Effect of Ba:Ti Molar Ratio and Sintering Temperature on the Structural and Electrical Properties of BaTiO3-Type Solid Solutions, Synthesized by the Hydrothermal Method
Abstract
1. Introduction
2. Materials and Methods
3. Results
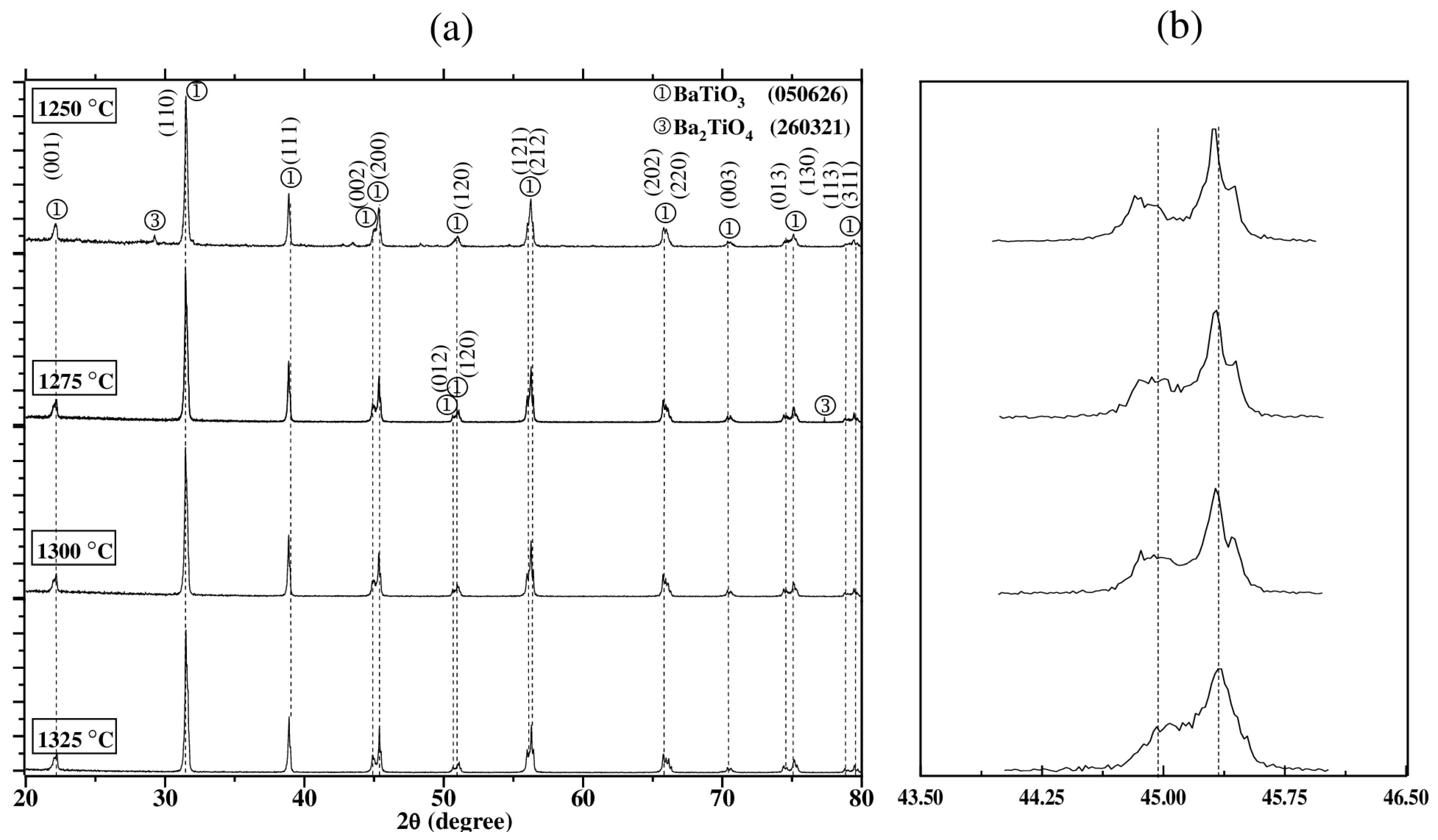
3.1. X-Ray Diffraction
3.1.1. Effect of the Ba:Ti Molar Ratio on the Synthesis of BT
3.1.2. Effect of Sintering Temperature
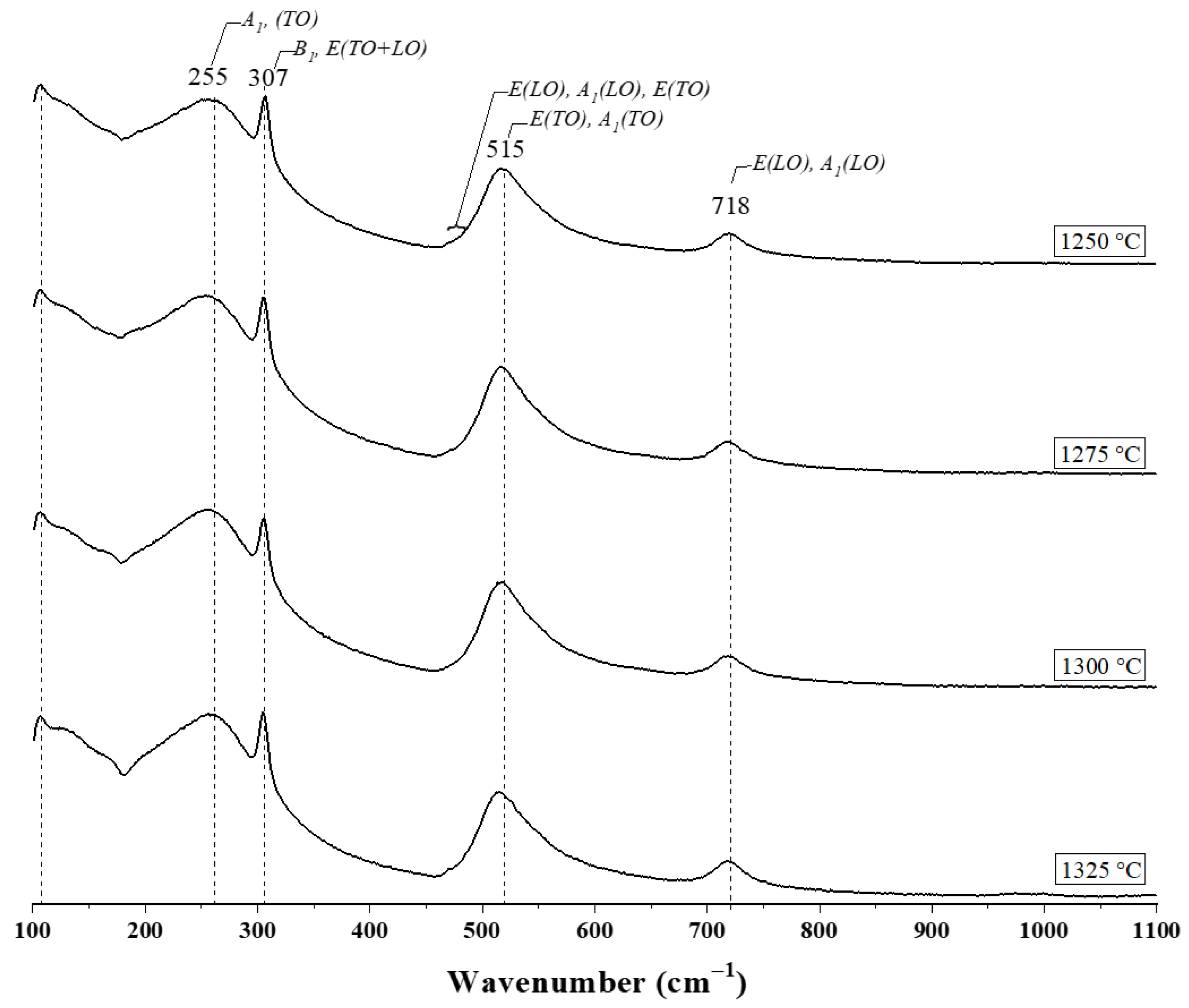
3.2. Raman Spectroscopy
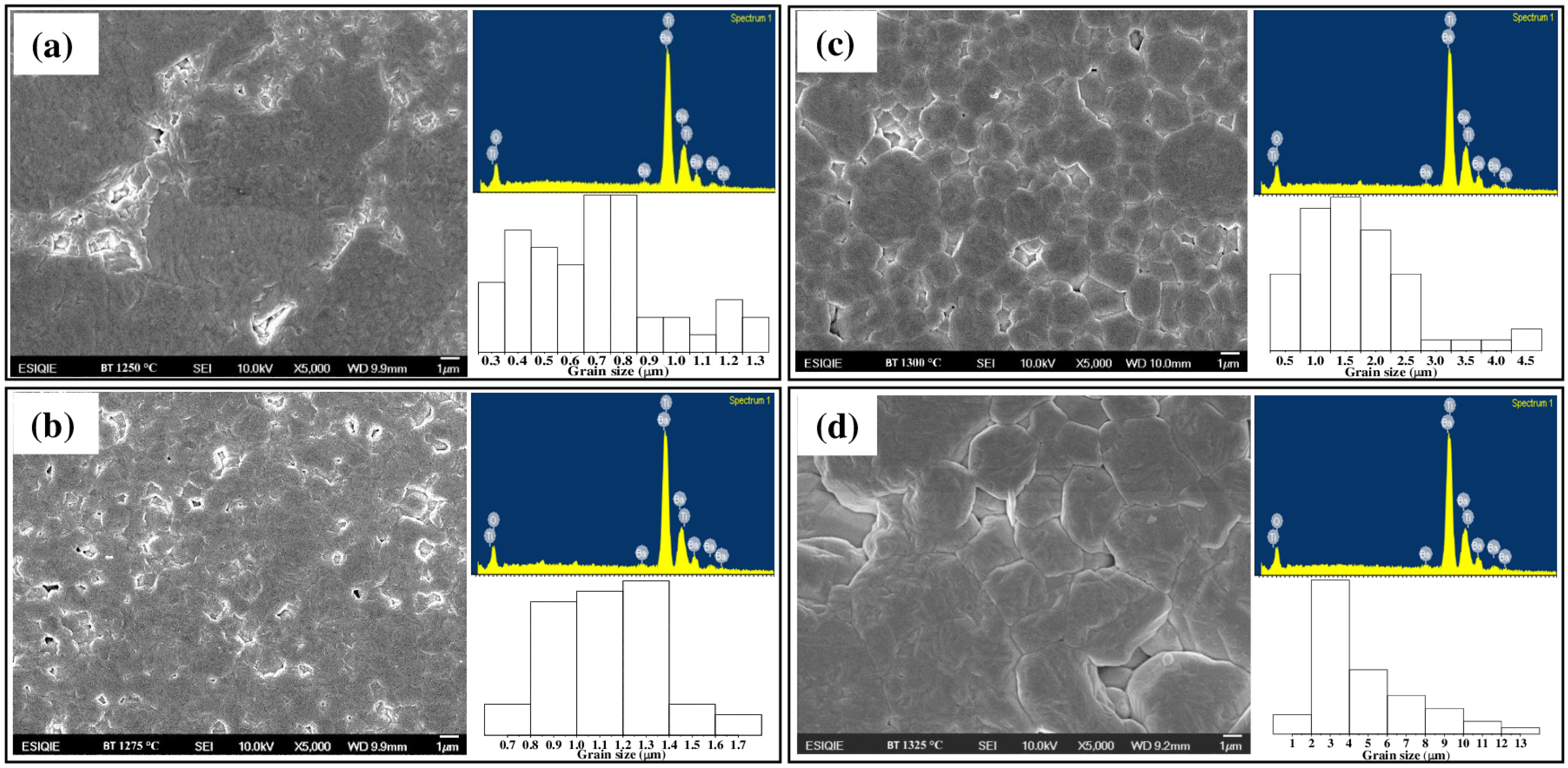
3.3. High-Resolution Scanning Electron Microscopy (HRSEM)
Density and Apparent Porosity
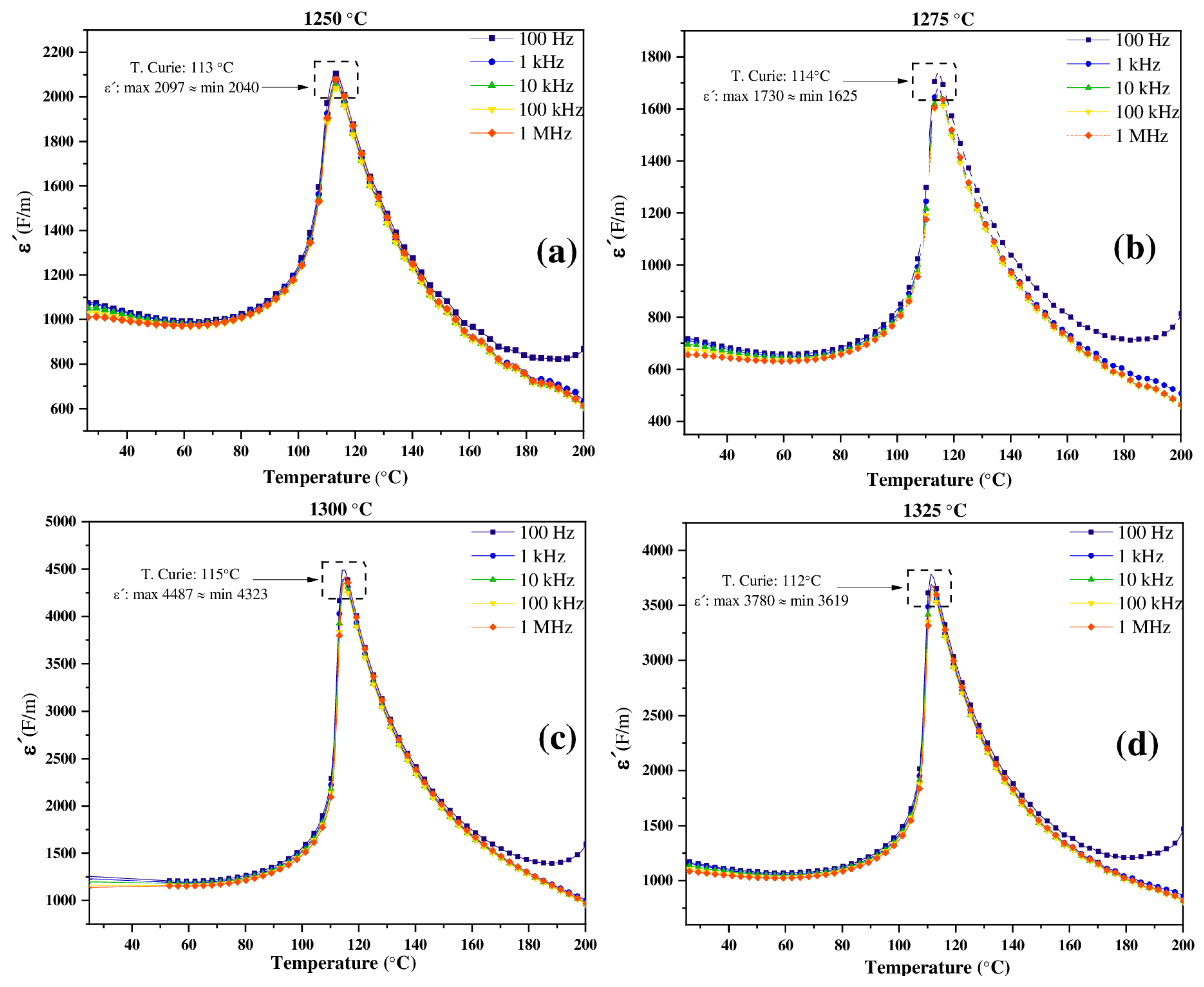
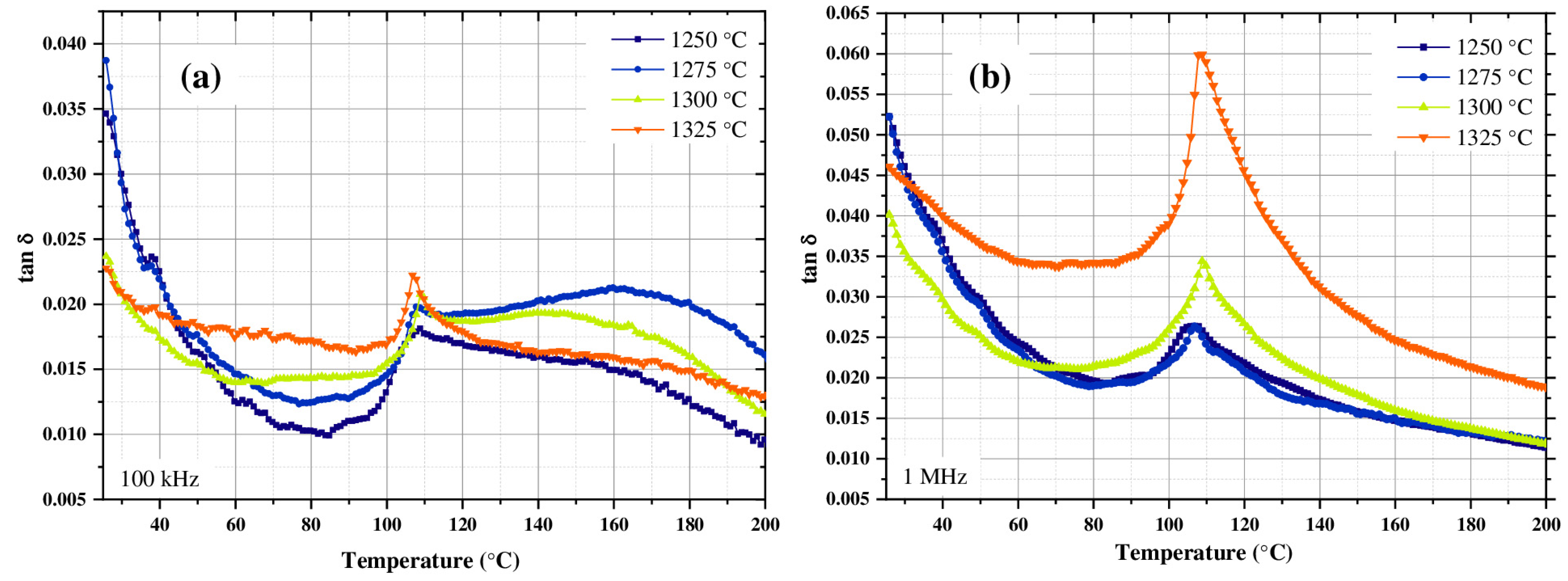
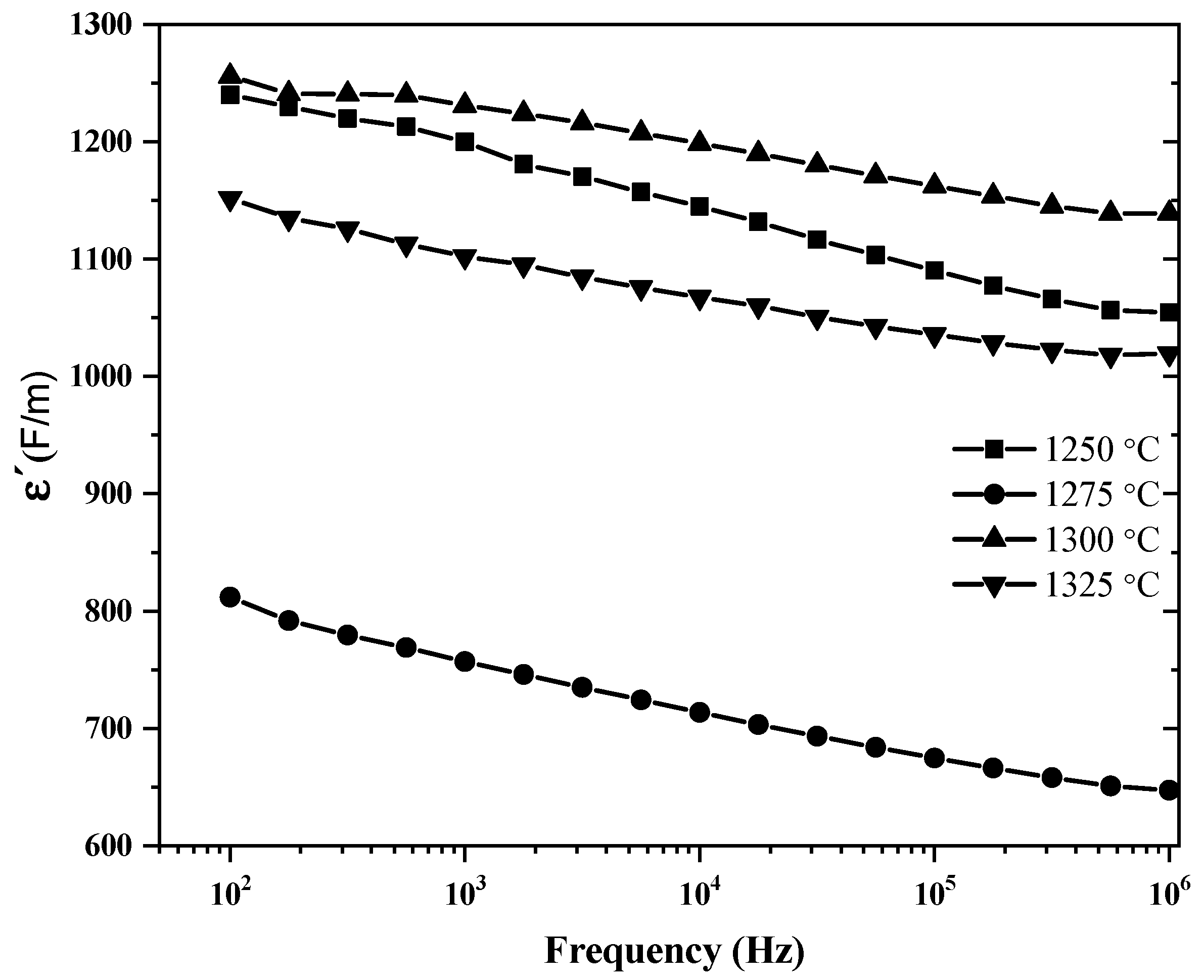
3.4. Dielectric Properties
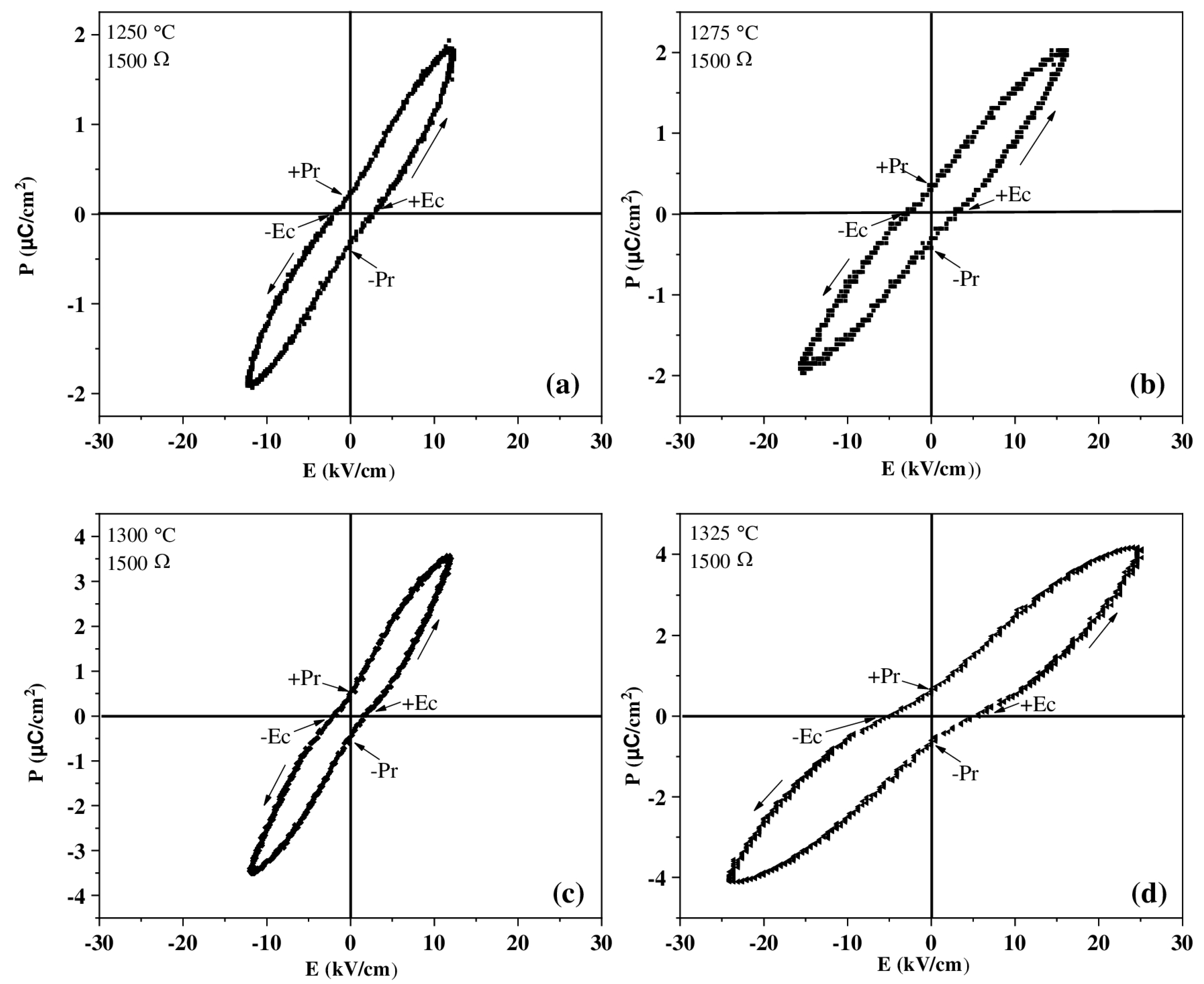
3.5. Ferroelectric Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yin, W.-J.; Yang, J.-H.; Kang, J.; Yan, Y.; Wei, S.-H. Halide perovskite materials for solar cells: A theoretical review. J. Mater. Chem. A 2015, 3, 8926–8942. [Google Scholar] [CrossRef]
- Mitchell, R.H.; Welch, M.D.; Chakhmouradian, A.R. Nomenclature of the perovskite supergroup: A hierarchical system of classification based on crystal structure and composition. Miner. Mag. 2017, 81, 411–461. [Google Scholar] [CrossRef]
- Santomauro, F.G.; Grilj, J.; Mewes, L.; Nedelcu, G.; Yakunin, S.; Rossi, T.; Capano, G.; Al Haddad, A.; Budarz, J.; Kinschel, D.; et al. Localized holes and delocalized electrons in photoexcited inorganic perovskites: Watching each atomic actor by picosecond X-ray absorption spectroscopy. Struct. Dyn. 2017, 4, 044002. [Google Scholar] [CrossRef] [PubMed]
- Husainat, A.; Ali, W.; Cofie, P.; Attia, J.; Fuller, J. Simulation and analysis of methylammonium lead iodide (CH3NH3PbI3) perovskite solar cell with Au contact using SCAPS 1D simulator. Am. J. Opt. Photonics 2019, 7, 33. [Google Scholar] [CrossRef]
- Oliveira, M.C.; Ribeiro, R.A.P.; Longo, E.; Bomio, M.R.D.; Motta, F.V.; de Lazaro, S.R. Temperature dependence on phase evolution in the BaTiO3 polytypes studied using ab initio calculations. Int. J. Quantum Chem. 2020, 120, e26054. [Google Scholar] [CrossRef]
- Zhou, D.; Zhou, T.; Tian, Y.; Zhu, X.; Tu, Y. Perovskite-based solar cells: Materials, methods, and future perspectives. J. Nanomater. 2018, 2018, 8148072. [Google Scholar] [CrossRef]
- Bae, H.; Lee, K.T. Effect of tetragonal to cubic phase transition on the upconversion luminescence properties of A/B site erbium-doped perovskite BaTiO3. RSC Adv. 2019, 9, 2451–2457. [Google Scholar] [CrossRef]
- Slimani, Y.; Erdemi, H.; Baykal, A.; Almessiere, M.A.; Thakur, A.; Batoo, K.M. Synergistic impact of metal/semiconductor (Co@ Co3O4) core-shell nanostructures on the structure, electrical, and dielectric properties of BaTiO3-based composites. J. Phys. Chem. Solids 2024, 195, 112292. [Google Scholar] [CrossRef]
- Lee, J.W.; Eom, K.; Paudel, T.R.; Wang, B.; Lu, H.; Huyan, H.X.; Lindemann, S.; Ryu, S.; Lee, H.; Kim, T.H.; et al. In-plane quasi-single-domain BaTiO3 via interfacial symmetry engineering. Nat. Commun. 2021, 12, 6784. [Google Scholar] [CrossRef]
- Yu, P.; Liu, W.; Gao, P.; Shao, T.; Zhao, S.; Han, Z.; Gu, X.; Zhang, J.; Wang, Y. Investigation on synthesis of tetragonal BaTiO3 nanopowders by a new wet chemical method. J. Mater. Sci. Mater. Electron. 2022, 33, 10828–10840. [Google Scholar] [CrossRef]
- Varela, C.F.; Rivera, A.M.; Gutiérrez, S.S.; Agámez-Pertuz, Y.; Moreno-Aldana, L. Effect of the progressive Fe-substitution in the A-site of the BaTiO3: Wet chemical synthesis, systematic characterization and photocatalytic performance. Ceram. Int. 2025, 51, 26566–26580. [Google Scholar] [CrossRef]
- Jin, S.H.; Lee, H.W.; Kim, N.W.; Lee, B.-W.; Lee, G.-G.; Hong, Y.-W.; Nam, W.H.; Lim, Y.S. Sonochemically activated solid-state synthesis of BaTiO3 powders. J. Eur. Ceram. Soc. 2021, 41, 4826–4834. [Google Scholar] [CrossRef]
- Tihtih, M.; Ibrahim, J.E.F.; Basyooni, M.A.; Kurovics, E.; Belaid, W.; Hussainova, I.; Kocserha, I. Role of A-site (Sr), B-site (Y), and A, B sites (Sr, Y) substitution in lead-free BaTiO3 ceramic compounds: Structural, optical, microstructure, mechanical, and thermal conductivity properties. Ceram. Int. 2023, 49, 1947–1959. [Google Scholar] [CrossRef]
- Palmas-León, J.A.; Barrientos-Hernández, F.R.; Pérez-Labra, M.; Patiño-Cardona, F.; Ramajo, L.; Romero-Serrano, J.A.; Cobos-Murcia, J.A.; Reyes-Pérez, M.; Juárez-Tapia, J.C. Synthesis and Microstructural Characterization by the Pechini Method of BaTiO3 Co-Doped with Nb5+ and La3+. In Advances in Ceramic Materials and Processing, Proceedings of the TMS 2025, Las Vegas, NV, USA, 23–27 March 2025; TMS Annual Meeting & Exhibition; Springer Nature: Cham, Switzerland, 2025; pp. 179–188. [Google Scholar] [CrossRef]
- Khan, M.Q.; Alharthi, F.A.; Ahmad, K.; Kim, H. Hydrothermal synthesis of BaTiO3 perovskite for H2O2 sensing. Chem. Phys. Lett. 2022, 805, 139950. [Google Scholar] [CrossRef]
- Kumar, D.; Kumar, S.; Kumar, S.; Thakur, N.; Shandilya, M. A very low temperature growth of BaTiO3 nanoparticles by sol-hydrothermal method. Phys. Status Solidi A 2022, 219, 2200238. [Google Scholar] [CrossRef]
- On, D.V.; Vuong, L.D.; Van Chuong, T.; Quang, D.A.; Van Tuyen, H.; Tung, V.T. Influence of sintering behavior on the microstructure and electrical properties of BaTiO3 lead-free ceramics from hydrothermal synthesized precursor nanoparticles. J. Adv. Dielectr. 2021, 11, 2150014. [Google Scholar] [CrossRef]
- Pang, X.; Wang, T.; Liu, B.; Fan, X.; Liu, X.; Shen, J.; Zhong, C.; Hu, W. Effect of solvents on the morphology and structure of barium titanate synthesized by a one-step hydrothermal method. Int. J. Miner. Metall. Mater. 2023, 30, 1407–1416. [Google Scholar] [CrossRef]
- Zhang, M.; Falvey, J.; Hector, A.L.; Garcia-Araez, N. Effects of the reaction temperature and Ba/Ti precursor ratio on the crystallite size of BaTiO3 in hydrothermal synthesis. RSC Adv. 2022, 12, 27809–27819. [Google Scholar] [CrossRef]
- Bakken, K.; Pedersen, V.H.; Blichfeld, A.B.; Nylund, I.-E.; Tominaka, S.; Ohara, K.; Grande, T.; Einarsrud, M.-A. Structures and role of the intermediate phases on the crystallization of BaTiO3 from an aqueous synthesis route. ACS Omega 2021, 6, 9567–9576. [Google Scholar] [CrossRef]
- Wang, T.; Pang, X.; Liu, B.; Liu, J.; Shen, J.; Zhong, C. A facile and eco-friendly hydrothermal synthesis of high tetragonal barium titanate with uniform and controllable particle size. Materials 2023, 16, 4191. [Google Scholar] [CrossRef]
- Fatimah, S.; Ragadhita, R.; Al Husaeni, D.F.; Nandiyanto, A.B.D. How to calculate crystallite size from x-ray diffraction (XRD) using Scherrer method. ASEAN J. Sci. Eng. 2022, 2, 65–76. [Google Scholar] [CrossRef]
- Wu, H.; Sun, H.; Han, F.; Xie, P.; Zhong, Y.; Quan, B.; Zhao, Y.; Liu, C.; Fan, R.; Guo, Z. Negative permittivity behavior in flexible carbon nanofibers-polydimethylsiloxane films. Eng. Sci. 2021, 17, 113–120. [Google Scholar] [CrossRef]
- Hartig, S.M. Basic image analysis and manipulation in ImageJ. Curr. Protoc. Mol. Biol. 2013, 102, 14.15.1–14.15.12. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-C.; Ahn, S.-H. Bulk density measurement of porous functionally graded materials. Int. J. Precis. Eng. Manuf. 2018, 19, 31–37. [Google Scholar] [CrossRef]
- Westphal, E.; Seitz, H. Porosity and density measurement of additively manufactured components: A comparative analysis of measurement methods across processes and materials. Mater. Sci. Addit. Manuf. 2025, 4, 025090010. [Google Scholar] [CrossRef]
- Choi, S.H.; Lee, Y.-S.; Kwak, H.; Jung, H.J.; Kim, M.; Cho, S.; Yoon, J.H.; Choi, J.W.; Kim, M.S.; Kim, J.H.; et al. Major factors affecting the dielectric properties and reliability of solid stated reacted BaTiO3 powders for capacitor. J. Asian Ceram. Soc. 2022, 10, 713–721. [Google Scholar] [CrossRef]
- Chen, B.; Liao, F.-H.; Jiao, H.; Jing, X.-P. Thermal stability and phase transformation of barium orthotitanate (Ba2TiO4). Phase Transit. 2013, 86, 380–390. [Google Scholar] [CrossRef]
- Qi, J.Q.; Sun, L.; Wang, Y.; Chen, W.P.; Du, P.; Xu, Y.G.; Li, L.T.; Nan, C.W.; Chan, H.L.W. Excess titanium in barium titanate nanoparticles directly synthesized from solution. J. Phys. Chem. Solids 2010, 71, 1676–1679. [Google Scholar] [CrossRef]
- Pasuk, I.; Neațu, F.; Neațu, Ș.; Florea, M.; Istrate, C.M.; Pintilie, I.; Pintilie, L. Structural details of batio3 nano-powders deduced from the anisotropic xrd peak broadening. Nanomaterials 2021, 11, 1121. [Google Scholar] [CrossRef]
- Hao, T.; Shen, J.; Peng, Q.; Liu, J.; Hu, W.; Zhong, C. Solid-State Synthesis for High-Tetragonality, Small-Particle Barium Titanate. Materials 2024, 17, 5655. [Google Scholar] [CrossRef]
- Liu, Y.; Fu, Y.; Gu, F.; Yu, H.; Zhuang, L.; Chu, Y. Lattice-Distortion-Driven Reduced Lattice Thermal Conductivity in High-Entropy Ceramics. Adv. Sci. 2025, 12, 2501157. [Google Scholar] [CrossRef]
- Lu, D.; Sheptyakov, D.; Cao, Y.; Zhao, H.; Zhang, J.; Pi, M.; Ye, X.; Liu, Z.; Zhang, X.; Pan, Z.; et al. Magnetic-Field Controllable Displacement-Type Ferroelectricity Driven by Off-Center Fe2+ Ions in CaFe3Ti4O12 Perovskite. Adv. Funct. Mater. 2024, 34, 2411133. [Google Scholar] [CrossRef]
- Brzozowski, E.; Castro, M.; Foschini, C.; Stojanovic, B. Secondary phases in Nb-doped BaTiO3 ceramics. Ceram. Int. 2002, 28, 773–777. [Google Scholar] [CrossRef]
- Kløve, M.; Philippot, G.; Auxéméry, A.; Aymonier, C.; Iversen, B.B. Stabilizing tetragonal ZrO2 nanocrystallites in solvothermal synthesis. Nanoscale 2024, 16, 3185–3190. [Google Scholar] [CrossRef] [PubMed]
- Grendal, O.G.; Blichfeld, A.B.; Skjærvø, S.L.; Van Beek, W.; Selbach, S.M.; Grande, T.; Einarsrud, M.-A. Facile Low Temperature Hydrothermal Synthesis of BaTiO3 Nanoparticles Studied by In Situ X-ray Diffraction. Crystals 2018, 8, 253. [Google Scholar] [CrossRef]
- Mahmood, P.H.; Amiri, O.; Sajadi, S.M. BaTiO3 Nanoparticles for highly efficient piezocatalytic reduction of toxic hexavalent chromium: Synthesis, optimization, and kinetic study. J. Hazard. Mater. Adv. 2024, 16, 100468. [Google Scholar] [CrossRef]
- Vinita, V.S.; Rao, R.G.S.; Samuel, J.; Shabna, S.; Ananth, N.J.; Shinu, P.M.S.; Suresh, S.; Samson, Y.; Biju, C.S. Structural, Raman and optical investigations of barium titanate nanoparticles. Phosphorus Sulfur Silicon Relat. Elem. 2022, 197, 169–175. [Google Scholar] [CrossRef]
- Sá, F.G.; Silva, M.R.; Sierra, D.L.; Ivanov, M.; Tkach, A.; Vilarinho, P.M.; Ferreira, P. Comparative microwave-and conventional oven-assisted hydrothermal syntheses of BaTiO3 nanoparticles for improved electroceramics. Ceram. Int. 2024, 50, 9096–9104. [Google Scholar] [CrossRef]
- Asiaie, R.; Zhu, W.D.; Akbar, S.A.; Dutta, P.K. Characterization of submicron particles of tetragonal BaTiO3. Chem. Mater. 1996, 8, 226–234. [Google Scholar] [CrossRef]
- Lara, J.P.H.; Labra, M.P.; Hernández, F.R.B.; Serrano, J.A.R.; Dávila, E.O.Á.; Thangarasu, P.; Ramirez, A.H. Structural evolution and electrical properties of BaTiO3 doped with Gd3+. Mater. Res. 2017, 20, 538–542. [Google Scholar] [CrossRef]
- Jha, P.A.; Jha, A.K. Effect of sintering temperature on the grain growth and electrical properties of barium zirconate titanate ferroelectric ceramics. J. Mater. Sci. Mater. Electron. 2013, 24, 1511–1518. [Google Scholar] [CrossRef]
- Yan, J.; Fang, B.; Zhang, S.; Lu, X.; Ding, J. (Sb0.5Li0.5) TiO3-Doping Effect and Sintering Condition Tailoring in BaTiO3-Based Ceramics. Materials 2024, 17, 2085. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Lee, Y.-S.; Kim, J.; Seo, J.-H.; Cho, M.; Kwak, H.; Cheon, R.-S.; Cho, S.; Kim, Y.; Moon, K.-S.; et al. Controlling composite TiO2 powder characteristics in the solid-state synthesis of BaTiO3 powders for improved sintering and permittivity: A comparative study. Appl. Sci. 2023, 13, 9720. [Google Scholar] [CrossRef]
- Yusoff, N.H.; Osman, R.A.M.; Idris, M.S.; Muhsen, K.N.D.K.; Nor, N.I.M. Dielectric and structural analysis of hexagonal and tetragonal phase BaTiO3. In Proceedings of the 2nd International Conference on Applied Photonics and Electronic 2019 (InCAPE 2019), Putrajaya, Malaysia, 22 August 2019; AIP Publishing LLC: Melville, NY, USA, 2020; p. 020038. [Google Scholar] [CrossRef]
- Jiang, J.; Ontaneda, J.; Pal, S.; Guo, Z.; Forrester, C.; Zheng, K.; Wang, M.; Briscoe, J.; Titirici, M.-M.; Au, H. Enhanced polysulfide trapping in Li–S batteries by dipole alignment in ferroelectric BaTiO3. Energy Environ. Sci. 2024, 17, 6291–6301. [Google Scholar] [CrossRef]
- Gigli, L.; Veit, M.; Kotiuga, M.; Pizzi, G.; Marzari, N.; Ceriotti, M. Thermodynamics and dielectric response of BaTiO3 by data-driven modeling. npj Comput. Mater. 2022, 8, 209. [Google Scholar] [CrossRef]
- Uddin, A.S.M.I.; Lee, D.; Cho, C.; Kim, B. Impact of multi-walled CNT incorporation on dielectric properties of PVDF-BaTiO3 nanocomposites and their energy harvesting possibilities. Coatings 2022, 12, 77. [Google Scholar] [CrossRef]
- Yoon, S.-H.; Kim, M.-Y.; Kim, D. Correlation between tetragonality (c/a) and direct current (dc) bias characteristics of BaTiO3-based multi-layer ceramic capacitors (MLCC). J. Mater. Chem. C 2020, 8, 9373–9381. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, Z. High Permittivity, Low Dielectric Loss and Impedance Characteristics of Li0.5La0.5Cu3Ti4O12 Ceramics by a Sol–Gel Technique. J. Electron. Mater. 2019, 48, 5333–5341. [Google Scholar] [CrossRef]
- Kumar, B.P.; Kumar, H.; Kharat, D. Effect of porosity on dielectric properties and microstructure of porous PZT ceramics. Mater. Sci. Eng. B 2006, 127, 130–133. [Google Scholar] [CrossRef]
- Liang, P.; Chao, X.; Wang, F.; Liu, Z.; Yang, Z.; Alford, N. The Lowered Dielectric Loss and Grain-Boundary Effects in La-doped Y 2/3 Cu 3 Ti 4 O 12 Ceramics. J. Am. Ceram. Soc. 2013, 96, 3883–3890. [Google Scholar] [CrossRef]
- Shankar, J.; Kumar, A.S.; Kumar, R.S. Effect of sintering temperature on microstructure, dielectric and ferroelectric properties of BaTiO3 ceramics. Ferroelectrics 2023, 606, 207–218. [Google Scholar] [CrossRef]
- Cai, Z.; Feng, P.; Zhu, C.; Wang, X. Dielectric breakdown behavior of ferroelectric ceramics: The role of pores. J. Eur. Ceram. Soc. 2021, 41, 2533–2538. [Google Scholar] [CrossRef]
- Arya, B.B.; Choudhary, R.N.P. Studies of structural and electrical characteristics of multi-substituted Bi0.5Na0.5TiO3 ferroelectric ceramics. J. Mater. Sci. Mater. Electron. 2021, 32, 11547–11567. [Google Scholar] [CrossRef]
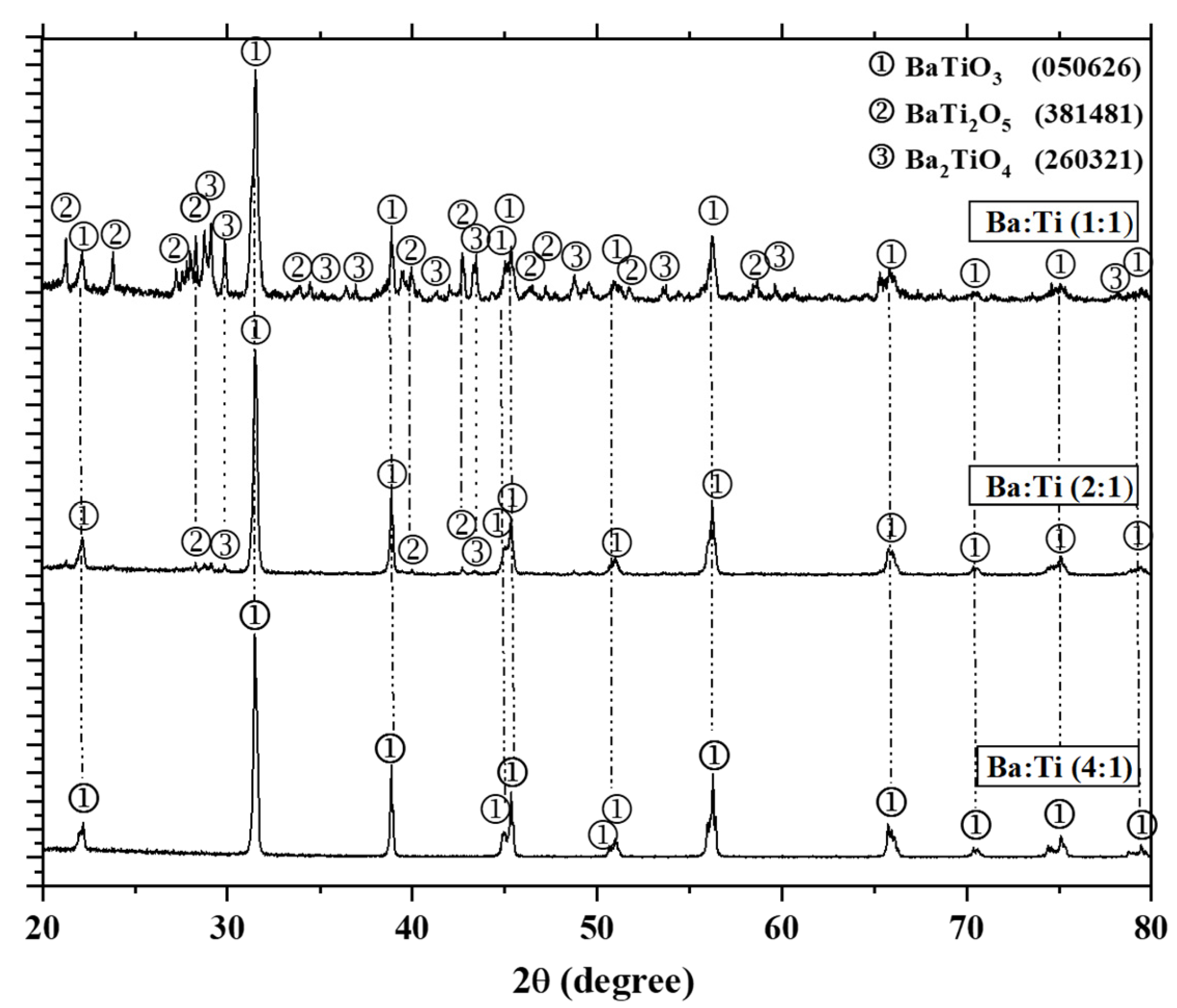


| Ba:Ti Molar Ratio | Barium Hydroxide (g) | Titanium Butoxide (g) | Sodium Hydroxide (g) | Triton X-100 (g) |
|---|---|---|---|---|
| 1:1 | 2.85 | 5.66 | 2.0 | 0.379 |
| 2:1 | 5.70 | 5.66 | 2.0 | 0.379 |
| 4:1 | 9.36 | 5.66 | 2.0 | 0.379 |
| Sintering Temperature (°C) | Phase | Lattice Parameters (A°) | c/a | Crystal Size (Å) | |
|---|---|---|---|---|---|
| a | C | ≈45° | |||
| 1250 | BaTiO3 (JCPDS 050626) | 3.995 | 4.023 | 1.0070 | 1025.27 |
| 1275 | 3.994 | 4.038 | 1.0110 | 1491.82 | |
| 1300 | 3.993 | 4.038 | 1.0113 | 3540.87 | |
| 1325 | 3.993 | 4.036 | 1.0108 | 5556.29 | |
| Peak (cm−1) | Phonon | Reference |
|---|---|---|
| 255 | A1(TO) | [30] |
| 307 | (B1, E(TO + LO)) | [12] |
| 515 | (E(TO), A1(TO)) | [12] |
| 718 | (E(LO), A1(LO)) | [31] |
| Sintering Temperature (°C) | Pr Estimate (µC/cm2) | Observations |
|---|---|---|
| 1250 | 0.8 | Tight curves, low material polarization |
| 1275 | 1.2 | Improvement in ferroelectric response |
| 1300 | 2.5 | Greater area on the curve and increased polarization |
| 1325 | 3.5 | Higher ferroelectric performance |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palmas Léon, J.A.; Ramajo, L.; Parra, R.; Pérez Labra, M.; Barrientos Hernández, F.R.; Cruz Ramírez, A.; Acosta Sanchez, V.; Teja Ruiz, A.M.; Ordoñez Hernández, S. Effect of Ba:Ti Molar Ratio and Sintering Temperature on the Structural and Electrical Properties of BaTiO3-Type Solid Solutions, Synthesized by the Hydrothermal Method. Materials 2025, 18, 4797. https://doi.org/10.3390/ma18204797
Palmas Léon JA, Ramajo L, Parra R, Pérez Labra M, Barrientos Hernández FR, Cruz Ramírez A, Acosta Sanchez V, Teja Ruiz AM, Ordoñez Hernández S. Effect of Ba:Ti Molar Ratio and Sintering Temperature on the Structural and Electrical Properties of BaTiO3-Type Solid Solutions, Synthesized by the Hydrothermal Method. Materials. 2025; 18(20):4797. https://doi.org/10.3390/ma18204797
Chicago/Turabian StylePalmas Léon, José Agustin, Leandro Ramajo, Rodrigo Parra, Miguel Pérez Labra, Francisco Raúl Barrientos Hernández, Alejandro Cruz Ramírez, Vanessa Acosta Sanchez, Aislinn Michelle Teja Ruiz, and Sayra Ordoñez Hernández. 2025. "Effect of Ba:Ti Molar Ratio and Sintering Temperature on the Structural and Electrical Properties of BaTiO3-Type Solid Solutions, Synthesized by the Hydrothermal Method" Materials 18, no. 20: 4797. https://doi.org/10.3390/ma18204797
APA StylePalmas Léon, J. A., Ramajo, L., Parra, R., Pérez Labra, M., Barrientos Hernández, F. R., Cruz Ramírez, A., Acosta Sanchez, V., Teja Ruiz, A. M., & Ordoñez Hernández, S. (2025). Effect of Ba:Ti Molar Ratio and Sintering Temperature on the Structural and Electrical Properties of BaTiO3-Type Solid Solutions, Synthesized by the Hydrothermal Method. Materials, 18(20), 4797. https://doi.org/10.3390/ma18204797








