, G. Melting intercalation method to prepare lauric acid/organophilic montmorillonite shape-stabilized phase change material. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2010, 25, 674–677. [Google Scholar] [CrossRef]
- Chen, J.; Li, J.; Wang, H.; Huang, W.; Sun, W.; Xu, T. Preparation and effectiveness of composite phase change material for performance improvement of Open Graded Friction Course. J. Clean. Prod. 2019, 214, 259–269. [Google Scholar] [CrossRef]
- Wang, X.; Ma, B.; Yu, M.; Mao, W.; Si, W. Testing and modeling of incomplete phase change heat storage and release of epoxy resin/microcapsule composite phase change materials for asphalt pavement. J. Energy Storage 2025, 105, 114672. [Google Scholar] [CrossRef]
- Tian, B.; Yang, W.; Luo, L.; Wang, J.; Zhang, K.; Fan, J.; Wu, J.; Xing, T. Synergistic enhancement of thermal conductivity for expanded graphite and carbon fiber in paraffin/EVA form-stable phase change materials. Sol. Energy 2016, 127, 48–55. [Google Scholar] [CrossRef]
- Amini, N.; Hayati, P. Effects of CuO nanoparticles as phase change material on chemical, thermal and mechanical properties of asphalt binder and mixture. Constr. Build. Mater. 2020, 251, 118996. [Google Scholar] [CrossRef]
- Cheng, C.; Cheng, G.; Gong, F.; Fu, Y.; Qiao, J. Performance evaluation of asphalt mixture using polyethylene glycol polyacrylamide graft copolymer as solid–solid phase change materials. Constr. Build. Mater. 2021, 300, 124221. [Google Scholar] [CrossRef]
- Guo, M.; Zhang, S.; Zhang, R.; Du, X. Study on temperature regulation capability of asphalt mixture modified by dual phase change material used in ultra-thin overlay. Int. J. Pavement Eng. 2024, 25, 2387748. [Google Scholar] [CrossRef]
- ISO 22007-2:2022; Plastics—Determination of Thermal Conductivity and Thermal Diffusivity—Part 2: Transient Plane Heat Source (Hot Disc) Method. International Organization for Standardization: Geneva, Switzerland, 2022.
- Pyzalski, M.; Bialoskórski, J.; Walasek, E. Reaction between carbon fibres and molten silicon: Heat determination using DTA. J. Therm. Anal. 1986, 31, 1193–1196. [Google Scholar] [CrossRef]







| Material Type | Phase Transition Temperature Range (°C) | Enthalpy of Phase Change (J/g) |
|---|---|---|
| PW-5 | −8.4–1.0 | 153.0 |
| PW-44H | 38.8–46.4 | 249.1 |
| Material Type | Heating Up | Cool Down | ||||||
|---|---|---|---|---|---|---|---|---|
| Initial Temperature (°C) | Peak Temperature (°C) | Termination Temperature (°C) | Enthalpy Value (°C) | Initial Temperature (°C) | Peak Temperature (°C) | Termination Temperature (°C) | Enthalpy Value (J/g) | |
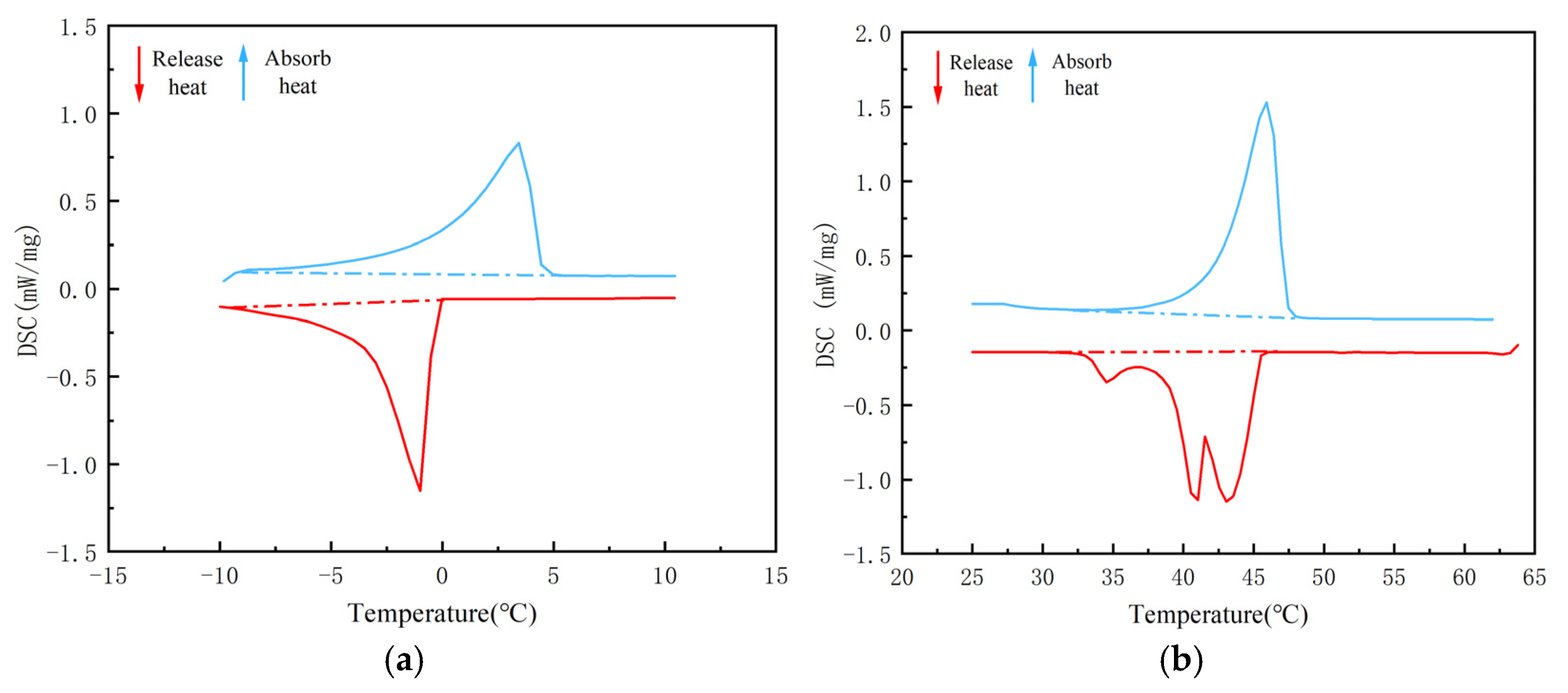
| 5/EVM | −9.8 | 3.4 | 5.0 | 96.9 | 0 | −1.0 | −9.5 | 104.1 |
| 44H/EVM | 37.1 | 47.9 | 50.5 | 156.4 | 44.0 | 41.0 | 30.5 | 160.1 |
| Material Type | Heating Up | Cool Down | ||||||
|---|---|---|---|---|---|---|---|---|
| Initial Temperature (°C) | Peak Temperature (°C) | Termination Temperature (°C) | Enthalpy Value (J/g) | Initial Temperature (°C) | Peak Temperature (°C) | Termination Temperature (°C) | Enthalpy Value (J/g) | |
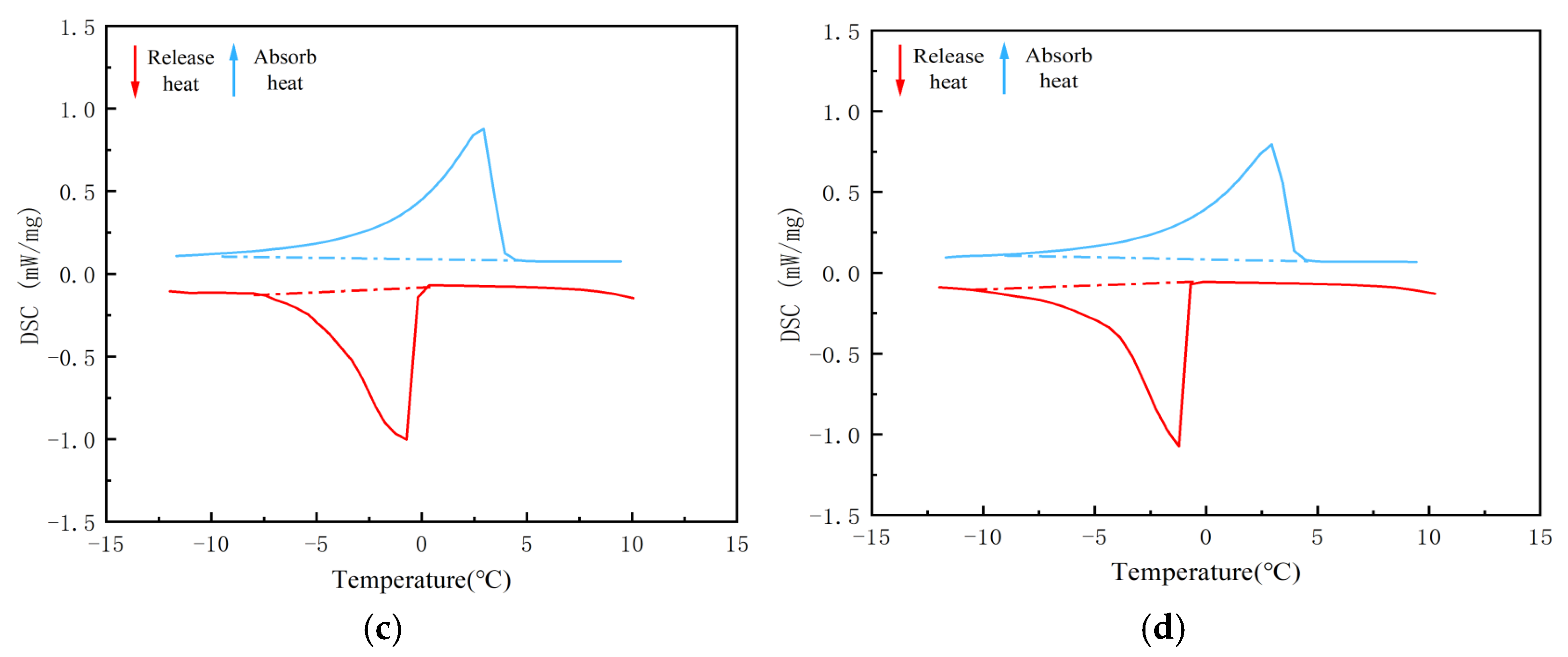
| 5/EVM-1 | −8.7 | 2.9 | 4.4 | 100.7 | 0 | −1.5 | −9.4 | 103.7 |
| 5/EVM-2 | −8.1 | 2.9 | 3.9 | 101.5 | 0.4 | −0.7 | −7.9 | 106.6 |
| 5/EVM-3 | −9.1 | 3.0 | 4.1 | 100.0 | 0.5 | −0.7 | −7.9 | 103.9 |
| 5/EVM-4 | −8.6 | 2.9 | 4.4 | 88.5 | 0 | −1.2 | −9.9 | 92.5 |
| 44H/EVM-1 | 36.1 | 44.9 | 46.9 | 149.5 | 43.0 | 39.5 | 34.0 | 155.6 |
| 44H/EVM-2 | 37.7 | 44.4 | 45.9 | 150.8 | 42.5 | 40.5 | 35.0 | 155.8 |
| 44H/EVM-3 | 35.5 | 44.4 | 46.5 | 151.3 | 43.0 | 39.5 | 33.5 | 155.3 |
| 44H/EVM-4 | 34.4 | 44.9 | 47.4 | 138.1 | 42.5 | 39.5 | 33.0 | 142.2 |
| Phase Change Material | After 20 Cycles | After 50 Cycles | ||
|---|---|---|---|---|
| Residual Mass (%) | Enthalpy of Phase Change (J/g) | Residual Mass (%) | Enthalpy of Phase Change (J/g) | |
| 44H-nTiO2/EVM | 94 | 140.3 | 93 | 139.2 |
| 5-nTiO2/EVM | 95 | 100.4 | 94 | 98.9 |
| Dosage of Double Phase Change Materials | Cool Down | Heating Up | ||||||
|---|---|---|---|---|---|---|---|---|
| Initial Temperature (°C) | Peak Temperature (°C) | Termination Temperature (°C) | Enthalpy Value (J/g) | Initial Temperature (°C) | Peak Temperature (°C) | Termination Temperature (°C) | Enthalpy Value (J/g) | |
| 0 | - | - | - | - | - | - | - | - |
| 10% | 0 | −2.5 | −4.0 | 5.4 | 38.5 | 42.5 | 44.5 | 8.4 |
| 20% | 0.5 | −1.0 | −3.5 | 8.2 | 39.4 | 43.5 | 44.9 | 18.8 |
| 30% | 0.3 | −0.6 | −4.0 | 13.4 | 40.5 | 43.5 | 45.0 | 28.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Cheng, X.; Wang, S.; Wei, S.; Guo, M.; Song, S.; Zhang, F. Study on the Thermal and Rheological Properties of Nano-TiO2-Modified Double Phase Change Asphalt. Materials 2025, 18, 4799. https://doi.org/10.3390/ma18204799
Liu X, Cheng X, Wang S, Wei S, Guo M, Song S, Zhang F. Study on the Thermal and Rheological Properties of Nano-TiO2-Modified Double Phase Change Asphalt. Materials. 2025; 18(20):4799. https://doi.org/10.3390/ma18204799
Chicago/Turabian StyleLiu, Xingming, Xiaojun Cheng, Shanshan Wang, Sishuang Wei, Meng Guo, Shanglin Song, and Fukui Zhang. 2025. "Study on the Thermal and Rheological Properties of Nano-TiO2-Modified Double Phase Change Asphalt" Materials 18, no. 20: 4799. https://doi.org/10.3390/ma18204799
APA StyleLiu, X., Cheng, X., Wang, S., Wei, S., Guo, M., Song, S., & Zhang, F. (2025). Study on the Thermal and Rheological Properties of Nano-TiO2-Modified Double Phase Change Asphalt. Materials, 18(20), 4799. https://doi.org/10.3390/ma18204799







